Biocatalytic Recycling of Polyethylene Terephthalate: From Conventional to Innovative Routes for Transforming Plastic and Textile Waste into Renewable Resources
Abstract
1. Introduction
2. Resources: Plastic and Textile Waste to PET
3. PET Characteristics and Recycling Context
| Approach | Mechanical Recycling | Chemical Recycling | Biological Recycling | Ref |
|---|---|---|---|---|
| Technology recycling of PET |
|
| Various microorganism is used to recycle PET | [26] |
| Product | New PET bottle | Dimethyl terephthalate, Bis(2-hydroxyethyl) terephthalate, TPA, MEG, and oligomers | Dimethyl terephthalate, Bis(2-hydroxyethyl) terephthalate, TPA, MEG | [26] |
| Rate of degradation | High speed | High speed | Moderate | [56] |
| Separation process | Applicable | Complicated to separate the product | Applicable | [56] |
| Temperature/Pressure | High | High | Moderate | [26] |
| Greenhouse gas | Low | High | Moderate | |
| Advantages |
|
|
| [26,57] |
| Drawbacks |
|
|
| [57,58] |
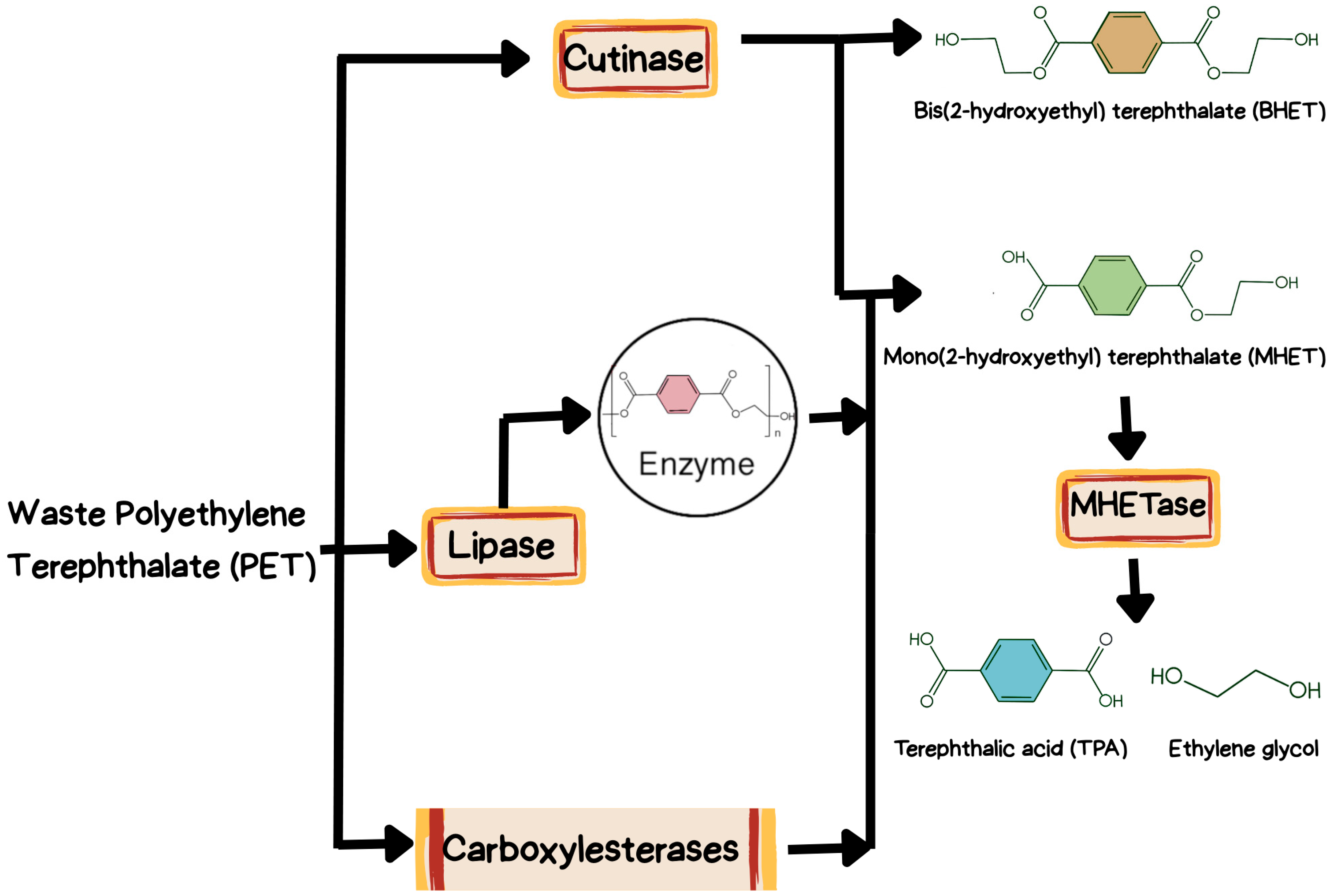
4. Classes of PET-Hydrolyzing Enzymes
4.1. Cutinase
4.2. Carboxylesterases
| Substrate | Type of Enzyme | Source | Condition Operations | PET Degradation, % | Ref | ||
|---|---|---|---|---|---|---|---|
| T, °C | t, h | pH | |||||
| PET package | LC-cutinase | Metagenome from leaf branch compost | 70 | 24 | 8 | ≤25 | [80] |
| Amorphous PET film | Variant of TfCut2 | T. f usca KW3 | 60 | 24 | 8 | ≤25 | [81] |
| Amorphized and micronized PET | LC-cutinase variant | Metagenome from leaf branch compost | 72 | 10 | 9 | 90 | [82] |
| PET film | Cut190**SS | Escherichia coli | 70 | 48 | 8.6 | 10.1 | [83] |
| Amorphous PET film | Thermobifida fusca cutinase TfCut2 | B. subtilis strain RH 11496 | 70 | 96 | 8 | 50 | [84] |
| Textile PET fibres | Cutinase ICCGDAQI | E. coli BL21 (DE3) | 70 | 14 | 9 | 97 | [85] |
| PET-GF | Variant of Cut190 | Saccharomonospora viridis AHK 90 | 63 | NA | ±8 | 33.6 ± 3.0 | [86] |
| PET-GF film | HiC (Novo) | Humicola insolens | 70 | 96 | 6.5–9.5 | 97 ± 3 | [87] |
| PET | ThcCut1-G63A/F210I/D205C /E254C/Q93G (ThcCut1-AICCG) | NA | 70 | 96 | NA | 96.2 | [68] |
| Post-industrial PET fibres | Cutinase ICCGDAQI | E. coli | 70 | 24 | 9 | 97.8 | [88] |
| Post-consumer PET bottles | Cutinase Est1_5M | Thermobifida alba AHK119 | 65 | 24–72 | 8 | 90.8 | [89] |
| PET fabrics | Tfu_0883 | Thermobififida fusca | 60 | 48 | 7.5 | 50 | [90] |
4.3. Lipases
| Substrate | Crystallinity, % | Type of Enzyme | Source | Condition Operations | Kinetic Parameter * | Product | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T, °C | t, h | pH | kcat | km | R2 | ||||||
| Post-consumer PET | 41.1 | Humicola insolens cutinase | Novozymes | 70 | 96 | 7 | 224.2 ± 34.0 (h−1) | 0.0041 ± 0.0010 (L g−1) | 0.981 | TPA + MHET + BHET | [108] |
| Bis(benzoyloxyethyl) terephthalate (3PET) with (p-nitrophenyl acetate (PNPA)) | NA | Tha_Cut1 | Escherichia coli BL21-Gold(DE3) | 50 | NA | 7 | 2.72 ± 0.2 (h−1) | 213 19 (μmol L−1) | NA | MHET, TPA, Benzoic acid, 2-hydroxyethyl benzoate | [109] |
| Bis(benzoyloxyethyl) terephthalate (3PET) with (p-nitrophenyl butyrate (PNPB)) | NA | Tha_Cut1 | E.coli BL21-Gold(DE3) | 50 | NA | 7 | 6.03 ± 0.59 (s−1) | 1933 ± 306 (μmol L−1) | NA | MHET, TPA, Benzoic acid, 2-hydroxyethyl benzoate | [109] |
| PET nanoparticles | 9.8 | TfCut2 | Thermobifida fusca | 60 | 1 | 8.5 | 147.673 ± 4.928 (min−1) | 0.010 ± 7.40 × 10−4 (mLcm−2) | 0.998 | BHET, MHET | [110] |
| Amorphous PET | NA | Cut190**SS | E. coli | 37 | 24 | 7 | 24.9 (s−1) | 0.082 (mM) | NA | NA | [111] |
| PET with (p-nitrophenyl acetate (PNPA)) | 37 | Thf42_Cut1 | Thermobifida fusca DSM44342 | 50 | 120 | 7 | 39.5 ± 3.0 (s−1) | 167 ± 29 (μmol L−1) | NA | BHET, MHET, TPA, Benzoic acid, 2-hydroxyethyl benzoate | [59] |
| PET with (p-nitrophenyl butyrate (PNPB)) | 37 | Thf42_Cut1 | Thermobifida fusca DSM44342 | 50 | 120 | 7 | 30.9 ± 8.6 (s−1) | 2100 ± 361 (μmol L−1) | NA | BHET, MHET, TPA, Benzoic acid, 2-hydroxyethyl benzoate | [59] |
4.4. PETase
| Raw Materials | Type of Enzymes | Organism | Condition Operations | Kinetic Parameter * | Product | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| T, °C | t, h | kcat | km | k | |||||
| Waste PET bottle | IsPETase | E. coli BL21 | 40 | 24 | 11.59 ± 0.16 s−1 | 0.19 ± 0.01 mmol.L−1 | NA | TPA, BHET, | [122] |
| Waste PET bottle | IsPETaseW159H/F229Y | E. coli BL21 | 40 | 24 | 9.64 ± 0.13 | 0.08 ± 0.01 | NA | TPA, BHET, | [122] |
| PET sheet | LCCICCG | Escherichia coli | 50 | 4.1 | NA | 0.11 ± 0.02 µM | NA | NA | [123] |
| Post-consumer recycled PET flakes | LCCICCG | E. coli BL21 | 65 | 48 | 4.66 g/(µmol⋅h) | 5.39 g/L | 0.036 h−1 | TPA, EG, 1.3 Propanediol, 1.4 Butanediol | [124] |
| Post-consumer PET bottle | ICCG-GS4-αSP | E. coli Shuffle T7 and E. coli BL21 (DE3) | 60 | 24 | 3.91 ± 0.81 s−1 | 5.8 ± 1.2 g/L | NA | TPA, BHET, MHET | [125] |
| PET Film | PETase | Polyester hydrolase TfCut2 | 60 | 1 | 0.31 ± 0.01 s−1 | 7.33 × 10−3 ± 3.62 × 10−4 mol L−1 | 147.673 ± 4.928 min−1 | MHET, BHET | [110] |
| Semi-crystalline PET powder | HiC [AAE13316.1] and | Humicola insolens and | 50 | 5 | 0.088 s−1 | 0.27 g/L | NA | BETEB, BHET, MHET | [126] |
| Semi-crystalline PET powder | TfC [AAZ54921.1] | Thermobifida fusca | 50 | 5 | 0.015 s−1 | 1.2 g/L | NA | BETEB, BHET, MHET | [126] |
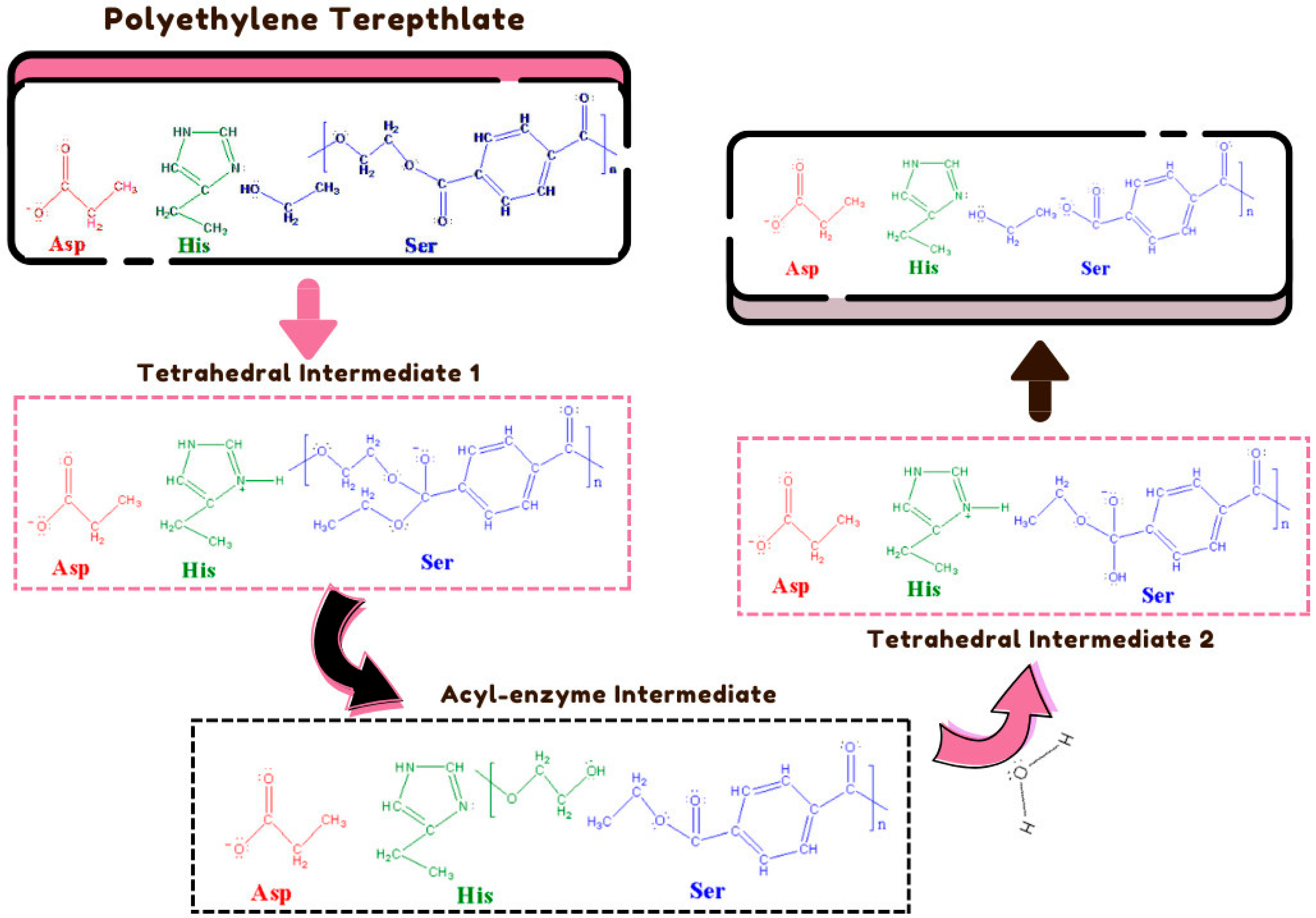
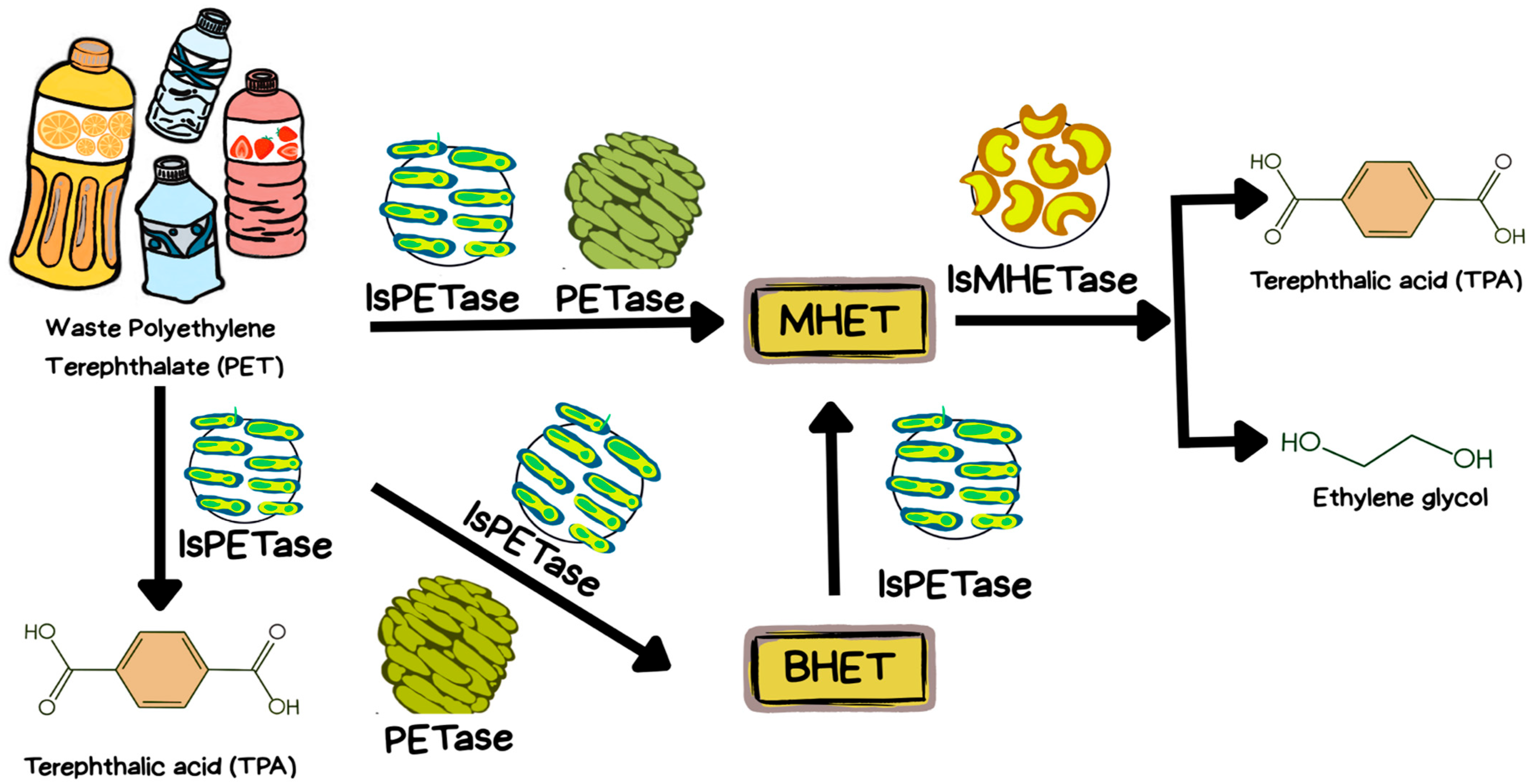
4.5. IsPETase
4.6. IsMHETase
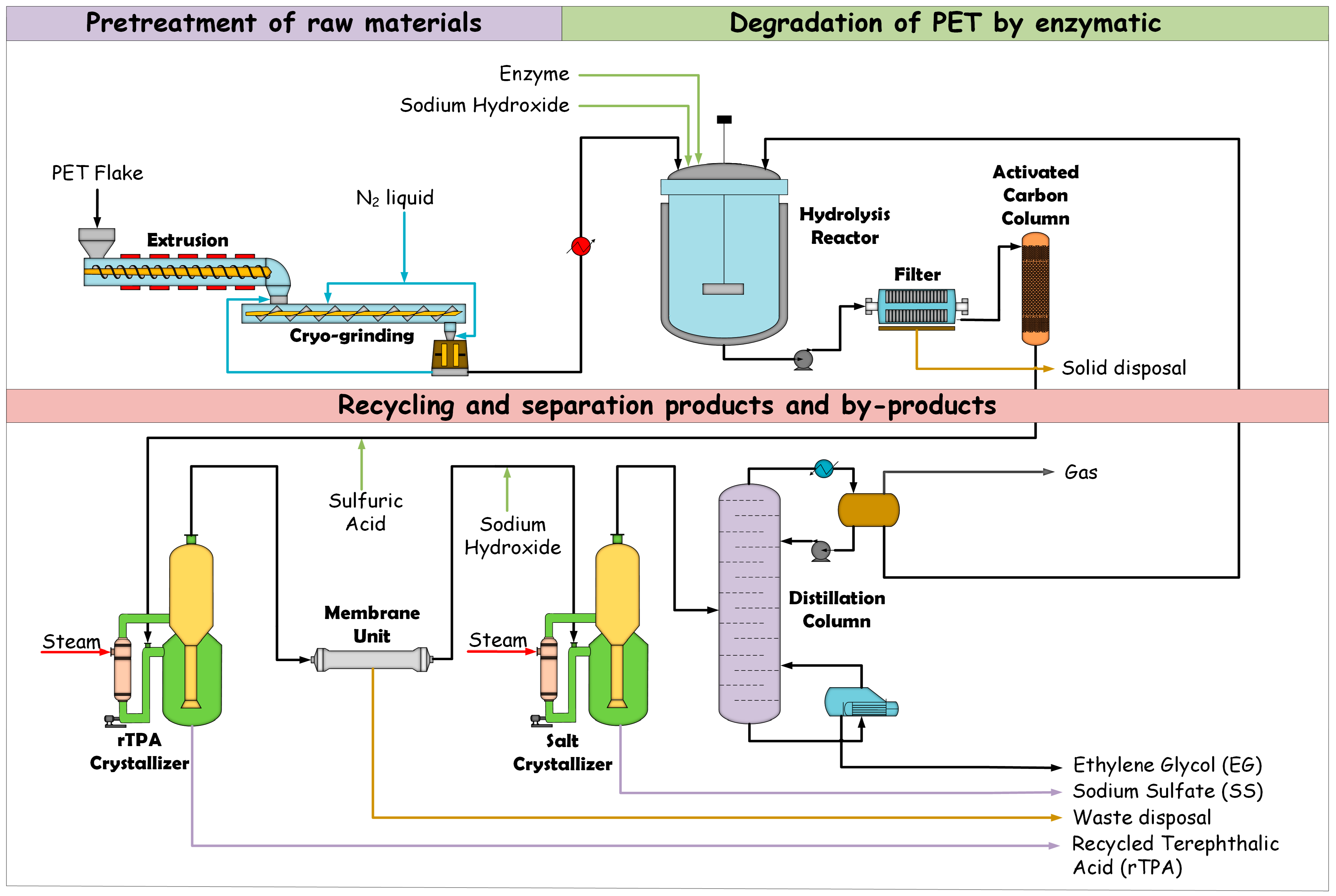
5. The Implementation of Biocatalytic PET for Large-Scale Industrial Applications
