Evaluating Alternative Oxidants for Artificial Chemical Oxygen Demand Removal Performance from Wastewater Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxychloride Reagents
2.2. Wastewater
2.2.1. Organic Matter Component
2.2.2. Inorganic Matter Component
2.2.3. Preparation of Synthetic Wastewaters
2.3. COD Analysis
2.3.1. Potassium Hydrogen Phthalate (KHP)
2.3.2. Preparation of COD Calibration Curve
2.3.3. Spectrophotometer
2.4. Experimental Procedure to Evaluate Oxychlorides Interference on COD Method
2.4.1. Using KHP as Chemical Standard
2.4.2. Using Synthetic Wastewaters
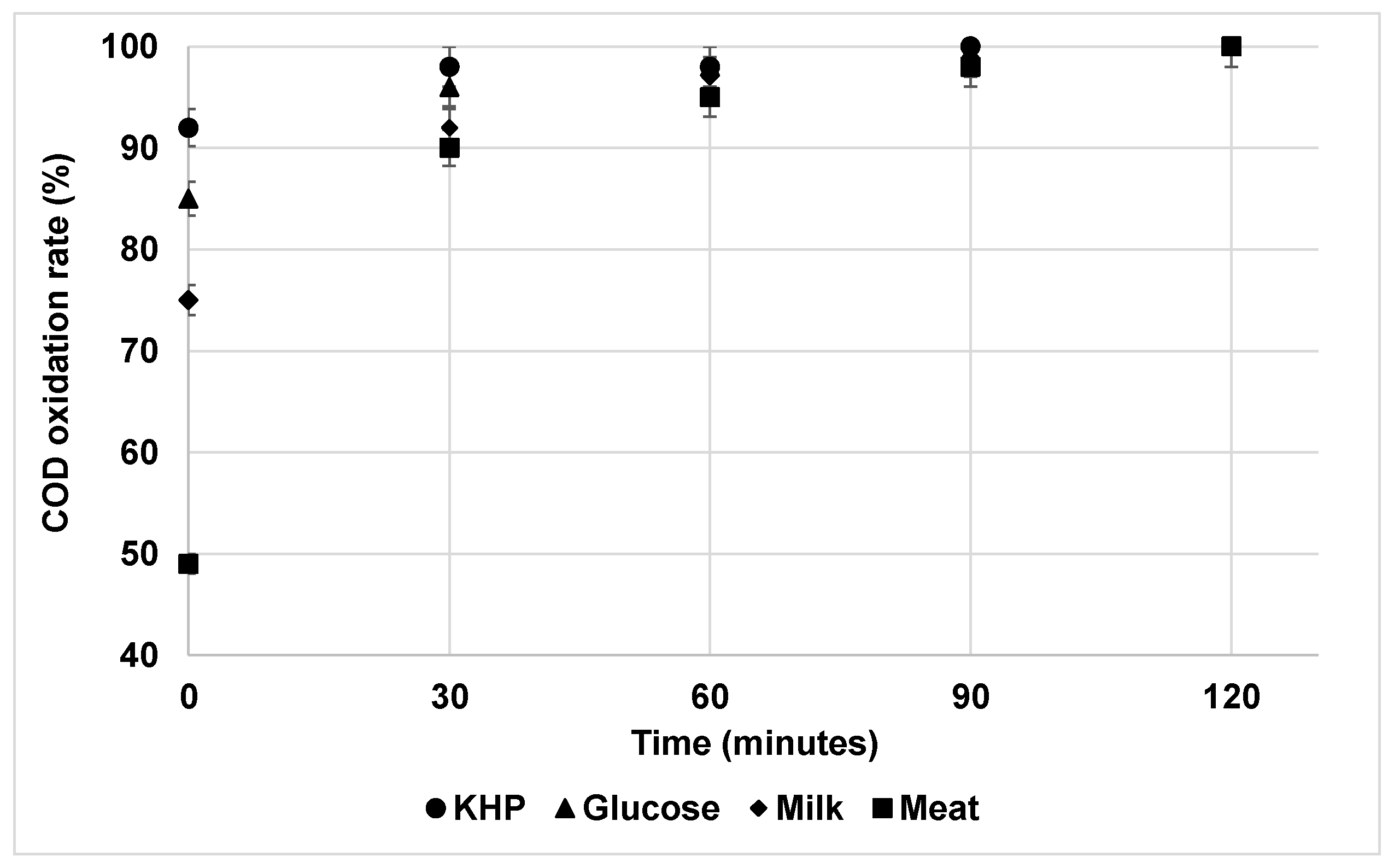
2.5. Experimental Procedure to Evaluate COD Oxidation Rate
2.6. Statistical Information
3. Results and Discussion
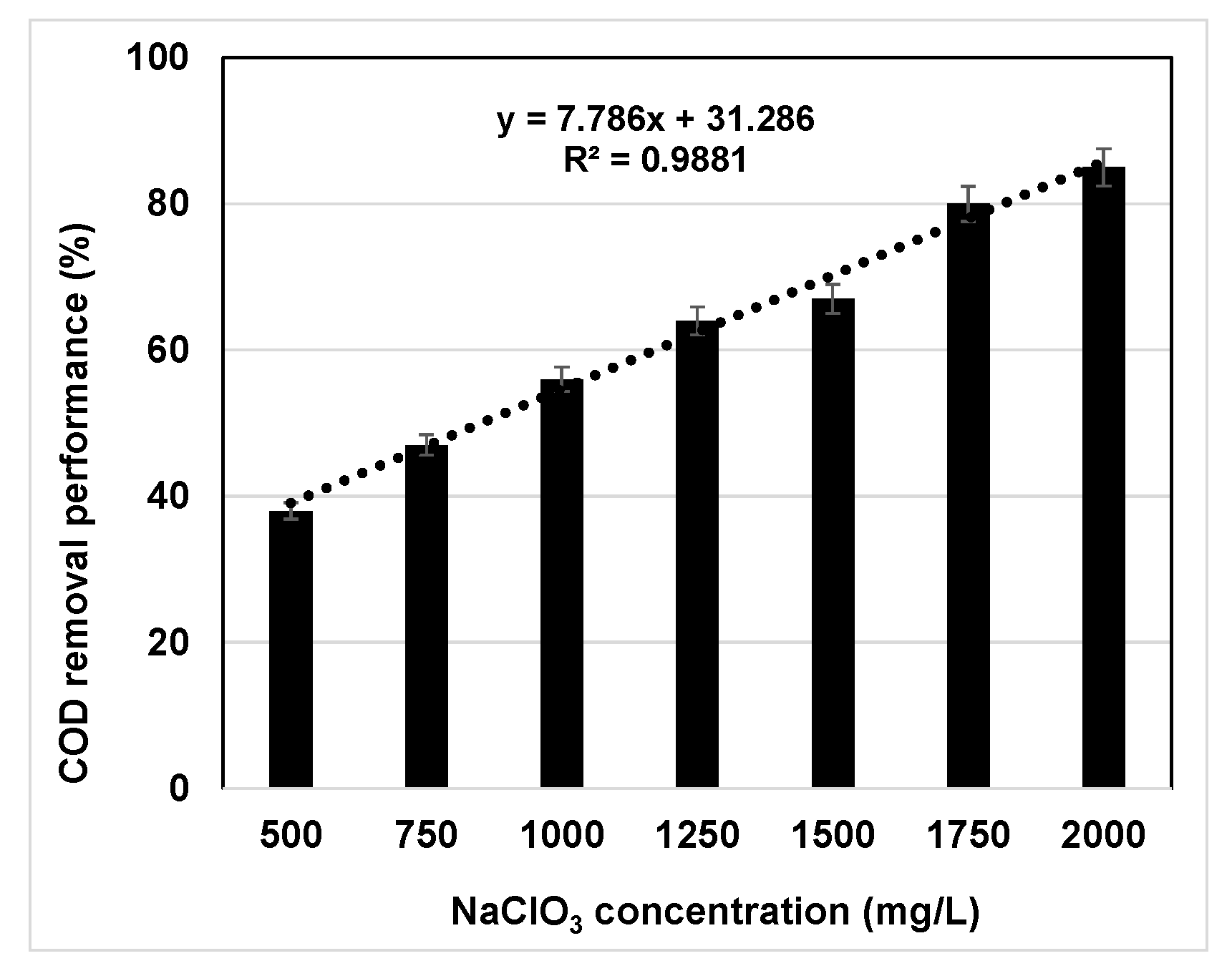
3.1. Evaluation of Oxychlorides Interference in COD Determination Using KHP as Chemical Standard
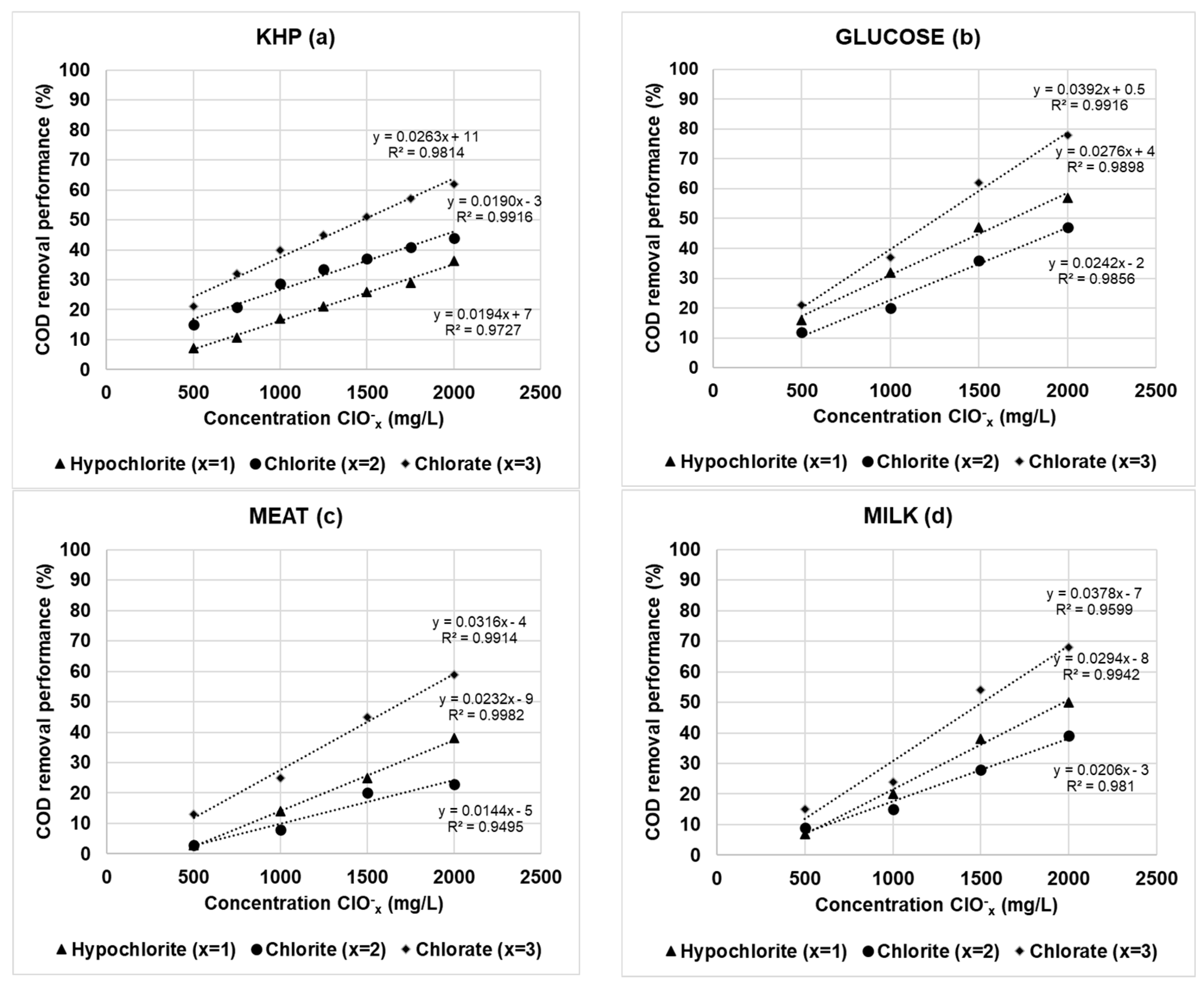
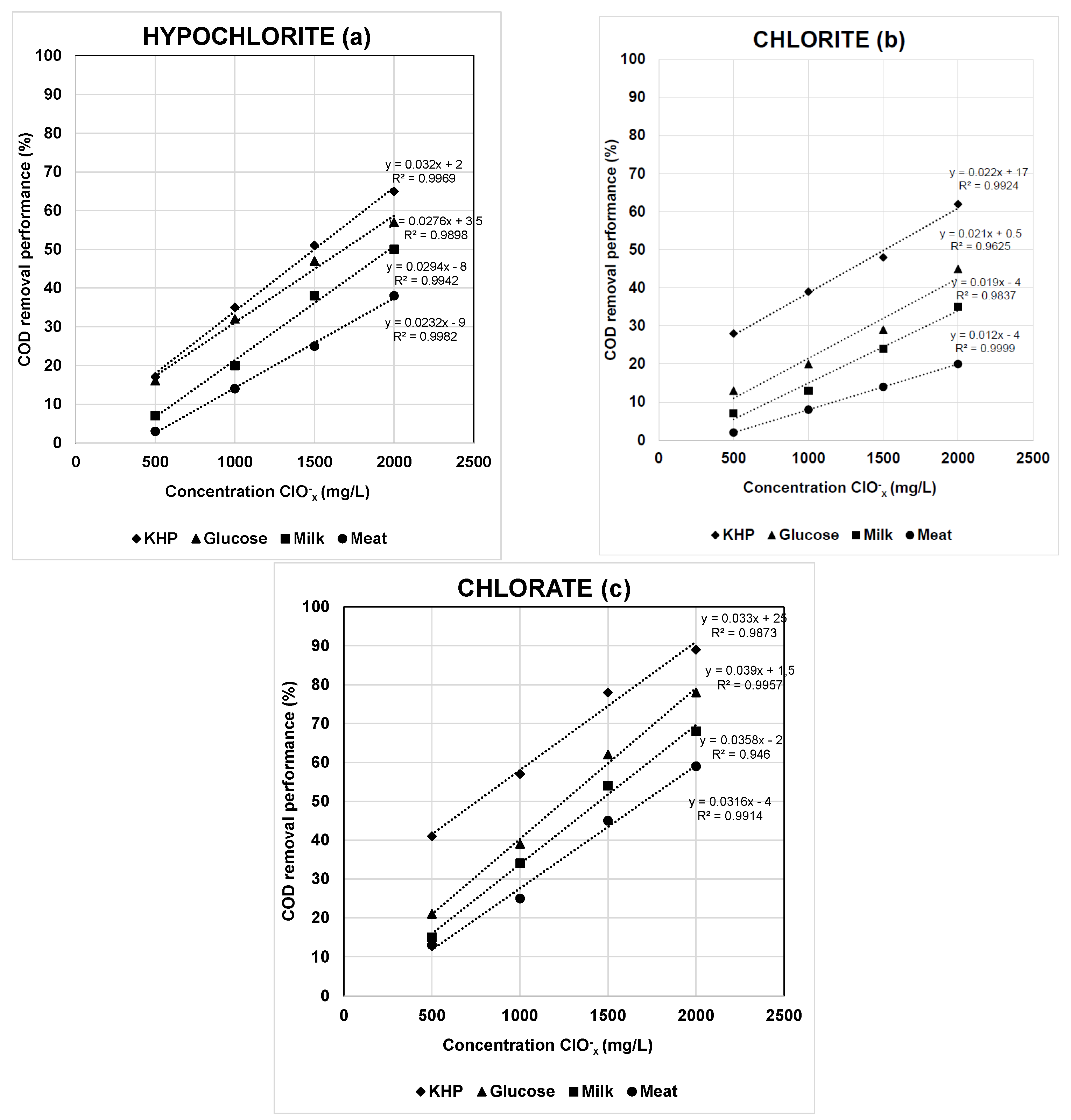
3.2. Evaluation of Oxychlorides Interference in COD Determination Using Synthetic Wastewaters
4. Conclusions
- The diverse ClOx− agents have different oxidizing capacity at the experimental conditions evaluated (at 150 °C for 120 min). However, ClO4− agent was not effective at all as a “COD remover”;
