Abstract
Climate change, through increased aridity, threatens ecosystems, including Morocco’s endemic Argania Spinosa L. Skeels. This study assesses the impact of aridity on argan trees by analyzing morphological, ecophysiological, and biochemical parameters across various regions and comparing them with historical data. Significant variations were observed in leaf area, leaf length, chlorophyll content, relative water content, polyphenols, flavonoids, soluble sugars, and antioxidant activity, while leaf width ratio and chlorophyll ratio remained stable. Tioughza exhibited the largest leaf area (136.07 mm2), the highest chlorophyll content (436.76 mg/m2), and superior water retention (52.27%). Conversely, Ezzaouite showed the smallest leaf area (85.76 mm2) and lowest water content (37.68%). Increased aridity has intensified these differences, revealing the argan tree’s vulnerability to climate change. The findings underscore the need for targeted conservation efforts, including reforestation, strengthened legislation, and enhanced genetic research, to sustain this vital species.
1. Introduction
Morocco, located in North Africa, faces significant climate change challenges, including rising temperatures, more frequent heatwaves, and altered rainfall patterns [1]. Its Mediterranean climate, characterized by dry, hot summers and occasional storms, puts crops under stress [2]. This arid to semi-arid climate places crops under significant heat and drought stress [3], with increasing climate variability threatening agriculture, especially in developing regions. Adaptation strategies based on advanced climate models and localized research are vital for maintaining food security and resilience, while continued research is crucial for developing targeted policies and practices [4]. Morocco has experienced faster warming and drying trends than the global average, with more frequent droughts in the northern regions, which future models predict will worsen with decreased annual precipitation [5]. While extreme precipitation trends are not yet evident, projections suggest a decline by the end of the century [6]. Drought, a key global agricultural challenge, has caused significant water shortages in Morocco, affecting agriculture, water supplies, and electricity, as seen in past droughts [5]. It also triggers various physiological, biochemical, and molecular reactions in plants which regulate growth and productivity, disrupting essential plant processes [7].
Plants manage stress through morphological, biochemical, and molecular changes, including the production of metabolites like proline, polyamines, and carbohydrates. Drought-induced water stress triggers osmoregulatory responses, such as the accumulation of sugars and amino acids to maintain turgor [8,9]. Phenolic compounds, particularly flavonoids, play a crucial role as secondary metabolites in plants, supporting various physiological processes and acting as stress biomarkers, enabling plants to survive in diverse environmental conditions [10]. Exposure to drought has been shown to promote higher production of various classes of secondary metabolites (SMs), including terpenes, complex phenols, and alkaloids, during both in vitro and in vivo growth, through the induction of ionic or osmotic stress [11].
The argan tree (Argania spinosa L.), a key species in Morocco, is recognized for its resilience to arid conditions. UNESCO declared its woodlands a Biosphere Reserve in 1998, and it is celebrated annually on 10 May [1,12]. Native to southwestern Morocco, where water scarcity is severe, the argan tree has faced a significant decline due to intensified overgrazing and aridity [13,14]. This raises questions about the tree’s physiological and biochemical responses to drought and its sustainability.
Vegetation in west-central Morocco, despite its significance, is highly susceptible to degradation, with the argan forest losing nearly 50% of its area during the 20th century [15,16]. Argan tree density fell from 300 to fewer than 100 trees per hectare by the 1990s, losing two-thirds of its natural heritage [2]. From 1987 to 2014, the forest declined by 5%, with 87% converted to cropland or rangeland, and 11% to agriculture [17]. A case study in Aoulouz showed a 44.5% reduction in forest density between 1970 and 2007 due to fuelwood extraction and increasing aridity [16,18].
Argan forest regeneration is nearly nonexistent due to excessive grazing and year-round human use [17,19]. Pastoral activities deplete scarce natural resources, and worsening aridity is expected to intensify the situation [20]. A study highlighted indicators of argan land degradation, with projections showing declining rainfall and rising temperatures, threatening the agricultural sector and subsistence farming [14,21]. While argan trees could serve as an alternative crop in low-rainfall areas, challenges in domestication and genetic variability persist, limiting their exploitation to natural ranges [12,22]. Urgent conservation efforts are needed to protect this endemic species, with a focus on identifying drought-resistant argan types to combat desertification [13,23].
Recent conservation efforts, including collaboration between the National Agency for the Development of Oasis Areas and the Argan (ANDZOA) and the High Commission for Water and Forests, have led to the restoration of over 100,000 hectares by 2017 [17]. The “arganiculture” initiative under the Development of Argan Orchards in Degraded Environments (DARED) project, financed by the Green Climate Fund and carried out by the National Agency of Development (ADA), the National Agency for the Development of Oasian Zones and the Argan Tree (ANDZOA), and the National Institute of Agronomical Research (INRA), aims to increase productivity on 10,000 hectares, promote co-management of natural forests, and enhance research [23,24].