5.1. Process Engineering and Reactor Design
5.2. Techno-Economic and Environmental Considerations
- (i)
- Mechanical Recycling: Lowest Energy & Cost but Quality Loss and Limited Cycles
- (ii)
- Chemical Recycling: Flexibility and Quality at Cost of Energy Intensity
- (iii)
- Biocatalytic Recycling: Promising Resource Efficiency, But High Uncertainty & Scale Challenges
6. Limitations of Biocatalytic PET
| Biocatalyst | Parameters | Limitations | Ref |
|---|---|---|---|
| Cutinase |
| The smooth hydrophobic surface of PET inhibits the binding of cutinase to PET, consequently limiting the rate of the PET degradation process. | [159] |
| Carboxylesterases |
| For applications in industrial polyester and plastic recycling, the stability and hydrolytic activity of known natural esterases toward synthetic polyesters are often inadequate. | [139,160,161] |
| Lipases |
| The challenges to depolymerization of PET by lipases in biocatalytic systems are the consistent product inhibition by hydrolysis byproducts, such as MHET, and another limitation is that it is tough to degrade the crystalline structure of PET | [162,163] |
| PETase |
| PETase is heat sensitive, and lower temperatures might reduce the specific volume of polymeric materials. Meanwhile, it potentially reduces its degrading activity at mild temperatures. | [160,164] |
| IsPETase |
| IsPETase’s poor catalytic effectiveness and inherent lack of thermostability are two of its drawbacks for PET degradation. | [114,116] |
| IsMHETase |
| Low catalytic efficiency, poor thermostability, possible product inhibition from degradation intermediates, and the intrinsic stability and crystallinity of the PET polymer itself are the drawbacks of biocatalytic PET depolymerization employing enzymes such as IsMHETase. | [47,160] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHET | Bis-(2-hydroxyethyl) terephthalate |
| MHET | Mono(2-hydroxyethyl) terephthalic acid |
| MEG | Monoethylene glycol |
| PET | Polyethylene terephthalate |
| TPA | Terephthalic acid |
| Tg | Glass transition temperature |
References
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.-S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef]
- Gabriel, V.H.; Schaffernak, A.; Pfitzner, M.; Fellner, J.; Tacker, M.; Apprich, S. Rigid Polyethylene Terephthalate Packaging Waste: An Investigation of Waste Composition and Its Recycling Potential in Austria. Resources 2023, 12, 128. [Google Scholar] [CrossRef]
- Ding, Q.; Zhu, H. The Key to Solving Plastic Packaging Wastes: Design for Recycling and Recycling Technology. Polymers 2023, 15, 1485. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-Y.; Lee, Y.-M. Management strategy of plastic wastes in Taiwan. Sustain. Environ. Res. 2022, 32, 11. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Oberoi, G.; Garg, A. Single-use plastics: A roadmap for sustainability? Supremo Amic. 2021, 24, 585. [Google Scholar]
- Masnadi, M.S.; El-Houjeiri, H.M.; Schunack, D.; Li, Y.; Englander, J.G.; Badahdah, A.; Monfort, J.-C.; Anderson, J.E.; Wallington, T.J.; Bergerson, J.A. Global carbon intensity of crude oil production. Science 2018, 361, 851–853. [Google Scholar] [CrossRef]
- Hamilton, L.A.; Feit, S.; Muffett, C.; Kelso, M.; Rubright, S.M.; Bernhardt, C.; Schaeffer, E.; Moon, D.; Morris, J.; Labbé-Bellas, R. Plastic and Climate: The Hidden Costs of a Plastic Planet; CIEL: Washington, DC, USA, 2019. [Google Scholar]
- Royer, S.-J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef]
- Egan, J.; Salmon, S. Strategies and progress in synthetic textile fiber biodegradability. SN Appl. Sci. 2022, 4, 22. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef]
- Mäkelä, M.; Rissanen, M.; Sixta, H. Machine vision estimates the polyester content in recyclable waste textiles. Resour. Conserv. Recycl. 2020, 161, 105007. [Google Scholar] [CrossRef]
- MacArthur, E. A New Textiles Economy: Redesigning Fashion’s Future (2017); Ellen MacArthur Foundation: Cowes, UK, 2021. [Google Scholar]
- Koszewska, M. Circular economy—Challenges for the textile and clothing industry. Autex Res. J. 2018, 18, 337–347. [Google Scholar] [CrossRef]
- Pristiani, M.; Damayanti, D.; Wu, H.-S. Microwave-Assisted Acid Hydrolysis of PA6 Wastes in PA6 Process: Kinetics, Activation Energies, and Monomer Recovery. Processes 2025, 13, 3175. [Google Scholar] [CrossRef]
- Moroni, M.; Mei, A.; Leonardi, A.; Lupo, E.; La Marca, F. PET and PVC separation with hyperspectral imagery. Sensors 2015, 15, 2205–2227. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, A.; Kaghazchi, T.; Soleimani, M. Preparation and evaluation of activated carbons obtained by physical activation of polyethyleneterephthalate (PET) wastes. J. Taiwan Inst. Chem. Eng. 2012, 43, 631–637. [Google Scholar] [CrossRef]
- Exchange, T. Preferred Fiber & Materials Market Report 2021; Textile Exchange: Lamesa, TX, USA, 2021; Volume 4. [Google Scholar]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1. [Google Scholar] [CrossRef]
- Shen, L.; Nieuwlaar, E.; Worrell, E.; Patel, M.K. Life cycle energy and GHG emissions of PET recycling: Change-oriented effects. Int. J. Life Cycle Assess. 2011, 16, 522–536. [Google Scholar] [CrossRef]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and consolidated review of plastic waste management and bio-based biodegradable plastics: Challenges and opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Nanoparticle decorated cellulose nanocrystals (CNC) composites for energy, catalysis, and biomedical applications. Adv. Funct. Mater. 2025, 35, 2412869. [Google Scholar] [CrossRef]