- In general, the experimental values of COD removal performance should be considered as dual concentration dependent. On the one hand, for each oxychloride oxidizing agent the COD reduction performance is directly proportional to the dosage used in the experiment (the higher the ClOx− concentration, the greater the COD removal performance). On the other hand, the COD removal performance was inversely proportional to the OM concentration present in the wastewater (the higher the OM concentration, the lower the COD removal performance);
- In addition, using simulated wastewater at a concentration of about 450 mg O2/L, the interference extent of ClOx− agents on COD measurements decreased in the order of KHP > glucose > milk > meat.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Public Health Association; American Water Works Association, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Barbosa Segundo, I.D.; Cardozo, J.C.; Castro, P.S.; Gondim, A.D.; dos Santos, E.V.; Martínez-Huitle, C.A. Cost-effective smartphone-based method for low range chemical oxygen demand analysis. MethodsX 2023, 11, 102300. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; de la Rubia, M.A.; Borja, R.; Alaíz, M. Assessment of a modified and optimized method for determining Chemical Oxygen Demand of solid substrates and solutions with high suspended solid content. Talanta 2008, 76, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Duan, N.; Wu, X.; Fang, H. COD discharge limits for urban wastewater treatment plants in China based on statistical methods. Water 2018, 10, 777. [Google Scholar] [CrossRef]
