The Contribution of Arbuscular Mycorrhizal Fungi to Soil Enzyme Activity and the Performance of Mimosa caesalpiniaefolia in Soil Degraded by Scheelite Mining: Implications for Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions, Experimental Design, and Plant Species
2.2. Experimental Process
2.3. Plant Growth Parameters
2.4. Dickson Quality Index
2.5. Soil AMF Spores and Mycorrhizal Colonization
2.6. Soil Enzymatic Activity and Its Stoichiometry
2.7. Statistical Analysis
3. Results
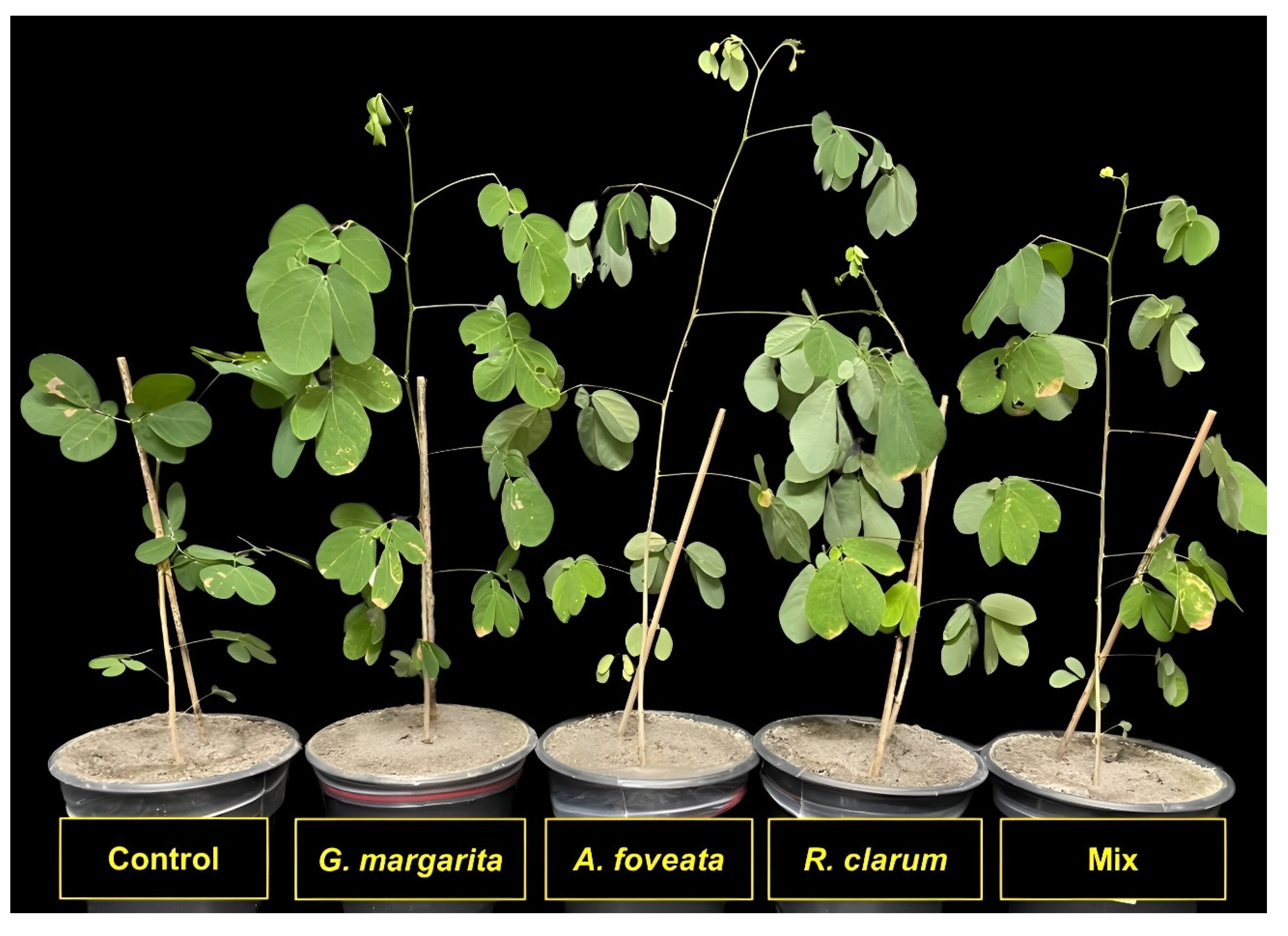
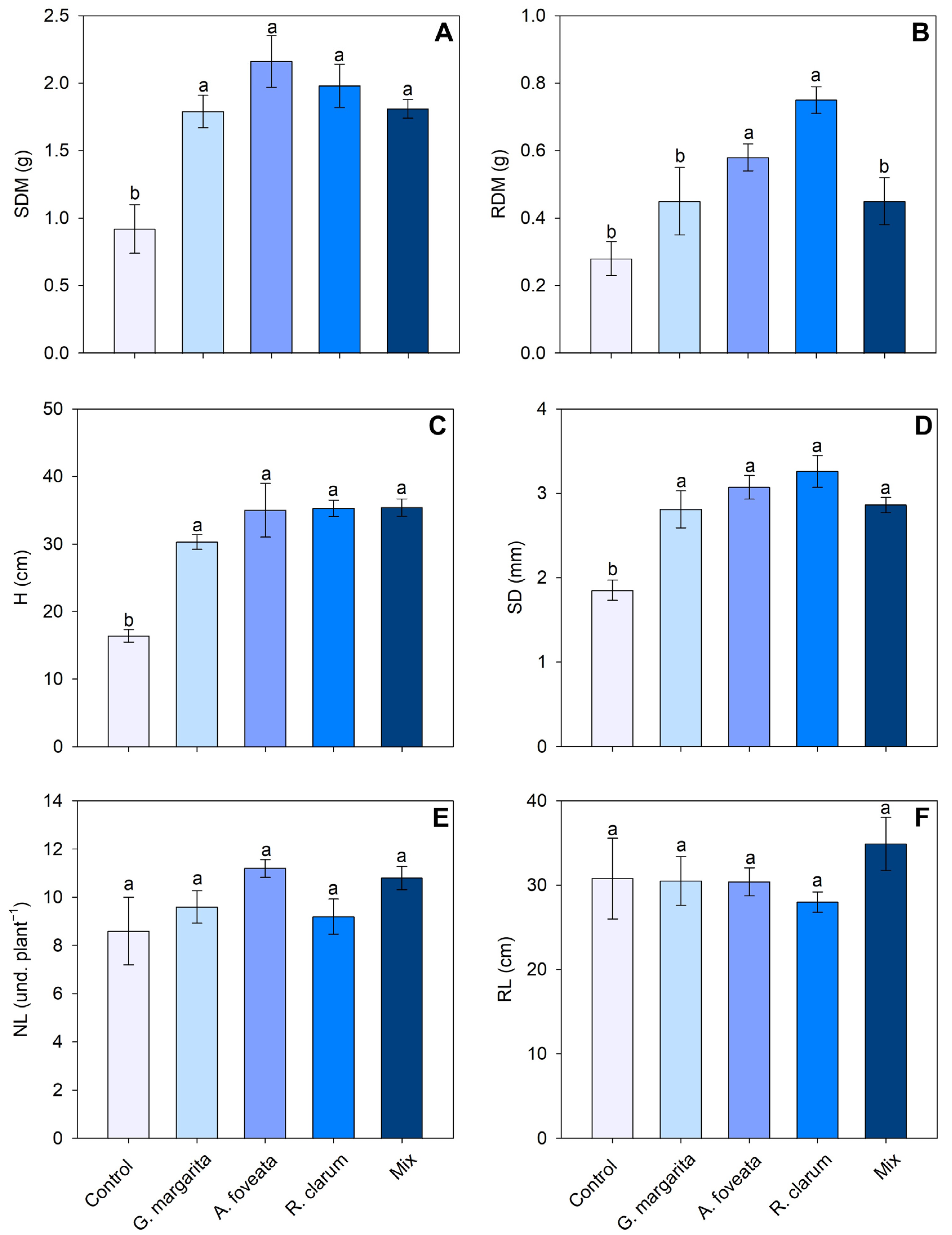
3.1. Plant Growth Parameters
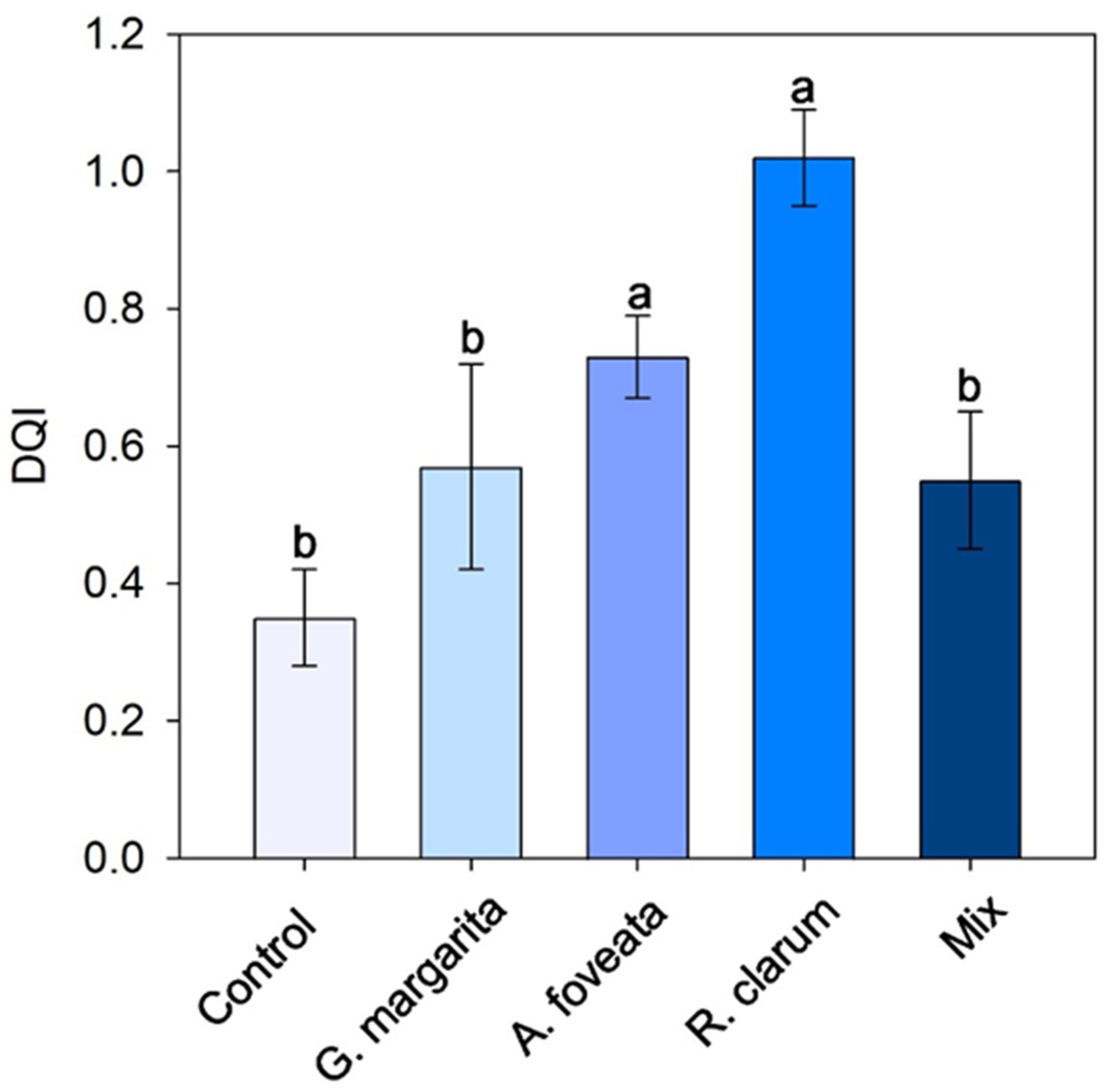
3.2. Dickson Quality Index
3.3. AMF Spore Number and Mycorrhizal Colonization
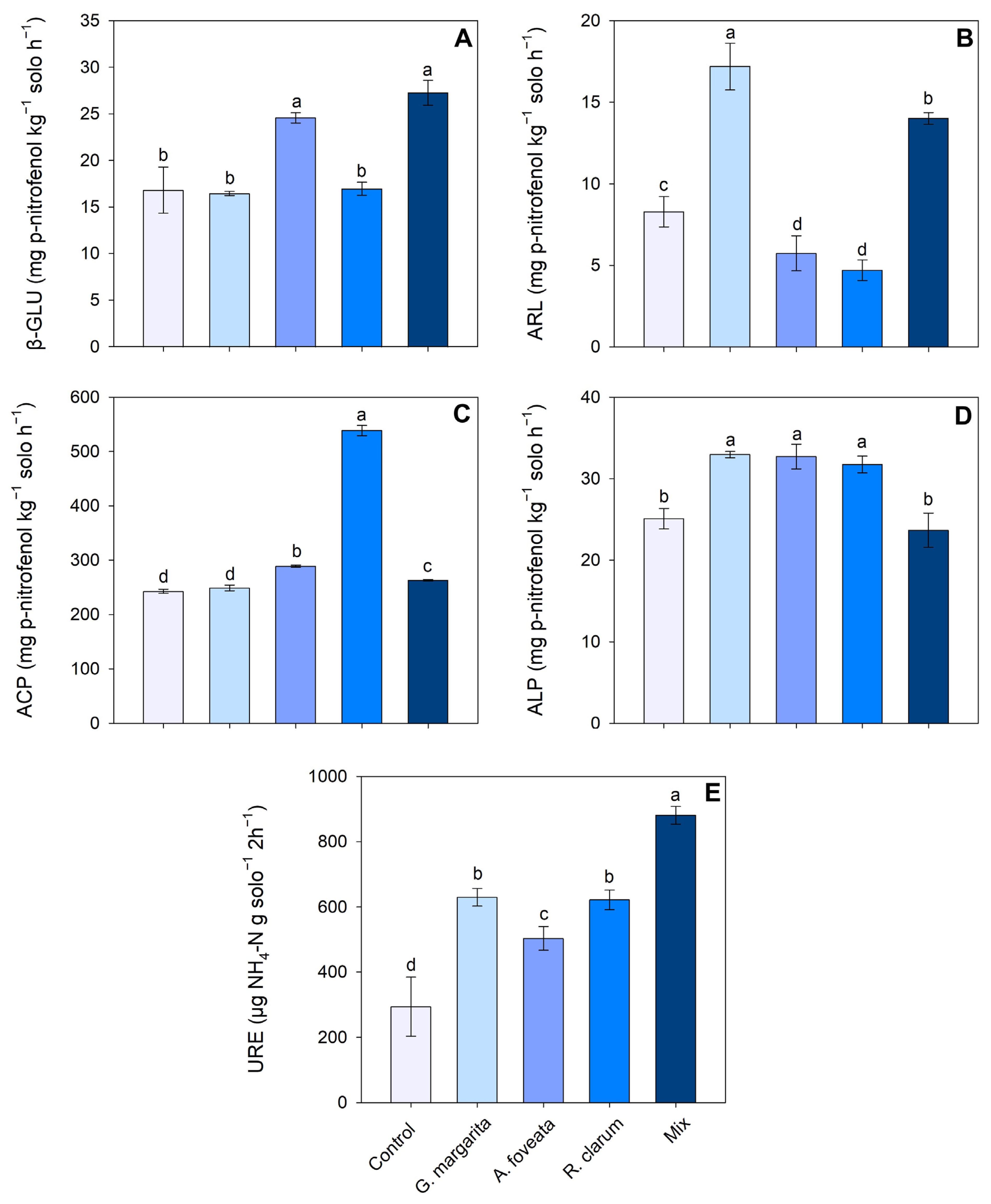
3.4. Soil Enzyme Activity
3.5. Enzyme Stoichiometry
3.6. Relationships Between Soil Enzyme Activity and Mycorrhizal Attributes on Plant Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rehman, G.; Khattak, I.; Hamayun, M.; Rahman, A.; Haseeb, M.; Umar, M.; Ali, S.; Iftikhar; Shams, W.A.; Pervaiz, R. Impacts of Mining on Local Fauna of Wildlife in District Mardan & District Mohmand Khyber Pakhtunkhwa Pakistan. Braz. J. Biol. 2021, 84, e251733. [Google Scholar] [CrossRef]
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Dantas, J.R.A.; Barros, L.B.; Souza, V.C.; Mont’alverne, A.A.F. Distritos Mineiros do Nordeste Oriental; Diretoria de Fiscalização Mineral—DIFIS; 4º Distrito Pernambuco; Departamento Nacional de Produção Mineral (DNPM): Brasília, DF, Brasil, 2000; p. 90. [Google Scholar]
- Nascimento, A.R.V.J.; Cunha, G.K.G.; do Nascimento, C.W.A.; da Cunha, K.P.V. Assessing Soil Quality and Heavy Metal Contamination on Scheelite Mining Sites in a Tropical Semi-Arid Setting. Water Air Soil. Pollut. 2021, 232, 375. [Google Scholar] [CrossRef]