- Mancini, S.D.; Schwartzman, J.A.S.; Nogueira, A.R.; Kagohara, D.A.; Zanin, M. Additional steps in mechanical recyling of PET. J. Clean. Prod. 2010, 18, 92–100. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Likozar, B.; Saha, B. Chemical recycling processes of waste polyethylene terephthalate using solid catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.-S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Sevigné-Itoiz, E.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X. Contribution of plastic waste recovery to greenhouse gas (GHG) savings in Spain. Waste Manag. 2015, 46, 557–567. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; Van Harmelen, T.; De Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed]
- García, J.L. Enzymatic recycling of polyethylene terephthalate through the lens of proprietary processes. Microb. Biotechnol. 2022, 15, 2699–2704. [Google Scholar] [CrossRef]
- Hiraga, K.; Taniguchi, I.; Yoshida, S.; Kimura, Y.; Oda, K. Biodegradation of waste PET: A sustainable solution for dealing with plastic pollution. EMBO Rep. 2019, 20, e49365. [Google Scholar] [CrossRef]
- Kawai, F. Emerging strategies in polyethylene terephthalate hydrolase research for biorecycling. ChemSusChem 2021, 14, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Khan, F.U.; Hussain, M.; Malik, R.N. Sustainability assessment of home textiles made of recycled PET fibre using life cycle assessment and life cycle costing analyses. Sci. Total Environ. 2025, 982, 179652. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene terephthalate (PET) bottle-to-bottle recycling for the beverage industry: A review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Mohtaram, F.; Fojan, P. From Waste to Value: Advances in Recycling Textile-Based PET Fabrics. Textiles 2025, 5, 24. [Google Scholar] [CrossRef]
- Majumdar, A.; Shukla, S.; Singh, A.A.; Arora, S. Circular fashion: Properties of fabrics made from mechanically recycled poly-ethylene terephthalate (PET) bottles. Resour. Conserv. Recycl. 2020, 161, 104915. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Lerici, L.C.; Renzini, M.S.; Pierella, L.B. Chemical catalyzed recycling of polymers: Catalytic conversion of PE, PP and PS into fuels and chemicals over HY. Procedia Mater. Sci. 2015, 8, 297–303. [Google Scholar] [CrossRef]
- Valerio, O.; Muthuraj, R.; Codou, A. Strategies for polymer to polymer recycling from waste: Current trends and opportunities for improving the circular economy of polymers in South America. Curr. Opin. Green Sustain. Chem. 2020, 25, 100381. [Google Scholar] [CrossRef]
- Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Muringayil Joseph, T.; Azat, S.; Ahmadi, Z.; Moini Jazani, O.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethylene terephthalate (PET) recycling: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Roohi; Bano, K.; Kuddus, M.; Zaheer, M.R.; Zia, Q.; Khan, M.F.; Ashraf, G.M.; Gupta, A.; Aliev, G. Microbial enzymatic degradation of biodegradable plastics. Curr. Pharm. Biotechnol. 2017, 18, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Fiorillo, C.; Trossaert, L.; Bezeraj, E.; Debrie, S.; Ohnmacht, H.; Van Steenberge, P.H.; D’hooge, D.R.; Edeleva, M. Molecular and material property variations during the ideal degradation and mechanical recycling of PET. RSC Sustain. 2024, 2, 3596–3637. [Google Scholar] [CrossRef]
- Cusano, I.; Campagnolo, L.; Aurilia, M.; Costanzo, S.; Grizzuti, N. Rheology of recycled PET. Materials 2023, 16, 3358. [Google Scholar] [CrossRef] [PubMed]
- Elamri, A.; Zdiri, K.; Harzallah, O.; Lallam, A. Progress in polyethylene terephthalate recycling. In Polyethylene Terephthalate: Uses, Properties and Degradation; Nova Science: Hauppauge, NY, USA, 2017. [Google Scholar]
- Carta, D.; Cao, G.; D’Angeli, C. Chemical recycling of poly (ethylene terephthalate)(PET) by hydrolysis and glycolysis. Environ. Sci. Pollut. Res. 2003, 10, 390–394. [Google Scholar] [CrossRef]
- Wei, R.; von Haugwitz, G.; Pfaff, L.; Mican, J.; Badenhorst, C.P.S.; Liu, W.; Weber, G.; Austin, H.P.; Bednar, D.; Damborsky, J.; et al. Mechanism-Based Design of Efficient PET Hydrolases. ACS Catal. 2022, 12, 3382–3396. [Google Scholar] [CrossRef]
- Wei, R.; Westh, P.; Weber, G.; Blank, L.M.; Bornscheuer, U.T. Standardization guidelines and future trends for PET hydrolase research. Nat. Commun. 2025, 16, 4684. [Google Scholar] [CrossRef] [PubMed]
- Dubdub, I.; Alhulaybi, Z. Catalytic pyrolysis of PET polymer using nonisothermal thermogravimetric analysis data: Kinetics and artificial neural networks studies. Polymers 2022, 15, 70. [Google Scholar] [CrossRef]
- Daggubati, L.; Sobhani, Z.; Carbery, M.; Ramadass, K.; Palanisami, T. Fingerprinting risk from recycled plastic products using physical and chemical properties. J. Hazard. Mater. 2025, 488, 137507. [Google Scholar] [CrossRef] [PubMed]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Pérez-Maqueda, L.A. Determination of the activation energy under isothermal conditions: Revisited. J. Therm. Anal. Calorim. 2023, 148, 1679–1686. [Google Scholar] [CrossRef]
- Martín-Gullón, I.; Esperanza, M.; Font, R. Kinetic model for the pyrolysis and combustion of poly-(ethylene terephthalate)(PET). J. Anal. Appl. Pyrolysis 2001, 58, 635–650. [Google Scholar] [CrossRef]
