Animal Fats and Vegetable Oils—Promising Resources for Obtaining Effective Corrosion Inhibitors for Oil Refinery Equipment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
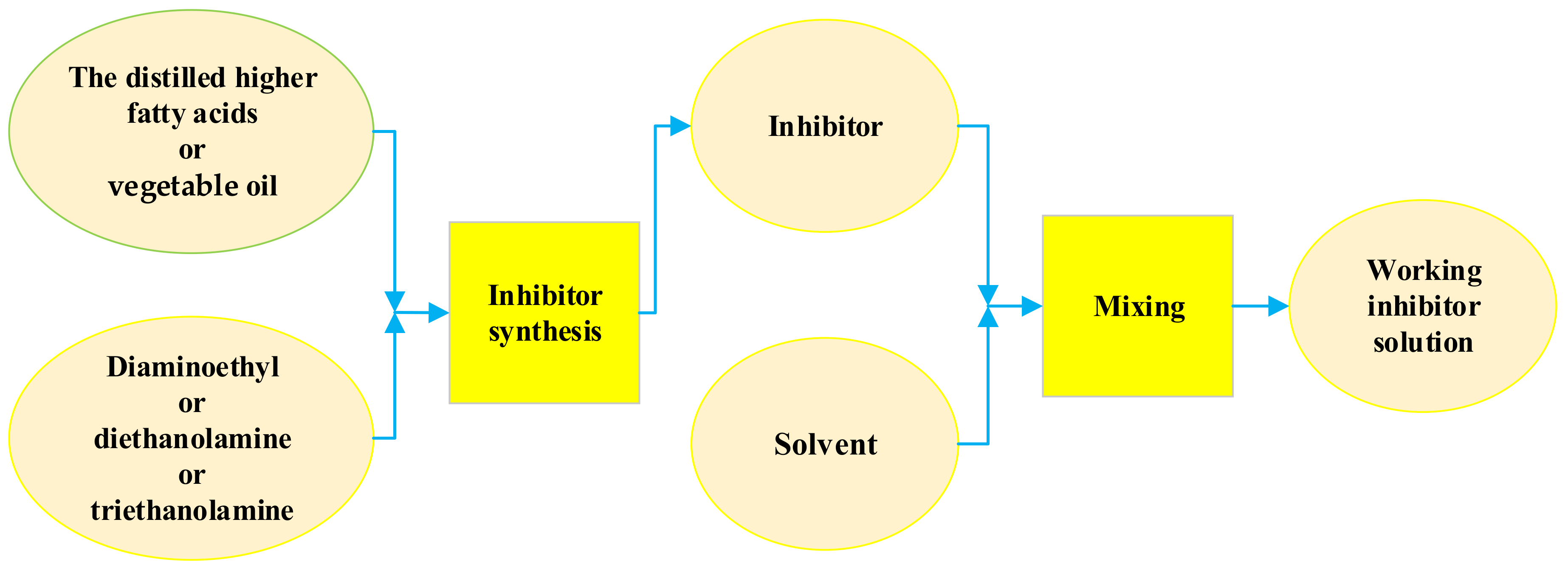
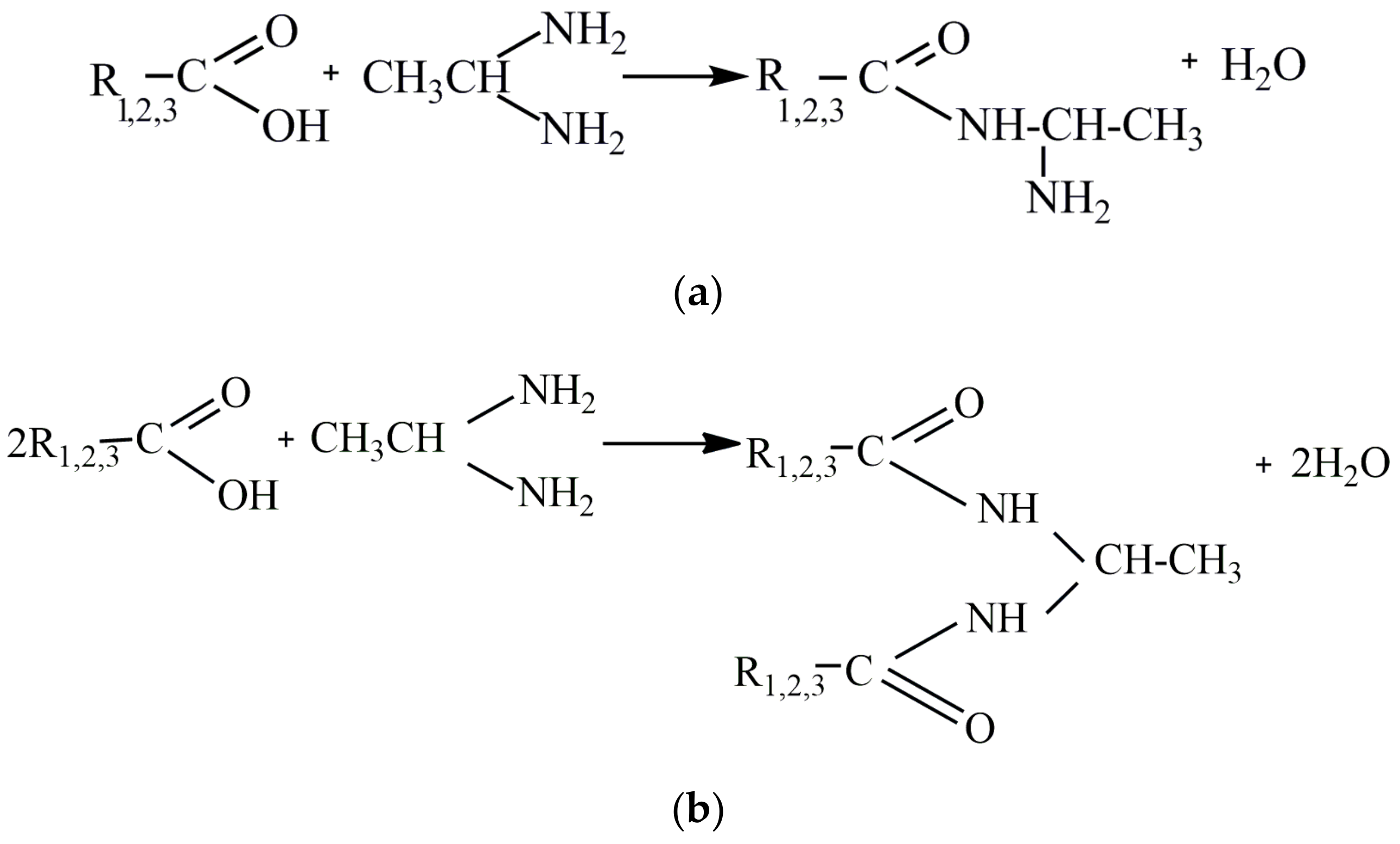
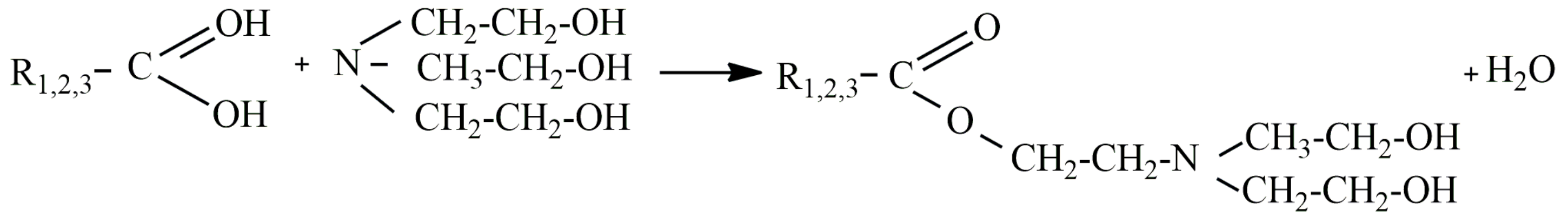
2.2. Synthesis of Corrosion Inhibitors and Preparation of Working Solution
2.3. Methods of Analysis
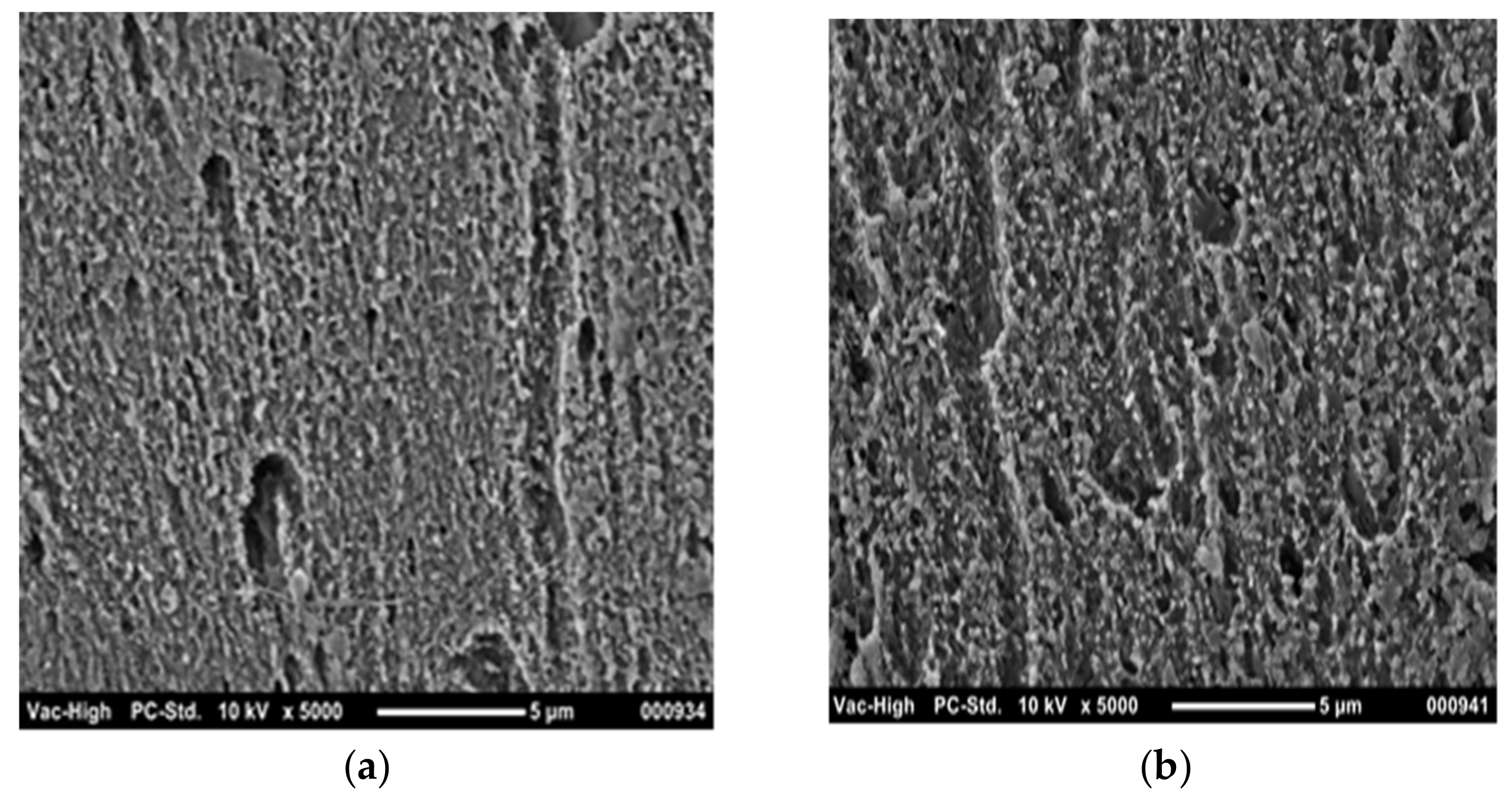
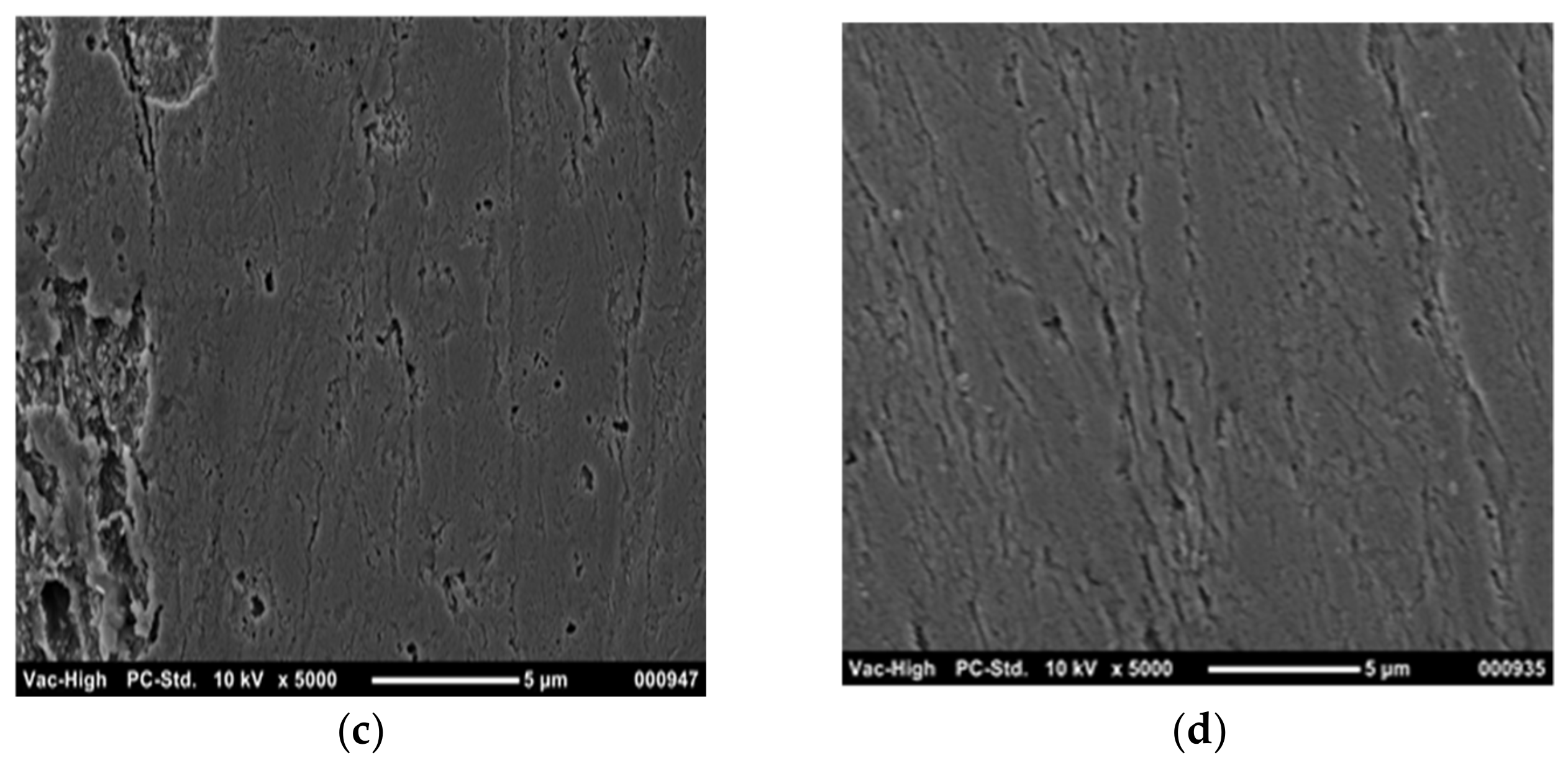
3. Results and Discussion
- -
- with increasing temperature, the density (and, accordingly, the molecular weight) of the products increases;
- -
- the temperature of 130 °C is optimal for achieving maximum molecular activity, which contributes to forming a stable protective film.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pyshyev, S.; Miroshnichenko, D.; Chipko, T.; Donchenko, M.; Bogoyavlenska, O.; Lysenko, L.; Miroshnychenko, M.; Prysiazhnyi, Y. Use of Lignite Processing Products as Additives to Road Petroleum Bitumen. Chem. Eng. 2024, 8, 27. [Google Scholar] [CrossRef]