- Sousa, S.S.; Freitas, D.A.F.; Latini, A.O.; Silva, B.M.; Viana, J.H.M.; Campos, M.P.; Peixoto, D.S.; Botula, Y.D. Iron Ore Mining Areas and Their Reclamation in Minas Gerais State, Brazil: Impacts on Soil Physical Properties. SN Appl. Sci. 2020, 2, 1659. [Google Scholar] [CrossRef]
- Araújo, B.D.; Maia, R.A.; Barbosa, M.; Silva, T.F.; Modolo, L.V.; Negreiros, D.; Fernandes, G.W. Mining Tailings Effects on Soil Quality and Performance of Two Native Species of Atlantic Forest: Implications for Restoration. Water Air Soil Pollut. 2024, 235, 687. [Google Scholar] [CrossRef]
- Ramos, L.; Negreiros, D.; Ferreira, B.S.S.; Figueiredo, J.C.G.; Paiva, D.C.; Oki, Y.; De Souza Justino, W.; Dos Santos, R.M.; Aguilar, R.; Nunes, Y.R.F.; et al. Strong Relationships between Soil and Vegetation in Reference Ecosystems of a Riparian Atlantic Rainforest in the Upper Doce River Watershed, Southeastern Brazil. IForest 2023, 16, 226. [Google Scholar] [CrossRef]
- Garcia, K.G.V.; de Souza Oliveira Filho, J.; de Araújo Pereira, A.P.; Mendes Filho, P.F. Can Inoculation of Native Arbuscular Mycorrhizal Fungi from a Mining Area Attenuate Stress of Acacia Mangium Willd. to Excess Manganese? J. Soils Sediments 2024, 24, 3252–3264. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Tomazelli, D.; Costa, M.D.; Primieri, S.; Rech, T.D.; Santos, J.C.P.; Klauberg-Filho, O. Inoculation of Arbuscular Mycorrhizal Fungi Improves Growth and Photosynthesis of Ilex Paraguariensis (St. Hil) Seedlings. Braz. Arch. Biol. Technol. 2022, 65, e22210333. [Google Scholar] [CrossRef]
- Garcia, K.G.V.; Mendes Filho, P.F.; Pinheiro, J.I.; do Carmo, J.F.; de Araújo Pereira, A.P.; Martins, C.M.; de Abreu, M.G.P.; de Souza Oliveira Filho, J. Attenuation of Manganese-Induced Toxicity in Leucaena Leucocephala Colonized by Arbuscular Mycorrhizae. Water Air Soil. Pollut. 2020, 231, 22. [Google Scholar] [CrossRef]