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste–A review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Zimmermann, W. Biocatalytic recycling of plastics: Facts and fiction. Chem. Sci. 2025, 16, 6573–6582. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Sambyal, P.; Najmi, P.; Sharma, D.; Khoshbakhti, E.; Hosseini, H.; Milani, A.S.; Arjmand, M. Plastic recycling: Challenges and opportunities. Can. J. Chem. Eng. 2025, 103, 2462–2498. [Google Scholar] [CrossRef]
- Mwanza, B.G. Introduction to recycling. In Recent Developments in Plastic Recycling; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–13. [Google Scholar]
- Herrero Acero, E.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Wei, R.; Zimmermann, W.; Zinn, M. Enzymatic surface hydrolysis of PET: Effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules 2011, 44, 4632–4640. [Google Scholar] [CrossRef]
- Tournier, V.; Duquesne, S.; Guillamot, F.; Cramail, H.; Taton, D.; Marty, A.; André, I. Enzymes’ power for plastics degradation. Chem. Rev. 2023, 123, 5612–5701. [Google Scholar] [CrossRef]
- Ruginescu, R.; Purcarea, C. Plastic-Degrading Enzymes from Marine Microorganisms and Their Potential Value in Recycling Technologies. Mar. Drugs 2024, 22, 441. [Google Scholar] [CrossRef]
- Castro-Rodríguez, J.A.; Rodríguez-Sotres, R.; Farrés, A. Determinants for an efficient enzymatic catalysis in poly (ethylene terephthalate) degradation. Catalysts 2023, 13, 591. [Google Scholar] [CrossRef]
- Carniel, A.; de Abreu Waldow, V.; de Castro, A.M. A comprehensive and critical review on key elements to implement enzymatic PET depolymerization for recycling purposes. Biotechnol. Adv. 2021, 52, 107811. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, R.; de Oliveira Veloso, C.; de Castro, A.M.; Langone, M.A.P. Enzymatic post-consumer poly (ethylene terephthalate)(PET) depolymerization using commercial enzymes. 3 Biotech 2023, 13, 135. [Google Scholar] [CrossRef]
- Amanna, R.; Rakshit, S.K. Review of nomenclature and methods of analysis of polyethylene terephthalic acid hydrolyzing enzymes activity. Biodegradation 2024, 35, 341–360. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Saeki, T.; Yamazaki, F.; Koyama, N.; Okubo, T.; Hombe, D.; Ogura, Y.; Hashino, Y.; Tatsumi-Koga, R.; Koga, N. Development and Production of Moderate-Thermophilic PET Hydrolase for PET Bottle and Fiber Recycling. ACS Sustain. Chem. Eng. 2025, 13, 10404–10417. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, S.; Cai, D.; Shao, C.; Zhang, C.; Zhou, J.; Cui, Z.; He, T.; Chen, C.; Chen, B. Depolymerization of post-consumer PET bottles with engineered cutinase 1 from Thermobifida cellulosilytica. Green Chem. 2022, 24, 5998–6007. [Google Scholar] [CrossRef]
- Abokitse, K.; Grosse, S.; Leisch, H.; Corbeil, C.R.; Perrin-Sarazin, F.; Lau, P.C. A novel actinobacterial cutinase containing a noncatalytic polymer-binding domain. Appl. Environ. Microbiol. 2022, 88, e01522-21. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.V.D.; Calandrini, G.; da Costa, C.H.S.; da Silva de Souza, C.G.; Alves, C.N.; Silva, J.R.A.; Lima, A.H.; Lameira, J. Evaluating cutinase from Fusarium oxysporum as a biocatalyst for the degradation of nine synthetic polymer. Sci. Rep. 2025, 15, 2887. [Google Scholar] [CrossRef]
- Ellen MacArthur Found. Redesigning Fashion’s Future; Ellen MacArthur Found: Cowes, UK, 2017; pp. 1–150. [Google Scholar]
- Won, S.-J.; Yim, J.H.; Kim, H.-K. Functional production, characterization, and immobilization of a cold-adapted cutinase from Antarctic Rhodococcus sp. Protein Expr. Purif. 2022, 195, 106077. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yoo, W.; Shin, S.C.; Kim, K.K.; Kim, H.-W.; Do, H.; Lee, J.H. Structural and biochemical insights into Bis (2-hydroxyethyl) terephthalate degrading carboxylesterase isolated from Psychrotrophic bacterium Exiguobacterium antarcticum. Int. J. Mol. Sci. 2023, 24, 12022. [Google Scholar] [CrossRef]
- Johan, U.U.M.; Rahman, R.N.Z.R.A.; Kamarudin, N.H.A.; Ali, M.S.M. An integrated overview of bacterial carboxylesterase: Structure, function and biocatalytic applications. Colloids Surf. B Biointerfaces 2021, 205, 111882. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.R. Phase I Biotransformation Reactions-Esterases, and Amidases; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Hitch, T.C.; Clavel, T. A proposed update for the classification and description of bacterial lipolytic enzymes. PeerJ 2019, 7, e7249. [Google Scholar] [CrossRef]
- Wongsatit, T.; Srimora, T.; Kiattisewee, C.; Uttamapinant, C. Enzymes, auxiliaries and cells for the recycling and upcycling of polyethylene terephthalate (PET). Curr. Opin. Syst. Biol. 2024, 38, 100515. [Google Scholar] [CrossRef]
- Ghodke, V.M.; Punekar, N.S. Environmental role of aromatic carboxylesterases. Environ. Microbiol. 2022, 24, 2657–2668. [Google Scholar] [CrossRef]
- Wawer, J.; Panuszko, A.; Kozłowski, D.; Juniewicz, J.; Szymikowski, J.; Brodnicka, E. Sustainable Management of Microplastic Pollutions from PET Bottles: Overview and Mitigation Strategies. Appl. Sci. 2025, 15, 5322. [Google Scholar] [CrossRef]
- Sulaiman, S.; You, D.-J.; Kanaya, E.; Koga, Y.; Kanaya, S. Crystal structure and thermodynamic and kinetic stability of metagenome-derived LC-cutinase. Biochemistry 2014, 53, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Honak, A.; Oeser, T.; Wei, R.; Belisário-Ferrari, M.R.; Then, J.; Schmidt, J.; Zimmermann, W. A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol. J. 2016, 11, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Guo, X.; Tong, K.; Niu, Y.; Shen, Z.; Weng, H.; Zhang, F.; Wu, J. Enhanced degradation activity of PET plastics by fusion protein of anchor peptide LCI and Thermobifida fusca cutinase. Enzym. Microb. Technol. 2025, 184, 110562. [Google Scholar] [CrossRef]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef]