- Casau, M.; Dias, M.F.; Matias, J.C.O.; Nunes, L.J.R. Residual Biomass: A Comprehensive Review on the Importance, Uses and Potential in a Circular Bioeconomy Approach. Resources 2022, 11, 35. [Google Scholar] [CrossRef]
- Pstrowska, K.; Łużny, R.T.; Fałtynowicz, H.; Jaroszewska, K.; Postawa, K.; Pyshyev, S.; Witek-Krowiak, A. Unlocking sustainability: A comprehensive review of up-recycling biomass waste into biochar for environmental solutions. Chem. Chem. Technol. 2024, 18, 211–231. [Google Scholar] [CrossRef]
- Filho, J.J.d.S.; Gaspar, P.D.; Souza, A.C.d.; Paço, A.d. Circular Economy in Guaiamum and Uçá Crab Waste in Brazil: Potential By-Products—A Systematic Literature Review. Resources 2024, 13, 46. [Google Scholar] [CrossRef]
- Szpilko, D.; de la Torre Gallegos, A.; Jimenez Naharro, F.; Rzepka, A.; Remiszewska, A. Waste Management in the Smart City: Current Practices and Future Directions. Resources 2023, 12, 115. [Google Scholar] [CrossRef]
- Rai, M.; Pant, G.; Pant, K.; Aloo, B.N.; Kumar, G.; Singh, H.B.; Tripathi, V. Microplastic Pollution in Terrestrial Ecosystems and Its Interaction with Other Soil Pollutants: A Potential Threat to Soil Ecosystem Sustainability. Resources 2023, 12, 67. [Google Scholar] [CrossRef]
- Bannikov, L.; Miroshnichenko, D.; Pylypenko, O.; Pyshyev, S.; Fedevych, O.; Meshchanin, V. Coke quenching plenum equipment corrosion and its dependents on the quality of the biochemically treated water of the coke-chemical production. Chem. Chem. Technol. 2022, 16, 328–336. [Google Scholar] [CrossRef]
- Saji, V.S.; Umoren, S.A. (Eds.) Corrosion Inhibitors in the Oil and Gas Industry; John Wiley & Sons: Hoboken, NJ, USA, 2020; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527822140 (accessed on 14 February 2020).
- Sriplai, N.; Sombatmankhong, K. Corrosion inhibition by imidazoline and imidazoline derivatives: A review. Corros. Rev. 2023, 41, 237–262. [Google Scholar] [CrossRef]
- Kousar, K.; Ljungdahl, T.; Show, A.W. An Exemplar Imidazoline Surfactant for Corrosion Inhibitor Studies: Synthesis, Characterization, and Physicochemical Properties. JSD 2019, 23, 225–234. [Google Scholar] [CrossRef]
- Jalab, R.; Saad, M.; Benali, A.; Hussein, I.A.; Khaled, M. Biodegradable polysaccharide grafted polyacrylamide inhibitor for corrosion in CO2- saturated saline solution. Heliyon 2023, 9, e20304. [Google Scholar] [CrossRef]
- Lavanya, M.; Avryl, A. Surfactants as biodegradable sustainable inhibitors for corrosion control in diverse media and conditions: A comprehensive review. Sci. Total Environ. 2024, 908, 168407. [Google Scholar] [CrossRef]
- Obot, I.B.; Moses, M.S.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. JIEC 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Obot, I.B.; Ikenna, B.; Onyeachu, S.A.; Umoren, M.A.; Quraishi, A.A.; Sorour, T.; Aljeaban, N.; Wang, Q. High temperature sweet corrosion and inhibition in the oil and gas industry: Progress, challenges and future perspectives. J. Pet. Eng. 2020, 185, 106469. [Google Scholar] [CrossRef]
- Goni, L.; Mazumder, M.; Green Corrosion Inhibitors. 30 April 2019. Available online: https://www.intechopen.com/chapters/63755 (accessed on 30 April 2019).
