Abstract
The accurate characterization of pollution in terms of chemical oxygen demand (COD) in wastewater treatment plants is considered as a key topic for their monitoring. In this research work, the negative interference of oxychlorides in COD measurements has been evaluated at a laboratory scale. Specifically, the role of oxychlorides as alternative oxidizing agents in competition with dichromate has been assessed. The extent of COD reduction performance varied widely (40–100%) according to the particular oxychloride oxidizing reagent used and its concentration, as well as the organic carbon source and amount present in the wastewater. The experimental values of COD removal performance should be considered as dual concentration dependent. On the one hand, for each oxidizing agent, the COD reduction performance is directly proportional to the dosage used in the experiment. On the other hand, the influence of organic matter concentration on COD removal performance was inversely proportional. In addition, chlorate can be considered the strongest oxidizing agent and the principal interferent responsible for the overevaluation of COD removal performance. Furthermore, the interference extent of oxychlorides on COD determination decreased in the order of phthalate > hydrocarbons > proteins. These results can be useful to appropriately evaluate the performance of wastewater treatment plants.
1. Introduction
Chemical oxygen demand (COD) is defined, according to Standard Methods for the Examination of Water and Wastewater, as the quantity of a specified oxidant that reacts with the sample under controlled conditions [1]. The amount of oxidant expended is expressed in terms of its oxygen equivalence, commonly as the mass of oxygen consumed over volume of solution [2]. Alternatively, COD can be described as a measure of the oxygen equivalent of the organic matter (OM) content of a sample that is susceptible to oxidation by a strong chemical oxidant [3]. Conventionally, potassium dichromate (K2Cr2O7) is used as a chemical oxidant by a refluxing procedure. Furthermore, silver sulphate (AgSO4) in sulphuric acid (H2SO4) is added as a catalyst to increase the oxidation of relatively recalcitrant organic compounds. After the digestion, the reduced amount of K2Cr2O7 can be considered proportional to the oxidizable OM present in the sample. Additionally, COD is considered the most used analytical parameter to characterize wastewater in terms of water quality by providing an indirect measure of the pollutants present in a sample. It is important to note that COD parameters are used worldwide, particularly in the operation of wastewater treatment plants (WWTPs) because many environmental supervisory agencies impose strict regulations regarding the COD values of wastewater effluents before their discharge into the environment [4].
One objective of the WWTPs is to remove the maximum amount of pollutants because standard regulations fixed discharge limits between 100 and 125 mg O2/L [5]. The suitability of judgements based on COD values depends on the quality of measurements [6]. Therefore, reliable analysis of COD is required for accurately monitoring the OM content in the WWTPs [7]. However, a common problem relating to COD analysis is the interference effects of some inorganic substances potentially present in samples such as halide ions (mainly chlorides), nitrites (NO2−), and reduced inorganic species (Fe+2, Mn+2, sulphide). This subject has been widely described in the scientific literature, including the APHA-Standard Methods [1].
In spite of its treatment, it is possible that some COD values for wastewater may not reach the established discharge limit standard. Therefore, further treatment methods, such as physical and/or chemical, are used to treat the remaining OM content present in the wastewater. In this sense, advanced oxidation processes (AOPs) refer to a set of chemical treatments planned to remove organic (and sometimes inorganic) materials considered as pollutants using oxidation through reactions with hydroxyl radicals [8]. Unfortunately, some of the chemical species used in AOP have not been mentioned as interferent elements for COD determination in the APHA-Standard Methods [1]. However, the scientific literature has plenty of information related to this situation. In this way, the positive interference of H2O2 on COD measurements was previously reported [9,10]. Similarly, potassium persulfate (K2S2O8) could also cause a positive error in the COD determination. This interference can be eliminated based on the addition of sodium sulphite (Na2SO3), as well as heating at 90 °C for 60 min [11]. More recently, an alternative method to artificially remove the OM in industrial WWTPs has been reported at industrial level. This is a very simple system, which involves adding a sodium chlorate-based product ambiguously labelled as a “COD remover” [5,12,13] at the intermediate or final stages of the WWTPs, where samples are normally taken for compliance COD testing. However, this “COD remover” product does not really react with the OM content; it just serves as an alternative oxidant versus dichromate during the analysis technique [14]. In fact, “COD remover” should be considered a negative interfering element in COD determination. This analytical interference has not been specified in the APHA-Standard Methods [1]. Moreover, a new AOP based on the electrochemical technology has recently been developed. This technology is the so-called electrochemical advanced oxidation process (EAOP), and it has been proposed as a way to prevent and remediate pollution problems [15,16]. Unfortunately, it has been reported that some oxychloride compounds (ClOx−) are formed during the application of this kind of electrochemical treatment system of wastewater. They can be considered as interfering elements for COD determination and, similarly to the so-called “COD remover”, they are responsible for the false COD reduction [17,18,19,20]. This way, they would result in the wrong appearance of wastewater effluents reaching the COD discharge limit standard.