- Fritzsche, S.; Popp, M.; Spälter, L.; Bonakdar, N.; Vogel, N.; Castiglione, K. Recycling the recyclers: Strategies for the immobilisation of a PET-degrading cutinase. Bioprocess Biosyst. Eng. 2025, 48, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Yamagami, Y.; Inaba, S.; Oida, T.; Yamamoto, M.; Kitajima, S.; Kawai, F. Enzymatic hydrolysis of PET: Functional roles of three Ca2+ ions bound to a cutinase-like enzyme, Cut190*, and its engineering for improved activity. Appl. Microbiol. Biotechnol. 2018, 102, 10067–10077. [Google Scholar] [CrossRef] [PubMed]
- Ronkvist, Å.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Fritzsche, S.; Hübner, H.; Oldiges, M.; Castiglione, K. Comparative evaluation of the extracellular production of a polyethylene terephthalate degrading cutinase by Corynebacterium glutamicum and leaky Escherichia coli in batch and fed-batch processes. Microb. Cell Factories 2024, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, Y.; Jin, S.; Wu, Q.; Wu, J.; Liu, J.; Wang, F.; Deng, L.; Nie, K. The evolution of cutinase Est1 based on the clustering strategy and its application for commercial PET bottles degradation. J. Environ. Manag. 2024, 368, 122217. [Google Scholar] [CrossRef]
- Silva, C.; Da, S.; Silva, N.; Matamá, T.; Araújo, R.; Martins, M.; Chen, S.; Chen, J.; Wu, J.; Casal, M. Engineered Thermobifida fusca cutinase with increased activity on polyester substrates. Biotechnol. J. 2011, 6, 1230–1239. [Google Scholar] [CrossRef]
- Han, Y.; Wang, R.; Wang, D.; Luan, Y. Enzymatic degradation of synthetic plastics by hydrolases/oxidoreductases. Int. Biodeterior. Biodegrad. 2024, 189, 105746. [Google Scholar] [CrossRef]
- Freije García, F.; García Liñares, G. Use of Lipases as a Sustainable and Efficient Method for the Synthesis and Degradation of Polymers. J. Polym. Environ. 2024, 32, 2484–2516. [Google Scholar] [CrossRef]
- Carniel, A.; Valoni, É.; Junior, J.N.; da Conceição Gomes, A.; De Castro, A.M. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Singhvi, M.; Kim, B.S. Biodegradation of poly (ethylene terephthalate): Mechanistic insights, advances, and future innovative strategies. Chem. Eng. J. 2023, 457, 141230. [Google Scholar] [CrossRef]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly (ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Eberl, A.; Heumann, S.; Brückner, T.; Araujo, R.; Cavaco-Paulo, A.; Kaufmann, F.; Kroutil, W.; Guebitz, G.M. Enzymatic surface hydrolysis of poly (ethylene terephthalate) and bis (benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar] [CrossRef]
- Boneta, S.; Arafet, K.; Moliner, V. QM/MM study of the enzymatic biodegradation mechanism of polyethylene terephthalate. J. Chem. Inf. Model. 2021, 61, 3041–3051. [Google Scholar] [CrossRef]
- Swiderek, K.; Velasco-Lozano, S.; Galmés, M.; Olazabal, I.; Sardon, H.; López-Gallego, F.; Moliner, V. Mechanistic studies of a lipase unveil a dramatic effect of pH on the selectivity toward the hydrolysis of PET oligomers. Nat. Commun. 2022, 14, 3556. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-h.; Jin, J.-l.; Sun, H.-t.; Li, S.; Zhang, F.-f.; Yu, X.-h.; Cao, Q.-z.; Song, Y.-x.; Li, N.; Lu, Z.-h. Perspectives on the microorganisms with the potentials of PET-degradation. Front. Microbiol. 2025, 16, 1541913. [Google Scholar] [CrossRef]
- De Jesus, R.; Alkendi, R. A minireview on the bioremediative potential of microbial enzymes as solution to emerging microplastic pollution. Front. Microbiol. 2023, 13, 1066133. [Google Scholar] [CrossRef]
- Guo, R.-T.; Li, X.; Yang, Y.; Huang, J.-W.; Shen, P.; Liew, R.K.; Chen, C.-C. Natural and engineered enzymes for polyester degradation: A review. Environ. Chem. Lett. 2024, 22, 1275–1296. [Google Scholar] [CrossRef]
- Safdar, A.; Ismail, F.; Imran, M. Biodegradation of synthetic plastics by the extracellular lipase of Aspergillus niger. Environ. Adv. 2024, 17, 100563. [Google Scholar] [CrossRef]
- Heydemann, P.; Guicking, H. Specific volume of polymers as a function of temperature and pressure. Kolloid-Z. Und Z. Für Polym. 1963, 193, 16–25. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, H.; Hong, H.; Park, J.; Ki, D.; Kim, M.; Kim, H.-J.; Kim, K.-J. Three-directional engineering of IsPETase with enhanced protein yield, activity, and durability. J. Hazard. Mater. 2023, 459, 132297. [Google Scholar] [CrossRef]
- Hu, Z.; Klupt, K.; Zechel, D.L.; Jia, Z.; Howe, G. Mining Thermophile Genomes for New PETases with Exceptional Thermostabilities Using Sequence Similarity Networks. Chembiochem 2025, 26, e202500065. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- García-Meseguer, R.; Ortí, E.; Tuñón, I.; Ruiz-Pernía, J.J.; Aragó, J. Insights into the enhancement of the poly (ethylene terephthalate) degradation by FAST-PETase from computational modeling. J. Am. Chem. Soc. 2023, 145, 19243–19255. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, E.d.Q.; Campisano, I.S.P.; de Castro, A.M.; Coelho, M.A.Z.; Langone, M.A.P. Kinetic modeling of the post-consumer poly (ethylene terephthalate) hydrolysis catalyzed by cutinase from Humicola insolens. J. Polym. Environ. 2022, 30, 1627–1637. [Google Scholar] [CrossRef]