- Shang, Z.; Zhu, J. Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol. 2021, 15, 5078–5094. [Google Scholar] [CrossRef]
- Topilnytskyy, P.; Romanchuk, V.; Yarmola, T. Production of Corrosion Inhibitors for Oil Refining Equipment Using Natural Components. Chem. Chem. Tech. 2018, 12, 400–404. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Verotta, L. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.S.; de Almeida, T.F.; Santos, A.d.M.; Tokumoto, M.S.; Cotting, F.; Capelossi, V.R. Evaluation of vegetables residues as corrosion inhibitors. Res. Soc. 2021, 10, 2525–3409. [Google Scholar] [CrossRef]
- Sun, Z.; Singh, A.; Xu, X.; Chen, S.; Liu, W.; Lin, Y. Inhibition effect of pomelo peel extract for N80 steel in 3.5% NaCl saturated with CO2 solution. Rev. Chem. Intermed. 2017, 11, 6719–6736. [Google Scholar] [CrossRef]
- Su, F.; Gu, M.; Zhao, J. Inhibition of carbon steel corrosion in solution saturated with CO2 by ginkgo leaves extract. J. Hebei Univ. Eng. Nat. Sci. Ed. 2016, 36, 264–268. [Google Scholar]
- Pogrebova, I.S. Monograph: Model of Adsorption of Organic Compounds on Metals, Based on the Concept of Formation of Complexes with Charge Transfer, and Their Use in the Selection of Corrosion Inhibitors. Promising Materials and Processes in Applied Electrochemistry; KNUTD: Kyiv, Ukraine, 2018; pp. 195–214. (In Ukrainian) [Google Scholar]
- Diaz, E.F.; Gonzalez-Rodriguez, J.G.; Martinez-Villafañe, A.; Gaona-Tiburcio, C. H2S corrosion inhibition of an ultra high strength pipeline by carboxyethyl-imidazoline. J. Appl. Electrochem. 2010, 40, 1633. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Palimi, M.J.; Tang, Y.; Alvarez, V.; Kuru, E.; Li, D.Y. Green corrosion inhibitors for drilling operation: New derivatives of fatty acid-based inhibitors in drilling fluids for 1018 carbon steel in CO2-saturated KCl environments. Mater. Chem. Phys. 2022, 288, 126406. [Google Scholar] [CrossRef]
- Swathi, P.N.; Rasheeda, K.; Samshuddin, S.; Alva, V. Fatty acids and its derivatives as corrosion inhibitors for mild steel—An overview. Asian Sci. Res. 2017, 7, 301–308. [Google Scholar] [CrossRef]
- Elabar, D.; Elgazali, A.; Gebril, M.; Haidar, F.; Esmaael, R.; Ahmad, I. The Comparative Corrosion Inhibition tendency of Imidazolines Prepared from Fatty Acids of Carbon Chain 16 or 18 with Diethylene Triamine. IJCS 2023, 13, 86–93. [Google Scholar]
- Desimone, M.P.; Gordillo, G.; Simison, S.N. The effect of temperature and concentration on the corrosion inhibition mechanism of an amphiphilic amido-amine in CO2 saturated solution. Corros. Sci. 2011, 53, 4033–4043. [Google Scholar] [CrossRef]
- Solmaz, R.; Salc, A.; Dursun, Y.A.; Kardaş, G. A comprehensive study on the adsorption, corrosion inhibition efficiency and stability of acriflavine on mild steel in 1 M HCl solution. Colloids Surf. A: Physicochem. Eng. Asp. 2023, 674, 131908. [Google Scholar] [CrossRef]
- Huang, W.; Tang, Z.; Liu, X.; Liu, L.; Zhong, H.; Yu, Y.; Chen, H.; Wang, C.; Jiang, Q.; Ye, Y.W.; et al. A green and effective anti-corrosion and anti-microbial inhibitor of citric acid-based carbon dots: Experiment and mechanism analysis. J. Mater. Res. Technol. 2024, 32, 2149–2159. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Zou, Y.; Zhao, H.; Chen, H. A feasible method to improve the protection ability of metal by functionalized carbon dots as environment-friendly corrosion inhibitor. J. Clean. Prod. 2020, 264, 121682. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, W.; Liu, L.; Li, H.; Meng, H.; Zeng, T.; Ye, X.; Jiang, Q.; Ye, Y.W.; Liu, Y. Study on structure and molecular scale protection mechanism of green Ce, N-CDs anti-bacterial and anti-corrosive inhibitor. J. Mater. Res. Technol. 2024, 28, 3868–3881. [Google Scholar] [CrossRef]
- Zou, C.J.; Tang, Q.W.; Zhao, P.W.; Guan, E.D.; Wu, X.; Ye, H. Further study on the inclusion complex of 2-phosphonobutane-1,2,4-tricarboxylic acid with bcyclodextrin: A new insight of high inhibition efficiency for protecting steel corrosion. J. Petrol. Sci. Eng. 2013, 103, 29–35. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, Y. Experimental and theoretical studies of benzaldehyde thiosemicarbazone derivatives as corrosion inhibitors for mild steel in acid media. J. Mol. Str. 2019, 1177, 90–100. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L.; Kaya, S.; Katin, K.P.; Verma, D.K.; Rbaa, M.; Dagdag, O.; Haldhar, R. Novel bromide–cucurbit [7] uril supramolecular ionic liquid as a green corrosion inhibitor for the oil and gas industry. JEAC. 2021, 901, 115794. [Google Scholar] [CrossRef]
- Haldhar, R.; Raorane, C.J.; Mishra, V.K.; Periyasamy, T.; Berisha, A.; Kim, S.-C. Development of different chain lengths ionic liquids as green corrosion inhibitors for oil and gas industries: Experimental and theoretical investigations. J. Mol. Liq. 2023, 372, 121168. [Google Scholar] [CrossRef]
- OEC. Available online: https://oec.world/en/profile/hs/animal-fat?latestTrendsYAxisSelector=log (accessed on 1 December 2024).
- ReportLinker. Available online: https://www.reportlinker.com/clp/global/457161#block-indicator (accessed on 1 December 2024).
- USDA. Available online: https://fas.usda.gov/data/production/commodity-group/oilseeds%2C%20oil (accessed on 1 December 2024).