- Qiu, L.; Bi, Y.; Jiang, B.; Wang, Z.; Zhang, Y.; Zhakypbek, Y. Arbuscular Mycorrhizal Fungi Ameliorate the Chemical Properties and Enzyme Activities of Rhizosphere Soil in Reclaimed Mining Subsidence in Northwestern China. J. Arid. Land 2019, 11, 135–147. [Google Scholar] [CrossRef]
- Song, Z.; Bi, Y.; Zhang, J.; Gong, Y.; Yang, H. Arbuscular Mycorrhizal Fungi Promote the Growth of Plants in the Mining Associated Clay. Sci. Rep. 2020, 10, 2663. [Google Scholar] [CrossRef]
- Moura, M.L.A.; Oki, Y.; Arantes-Garcia, L.; Cornelissen, T.; Nunes, Y.R.F.; Fernandes, G.W. Mycorrhiza Fungi Application as a Successful Tool for Worldwide Mine Land Restoration: Current State of Knowledge and the Way Forward. Ecol. Eng. 2022, 178, 106580. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Ren, T.; Wang, H.; Xu, Q.; Zhang, G. Assessment of Soil Fertility Degradation Affected by Mining Disturbance and Land Use in a Coalfield via Machine Learning. Ecol. Indic. 2021, 125, 107608. [Google Scholar] [CrossRef]
- Maia, E.P.V.; Garcia, K.G.V.; de Souza Oliveira Filho, J.; Pinheiro, J.I.; Filho, P.F.M. Co-Inoculation of Rhizobium and Arbuscular Mycorrhiza Increases Mimosa Caesalpiniaefolia Growth in Soil Degraded by Manganese Mining. Water Air Soil. Pollut. 2023, 234, 289. [Google Scholar] [CrossRef]
- De Medeiros, A.V.S.; da Guedes, R.S.; de Souza, P.F.; Zanella, F.C.V. Phytosociology of an Open Arboreal Caatinga with High Basal Area in the Seridó Desertification Region, Brazil Fitossociologia de Caatinga Arbórea Aberta de Elevada Área Basal Em Núcleo de Desertificação Do Seridó. For. Sci. 2023, 36, 601–611. [Google Scholar] [CrossRef]
- Baptista, M.S.P.; Assunção, V.A.; Bueno, M.L.; Casagrande, J.C.; Sartori, Â.L.B. Species Representativeness of Fabaceae in Restrictive Soils Explains the Difference in Structure of Two Types of Chaco Vegetation. Acta Bot. Bras. 2020, 34, 559–569. [Google Scholar] [CrossRef]