- Ribitsch, D.; Acero, E.H.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Freddi, G.; Schwab, H.; Guebitz, G.M. Characterization of a new cutinase from Thermobifida alba for PET-surface hydrolysis. Biocatal. Biotransform. 2012, 30, 2–9. [Google Scholar] [CrossRef]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Kondo, F.; Kamiya, N.; Bekker, G.-J.; Nagao, S.; Numoto, N.; Sekiguchi, H.; Ito, N.; Oda, M. Structure-activity relationship of PET-degrading cutinase regulated by weak Ca2+ binding and temperature. Biophys. Physicobiol. 2025, 22, e220009. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jaafar, N.R.; Ling, J.G.; Huyop, F.; Bakar, F.D.A.; Rahman, R.A.; Illias, R.M. Cross-linked enzyme aggregates of polyethylene terephthalate hydrolyse (PETase) from Ideonella sakaiensis for the improvement of plastic degradation. Int. J. Biol. Macromol. 2024, 263, 130284. [Google Scholar] [CrossRef]
- Chen, C.C.; Han, X.; Ko, T.P.; Liu, W.; Guo, R.T. Structural studies reveal the molecular mechanism of PET ase. FEBS J. 2018, 285, 3717–3723. [Google Scholar] [CrossRef]
- Ermis, H. A mini-review on the role of PETase in polyethylene terephthalate degradation. Rev. Environ. Sci. Bio/Technol. 2025, 24, 545–555. [Google Scholar] [CrossRef]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef]
- Stevensen, J.; Janatunaim, R.Z.; Ratnaputri, A.H.; Aldafa, S.H.; Rudjito, R.R.; Saputro, D.H.; Suhandono, S.; Putri, R.M.; Aditama, R.; Fibriani, A. Thermostability and Activity Improvements of PETase from Ideonella sakaiensis. ACS Omega 2025, 10, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Britton, D.; Liu, C.; Xiao, Y.; Jia, S.; Legocki, J.; Kronenberg, J.; Montclare, J.K. Protein-engineered leaf and branch compost cutinase variants using computational screening and IsPETase homology. Catal. Today 2024, 433, 114659. [Google Scholar] [CrossRef]
- Dissanayake, L.; Jayakody, L.N. Engineering microbes to bio-upcycle polyethylene terephthalate. Front. Bioeng. Biotechnol. 2021, 9, 656465. [Google Scholar] [CrossRef] [PubMed]
- Palm, G.J.; Reisky, L.; Böttcher, D.; Müller, H.; Michels, E.A.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 2019, 10, 1717. [Google Scholar] [CrossRef]
- Burgin, T.; Pollard, B.C.; Knott, B.C.; Mayes, H.B.; Crowley, M.F.; McGeehan, J.E.; Beckham, G.T.; Woodcock, H.L. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun. Chem. 2024, 7, 65. [Google Scholar] [CrossRef]
- Erickson, E.; Shakespeare, T.J.; Bratti, F.; Buss, B.L.; Graham, R.; Hawkins, M.A.; König, G.; Michener, W.E.; Miscall, J.; Ramirez, K.J. Comparative performance of PETase as a function of reaction conditions, substrate properties, and product accumulation. ChemSusChem 2022, 15, e202101932. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Liu, H.; Li, Q.; Xu, G.; Zhang, Y.; Guan, F.; Zhang, Y.; Zhang, W.; Wu, N. Protein engineering of stable IsPETase for PET plastic degradation by Premuse. Int. J. Biol. Macromol. 2021, 180, 667–676. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Radmer, T.S.; Meyer, A.S. Enzymatic degradation of poly (ethylene terephthalate)(PET): Identifying the cause of the hypersensitive enzyme kinetic response to increased PET crystallinity. Enzym. Microb. Technol. 2024, 173, 110353. [Google Scholar] [CrossRef] [PubMed]
- Abid, U.; Sun, G.; Soong, Y.-H.V.; Williams, A.; Chang, A.C.; Ayafor, C.; Patel, A.; Wong, H.-W.; Sobkowicz, M.J.; Xie, D. Evaluation of enzymatic depolymerization of PET, PTT, and PBT polyesters. Biochem. Eng. J. 2023, 199, 109074. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, Z.; Su, L.; Bai, S.; Cai, D.; Chen, C.; Yu, L.; Sun, Y. Enhanced degradation of post-consumer polyethylene terephthalate (PET) wastes by fusion cutinase: Effects of anchor and linker peptides. Process Biochem. 2024, 145, 1–12. [Google Scholar] [CrossRef]
- Bååth, J.A.; Borch, K.; Jensen, K.; Brask, J.; Westh, P. Comparative biochemistry of four polyester (PET) hydrolases. ChemBioChem 2021, 22, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, J.M.F. Computational Development of New Biocatalyst for Plastic Degradation Through QM/MM Methods. Master’s Thesis, Universidade do Minho (Portugal), Braga, Portugal, 2021. [Google Scholar]
- Chen, X.-Q.; Rao, D.-M.; Zhu, X.-Y.; Zhao, X.-M.; Huang, Q.-S.; Wu, J.; Yan, Z.-F. Current state and sustainable management of waste polyethylene terephthalate bio-disposal: Enzymatic degradation to upcycling. Bioresour. Technol. 2025, 429, 132492. [Google Scholar] [CrossRef]
- Kan, Y.; He, L.; Luo, Y.; Bao, R. IsPETase is a novel biocatalyst for poly (ethylene terephthalate)(PET) hydrolysis. ChemBioChem 2021, 22, 1706–1716. [Google Scholar] [CrossRef]
- Seo, H.; Kim, S.; Son, H.F.; Sagong, H.-Y.; Joo, S.; Kim, K.-J. Production of extracellular PETase from Ideonella sakaiensis using sec-dependent signal peptides in E. coli. Biochem. Biophys. Res. Commun. 2019, 508, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, C.; Zhu, S.; Wei, R.; Yin, C.-C. Structural and functional characterization of polyethylene terephthalate hydrolase from Ideonella sakaiensis. Biochem. Biophys. Res. Commun. 2019, 508, 289–294. [Google Scholar] [CrossRef]
- Magalhães, R.P.; Fernandes, H.S.; Sousa, S.F. The critical role of Asp206 stabilizing residues on the catalytic mechanism of the Ideonella sakaiensis PETase. Catal. Sci. Technol. 2022, 12, 3474–3483. [Google Scholar] [CrossRef]
- Bell, E.L.; Smithson, R.; Kilbride, S.; Foster, J.; Hardy, F.J.; Ramachandran, S.; Tedstone, A.A.; Haigh, S.J.; Garforth, A.A.; Day, P.J. Directed evolution of an efficient and thermostable PET depolymerase. Nat. Catal. 2022, 5, 673–681. [Google Scholar] [CrossRef]