- Ukrainian Research Institute of Oils and Fats of the National Academy of Agrarian Sciences of Ukraine. Oil and Fat Industry of Ukraine. Information and Analytical Bulletin of the Oil and Fat Industry of Ukraine. Scientific publication. Kharkiv, Ukraine. 2021. Available online: https://www.fatoil.com.ua/images/document/9187574/52021.pdf (accessed on 15 December 2021).
- Romanchuk, V.; Topilnytskyy, P. Investigation of Reagents with Different Chemical Compositions for Protection of Oil Primary Refining Equipment. Chem. Chem. Technol. 2010, 3, 231–236. [Google Scholar] [CrossRef]
- DSTU 7809; Rolled long products, calibrated with special surface treatment of high-quality carbon structural steel. General technical conditions: DP “UkrNDC”, Kyiv, Ukraine, 2016. (In Ukrainian)
- Ibrahimi, B.; Jmiai, L.; Bazzi, S. El Issami Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Roscher, J.; Liu, D.; Xie, X.; Holze, R. Aromatic Metal Corrosion Inhibitors. Corros. Mater. Degrad. 2024, 5, 513–560. [Google Scholar] [CrossRef]
- Abdel-Fatah, T.M.; Abdel-Samad, H.S.; Hassan, A.A.M.; El-Sehiety, H.E.E. Effect of variation of the structure of amino acids on inhibition of the corrosion of low-alloy steel in ammoniated citric acid solutions. Res. Chem. Intermed. 2014, 40, 1675–1690. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Nasser, N.M.; El-Azabawy, O.E.; Tabey, A.E.E. Corrosion inhibition behavior of new synthesized nonionic surfactants based on amino acid on carbon steel in acid media. J. Mol. Liq. 2016, 219, 1078–1088. [Google Scholar] [CrossRef]
- ASTM D97; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D86; Standard Test Method for Distillation of Petroleum Products at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D1298; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D92; Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM International: West Conshohocken, PA, USA, 2024.
- Pat. 64703 Ukraine MPK51 C23F 11/10, C10 G 75/00, C10 G 7/00 Method for determining the degree of protection of corrosion inhibitors for oil refinery equipment/Romanchuk V.V.; Topilnytskyi P.I.; applicant and patent owner National University “Lviv Polytechnic”. - No. u 2011 06534; appl. 24.05.2011, publ. 10.11.2011, bull. No. 21. Available online: https://base.uipv.org/searchInvRevoke/search.php?action=showsearchresults&page=1&lang=ukr&qp=claims_per_page%3D10%3Bch_0%3Don%3Bqe0%3D64703%3B (accessed on 10 November 2011).
- Ha, M.-G.; Heo, C.-J.; Ahn, J.-H. Monitoring for relative effect of corrosive environmental factor on corrosion rate for steel structural details. J. Build. Eng. 2023, 78, 107565. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Lu, X.; Wang, Z. Effect of temperature and ultraviolet radiation on corrosion behavior of carbon steel in high humidity tropical marine atmosphere. Mater. Chem. Phys. 2022, 277, 124962. [Google Scholar] [CrossRef]


| Raw Material | Content of Acids, wt% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lauric C12H24O2 | Myristic C14H28O2 | Palmitic C16H32O2 | Stearic C18H36O2 | Oleic C18H34O2 | Erucic C22H42O2 | Linoleic C18H32O2 | Linolenic C18H30O2 | Average Amount of Unsaturated Acids | |
| Corn oil | - | - | 9–19 | 1–3 | 40 | - | 40 | 1 | 80 |
| Sunflower oil | - | - | - | 6 | 35 | - | 56 | - | 91 |
| Coconut oil | 49 | 16 | 9 | 2 | 6 | - | 2 | - | 8 |
| Beef fat | - | - | 32 | 14 | 48 | - | 3 | - | 51 |
| Sample No. | Manufacturer | Active Substance | Solvent | Product Name |