On the other hand, depending on the wastewater itself, the overall chemical composition and the type of pollutant may vary [21]. Most of the previously published studies regarding the negative interference of oxychlorides in COD determination have been carried out by evaluating refractory organic matter in the form of benzene derived compounds such as benzohydroxamic acid (BHA) [5], phenol [19], and its degradation intermediate products such as benzoquinone, catechol, and clorophenols [17,20]. It is important to take into account that some of these compounds cannot be totally oxidized according to their relationship between experimental COD and theoretical oxygen demand [22]. However, the interference effects on COD determination of some totally oxidizable organic components such as carbohydrates and proteins, commonly present as OM in the agro-industrial and municipal wastewaters, have not been previously reported.
The main objective of this research study was to be useful to form accurate COD measurements in case of fraudulent treatment by using interferent oxidant reagents, as has been previously described in industrial wastewater treatment plants [13]. This study was carried out from a novel viewpoint because the effects of the interferent reagents have been assessed more deeply, and the influence of different OM sources has also been included. Therefore, two main aims were established. Firstly, the influence of different oxychloride oxidizing agents was evaluated according to phthalate as a conventional reference organic compound and individually tested at each concentration level, as well as through the complete calibration range. Secondly, the role of different oxychloride reagents was assessed by considering the organic carbon source of wastewater.
2. Materials and Methods
2.1. Oxychloride Reagents
Four oxidizing agents were used in this research work: (i) Sodium hypochlorite (NaClO) was provided by Panreac AppliChem (Barcelona, Spain) as 5% w/v solution (certified value of 5.5% w/v); (ii) Sodium chlorite (NaClO2) was provided by Panreac AppliChem (Barcelona, Spain) as 25% w/w solution (certified value of 31% w/w); (iii) Sodium Chlorate (NaClO3) was provided by Roth (Karlsruhe, Germany) in solid form (certified value of 100% w/w); (iv) Sodium perchlorate (NaClO4) was provided by Alfa Aesar (Heysham, United Kingdom) in solid form (certified value of 99.9% w/w). These concentrate reagents were used to prepare stock solutions in the range of 2.5–20.0 g/L. Finally, appropriate volumes of these solutions were taken to achieve the interferent level range of the study (0.5–2.0 g/L).
2.2. Wastewater
The wastewater used for this experimentation can be collected theoretically from a wastewater treatment plant or prepared at the laboratory level because there is no difference from the COD viewpoint. In this study, the most important characteristic of wastewater is the OM content, according to both the COD concentration and the carbon source. As a consequence of the evaluation of the organic carbon origin, the use of synthetic wastewater provides an advantage to the work as using a fixed formulation avoids the inherent variability of real wastewaters [23,24].
In addition, the macro and micronutrient composition was evaluated to assess the possible interference in COD removal performance. However, this mineral formulation was checked experimentally with the results considered as interference free.
2.2.1. Organic Matter Component
The selected products were purchased in solid form: (i) Glucose (C6H6O12) was provided by Panreac AppliChem (Barcelona, Spain); (ii) Meat extract (Lab-Lemco powder) was provided by Oxoid (Sasingstoke, United Kingdom); (iii) Milk (Non-fat dried milk powder) was provided by Panreac AppliChem (Barcelona, Spain) as a mixture of lactose (52% w/w), proteins (33.3% w/w), and fats (0.7% w/w). Table 1 summarizes the main characteristics of these products in terms of loss on drying and dry matter (105 °C), and OM and ash (550 °C).