In this article, we investigated the effect of aridity on argan trees in five vulnerable areas in southern Morocco by examining the morphometric, phytochemical, and eco-physiological characteristics. We hypothesize that drought and aridity significantly impact the physiological and biochemical responses of the argan tree, particularly in the production of secondary metabolites such as phenolic compounds and flavonoids, which are crucial for the tree’s survival under adverse environmental conditions. This study aims to emphasize the critical impact of aridity on argan trees, regional biodiversity, and ecosystem resilience. Additionally, it seeks to highlight the urgent need for reforestation and conservation measures to ensure the long-term sustainability of the species.
2. Materials and Methods
2.1. Study Site
The study was conducted in five different sites across vulnerable areas and borders of the RBA forests (Figure 1) falling under the following regions: Marrakech-Safi, Chtouka Ait Baha, Tiznit, Guelmim Oued Noun, and Sidi Ifni.
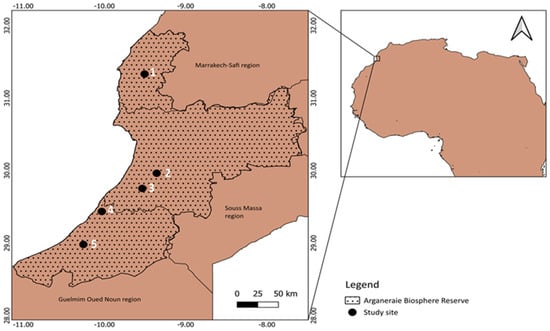
Figure 1.
Study site locations within the Argan Biosphere Reserve, Morocco. Ezzaouite (1); Tamjlojt (2); Rasmouka (3); Tioughza (4); Laqsabi (5).
These sites are characterized by not having any land management practices and fall under three climatic zones: semi-arid, arid, and Saharan. Altitude (m), annual mean temperature (°C), and precipitation (mm/day) range from 181 to 377, 19.77 to 21.11, and 0.41 to 0.76, respectively. The locations had different soil textures ranging from sandy loam for Ezzaouite, loamy sand for Tamjlojt, Rasmouka, and Laqsabi, and sandy clay loam for Tioughza. Soil properties are reported in Table 1.

Table 1.
Soil properties of the five studied sites.
To compare the phytochemical properties of argan trees across the sites, we selected ten trees per site, all of which are naturally regenerated and estimated to be over a hundred years old (Figure 2).
Figure 2.
Representative images of the states of the study sites.
All the trees were in healthy condition, with heights ranging from 2.51 to 8.2 m and diameters at breast height (DBH) ranging from 16.44 to 48.35 cm (Table 2). The leaf sampling was performed in spring March 2023, and leaves were gathered from three levels of the tree (bottom, middle, and top) in four directions, considering the exposure factor. This sampling method ensured the homogenization of samples to make them representative of the area. The collected plant material was stored in a cold room at 8 °C until use. Some of the leaves were dehydrated in an oven at 40 °C for 48 h and subsequently ground into a fine powder. The remaining leaves were preserved in a freezer for future examination. For the morphometric and chlorophyll content, we had ninety replicates per tree, while for the rest we went with three replicates per tree. Additionally, to highlight the impact of aridity, a comparison was made with a previous study conducted in 2013 by Fahmi et al. [25,26].

Table 2.
Geographic, climatic, and tree data of argan tree sampling sites.
2.2. Morphometric Characteristics
The measurements of the morphometric characteristics of the leaves (surface (mm2), width (mm), length (mm), and ratio (L/l)) were carried out using a portable leaf area meter (AM350, ADC BioScientific, Hoddesdon, UK It measures these parameters by scanning the leaf and calculating its dimensions. It uses advanced optical sensors to capture precise data, allowing for accurate assessments of leaf size and shape, with (n = 90).
2.3. Relative Water Content (RWC)
The relative water content of the leaves was evaluated using the equation by Weatherley [27]:
RWC (%) = (FW − DW)/(TW − DW) × 100
FW refers to the weight of the leaves in their fresh state. TW is the weight of the leaves after they have been soaked in distilled water in the dark for 24 h at 4 °C. DW is the weight of the leaves after they have been dried in an oven for 48 h at 70 °C.
2.4. Chlorophyll Content
Chlorophyll content was measured using a chlorophyll content meter (CCM-30, OPTI-SCIENCES, Hudson, East Rutherford, NJ, USA). It measures chlorophyll content and ratios by emitting specific wavelengths of light through the leaf and detecting the light absorbed by chlorophyll. This non-destructive method provides real-time data, with (n = 90).
2.5. Polyphenols Content
The determination of the total polyphenol content in argan tree extracts was carried out using the Folin–Ciocalteu reagent following the modified protocol cited by Fahmi et al. [26].