- Meng, X.; Khoso, S.A.; Lyu, F.; Wu, J.; Kang, J.; Liu, H.; Zhang, Q.; Han, H.; Sun, W.; Hu, Y. Study on the influence and mechanism of sodium chlorate on COD reduction of minerals processing wastewater. Miner. Eng. 2019, 134, 1–6. [Google Scholar] [CrossRef]
- Viana da Silva, A.M.E.; Bettencourt da Silva, R.J.N.; Camões, M.F.G.F.C. Optimization of the determination of chemical oxygen demand in wastewaters. Anal. Chim. Acta 2011, 699, 161–169. [Google Scholar] [CrossRef]
- Ma, J. Determination of chemical oxygen demand in aqueous samples with non-electrochemical methods. Trends Environ. Anal. Chem. 2017, 14, 37–43. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Talinli, I.; Anderson, G.K. Interference of hydrogen peroxide on the standard COD test. Water Res. 1992, 26, 107–110. [Google Scholar] [CrossRef]
- Kang, Y.W.; Cho, M.J.; Hwang, K.Y. Correction of hydrogen peroxide interference on standard chemical oxygen demand test. Water Res. 1999, 33, 1247–1251. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Zeng, Z.; Huang, Z.; Cui, Y. A method for removing persulfate interference in the analysis of the chemical oxygen demand in wastewater. Environ. Chem. Lett. 2019, 17, 1085–1089. [Google Scholar] [CrossRef]
- Htet, T.T.; Zeng, D. Preparation and application of a new composite COD remover. N. Am. Acad. Res. 2020, 3, 118–140. [Google Scholar] [CrossRef]