Table 1.
Main characteristics of organic matter constituents in synthetic wastewater.
2.2.2. Inorganic Matter Component
The selected products were provided in solid form by Panreac Applichem. They were used to prepare individual stock solutions at different concentrations: (i) Sodium bicarbonate (NaHCO3) at 25 g/L; (ii) Ammonium chloride (NH4Cl) at 7 g/L; (iii) Potassium hydrogen phosphate (K2HPO4) at 6.25 g/L; (iv) Magnesium sulphate (MgSO4·7H2O) at 5 g/L; (v) Calcium Chloride (CaCl2·2H2O) at 2.5 g/L. Later, a multicomponent stock solution was prepared by mixing appropriate volumes of each individual stock solution. Specifically, 100 mL/L was used for (i–iii), 50 mL/L for (iv), and 20 mL/L for (v).
2.2.3. Preparation of Synthetic Wastewaters
The corresponding amount of glucose, meat extract, and milk were weighed and mixed with 400 mL of distilled water in a 500 mL volumetric flask. Later, a volume of 50 mL of the multicomponent stock solution of nutrients was added and finally mixed appropriately with more deionized water to fill up the flask. The general composition of simulated wastewater was as follows: COD (500 mg O2/L); NaHCO3 (250 mg/L); NH4Cl (70 mg/L); K2HPO4 (62.5 mg/L); MgSO4·7H2O (25 mg/L); CaCl2·2H2O (5 mg/L). It should be pointed out that these COD and mineral contents were selected considering the average value of published wastewater recipes [24] and also considering the intermediate level in relation to the COD method calibration range.
2.3. COD Analysis
The COD measurements throughout this research work were carried out according to the 5220D APHA Standard Methods [1], which is basically a closed reflux and spectrophotometric method. This methodology is cost-effective according to both the chemicals used and the hazardous waste produced. For this purpose, 16 × 100 mm tubes were selected to add the three different components and achieve a total volume of 7.5 mL. Initially, 2.5 mL of sample was mixed with 1.5 mL of digestion solution (a mixture of K2Cr2O7, HgSO4, and H2SO4). Finally, a volume of 3.5 mL sulphuric acid reagent (a mixture of AgSO4 and H2SO4) was carefully added and completely mixed with the rest of the components. The tubes were digested by closed reflux (at 150 °C for 2 h) using a Selecta heater block digest.
2.3.1. Potassium Hydrogen Phthalate (KHP)
KPH was provided by Panreac Applichem as a powder product of high analytical purity. A KHP stock standard solution of 1000 mg O2/L was prepared, considering theoretical value of 1.171 g O2/g KHP, by dissolving 0.425 g of dried (at 105 °C for 2 h) product in 500 mL of deionized water.
2.3.2. Preparation of COD Calibration Curve
Seven standards of 50 mL were prepared from KHP stock standard solution through appropriate dilutions to provide COD equivalents of 0, 50, 100, 250, 500, 750, and 1000 mg O2/L. Duplicate absorbance measurements were performed. A linear regression was obtained from absorbance readings versus COD concentration values. Statistical evaluation of this regression was carried out using EXCEL-Data analysis.
2.3.3. Spectrophotometer
The instrument Genesys 10S UV-Vis (Thermo Scientific) was used to directly measure the absorbance of tubes at 600 nm. Calculation of COD values was carried out according to the calibration curve.
2.4. Experimental Procedure to Evaluate Oxychlorides Interference on COD Method
2.4.1. Using KHP as Chemical Standard
For this purpose, the same concentration levels used for obtaining the COD calibration curve were, this time, set as mixtures including oxychloride reagents. For this reason, these standards were prepared from a more concentrated KHP standard solution of 2000 mg O2/L. Next, appropriate volumes of each individual oxychloride stock solutions (2.5, 5.0, 7.5, and 10.0 g/L) were taken to achieve seven predefined interferent dosages in the range of 500–2000 mg/L. After mixing (e.g., 5–10 min), the samples were taken for COD determination. The absorbance values obtained were used to obtain the corresponding COD results. The results were evaluated according to the different concentrations of standard analyte (KHP) and interferent oxychloride reagents. Moreover, to appropriately evaluate the full working range of KHP in terms of COD, a complementary test of calibration curves was obtained by using the COD absorbance values from KHP solutions spiked with oxychloride reagents. These results were expressed as slope ratios between spiked and not spiked (standard calibration curve).