To extract the total phenolic compounds, 50 mg of powdered plant material was immersed in 2.5 mL of 95% ethanol and then placed in the freezer for 48 h. After homogenization, the mixture was centrifuged 13,000 times in 10 min. A portion of the liquid remaining after centrifugation (0.3 mL) was combined with 0.3 mL of 95% pure ethanol, 1.5 mL of distilled water, and 0.150 mL of the Folin–Ciocalteu reagent. After allowing the mixture to stand for 5 min, 0.3 mL of a 5% sodium carbonate (Na2CO3) solution was added. Following agitation, the reaction mixture was incubated in the dark for 1 h. The optical density of the mixture was then determined at a wavelength of 725 nm using T80+ UV-VIS spectrophotometer (PG instruments, Wibtoft, UK). The quantification of polyphenols was determined by measuring the amount of Gallic acid, reported in milligrams per gram of dry weight (DW).
2.6. Flavonoids Content
The determination of the total flavonoid content (TFC) was conducted using the method outlined by Lfitat et al. [28]. This method relies on the development of a complex between aluminum and flavonoids. At first, 1.5 mL of the extract was combined with 1.5 mL of a solution containing 2% aluminum chloride (AlCl3). The combination was incubated at room temperature for 30 min, and then the absorbance was measured at 430 nm relative to the blank. The flavonoid concentrations were measured by using a quercetin standard curve. The quantification of flavonoids was measured in milligrams of quercetin equivalents per gram of dry weight (mg QE/g DW).
2.7. Antioxidant Activity
Antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, according to the protocol described by Fahmi et al. [26], with modifications. Initially, a 0.1 mM DPPH solution was freshly prepared in methanol and kept protected from light. Then, 2850 µL of this solution was added to a test tube containing an aliquot of 150 µL of samples. The mixture was vortexed for 1 min and kept at room temperature for 30 min. Following this time frame, the absorbance was quantified at a wavelength of 517 nm relative to a control sample, which consisted of the DPPH solution without the extract. The percentage inhibition of the DPPH free radical was determined by using the following equation:
P = (A1 − A2) × 100/A1
P represents the percentage of DPPH radical scavenging. A1 represents the absorbance of the control reaction, namely the DPPH solution without the extract. A2 denotes the level of absorbance seen in the presence of the sample.
2.8. Soluble Sugar Content
The technique of Dubois et al. [29] was used to determine the soluble sugar concentration. A total of 0.02 g of dehydrated plant material was combined with 2 mL of 70% ethanol (v/v) and thereafter subjected to centrifugation at a speed of 2000 revolutions per minute for a duration of 10 min. The liquid portion was collected, and the process of removing substances was performed two more times on the solid residue. The liquid obtained after two extractions was mixed together, and 16 mL of distilled water was added. Next, 1 mL of the extract was combined with 1 mL of 5% phenol and 5 mL of concentrated sulfuric acid. The mixture was agitated vigorously and left undisturbed for a duration of 10 min, after which it was immersed in a water bath maintained at a temperature of 30 °C for a period of 20 min. The T80+ UV-VIS spectrophotometer (PG instruments, Wibtoft, UK) was used to measure the absorbance at a wavelength of 490 nm. The soluble sugar contents were quantified by using a glucose standard curve and represented as milligrams per gram of dry weight.
2.9. Bioclimatic Synthesis
The bioclimatic analysis was conducted over a period of 31 years (1991–2022) using meteorological data, particularly temperatures and precipitation, obtained from the NASA Power | Data Access Viewer database. Also, for the principal component analysis (PCA), we used the data of 2023 to obtain annual mean precipitation and temperature. In this context, the De Martonne aridity index was calculated for all the sites using the formula cited by Ionac and Grigore [30].
2.10. Statistical Analysis
The morphometric data were collected using 900 duplicates per site, whereas the other analyses were conducted using 30 replicates per site. The data were subjected to a one-way analysis of variance (ANOVA) using SPSS software version 25. The Newman–Keuls post hoc test was used to identify significant differences between groups at a significance level of p < 0.05. Data obtained on all parameters were analyzed using R.3.6.3 software, with principal component analysis using the FactoMineR and factoextra packages.
3. Results
The analysis of soil properties and tree growth across the five sites reveals notable patterns. At the Tioughza site, where soil phosphorus (P2O5) levels are highest, trees are significantly taller, suggesting that phosphorus plays a crucial role in promoting vertical growth in the argan tree. Additionally, sites with slightly lower pH values, such as Tioughza and Ezzaouite, tend to have trees with thicker trunks, indicating that more acidic soils might support stronger trunk development. Higher organic matter content at Ezzaouite is associated with relatively taller trees, highlighting the importance of soil fertility and moisture retention in tree growth. Geographical and climatic conditions also interact with these soil properties to influence tree growth patterns.