|---|---|---|---|---|
| 1 | Chimec S.p.A. | Alkyl imidazoline | Heavy aromatic hydrocarbons | Chimec 1839W |
| 2 | SUEZ Group | n-9-octadecyl 1,3-propane diamine | C10 hydrocarbons, heavy aromatic hydrocarbons | PhilmPlus 5068E |
| 3 | Clariant AG | Amides of polyamine naphthenic acids | C10 hydrocarbons, heavy aromatic hydrocarbons | Dodigen 481 |
| 4 | Nalco Water | Tallow oil hydroxyethyl imidazoline | Heavy aromatic hydrocarbons | Nalco EC1021A |
| 5 | Barva LLC | Diethanol-aminoethyl heptadecenyl imidazoline | C8 aromatic hydrocarbons | Carbosoline OT-2 |
| Index | Sample No. According to Table 2 | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Physical state | liquid | liquid | liquid | liquid | liquid |
| Pour point, °C | −31 | −34 | −32 | −25 | −20 |
| Initial boiling point, °C | 180 | 177 | 200 | 180 | 139 |
| Density at 20 °C, kg/m3 | 980 | 890 | 940 | 918 | 910 |
| Viscosity at 20 °C, mm2/s | 100 | 86 | 94 | 15 | 92 |
| Flashpoint, °C | >61 | 61 | 85 | 68 | 35 |
| C | Si | Mn | Ni | Cr | Cu | P | S | As | |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.17–0.24 | 0.17–0.37 | 0.35–0.65 | ≤0.3 | ≤0.25 | ≤0.3 | ≤0.035 | ≤0.04 | ≤0.08 |
| Sample No. | Synthesis Temperature, °C | Oil: Triethanolamine Ratio, wt/wt |
|---|---|---|
| Sunflower oil | ||
| 6.1 | 120 | 69.3:30.7 |
| 6.2 | 130 | 69.3:30.7 |
| 6.3 | 140 | 69.3:30.7 |
| 6.4 | 150 | 69.3:30.7 |
| Corn oil | ||
| 7.1 | 120 | 65.3:34.7 |
| 7.2 | 130 | 65.3:34.7 |
| 7.3 | 140 | 65.3:34.7 |
| 7.4 | 150 | 65.3:34.7 |
| Coconut oil | ||
| 8.1 | 120 | 67.0:33.0 |
| 8.2 | 130 | 67.0:33.0 |
| 8.3 | 140 | 67.0:33.0 |
| 8.4 | 150 | 67.0:33.0 |
| Sample No. | Synthesis Temperature,°C | DHFA: Amine Ratio, wt/wt |
| DHFA: diaminoethyl | ||
| 9.1 | 120 | 82:18 |
| 9.2 | 130 | 82:18 |
| 9.3 | 140 | 82:18 |
| 10.1 | 120 | 90:10 |
| 10.2 | 130 | 90:10 |
| 10.3 | 140 | 90:10 |
| DHFA: diethanolamine | ||
| 11.1 | 120 | 72:28 |
| 11.2 | 130 | 72:28 |
| 11.3 | 140 | 72:28 |
| Sample No. | Reaction Medium Density, kg/m3 | V, g/(m2·h) | Z, % |
|---|---|---|---|
| Blank (V0) | - | 1.1470 ± 5% | - |
| Sunflower oil | |||
| 6.1 | 919 ± 1 | 1.0360 ± 5% | 9.7 ± 5% |
| 6.2 | 970 ± 1 | 0.3292 ± 5% | 71.3 ± 5% |
| 6.3 | 978 ± 1 | 0.2799 ± 5% | 75.6 ± 5% |
| 6.4 | 984 ± 1 | 0.8820 ± 5% | 23.1 ± 5% |
| Corn oil | |||
| 7.1 | 975 ± 1 | 0.9956 ± 5% | 13.2 ± 5% |
| 7.2 | 981 ± 1 | 0.3498 ± 5% | 69.5 ± 5% |
| 7.3 | 982 ± 1 | 0.5597 ± 5% | 51.2 ± 5% |
| 7.4 | 985 ± 1 | 0.7456 ± 5% | 35.0 ± 5% |
| Coconut oil | |||
| 8.1 | 940 ± 1 | 0.6331 ± 5% | 44.8 ± 5% |
| 8.2 | 958 ± 1 | 0.3452 ± 5% | 69.9 ± 5% |
| 8.3 | 975 ± 1 | 0.6022 ± 5% | 47.5 ± 5% |
| 8.4 | 980 ± 1 | 0.4003 ± 5% | 65.1 ± 5% |
| Sample No. | Reaction Medium Density, kg/m3 | V, g/(m2·h) | Z, % |
|---|---|---|---|
| Blank (V0) | - | 1.1470 ± 5% | - |
| DHFA: diaminoethyl | |||
| 9.1 | 974 ± 1 | 0.0734 ± 5% | 93.6 ± 5% |
| 9.2 | 979 ± 1 | 0.0677 ± 5% | 94.1 ± 5% |
| 9.3 | 980 ± 1 | 0.0860 ± 5% | 92.5 ± 5% |
| 10.1 | 982 ± 1 | 0.2053 ± 5% | 82.1 ± 5% |
| 10.2 | 984 ± 1 | 0.1915 ± 5% | 83.3 ± 5% |
| 10.3 | 989 ± 1 | 0.2110 ± 5% | 81.6 ± 5% |
| DHFA: diethanolamine | |||
| 11.1 | 975 ± 1 | 0.0631 ± 5% | 94.5 ± 5% |
| 11.2 | 978 ± 1 | 0.0505 ± 5% | 95.6 ± 5% |
| 11.3 | 979 ± 1 | 0.0803 ± 5% | 93.0 ± 5% |
| Sample No. According to Table 5 | 50 °C | 60 °C | 70 °C | |||
|---|---|---|---|---|---|---|
| V, g/(m2·h) | Z, % | V, g/(m2·h) | Z, % | V, g/(m2·h) | Z, % | |