2.4.2. Using Synthetic Wastewaters
Three different organic carbon sources were considered for this study. The composition of the simulated wastewaters was similar, with the only difference in the organic compound providing the wastewater COD value (500 mg O2/L). The working scheme was to evaluate a mixture of 90%–10% v/v. Therefore, 45 mL of synthetic wastewater (90% v/v) and 5 mL of oxychloride stock solutions (5.0, 10.0, 15.0, and 20.0 g/L) were mixed to evaluate four interferent dosages (500, 1000, 1500, and 2000 mg/L). It is important to specify that after mixing, the synthetic wastewaters were diluted 10-fold. In addition, a control value (obtained without the effect of any interference in COD determination) was also included by replacing the volume of oxidizing agents by 5 mL of distilled water. After mixing (e.g., 5–10 min), the samples were taken for COD analyses. The COD ratio values of spiked versus not spiked (control) oxychloride reagents were used to calculate the COD removal performance according to the experimental conditions.
2.5. Experimental Procedure to Evaluate COD Oxidation Rate
Synthetic wastewater samples were analyzed for COD before heating them at 150 °C (zero time) and through the digestion time at 30, 60, 90, and 120 min. The experimental results at different digestion times were evaluated as % COD ratio against the maximum COD theoretical values.
2.6. Statistical Information
Each oxychloride reagent was checked as a COD interfering agent by carrying out oxidizing tests at least in duplicate, and the experimental results obtained were expressed as average and standard deviation or relative standard deviation (%). Statistical significance of trial results was evaluated at a 95% confidence interval.
3. Results and Discussion
3.1. Evaluation of Oxychlorides Interference in COD Determination Using KHP as Chemical Standard
Taking into account that sodium perchlorate is a strong oxidizing agent (only slightly weaker than dichromate), it was previously suggested that it could be used to reduce COD in fraudulent form as sodium chlorate [5]. However, it is important to highlight the unusual lack of ClO4− reactivity observed in chemical systems [25]. In fact, in this study, ClO4− was not effective at all as an oxidizing agent in the experimental conditions reported for COD determination. Therefore, in the working range evaluated of 500–2000 mg/L, ClO4− cannot be considered as a “COD remover” active agent. The same negative results were reported by Xiao et al. [18,19]. They justified this fact due to the high activation energy necessary for ClO4− reduction [26].
The effect of oxychlorides on the COD removal performance varied widely according to the experimental concentrations of both the ClOx− interferent agent and the KHP chemical standard. Therefore, the experimental values of COD reduction should be considered as dual concentration dependent. On the one hand, as expected, the COD removal performance observed was directly proportional to the ClOx− interferent concentration. As an example, Figure 1 shows the ClO3− concentration effect over the COD removal performance at 500 mg O2/L. A similar trend, seen in decreasing COD values while interference concentration increased, was previously reported by Xiao et al. [14]. They used KHP water samples at 150 mg O2/L and ClO3− at a concentration range between 0 and 20 mM. Specifically, COD values dropped from 150 mg O2/L to 80 mg O2/L, 50 mg O2/L, 40 mg O2/L, and 33 mg O2/L at interferent concentrations of 5, 10, 15, and 20 mM, respectively; this means a 47%, 67%, 73%, and 78% COD removal performance, respectively.
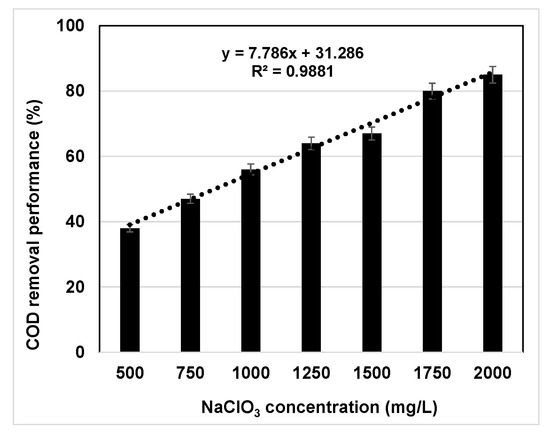
Figure 1.