- Brott, S.; Pfaff, L.; Schuricht, J.; Schwarz, J.N.; Böttcher, D.; Badenhorst, C.P.; Wei, R.; Bornscheuer, U.T. Engineering and evaluation of thermostable IsPETase variants for PET degradation. Eng. Life Sci. 2022, 22, 192–203. [Google Scholar] [CrossRef]
- Feng, S.; Yue, Y.; Zheng, M.; Li, Y.; Zhang, Q.; Wang, W. Is PETase-and Is MHETase-catalyzed cascade degradation mechanism toward polyethylene terephthalate. ACS Sustain. Chem. Eng. 2021, 9, 9823–9832. [Google Scholar] [CrossRef]
- Sagong, H.-Y.; Seo, H.; Kim, T.; Son, H.F.; Joo, S.; Lee, S.H.; Kim, S.; Woo, J.-S.; Hwang, S.Y.; Kim, K.-J. Decomposition of the PET film by MHETase using Exo-PETase function. ACS Catal. 2020, 10, 4805–4812. [Google Scholar] [CrossRef]
- Duan, S.; Chao, T.; Wu, Y.; Wei, Z.; Cao, S. Improvement in the Thermal Stability of Is MHETase by Sequence and Structure-Guided Calculation. Molecules 2025, 30, 988. [Google Scholar] [CrossRef]
- Buhari, S.B.; Nezhad, N.G.; Normi, Y.M.; Shariff, F.M.; Leow, T.C. Insight on recently discovered PET polyester-degrading enzymes, thermostability and activity analyses. 3 Biotech 2024, 14, 31. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Almdal, K.; Meyer, A.S. Significance of poly (ethylene terephthalate)(PET) substrate crystallinity on enzymatic degradation. New Biotechnol. 2023, 78, 162–172. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, D.O.; In Shim, K.; Kim, J.K.; Pelton, J.G.; Ryu, M.H.; Joo, J.C.; Han, J.W.; Kim, H.T.; Kim, K.H. One-pot chemo-bioprocess of PET depolymerization and recycling enabled by a biocompatible catalyst, betaine. ACS Catal. 2021, 11, 3996–4008. [Google Scholar] [CrossRef]
- Brizendine, R.K.; Erickson, E.; Haugen, S.J.; Ramirez, K.J.; Miscall, J.; Salvachúa, D.; Pickford, A.R.; Sobkowicz, M.J.; McGeehan, J.E.; Beckham, G.T. Particle size reduction of poly (ethylene terephthalate) increases the rate of enzymatic depolymerization but does not increase the overall conversion extent. ACS Sustain. Chem. Eng. 2022, 10, 9131–9140. [Google Scholar] [CrossRef]
- Zimmermann, W. Biocatalytic recycling of polyethylene terephthalate plastic. Philos. Trans. R. Soc. A 2020, 378, 20190273. [Google Scholar] [CrossRef]
- Lu, D.; Wu, J.; Jin, S.; Wu, Q.; Deng, L.; Wang, F.; Nie, K. The enhancement of waste PET particles enzymatic degradation with a rotating packed bed reactor. J. Clean. Prod. 2024, 434, 140088. [Google Scholar] [CrossRef]
- Rosevear, A. Immobilised biocatalysts—A critical review. J. Chem. Technol. Biotechnol. 1984, 34, 127–150. [Google Scholar] [CrossRef]
- Fukui, S.; Tanaka, A. Bioconversion of lipophilic compounds by immobilized microbial cells in organic solvents. Acta Biotechnol. 1981, 1, 339–350. [Google Scholar] [CrossRef]
- Brink, L.; Tramper, J.; Luyben, K.C.A.; Van’t Riet, K. Biocatalysis in organic media. Enzym. Microb. Technol. 1988, 10, 736–743. [Google Scholar] [CrossRef]
- Barth, M.; Wei, R.; Oeser, T.; Then, J.; Schmidt, J.; Wohlgemuth, F.; Zimmermann, W. Enzymatic hydrolysis of polyethylene terephthalate films in an ultrafiltration membrane reactor. J. Membr. Sci. 2015, 494, 182–187. [Google Scholar] [CrossRef]
- Yang, S.; Ding, W.; Chen, H. Enzymatic hydrolysis of corn stalk in a hollow fiber ultrafiltration membrane reactor. Biomass Bioenergy 2009, 33, 332–336. [Google Scholar] [CrossRef]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol. Adv. 2010, 28, 308–324. [Google Scholar] [CrossRef]
- Allen, R.D.; James, M.I. Chemical Recycling of PET. In Circular Economy of Polymers: Topics in Recycling Technologies; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1391, pp. 61–80. [Google Scholar]
- Bezeraj, E.; Debrie, S.; Arraez, F.J.; Reyes, P.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Edeleva, M. State-of-the-art of industrial PET mechanical recycling: Technologies, impact of contamination and guidelines for decision-making. RSC Sustain. 2025, 3, 1996–2047. [Google Scholar] [CrossRef]
- Uekert, T.; Singh, A.; DesVeaux, J.S.; Ghosh, T.; Bhatt, A.; Yadav, G.; Afzal, S.; Walzberg, J.; Knauer, K.M.; Nicholson, S.R.; et al. Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics. ACS Sustain. Chem. Eng. 2023, 11, 965–978. [Google Scholar] [CrossRef]
- Uusitalo, T.; Toukoniitty, E. Environmental Impacts of Plastic Recycling—A Literature Review; VTT Technical Research Centre: Espoo, Finland, 2024. [Google Scholar]
- Huang, P.; Ahamed, A.; Sun, R.; De Hoe, G.X.; Pitcher, J.; Mushing, A.; Lourenço, F.; Shaver, M.P. Circularizing PET-G Multimaterials: Life Cycle Assessment and Techno-Economic Analysis. ACS Sustain. Chem. Eng. 2023, 11, 15328–15337. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rorrer, N.A.; Nicholson, S.R.; Erickson, E.; Desveaux, J.S.; Avelino, A.F.T.; Lamers, P.; Bhatt, A.; Zhang, Y.; Avery, G.; et al. Techno-economic, life-cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate). Joule 2021, 5, 2479–2503. [Google Scholar] [CrossRef]
- Uekert, T.; Desveaux, J.S.; Singh, A.; Nicholson, S.R.; Lamers, P.; Ghosh, T.; McGeehan, J.E.; Carpenter, A.C.; Beckham, G.T. Life cycle assessment of enzymatic poly(ethylene terephthalate) recycling. Green Chem. 2022, 24, 6531–6543. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, Z.; Wei, R.; Dong, W.; Jiang, M. Interfacial catalysis in enzymatic PET plastic depolymerization. Trends Chem. 2025, 7, 175–185. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, G.; Chen, Y.; Yi, Z.; Jin, W.; Zhang, G. A versatile tag for simple preparation of cutinase towards enhanced biodegradation of polyethylene terephthalate. Int. J. Biol. Macromol. 2023, 225, 149–161. [Google Scholar] [CrossRef]