3.1. Morphometric Characteristics
3.1.1. Leaf Area
The statistical analysis (ANOVA) of argan tree leaf surface data showed a highly significant difference (p < 0.001) among the study sites (Table 3). The results presented in Table 4 demonstrate that leaf surface varies significantly between sites. Leaves from trees in Tioughza exhibit the highest leaf surface area with a value of 136.07 mm2, whereas leaves from trees at Ezzaouite and Tamjlojt stations recorded the lowest values of 85.76 mm2 and 85.53 mm2, respectively. It was also noted that Tioughza, with sandy clay loam, had a slightly acidic pH (7.21), and an available phosphorus content of (6.7 ± 2.75 mg/g soil). Meanwhile, Ezzaouite had a slightly alkaline pH (7.69) and a phosphorus content of (0.37 ± 0.32 mg/g soil).

Table 3.
ANOVA results for the study parameters.

Table 4.
Leaf area, leaf width, length, ratio, chlorophyll content, and chlorophyll ratio in argan leaves from the five studied sites (n = 900, mean ± SD).
3.1.2. Leaf Width
Leaf width is fairly consistent across most sites, except for Tioughza, which has slightly broader leaves (8.52 ± 1.34 mm) (Table 4). The Anova results showed no significant difference between the leaf width averages of the various studied stations.
3.1.3. Leaf Length
The analysis of averages shows that the highest leaf length was 24.54 ± 3.95 mm for trees in Rasmouka, followed by Tioughza (23.75 ± 3.7 mm), and Tamjlojt (22.86 ± 4.89 mm). The lowest values were observed in Ezzaouite (20.58 ± 2.97 mm) and Laqsabi (20.7 ± 2.8 mm). No significant difference was observed between the length of the leaves (Table 3).
3.1.4. Leaf Surface Ratio
The ratio of leaf length to width shows little variation, indicating that the overall shape of the leaves is relatively consistent, despite differences in absolute size.
3.2. Chlorophyll Content
The results indicate a highly significant difference among the study sites regarding the total chlorophyll content. Tioughza again stands out with the highest chlorophyll content (436.76 ± 85.41 mg/m2), while Tamjlojt has the lowest (374.78 ± 102.71 mg/m2).
3.3. Chlorophyll Content Ratio
There is no apparent significant difference among the study locations concerning the results of the chl a/chl b ratio. The chl a/chl b ratio remains consistently below one for all studied stations. The results show that chlorophyll “b” is generally more abundant than chlorophyll “a” and that argan trees from different locations appear to respond similarly to climatic conditions, suggesting that there is a consistent adaptation to light conditions across all sites.
3.4. Relative Water Content
RWC is an indicator of the plant’s water status and is important for understanding its ability to tolerate drought. The results of the water status of trees from the different sites are reported in Table 5. The Tioughza site has the highest RWC (52.27 ± 11.8%), suggesting better water retention and possibly less stress from water scarcity. Ezzaouite, on the other hand, has the lowest RWC (37.68 ± 11.26%), indicating that plants in this area may be under more significant water stress. Statistical analysis of the relative water content of argan tree leaves shows a highly significant difference between the study stations (Table 3).

Table 5.
Relative water, soluble sugars, polyphenols, flavonoids, and antioxidant activity in argan leaves from the five studied sites (n = 30, mean ± SD).
3.5. Soluble Sugar Content
Soluble sugars play a crucial role in osmotic adjustment and energy storage, especially under stress conditions. The analysis of variance followed by the S.N.K test (p = 0.05) reveals a highly significant difference regarding the soluble sugar content among the different studied stations. The highest value was noted in trees from the Tioughza station (75.03 ± 24.07 mg/g dry weight), while the lowest value was recorded in argan tree leaves from the Ezzaouite station (25.52 mg/g dry weight).
3.6. Total Polyphenol Content
Polyphenols are secondary metabolites that have antioxidant properties and help plants manage stress. All leaf extracts contained a considerable amount of phenolic compound (Table 3) with significant differences observed between sites. The highest content of polyphenols was observed in leaves from Tamjlojt (21.32 ± 3.74 mg EGA/g dry weight).
3.7. Flavonoids Content
Flavonoids are a type of polyphenol that also contribute to antioxidant defense. The statistical analysis of mean flavonoid content values revealed a highly significant difference among the study sites. Analyzing these means, referencing the standard curve of quercetin, showed that the recorded values of flavonoid content are relatively close for trees from four stations: Rasmouka, Tioughza, Tamjlojt, and Laqsabi (18.55 ± 5.99 mg EQ/g dry weight, 17.98 ± 4.46 mg EQ/g dry weight, 17.04 ± 3.07 mg EQ/g dry weight, and 16.96 ± 3.18 mg EQ/g dry weight, respectively). As for trees from the Ezzaouite station, the total flavonoid content is 13.18 ± 3.05 mg EQ/g dry weight, respectively (Table 5).
3.8. Antioxidant Activity
The results of the DPPH test revealed that argan tree leaf extracts exhibit very significant antioxidant activity. The DPPH test results (Table 5) demonstrate that the highest DPPH radical scavenging capacity was observed at the Tioughza station (92.27%), while the lowest was at the Laqsabi station (90.86%). Comparing the means showed highly significant differences between the study stations for this parameter.