- Teixeira, P.; Donagemma, G.; Fontana, A.; Teixeira, W. Manual de Métodos de Análise de Solo; Embrapa Solos: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Hungria, M.; Araujo, R.S. Manual de Métodos Empregados em Estudos de Microbiologia Agrícola, 1st ed.; Embrapa: Brasilia, DF, Brazil, 1994. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Jenkins, W. A Rapid Centrifugal-Flotation Technique for Separating Nematodes from Soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular—Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Spencer, B. Studies on Sulphatases. 20. Enzymic Cleavage of Aryl Hydrogen Sulphates in the Presence of H218O. Biochem. J. 1958, 69, 155–159. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector Analysis of Ecoenzyme Activities Reveal Constraints on Coupled, C, N and P Dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plot type designs. Braz. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Santiago, F.L.d.A.; Silva, A.O.; Batista, É.R.; Kemmelmeier, K.; Gastauer, M.; Ramos, S.J.; Siqueira, J.O.; Carneiro, M.A.C. Rehabilitation Promotes Rapid Recovery of Arbuscular Mycorrhizal Fungi in Iron Mining Areas. Pedobiologia 2022, 95, 150838. [Google Scholar] [CrossRef]
- Alam, M.Z.; Anamul Hoque, M.; Ahammed, G.J.; Carpenter-Boggs, L. Arbuscular Mycorrhizal Fungi Reduce Arsenic Uptake and Improve Plant Growth in Lens Culinaris. PLoS ONE 2019, 14, e0211441. [Google Scholar] [CrossRef]
- Gomes, J.M.; Couto, L.; Leite, H.G.; Xavier, A.; Garcia, S.L.R. Parâmetros morfológicos na avaliação de qualidade de mudas de Eucalyptus grandis. Rev. Árvore. 2002, 26, 655–664. [Google Scholar] [CrossRef]