- Jiang, C.; Zhai, K.; Wright, R.C.; Chen, J. Engineered Yeasts Displaying PETase and MHETase as Whole-Cell Biocatalysts for the Degradation of Polyethylene Terephthalate (PET). ACS Synth. Biol. 2025, 14, 2810–2820. [Google Scholar] [CrossRef]
- Williams, G.B.; Ma, H.; Khusnutdinova, A.N.; Yakunin, A.F.; Golyshin, P.N. Harnessing extremophilic carboxylesterases for applications in polyester depolymerisation and plastic waste recycling. Essays Biochem. 2023, 67, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Świderek, K.; Velasco-Lozano, S.; Galmés, M.À.; Olazabal, I.; Sardon, H.; López-Gallego, F.; Moliner, V. Mechanistic studies of a lipase unveil effect of pH on hydrolysis products of small PET modules. Nat. Commun. 2023, 14, 3556. [Google Scholar] [CrossRef]
- Arnal, G.; Anglade, J.; Gavalda, S.; Tournier, V.; Chabot, N.; Bornscheuer, U.T.; Weber, G.; Marty, A. Assessment of four engineered PET degrading enzymes considering large-scale industrial applications. ACS Catal. 2023, 13, 13156–13166. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Duan, R.; Xiao, Y.; Wei, Y.; Zhang, H.; Sun, X.; Wang, S.; Cheng, Y.; Wang, X.; Tong, S. Biodegradation of highly crystallized poly (ethylene terephthalate) through cell surface codisplay of bacterial PETase and hydrophobin. Nat. Commun. 2022, 13, 7138. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Hunt, C.J.; Meyer, A.S. Influence of substrate crystallinity and glass transition temperature on enzymatic degradation of polyethylene terephthalate (PET). New Biotechnol. 2022, 69, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Bhattacharya, A.; Khare, S.K. Enzymatic remediation of polyethylene terephthalate (PET)–based polymers for effective management of plastic wastes: An overview. Front. Bioeng. Biotechnol. 2020, 8, 602325. [Google Scholar] [CrossRef] [PubMed]
- Ahmaditabatabaei, S.; Kyazze, G.; Iqbal, H.M.; Keshavarz, T. Fungal enzymes as catalytic tools for polyethylene terephthalate (PET) degradation. J. Fungi 2021, 7, 931. [Google Scholar] [CrossRef]
- Faridkhou, A.; Tourvieille, J.-N.; Larachi, F. Reactions, hydrodynamics and mass transfer in micro-packed beds—Overview and new mass transfer data. Chem. Eng. Process. Process Intensif. 2016, 110, 80–96. [Google Scholar] [CrossRef]
- Sun, C.; Meng, X.; Sun, F.; Zhang, J.; Tu, M.; Chang, J.-S.; Reungsang, A.; Xia, A.; Ragauskas, A.J. Advances and perspectives on mass transfer and enzymatic hydrolysis in the enzyme-mediated lignocellulosic biorefinery: A review. Biotechnol. Adv. 2023, 62, 108059. [Google Scholar] [CrossRef]
- De Santis, P.; Meyer, L.-E.; Kara, S. The rise of continuous flow biocatalysis–fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Wegner, J.; Ceylan, S.; Kirschning, A. Ten key issues in modern flow chemistry. Chem. Commun. 2011, 47, 4583–4592. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Shen, H.; Zhang, J.; Dong, W.; Yu, Z. Scalable nanoplastic degradation in water with enzyme-functionalized porous hydrogels. J. Hazard. Mater. 2025, 487, 137196. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Liu, Y.; Gu, Z.; Zhang, L.; Guo, Z. Deconstructing PET: Advances in enzyme engineering for sustainable plastic degradation. Chem. Eng. J. 2024, 497, 154183. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhou, J.; Li, Y.; Yan, X.; Xu, A.; Zhou, X.; Liu, W.; Xu, Y.; Su, T.; Wang, S. State-of-the-art advances in biotechnology for polyethylene terephthalate bio-depolymerization. Green Carbon 2025, 3, 303–319. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, M. Navigating the landscape of enzyme design: From molecular simulations to machine learning. Chem. Soc. Rev. 2024, 53, 8202–8239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damayanti, D.; Marpaung, D.S.S.; Kodarif, A.R.; Sanjaya, A.; Saputri, D.R.; Fahni, Y.; Rahmiyati, L.; Silvia, P.Z.; A’yuni, D.Q.; Imalia, C.L.; et al. Biocatalytic Recycling of Polyethylene Terephthalate: From Conventional to Innovative Routes for Transforming Plastic and Textile Waste into Renewable Resources. Resources 2025, 14, 176. https://doi.org/10.3390/resources14110176
Damayanti D, Marpaung DSS, Kodarif AR, Sanjaya A, Saputri DR, Fahni Y, Rahmiyati L, Silvia PZ, A’yuni DQ, Imalia CL, et al. Biocatalytic Recycling of Polyethylene Terephthalate: From Conventional to Innovative Routes for Transforming Plastic and Textile Waste into Renewable Resources. Resources. 2025; 14(11):176. https://doi.org/10.3390/resources14110176
Chicago/Turabian StyleDamayanti, Damayanti, David Septian Sumanto Marpaung, Abdul Rozak Kodarif, Andri Sanjaya, Desi Riana Saputri, Yunita Fahni, Lutfia Rahmiyati, Putri Zulva Silvia, Dewi Qurrota A’yuni, Calaelma Logys Imalia, and et al. 2025. "Biocatalytic Recycling of Polyethylene Terephthalate: From Conventional to Innovative Routes for Transforming Plastic and Textile Waste into Renewable Resources" Resources 14, no. 11: 176. https://doi.org/10.3390/resources14110176
APA StyleDamayanti, D., Marpaung, D. S. S., Kodarif, A. R., Sanjaya, A., Saputri, D. R., Fahni, Y., Rahmiyati, L., Silvia, P. Z., A’yuni, D. Q., Imalia, C. L., Janah, D. U., & Wu, H. S. (2025). Biocatalytic Recycling of Polyethylene Terephthalate: From Conventional to Innovative Routes for Transforming Plastic and Textile Waste into Renewable Resources. Resources, 14(11), 176. https://doi.org/10.3390/resources14110176