3.9. De Martonne Index Evolution
The De Martonne index results (Figure 3) show the fluctuation of the aridity throughout the years from 1991 to 2021. They suggest that over the 30-year period, all sites have transitioned towards arid to hyper-arid climates, a trend that intensified over the last 10 years (Index < 10 to <5) due to successive droughts, including the period of the current study.
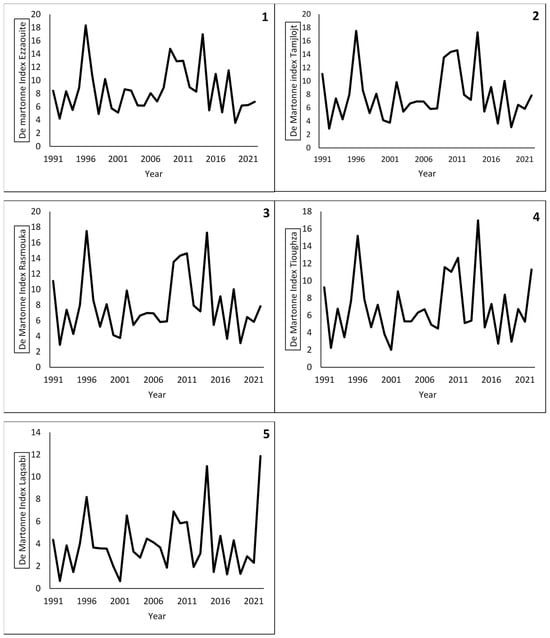
Figure 3.
Evolution of De Martonne Index from 1991 to 2021 for the five localities calculated using NASA Power data and the following formula: De Martonne Index = P/T + 10. Ezzaouite (1); Tamjlojt (2); Rasmouka (3); Tioughza (4); Laqsabi (5).
3.10. PCA and Correlation Results
The principal component analysis (Figure 4a) was conducted to assess the relationship between the parameters studied. According to the PCA results, PC1 and PC2 accounted for 45.8% and 28.3% of the data variability, respectively. The following variables—(relative water content, leaf area, flavonoids, soluble sugars, and nitrogen),contribute to the PC1, while electrical conductivity, pH, leaf and chlorophyll ratios, and annual mean temperature contribute to the PC2. It also shows that the sites can be distinguished into three groups: Ezzaouite, Tioughza, and a final group including Rasmouka, Laqsabi, and Tamjlojt.
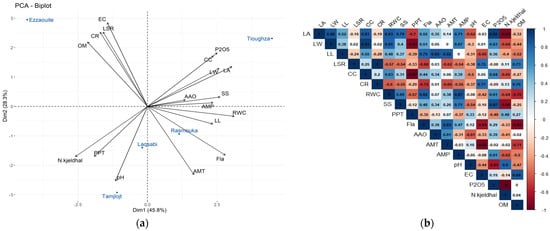
Figure 4.
Principal component analysis biplot (a) and correlation matrix (b) of sites based on environmental, soil properties, and biochemical variables. LA: leaf area, LW: leaf width, LL: leaf length, LSR: leaf surface area, CC: chlorophyll content, CR: chlorophyll ratio, RWC: relative water content, SS: soluble sugars, PPT: polyphenols, Fla: flavonoids, AAO: antioxidant activity, AMT: annual mean temperature, AMP: Annual mean precipitation, EC: conductive electricity, OM: organic matter.
Morphological parameters show a positive correlation with relative water content (RWC) and soluble sugars (SS). Leaf surface area (LA) showed a highly positive correlation with P2O5 (r = 0.92). It also showed a high correlation with annual mean precipitation (AMP) (R = 0.71), and a negative one with total phenolic content (PPT), pH, and nitrogen (N.Kjeldhal). LA and leaf width also showed a high positive correlation with chlorophyll content (CC) (r = 0.83, r = 0.93). Meanwhile, leaf length showed a positive correlation with flavonoids (r = 0.79), antioxidant activity (r = 0.85), and annual mean temperature (r = 0.82).
RWC and SS correlated positively with AMP; meanwhile, they showed a negative correlation with electrical conductivity (EC), nitrogen, and organic matter (OM). For secondary metabolites, PPT showed a negative correlation with a majority of parameters (LA, LW, LL, CC, RWC, and P2O5), yet a moderate and positive one to pH and nitrogen. Flavonoids, however, correlated positively with the mentioned parameters (LA, LW, LL, CC, RWC, and P2O5). It correlated negatively with EC and OM.
AMT showed a strong positive correlation with LL (r = 0.82), FLA (r = 0.83), AAO (r = 0.61), and a strong negative one with EC (r = −0.95) and OM (r = −0.71). AMP and P2O5 were observed to correlate negatively with nitrogen, pH, and P2O5.