COD removal performance (%) according to NaClO3 concentrations for KHP as chemical standard at 500 mg O2/L.
Similarly, it was reported that COD values dropped severely from 150 mg O2/L to below 100 mg O2/L at 5 mM of various ClOx− interferent agents and further decreased while interference concentration increased [18].
On the other hand, the influence of KHP concentration on COD reduction was inversely proportional. This effect has not been previously reported because ClOx− interference studies were carried out at single COD level where the only variable studied was the interfering concentration. As an example, Figure 2 shows the influence of KHP concentration on COD removal performance at chlorate concentration of 1000 mg/L. As it can be seen, the COD removal performance ranged from 100 to 40% depending on the initial KHP concentration, which was from 50 to 1000 mg O2/L, respectively.
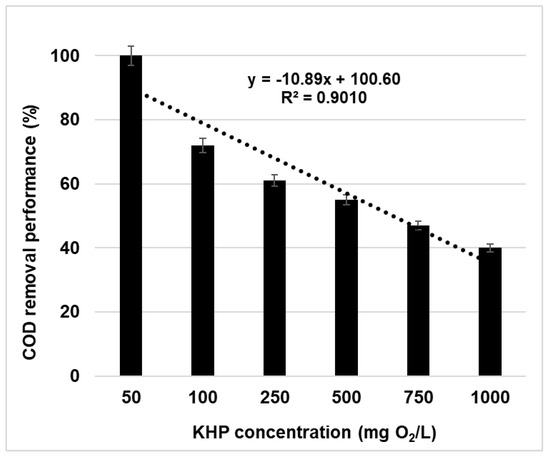
Figure 2.
COD removal performance (%) according to concentrations of KHP as chemical standard for NaClO3 concentration at 1000 mg/L.
The variable extent of these interference studies can be explained by considering the two oxidation reactions by K2Cr2O7 and ClOx− interfering agents. Depending on the amounts of organic compounds in the samples, the effect of the alternative oxidizing agent varies. At low KHP concentrations, there is an excess of ClOx−, so OM can be oxidized at a greater extent. At high KHP concentrations, the amount of ClOx− is comparatively lower, meaning that the alternate oxidation reaction is significantly lower. It must be pointed out that similar results are obtained from COD reduction when both concentrations are considered in the form of the same ratio value. In this line, the three concentration combinations (500, 750, and 1000 mg/L versus mg O2/L), giving a ratio value (ClOx−:COD) of one, provided the same COD removal performance of about 39%.
To try to simplify this dual concentration variability of the experimental results, the trial data was also assessed over the full calibration range through slope value ratios. For this purpose, slope values of testing calibration curves (spiked) were compared to the standard calibration curve (not spiked). Experimental results provided a general trend where the absorbance values and their corresponding slopes decreased while oxychlorides concentration increased (Figures S1–S3 and Tables S1–S3 of Supplementary Materials). Therefore, the extent of COD reduction was confirmed as directly proportional to the dosage of oxidizing agents. Figure 3a summarizes the results obtained in terms of overall COD removal performance versus ClOx− interferent concentration for KHP. The COD removal extent of ClO−, ClO2−, and ClO3− ranged from 7% to 36%, 15% to 44%, and 21% to 62%, respectively. According to the overall slope value relationships obtained by these linear regressions, the extent of COD removal for ClO3− was about 35–38% higher when compared to the other oxidizing compounds. Therefore, the effect of the degree of interference of oxychlorides on COD determination using KHP standard decreased in the order of ClO3− > ClO2− > ClO− (while ClO4− showed no interfering effect).