- Liu, L.; Jia, P.; Han, J.; Lichtfouse, E. The underground industry of wastewater adulteration: How to trick legal testing with COD removers. Environ. Chem. Lett. 2022, 20, 1–5. [Google Scholar] [CrossRef]
- Xiao, H.; Yan, W.; Zhao, Z.; Tang, Y.; Li, Y.; Yang, Q.; Luo, S.; Jiang, B. Chlorate induced false reduction in chemical oxygen demand (COD) based on standard dichromate method: Countermeasure and mechanism. Water Res. 2022, 221, 118732. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Zhu, J.; Ba, X.; Guo, X.; Zhang, Q.; Qi, Y.F.; Li, Y.; Wang, J.; Sun, H.; Jiang, B. Oxychlorides induced over-evaluation of electrochemical COD removal performance over dimensionally stable anode (DSA): The roles of cathode materials. Sep. Purif. Technol. 2022, 303, 122197. [Google Scholar] [CrossRef]
- Xiao, H.; Hao, Y.; Chen, J.; Feng, F.; Liu, Y.; Li, Y.; Luo, S.; Jiang, B. Overevaluation of electro-oxidation for Chemical Oxygen Demand removal using a boron-doped diamond anode: The roles of various electrogenerated oxychlorides and countermeasure. ACS EST Eng. 2023, 3, 283–294. [Google Scholar] [CrossRef]
- Xiao, H.; Xu, F.; Chen, J.; Hao, Y.; Guo, Y.; Zhu, C.; Luo, S.; Jiang, B. Electrogenerated oxychlorides induced overlooked negative effects on electro-oxidation wastewater treatment in terms of over-evaluated COD removal efficiency and biotoxicity. J. Hazard. Mater. 2023, 456, 131667. [Google Scholar] [CrossRef]