4. Discussion
The growth and productivity of plants are affected negatively by environmental stress conditions (drought and aridity). Plants face complex interactions with various environmental factors. When subjected to biotic and abiotic stress, they experience disruptions in their metabolic processes, which can lead to decreased growth and reduced productivity. Abiotic stress, in particular, has a significant impact on plant growth and plays a crucial role in causing substantial losses in agricultural products [31]. Consequently, plants respond to environmental stress through a series of morphological, anatomical, physiological, and biochemical modifications, which enable the maintenance of growth, development, and production. This response varies within populations and genotypes originating from different climatic regions [32]. Despite the abundant literature, there is controversy regarding the mechanisms of drought tolerance in plants. In addition to the complexity of drought itself, the behavioral responses of plants to drought are complex, and different mechanisms are adopted when they encounter drought.
4.1. Morphometric Characteristics
The study of leaf morphometric parameters revealed significant variability in surface area between sites, notably between Tioughza and Ezzaouite. Tioughza, with sandy clay loam and slightly acidic pH, had the largest leaf area, likely due to higher phosphorus content and better moisture retention [32,33]. In contrast, Ezzaouite’s sandy loam and slightly alkaline pH led to smaller leaf areas, likely due to lower phosphorus levels and faster soil drainage [33].
Leaf width and length showed no significant variation across sites, suggesting these traits are less influenced by soil and possibly more genetically determined. The stable leaf length-to-width ratio supports the idea that this trait is optimized for balancing light capture and water conservation [34]. These observations align with Zahidi and El Mousadik’s [35] findings, where morphometric traits explained nearly all leaf shape variability across regions. Understanding these variations is crucial for conserving and sustainably utilizing the argan tree.
4.2. Chlorophyll Content and Ratio
The results indicate a highly significant difference among the study sites regarding the total chlorophyll content. The studies conducted by Chakhchar et al. and Berka and Aïd [13,36] assert that the level of chlorophyll pigment decreases along an increasing aridity gradient (ranging from a semi-arid climate to an arid climate). Moreover, moderate and severe drought stress significantly affected the concentrations of chlorophylls. However, the quantity of chlorophyll in leaves can be influenced by several other factors, such as age and leaf position, as well as environmental factors like light, soil properties, temperature, and water availability [37]. These factors could explain the significant difference between the Tioughza, Rasmouka, and Laqsabi sites, given their similar climatic conditions. This difference could be attributed to factors like altitude, age, soil texture, and phosphorus content. Regarding the chlorophyll a/chlorophyll b ratio, no significant apparent difference was noted among the study locations. The study Ait Bihi et al. [23] conducted on argan trees revealed that there were significant differences in photosynthetic pigments, such as chlorophyll a and b, between two locations (north and south) and also within the same site in different seasons. Meanwhile, the study proved that there were no significant differences in the chlorophyll ratio between the stations.
4.3. Relative Water Content
RWC, an indicator of plant water status, varies significantly across sites (F = 9.1901, p < 0.0001). A study conducted by Meslem et al. [38] highlighted the remarkable ability of the argan tree to maintain its leaves in a turgid state for a long duration with minimal water input. This phenomenon was also observed in our study, where argan trees retain a certain level of water despite unfavorable climatic conditions (increased aridity and decreased precipitation). The results obtained showed that the relative water content is low at higher altitudes and higher in coastal areas, which is consistent with Fahmi et al. [26]. Ait bihi et al. [23] also found significant differences between sites (north/south), and between seasons. Keyvan [39] found that with an increase in the intensity of drought, there was a decrease in relative water content. This was also confirmed by Yavas et al. [40], who found that drought affected RWC significantly.
4.4. Total Polyphenols and Flavonoids Content
The lowest levels of phenolic compounds, namely polyphenols and flavonoids, were recorded at the Ezzaouite and Rasmouka stations, while the highest levels were observed in the other stations (Tamjlojt and Rasmouka), respectively. Plants respond to environmental stresses by increasing the production of polyphenols, especially flavonoids. These phenolic compounds can undergo significant fluctuations in response to such stresses [41]. Several studies have shown that drought stress induces an increase in secondary metabolites such as polyphenols and flavonoids [42,43,44,45]. However, it should be noted that in the case of the Argan tree, the factors influencing the induction of phenolic compound production cannot be definitively determined [46]. This could be explained by the involvement of various environmental factors that influence the phenolic compound content of A. spinosa, such as herbivore stress [47], radiation stress, thermal stress, and nutritional stress.
4.5. Antioxidant Activity
The argan tree employs several morphological and physiological strategies to withstand water stress, such as stomatal control in response to increasing temperatures or decreasing leaf water potential, leaf shedding, and the accumulation of antioxidant defense systems [48]. Several studies demonstrate that the antioxidant activity of leaf extracts is mainly attributed to their high content of phenolic compounds.