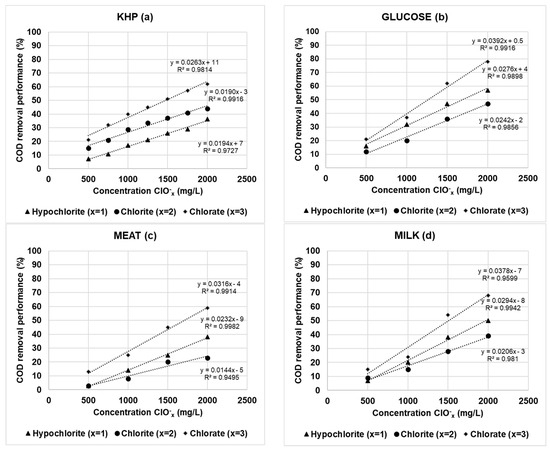
Figure 3.
COD removal performance (%) according to oxychloride concentrations for KHP (a), glucose (b), meat (c), and milk (d) as organic matter source.
3.2. Evaluation of Oxychlorides Interference in COD Determination Using Synthetic Wastewaters
Figure 3b–d shows the COD removal performance of simulated wastewater from different organic compounds (glucose, meat, and milk) where oxychloride reagents were used at different dosages (Supplementary Tables S4–S7). For the simulated wastewaters containing about 450 mg O2/L, in the same way as the results from KHP standard, the COD reduction values were directly proportional to the ClOx− interferent concentration. It is noteworthy to mention that ClO3− provided the maximum interference effect although the COD removal performance values were widely variable. For example, the ClO3− concentration effect over COD removal performance ranged between 13 and 59%, 15 and 68%, and 21 and 78% for meat, milk, and glucose, respectively. On the contrary, ClO− and ClO2− interchanged their relative interfering effect previously obtained for KHP. Therefore, the interference extent of oxychlorides on COD determination decreased in the order of ClO3− > ClO− > ClO2−.
The COD removal performance for the oxychlorides reagents was evaluated from the organic carbon source viewpoint (Figure 4 and Supplementary Tables S8–S10). It is very interesting to highlight the great influence of the OM nature in the results obtained. In this sense, the interference extent of oxychlorides on COD determination decreased in the order of KHP > glucose > milk > meat. These results can be explained by taking into account the COD oxidation rate (experimental COD values at different times versus theoretical ones) obtained by the different organic substrates evaluated. As can be seen in Figure 5, the % COD oxidation rate at 2 h of digestion achieved maximum values in all cases, confirming the total oxidation of the selected organic substrates. In addition, some of them are more easily oxidized than others under the test conditions. For example, after the addition of the reagents and without heating (zero time), the % COD oxidation rate values were 92(2)%, 85(1)%, 75(1)%, and 49(1)%, respectively. The higher oxidative rate of hydrocarbon versus protein like substrates has been demonstrated. In this study, the COD oxidation rate varied in the decreasing order of KHP > glucose > milk > meat. Therefore, the faster the oxidation rate, the higher the negative interference provided by ClOx− agents and their corresponding COD removal performance.
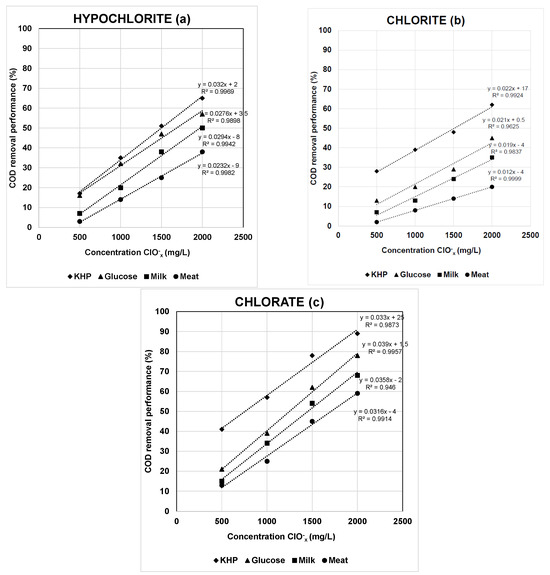
Figure 4.
COD removal performance (%) according to oxychloride concentrations for simulated wastewater. Effect of organic matter source.