- Yan, W.; Chen, J.; Wu, J.; Li, Y.; Liu, Y.; Yang, Q.; Tang, Y.; Jiang, B. Investigation on the adverse impacts of electrochemically produced ClOx− on assessing the treatment performance of dimensionally stable anode (DSA) for Cl−-containing wastewater. Chemosphere 2023, 310, 136848. [Google Scholar] [CrossRef]
- Sophonsiri, C.; Morgenroth, E. Chemical composition associated with different particle size fractions in municipal, industrial, and agricultural wastewaters. Chemosphere 2004, 55, 691–703. [Google Scholar] [CrossRef]
- Baker, J.R.; Milke, M.W.; Mihelcic, J.R. Relationship between chemical and theoretical oxygen demand for specific classes of organic chemicals. Water Res. 1999, 33, 327–334. [Google Scholar] [CrossRef]
- Kargol, A.K.; Burrell, S.R.; Chakraborty, I.; Gough, H.L. Synthetic wastewater prepared from readily available materials: Characteristics and economics. PLoS Water 2023, 2, e0000178. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Gray, N.F. A comparative analysis of the characteristics of a range of real and synthetic wastewaters. Environ. Sci. Pollut. Res. 2013, 20, 8813–8830. [Google Scholar] [CrossRef] [PubMed]
- Urbansky, E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 2002, 9, 187–192. [Google Scholar] [CrossRef]
- Clark, J.A.; Yang, Y.; Ramos, N.C.; Hillhouse, H.W. Selective oxidation of pharmaceuticals and suppression of perchlorate formation during electrolysis of fresh human urine. Water Res. 2021, 198, 117106. [Google Scholar] [CrossRef]

| D-Glucose | Meat | Milk | |
|---|---|---|---|