- Lin, K.H.; Wu, C.W.; Chang, Y. Sen Applying Dickson Quality Index, Chlorophyll Fluorescence, and Leaf Area Index for Assessing Plant Quality of Pentas Lanceolata. Not. Bot. Horti. Agrobot. 2019, 47, 169–176. [Google Scholar] [CrossRef]
- Hunt, G.A. Effect of Styroblock Design and Copper Treatment on Morphology of Conifer Seedlings. In Proceedings of the Target Seedling Symposium: Combined Meeting of the Western Forest Nursery Associations, Roseburg, OR, USA, 13–17 August 1990; p. 218. [Google Scholar]
- Gallegos-Cedillo, V.M.; Diánez, F.; Nájera, C.; Santos, M. Plant Agronomic Features Can Predict Quality and Field Performance: A Bibliometric Analysis. Agronomy 2021, 11, 2305. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, R.; Wang, X.; Yan, Z. Soil Tungsten Contamination and Health Risk Assessment of an Abandoned Tungsten Mine Site. Sci. Total Environ. 2022, 852, 158461. [Google Scholar] [CrossRef]
- Mitra, D.; Panneerselvam, P.; Senapati, A.; Chidambaranathan, P.; Nayak, A.K.; Mohapatra, P.K. Das Arbuscular Mycorrhizal Fungi Response on Soil Phosphorus Utilization and Enzymes Activities in Aerobic Rice under Phosphorus-Deficient Conditions. Life 2023, 13, 1118. [Google Scholar] [CrossRef]
- Wu, B.; Umer, M.; Guo, Y.; He, M.; Han, X.; Shen, K.; Xia, T.; He, Y.; He, X. Positive Responses of Soil Nutrients and Enzyme Activities to AM Fungus under Interspecific and Intraspecific Competitions When Associated with Litter Addition. Rhizosphere 2023, 27, 100728. [Google Scholar] [CrossRef]
- Gou, X.; Hu, Y.; Ni, H.; Wang, X.; Qiu, L.; Chang, X.; Shao, M.; Wei, G.; Wei, X. Arbuscular Mycorrhizal Fungi Alleviate Erosional Soil Nitrogen Loss by Regulating Nitrogen Cycling Genes and Enzymes in Experimental Agro-Ecosystems. Sci. Total Environ. 2024, 906, 167425. [Google Scholar] [CrossRef]