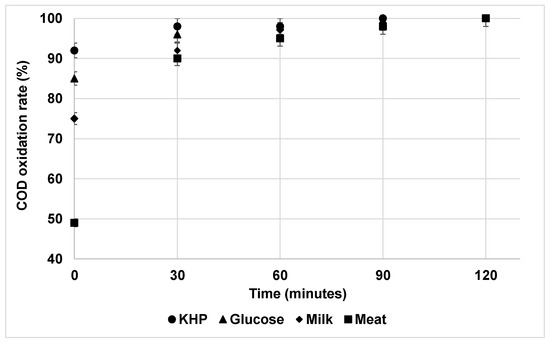
Figure 5.
COD oxidation rate for organic matter components of synthetic wastewater.
The influence of other organic carbon sources previously reported in the literature was considered as follows. BHA solutions were mixed with different dosages of NaClO3 at 500, 1000, and 1500 mg/L [5]. The COD values decreased from 220 mg O2/L to 100 mg O2/L, 60 mg O2/L, and 22 mg O2/L, respectively. Therefore, the COD reduction performance was about 55%, 73%, and 90%, respectively. These values were higher when compared to 40%, 60%, and 82% obtained on this study using KHP at 250 mg O2/L. In other research work, the higher COD removal performance of ClO3− was confirmed [17]. In this sense, a 0.5 mM phenol solution was evaluated and the experimental COD values dropped from 127 mg O2/L to 78 mg O2/L and 105 mg O2/L using ClO3− (5 mM) and ClO− (15 mM), respectively.
4. Conclusions
In this study, the negative interference of oxychloride reagents in the COD determination has been evaluated. Based on the results and discussion made, the following conclusions are described:
- The diverse ClOx− agents have different oxidizing capacity at the experimental conditions evaluated (at 150 °C for 120 min). However, ClO4− agent was not effective at all as a “COD remover”;
- In general, the experimental values of COD removal performance should be considered as dual concentration dependent. On the one hand, for each oxychloride oxidizing agent the COD reduction performance is directly proportional to the dosage used in the experiment (the higher the ClOx− concentration, the greater the COD removal performance). On the other hand, the COD removal performance was inversely proportional to the OM concentration present in the wastewater (the higher the OM concentration, the lower the COD removal performance);
- In addition, using simulated wastewater at a concentration of about 450 mg O2/L, the interference extent of ClOx− agents on COD measurements decreased in the order of KHP > glucose > milk > meat.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/resources14030046/s1, Figures S1–S3 and Tables S1–S11.
Author Contributions
Conceptualization, F.R.B.; investigation, J.A.G.G. and F.R.B.; writing—original draft preparation, F.R.B.; writing—review and editing, J.A.G.G., F.R.B., and A.F.M.; supervision, A.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- American Public Health Association; American Water Works Association, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Barbosa Segundo, I.D.; Cardozo, J.C.; Castro, P.S.; Gondim, A.D.; dos Santos, E.V.; Martínez-Huitle, C.A. Cost-effective smartphone-based method for low range chemical oxygen demand analysis. MethodsX 2023, 11, 102300. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; de la Rubia, M.A.; Borja, R.; Alaíz, M. Assessment of a modified and optimized method for determining Chemical Oxygen Demand of solid substrates and solutions with high suspended solid content. Talanta 2008, 76, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Duan, N.; Wu, X.; Fang, H. COD discharge limits for urban wastewater treatment plants in China based on statistical methods. Water 2018, 10, 777. [Google Scholar] [CrossRef]
- Meng, X.; Khoso, S.A.; Lyu, F.; Wu, J.; Kang, J.; Liu, H.; Zhang, Q.; Han, H.; Sun, W.; Hu, Y. Study on the influence and mechanism of sodium chlorate on COD reduction of minerals processing wastewater. Miner. Eng. 2019, 134, 1–6. [Google Scholar] [CrossRef]
- Viana da Silva, A.M.E.; Bettencourt da Silva, R.J.N.; Camões, M.F.G.F.C. Optimization of the determination of chemical oxygen demand in wastewaters. Anal. Chim. Acta 2011, 699, 161–169. [Google Scholar] [CrossRef]