Numerous studies have demonstrated a strong correlation between phenolic compound content and antioxidant activity. Phenolic compounds are a major class of antioxidant agents due to their free radical scavenging ability [49]. In our study, the free radical scavenging percentages of argan leaf extracts from different locations exceeded 90%, as also reported for the argan tree by Joguet and Maugard [50] and Fahmi et al. [26]. Generally, plants with high quantities of phenolic compounds exhibit very strong antioxidant capacity. However, the highest antioxidant activity in our study was recorded under conditions where phenolic compound levels were lowest.
4.6. Soluble Sugar Content
The results of soluble sugar content indicate that the argan tree is a species that accumulates sugars to a greater extent to resist stress. This accumulation is attributed to the degradation of starch, resulting in its conversion into soluble sugars. Similar findings have been observed in argan tree seedlings under water deficit conditions, where an increase in soluble sugar content was accompanied by a decrease in starch concentration. In the case of the argan tree, a rapid and significant accumulation (65%) of active soluble sugars is recorded during moderate stress [36]. In our study, this pattern was observed in trees from Tioughza, Tamjlojt, and Laqsabi stations where climatic conditions are more pronounced compared to other stations. Other studies also confirmed the effect of drought stress on soluble sugars [38,50,51].
Based on all these results, the argan tree is a species that exhibits resilience to stress conditions, even under favorable development conditions. While several authors acknowledge that stress memory could be an important strategy for coping with a changing climate with frequent drought events, the ecological memory capacity of stress in Argania spinosa is a mechanism of resistance to water stress in its habitat [48].
4.7. Aridity Effect
From our study, we have found the following findings, a relative water content from 37.68% to 59.25%, total polyphenols content from 14.04 to 21.32 mg/g dry weight, flavonoids content varying from 13.18 to 18.55 mg/g dry weight, antioxidant activity between 90.86% to 96.27% and a soluble sugars content ranging from 29.69 to 75.03 mg/g dry weight. Our results were different from those obtained by Fahmi et al. [25,26], who conducted a similar study in the same regions ten years ago and whose findings were as follows: 63.9% to 77.82%, 5.73 to 8.76 mg/g dry weight, 2.93 to 6.57 mg/g dry weight, and 93% and 7.24 to 9.64 mg/g dry weight, respectively.
The decreases observed in relative water content could be attributed to increased aridity, decreased precipitation, or differences in altitude among the sampling areas. The increase in polyphenol, flavonoid, and soluble sugar content in the study areas from 2013 to 2022 can be linked to the increased aridity gradient, as evidenced by the accumulation of phenolic and flavonoid compounds under drought stress [42]. Just as drought stress led to a rise in specific compounds and oxidative markers, the exacerbated aridity and water deficit in the argan tree’s environment likely triggered similar adaptive responses, resulting in higher concentrations of these beneficial compounds while simultaneously decreasing relative water content. This comparison highlights and underscores the exacerbated change that the argan tree is experiencing due to climate change, aridity, water deficit, and drought.
4.8. Principal Component Analysis
The PCA biplot and correlation analysis (Figure 4) reveal distinct site influences from environmental and soil variables. Tioughza stands out with low pH, medium EC, and high P2O5, indicating slightly alkaline, nutrient-rich soil that supports healthy plant growth. Tamjlojt and Laqsabi show more moderate conditions, with Tamjlojt having higher pH but lower EC and P2O5, reflecting balanced soil properties. Laqsabi has slightly higher EC and P2O5, suggesting increased salinity and nutrient levels, but these conditions are still not optimal for growth.
Ezzaouite has a slightly alkaline pH, the highest EC, low P2O5, and high OM, reflecting reduced nutrient availability and limited growth potential. Rasmouka, with acidic soil and low P2O5 and EC, shows poorer fertility but moderate-to-high LA and RWC, suggesting stress due to limited nutrients. In summary, the PCA biplot highlights how variations in soil properties, particularly pH, EC, and P2O5, influence plant growth across different sites. Tioughza benefits from more favorable soil conditions, while Ezzaouite and Rasmouka face nutrient limitations that stress plant growth, emphasizing the importance of soil management for optimizing plant health. The analysis highlights the differences in soil properties at each site and their impact on plant physiological traits under various environmental conditions.
5. Conclusions
The study reveals significant variability in argan tree traits across different sites, shaped by distinct environmental and soil conditions. Tioughza stands out with superior leaf size, chlorophyll content, and water retention, linked to favorable soil properties such as slightly high pH, the highest electrical conductivity, and moderate phosphorus levels. These factors also contribute to elevated soluble sugars (SS) and antioxidant activity (AAO), highlighting Tioughza’s strong stress adaptation and advantage in supporting healthy plant growth. In contrast, Ezzaouite and Rasmouka, with more alkaline soils and lower nutrient availability, show medium leaf size, chlorophyll content, and water retention, along with lower levels of polyphenols (PPT), SS, and AAO, indicating greater environmental stress and compromised vitality.