| Blank(V0) | 1.1470 ± 5% | - | 1.1960 ± 5% | - | 1.2007 ± 5% | - |
| 9.1 | 0.0734 ± 5% | 93.6 ± 5% | 0.1017 ± 5% | 91.5 ± 5% | 0.1237 ± 5% | 89.7 ± 5% |
| 9.2 | 0.0677 ± 5% | 94.1 ± 5% | 0.0945 ± 5% | 92.1 ± 5% | 0.1213 ± 5% | 89.9 ± 5% |
| 9.3 | 0.0860 ± 5% | 92.5 ± 5% | 0.1124 ± 5% | 90.6 ± 5% | 0.1381 ± 5% | 88.5 ± 5% |
| 10.1 | 0.2053 ± 5% | 82.1 ± 5% | 0.2380 ± 5% | 80.1 ± 5% | 0.2618 ± 5% | 78.2 ± 5% |
| 10.2 | 0.1915 ± 5% | 83.3 ± 5% | 0.2260 ± 5% | 81.1 ± 5% | 0.2642 ± 5% | 78.0 ± 5% |
| 10.3 | 0.2110 ± 5% | 81.6 ± 5% | 0.2404 ± 5% | 79.9 ± 5% | 0.2882 ± 5% | 76.0 ± 5% |
| 11.1 | 0.0631 ± 5% | 94.5 ± 5% | 0.0849 ± 5% | 92.9 ± 5% | 0.1105 ± 5% | 90.8 ± 5% |
| 11.2 | 0.0504 ± 5% | 95.6 ± 5% | 0.0730 ± 5% | 93.9 ± 5% | 0.0997 ± 5% | 91.7 ± 5% |
| 11.3 | 0.0802 ± 5% | 93.0 ± 5% | 0.1076 ± 5% | 91.0 ± 5% | 0.1417 ± 5% | 88.2 ± 5% |
| Sample No. According to Table 5 | 50 °C | 60 °C | 70 °C | |||
|---|---|---|---|---|---|---|
| V, g/(m2·h) | Z, % | V, g/(m2·h) | Z, % | V, g/(m2·h) | Z, % | |
| Blank (V0) | 1.1470 ± 5% | - | 1.1960 ± 5% | - | 1.2007 ± 5% | - |
| 9.2 | 0.0206 ± 5% | 98.2 ± 5% | 0.0466 ± 5% | 96.1 ± 5% | 0.0756 ± 5% | 93.7 ± 5% |
| 10.2 | 0.1503 ± 5% | 86.9 ± 5% | 0.1854 ± 5% | 84.5 ± 5% | 0.2149 ± 5% | 82.1 ± 5% |
| 11.2 | 0.0092 ± 5% | 99.2 ± 5% | 0.0347 ± 5% | 97.1 ± 5% | 0.0504 ± 5% | 95.8 ± 5% |
| Sample No. According to Table 2 and Table 5 | 50 °C | 60 °C | 70 °C | |||
|---|---|---|---|---|---|---|
| V, g/(m2·h) | Z, % | V, g/(m2·h) | Z, % | V, g/(m2·h) | Z, % | |
| Blank (V0) | 1.1470 ± 5% | - | 1.1960 ± 5% | - | 1.2007 ± 5% | - |
| 1 | 0.0963 ± 5% | 91.6 ± 5% | 0.1304 ± 5% | 89.1 ± 5% | 0.1561 ± 5% | 87.0 ± 5% |
| 2 | 0.0401 ± 5% | 96.5 ± 5% | 0.0610 ± 5% | 94.9 ± 5% | 0.0828 ± 5% | 93.1 ± 5% |
| 3 | 0.0057 ± 5% | 99.5 ± 5% | 0.0251 ± 5% | 97.9 ± 5% | 0.0504 ± 5% | 95.8 ± 5% |
| 4 | 0.0528 ± 5% | 95.4 ± 5% | 0.0742 ± 5% | 93.8 ± 5% | 0.0961 ± 5% | 92.0 ± 5% |
| 5 | 0.0252 ± 5% | 97.8 ± 5% | 0.0490 ± 5% | 95.9 ± 5% | 0.0756 ± 5% | 93.7 ± 5% |
| 11.2 | 0.0092 ± 5% | 99.2 ± 5% | 0.0347 ± 5% | 97.1 ± 5% | 0.0504 ± 5% | 95.8 ± 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyshyev, S.; Romanchuk, O.; Topilnytskyy, P.; Romanchuk, V.; Miroshnichenko, D.; Rohovyi, Y.; Omelianchuk, H.; Parkhomov, Y. Animal Fats and Vegetable Oils—Promising Resources for Obtaining Effective Corrosion Inhibitors for Oil Refinery Equipment. Resources 2025, 14, 30. https://doi.org/10.3390/resources14020030
Pyshyev S, Romanchuk O, Topilnytskyy P, Romanchuk V, Miroshnichenko D, Rohovyi Y, Omelianchuk H, Parkhomov Y. Animal Fats and Vegetable Oils—Promising Resources for Obtaining Effective Corrosion Inhibitors for Oil Refinery Equipment. Resources. 2025; 14(2):30. https://doi.org/10.3390/resources14020030
Chicago/Turabian StylePyshyev, Serhiy, Oleksandr Romanchuk, Petro Topilnytskyy, Viktoriya Romanchuk, Denis Miroshnichenko, Yurii Rohovyi, Hennadii Omelianchuk, and Yurii Parkhomov. 2025. "Animal Fats and Vegetable Oils—Promising Resources for Obtaining Effective Corrosion Inhibitors for Oil Refinery Equipment" Resources 14, no. 2: 30. https://doi.org/10.3390/resources14020030
APA StylePyshyev, S., Romanchuk, O., Topilnytskyy, P., Romanchuk, V., Miroshnichenko, D., Rohovyi, Y., Omelianchuk, H., & Parkhomov, Y. (2025). Animal Fats and Vegetable Oils—Promising Resources for Obtaining Effective Corrosion Inhibitors for Oil Refinery Equipment. Resources, 14(2), 30. https://doi.org/10.3390/resources14020030