- Ma, J. Determination of chemical oxygen demand in aqueous samples with non-electrochemical methods. Trends Environ. Anal. Chem. 2017, 14, 37–43. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Talinli, I.; Anderson, G.K. Interference of hydrogen peroxide on the standard COD test. Water Res. 1992, 26, 107–110. [Google Scholar] [CrossRef]
- Kang, Y.W.; Cho, M.J.; Hwang, K.Y. Correction of hydrogen peroxide interference on standard chemical oxygen demand test. Water Res. 1999, 33, 1247–1251. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Zeng, Z.; Huang, Z.; Cui, Y. A method for removing persulfate interference in the analysis of the chemical oxygen demand in wastewater. Environ. Chem. Lett. 2019, 17, 1085–1089. [Google Scholar] [CrossRef]
- Htet, T.T.; Zeng, D. Preparation and application of a new composite COD remover. N. Am. Acad. Res. 2020, 3, 118–140. [Google Scholar] [CrossRef]
- Liu, L.; Jia, P.; Han, J.; Lichtfouse, E. The underground industry of wastewater adulteration: How to trick legal testing with COD removers. Environ. Chem. Lett. 2022, 20, 1–5. [Google Scholar] [CrossRef]
- Xiao, H.; Yan, W.; Zhao, Z.; Tang, Y.; Li, Y.; Yang, Q.; Luo, S.; Jiang, B. Chlorate induced false reduction in chemical oxygen demand (COD) based on standard dichromate method: Countermeasure and mechanism. Water Res. 2022, 221, 118732. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Zhu, J.; Ba, X.; Guo, X.; Zhang, Q.; Qi, Y.F.; Li, Y.; Wang, J.; Sun, H.; Jiang, B. Oxychlorides induced over-evaluation of electrochemical COD removal performance over dimensionally stable anode (DSA): The roles of cathode materials. Sep. Purif. Technol. 2022, 303, 122197. [Google Scholar] [CrossRef]
- Xiao, H.; Hao, Y.; Chen, J.; Feng, F.; Liu, Y.; Li, Y.; Luo, S.; Jiang, B. Overevaluation of electro-oxidation for Chemical Oxygen Demand removal using a boron-doped diamond anode: The roles of various electrogenerated oxychlorides and countermeasure. ACS EST Eng. 2023, 3, 283–294. [Google Scholar] [CrossRef]
- Xiao, H.; Xu, F.; Chen, J.; Hao, Y.; Guo, Y.; Zhu, C.; Luo, S.; Jiang, B. Electrogenerated oxychlorides induced overlooked negative effects on electro-oxidation wastewater treatment in terms of over-evaluated COD removal efficiency and biotoxicity. J. Hazard. Mater. 2023, 456, 131667. [Google Scholar] [CrossRef]
- Yan, W.; Chen, J.; Wu, J.; Li, Y.; Liu, Y.; Yang, Q.; Tang, Y.; Jiang, B. Investigation on the adverse impacts of electrochemically produced ClOx− on assessing the treatment performance of dimensionally stable anode (DSA) for Cl−-containing wastewater. Chemosphere 2023, 310, 136848. [Google Scholar] [CrossRef]
- Sophonsiri, C.; Morgenroth, E. Chemical composition associated with different particle size fractions in municipal, industrial, and agricultural wastewaters. Chemosphere 2004, 55, 691–703. [Google Scholar] [CrossRef]
- Baker, J.R.; Milke, M.W.; Mihelcic, J.R. Relationship between chemical and theoretical oxygen demand for specific classes of organic chemicals. Water Res. 1999, 33, 327–334. [Google Scholar] [CrossRef]
- Kargol, A.K.; Burrell, S.R.; Chakraborty, I.; Gough, H.L. Synthetic wastewater prepared from readily available materials: Characteristics and economics. PLoS Water 2023, 2, e0000178. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Gray, N.F. A comparative analysis of the characteristics of a range of real and synthetic wastewaters. Environ. Sci. Pollut. Res. 2013, 20, 8813–8830. [Google Scholar] [CrossRef] [PubMed]
- Urbansky, E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 2002, 9, 187–192. [Google Scholar] [CrossRef]
- Clark, J.A.; Yang, Y.; Ramos, N.C.; Hillhouse, H.W. Selective oxidation of pharmaceuticals and suppression of perchlorate formation during electrolysis of fresh human urine. Water Res. 2021, 198, 117106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).