| Loss of drying (%) | 0.1 (0.0) | 6.1 (0.2) | 3.6 (0.1) |
| Dry matter—DM (%) | 99.9 (0.2) | 93.9 (0.2) | 96.4 (0.1) |
| Organic matter (% DM) | 100 (0.1) | 85.6 (0.1) | 89.7 (0.1) |
| Ash (% DM) | 0.0 (0.0) | 8.3 (0.1) | 6.8 (0.1) |
| COD (g O2/g DM) | 1.07 (0.01) | 1.27 (0.02) | 1.26 (0.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez González, J.A.; Fernández Mohedano, A.; Raposo Bejines, F. Evaluating Alternative Oxidants for Artificial Chemical Oxygen Demand Removal Performance from Wastewater Treatment Plants. Resources 2025, 14, 46. https://doi.org/10.3390/resources14030046
Gutiérrez González JA, Fernández Mohedano A, Raposo Bejines F. Evaluating Alternative Oxidants for Artificial Chemical Oxygen Demand Removal Performance from Wastewater Treatment Plants. Resources. 2025; 14(3):46. https://doi.org/10.3390/resources14030046
Chicago/Turabian StyleGutiérrez González, Julio Alejandro, Angel Fernández Mohedano, and Francisco Raposo Bejines. 2025. "Evaluating Alternative Oxidants for Artificial Chemical Oxygen Demand Removal Performance from Wastewater Treatment Plants" Resources 14, no. 3: 46. https://doi.org/10.3390/resources14030046
APA StyleGutiérrez González, J. A., Fernández Mohedano, A., & Raposo Bejines, F. (2025). Evaluating Alternative Oxidants for Artificial Chemical Oxygen Demand Removal Performance from Wastewater Treatment Plants. Resources, 14(3), 46. https://doi.org/10.3390/resources14030046