- Mao, Y.; Chang, D.; Cui, X.; Wu, Y.; Cai, B. Changes in Sulfur in Soybean Rhizosphere Soil and the Response of Microbial Flora in a Continuous Cropping System Mediated by Funneliformis Mosseae. Front Microbiol. 2023, 14, 1235736. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Z.; Liu, R.; Wang, L.; Cai, B. Enhancing Sulfur Absorption in Soybean Rhizosphere through Arbuscular Mycorrhizal Fungi Inoculation: Implications for Soil Health and Crop Growth. J. Clean Prod. 2024, 463, 142759. [Google Scholar] [CrossRef]
- Bhalla, S.; Bisht, A.; Garg, N. Silicon and Arbuscular Mycorrhizal Species Complement in Improving Soil Characteristics, Sulfur Metabolism and Antioxidant Defense Responses in Arsenic Stressed Cajanus Cajan (L.) Millsp. Arch. Agron. Soil Sci. 2023, 69, 2814–2832. [Google Scholar] [CrossRef]
- Ngosong, C.; Tatah, B.N.; Olougou, M.N.E.; Suh, C.; Nkongho, R.N.; Ngone, M.A.; Achiri, D.T.; Tchakounté, G.V.T.; Ruppel, S. Inoculating Plant Growth-Promoting Bacteria and Arbuscular Mycorrhiza Fungi Modulates Rhizosphere Acid Phosphatase and Nodulation Activities and Enhance the Productivity of Soybean (Glycine Max). Front. Plant Sci. 2022, 13, 934339. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, H.A.; Ferrera-Cerrato, R.; López-Delgado, H.A.; Sánchez-Rangel, J.C.; Alarcón, A.; Hernández-Ortega, H.A.; Ferrera-Cerrato, R.; López-Delgado, H.A.; Sánchez-Rangel, J.C.; Alarcón, A. Nutrient Status, Hydrogen Peroxide Content and Peroxidase Activity of Arbuscular Mycorrhizal Plants of Melilotus Albus Grown in Diesel-Contaminated Substrate. Sci. Fungorum 2021, 51, e1298. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-c.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular Mycorrhizal Fungi and Its Major Role in Plant Growth, Zinc Nutrition, Phosphorous Regulation and Phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Klauber-Filho, O.; Siqueira, J.O.; Moreira, F.M.d.S. Fungos Micorrízicos Arbusculares Em Solos de Área Poluída Com Metais Pesados. R. Bras. Ci. Solo 2002, 26, 125–134. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, A.G.; Dutta, P.; Sahoo, M.R.; Mohanty, S.; Kumar, S.; Choudhary, A.K.; Devi, E.L.; Sinyorita, S.; et al. Unlocking the Potential of Arbuscular Mycorrhizal Fungi: Exploring Role in Plant Growth Promotion, Nutrient Uptake Mechanisms, Biotic Stress Alleviation, and Sustaining Agricultural Production Systems. J. Plant Growth Regul. 2024, 2024, 1–39. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Wang, X.; Xu, X.; Ai, C.; He, P.; Liang, G.; Zhou, W.; Zhu, P. Effect of High Soil C/N Ratio and Nitrogen Limitation Caused by the Long-Term Combined Organic-Inorganic Fertilization on the Soil Microbial Community Structure and Its Dominated SOC Decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef]
- Qin, Z.; Tian, Y.; Hao, W.; Zhang, J.; Feng, G.; Christie, P.; Gai, J. Identifying the Predictors of Mycorrhizal Response under Multiple Fertilization Regimes. Agric. Ecosyst. Env. 2024, 365, 108926. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, H.; Zhao, M.; Monaco, T.A.; Rong, Y.; Huang, D.; Song, Q.; Zhao, K.; Wang, D. Soil Extracellular Enzyme Activities and the Abundance of Nitrogen-Cycling Functional Genes Responded More to N Addition than P Addition in an Inner Mongolian Meadow Steppe. Sci. Total Environ. 2021, 759, 143541. [Google Scholar] [CrossRef]



| pH | TOC | P | Ca2+ | Mg2+ | Na+ | K+ | N |
|---|---|---|---|---|---|---|---|
| (H2O) | (g kg−1) | (mg kg−1) | (cmolc kg−1) | (cmolc kg−1) | (cmolc kg−1) | (cmolc kg−1) | (g kg−1) |
| 7.38 | 2.75 | 22.50 | 2.89 | 2.00 | 3.19 | 0.11 | 0.25 |
| W | Cd | Cr | Cu | Mo | Pb | Zn | |
| (mg kg−1) | |||||||
| 1294.96 | 0.152 | 50.72 | 67.88 | 2.00 | 5.79 | 42.12 | |
| Bulk density | Sand | Silt | Clay | Textural Class | |||
| (g cm−3) | (%) | (%) | (%) | - | |||
| 1.37 | 52.48 | 18.70 | 28.82 | Sandy clay loam | |||
| AMF | Vector A | Vector L |
|---|---|---|
| Control | 44.94 ± 0.80 a | 0.47 ± 0.014 b |
| G. margarita | 41.99 ± 0.14 b | 0.45 ± 0.002 c |
| A. foveata | 43.29 ± 0.21 c | 0.49 ± 0.004 a |
| R. clarum | 44.57 ± 0.10 a | 0.43 ± 0.002 c |
| Mix | 41.36 ± 0.07 c | 0.49 ± 0.005 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, K.G.V.; Almeida, M.d.S.; Barbosa, F.L.A.; Pereira, A.P.d.A. The Contribution of Arbuscular Mycorrhizal Fungi to Soil Enzyme Activity and the Performance of Mimosa caesalpiniaefolia in Soil Degraded by Scheelite Mining: Implications for Restoration. Resources 2025, 14, 50. https://doi.org/10.3390/resources14030050
Garcia KGV, Almeida MdS, Barbosa FLA, Pereira APdA. The Contribution of Arbuscular Mycorrhizal Fungi to Soil Enzyme Activity and the Performance of Mimosa caesalpiniaefolia in Soil Degraded by Scheelite Mining: Implications for Restoration. Resources. 2025; 14(3):50. https://doi.org/10.3390/resources14030050
Chicago/Turabian StyleGarcia, Kaio Gráculo Vieira, Murilo de Sousa Almeida, Francisco Luan Almeida Barbosa, and Arthur Prudêncio de Araujo Pereira. 2025. "The Contribution of Arbuscular Mycorrhizal Fungi to Soil Enzyme Activity and the Performance of Mimosa caesalpiniaefolia in Soil Degraded by Scheelite Mining: Implications for Restoration" Resources 14, no. 3: 50. https://doi.org/10.3390/resources14030050
APA StyleGarcia, K. G. V., Almeida, M. d. S., Barbosa, F. L. A., & Pereira, A. P. d. A. (2025). The Contribution of Arbuscular Mycorrhizal Fungi to Soil Enzyme Activity and the Performance of Mimosa caesalpiniaefolia in Soil Degraded by Scheelite Mining: Implications for Restoration. Resources, 14(3), 50. https://doi.org/10.3390/resources14030050