Tamjlojt and Laqsabi, with more alkaline soil conditions, present the lowest values in traits like leaf size and chlorophyll content. However, Tamjlojt is notable for its higher levels of PPT and flavonoids (FLA), suggesting a unique biochemical response that enhances resilience to environmental challenges. The study underscores the intricate relationships between soil properties and plant traits, emphasizing how pH, phosphorus availability, and organic matter influence the argan tree’s physiological and biochemical characteristics, with key markers like PPT, FLA, SS, and AAO playing a crucial role in the tree’s adaptability to varying conditions.
Future research should explore the argan tree’s biochemical responses to drought and other environmental factors, such as altitude, sunlight, and precipitation, to optimize site-specific management practices and ensure the species’ long-term sustainability. Conservation efforts should include reforestation, stricter environmental regulations, and genetic research to identify and promote genotypes with optimal levels of these key bio-chemical markers.
Author Contributions
Conceptualization, C.A. and N.A.A.; Data curation, M.T., S.L. and N.C.; Formal analysis, C.A.; Funding acquisition, N.A.A.; Investigation, C.A.; Methodology, C.A. and N.A.A.; Resources, N.A.A.; Software, C.A. and S.L.; Supervision, N.A.A. and F.M.; Validation, C.A. and N.A.A.; Visualization, C.A. and N.A.A.; Writing—original draft, C.A.; Writing—review and editing, J.H., F.M. and N.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This publication has been financed with the support of the National Agency for the Development of Oasis and Argan Areas (ANDZOA) within the framework of the project for the Development of “Arganiculture” in Vulnerable Zones “DARED”, projects co-financed by the Green Climate Fund (GCF) with the grant number FP0022/Grant/DARED 2021-23.
Data Availability Statement
All data generated in this work are provided within this manuscript.
Acknowledgments
We extend our heartfelt gratitude to El Fiddaoui Keltouma, Oumasst Assma, Tiouidji Fatima Ezzahra, and the technical staff of INRA. A special thank you to Abdelaziz Mimouni, Abdelghani Tahiri, and Adelaaziz El Aasri for their invaluable assistance and support. We express our immense gratitude to ANDZOA, particularly Abderrahmane Aitlhaj, for providing the essential resources needed to conduct this study. Additionally, we offer our warm appreciation to the entire team of the DARED Project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hammoudy, W.; Ilmen, R.; Sinan, M. Impact of climate change on extremes events in Morocco. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012034. [Google Scholar] [CrossRef]
- El Ghazali, H.; Daoud, S.; Benoada Tlemçani, N. Impact of climate change on the Argan Biosphere Reserve (ABR) in Morocco. Preprints 2021, 2021050536. [Google Scholar] [CrossRef]
- Walters, S.A.; Mimouni, A.; Bouharroud, R. Local melon and watermelon crop populations to moderate yield responses to climate change in North Africa. Climate 2021, 9, 129. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Chaowiwat, W.; Wand, C. Climate change impact on major crop yield and water footprint under CMIP6 climate projections in repeated drought and flood areas in Thailand. Sci. Total Environ. 2022, 807, 150741. [Google Scholar] [CrossRef] [PubMed]
- Driouech, F.; Statfi, H.; Khouakhi, A.; Moutia, S.; Badi, W.; ElRhaz, K.; Chehbouni, A. Recent observed country-wide climate trends in Morocco. Int. J. Climatool. 2021, 41, E855–E874. [Google Scholar] [CrossRef]
- Chaqdid, A.; Tuel, A.; El Fatimy, A.; El Moçayad, N. Extreme rainfall events in Morocco: Spatial dependence and climate drivers. Weather Clim. Exterm. 2023, 40, 100556. [Google Scholar] [CrossRef]
- Chakhchar, A.; Lamaoui, M.; Aissam, S.; Ferradous, A.; Wahbi, S.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, A. Physiological and Biochemical Mechanisms of Drought Stress Tolerance in the Argan Tree. In Plant Metabolites and Regulation under Environmental Stress; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 311–322. [Google Scholar] [CrossRef]
- Punetha, A.; Kumar, D.; Suryavanshi, P.; Padalia, R.C.; Katanapalya Thimmaiah, V. Environmental Abiotic Stress and Secondary Metabolites Productions in Medical Plants: A Review. Tarim. Bilim. Derg. 2022, 28, 351–362. [Google Scholar]
- LE, A.; Torre, S.; Olsen, J.; Tanino, K. Stomatal Responses to Drought Stress and Air Humidity. In Abiotic Stress in Plants—Mechanisms and Adaptations; IntechOpen: Houston, TX, USA, 2011; 428p. [Google Scholar]
- Noori, M.; Dehshiri, M.-M.; Mehrdost, N. Root Flavonoids of some Iranian scirpus L. (Cyperaceae) Members. Int. J. Bot. 2012, 8, 165–169. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Ait Aabd, N.; Tahiri, A.; Qessaoui, R.; Mimouni, A.; Bouharroud, R. Self- and Cross-Pollination in Argane Tree and their Implications on Breeding Programs. Cells 2022, 11, 828. [Google Scholar] [CrossRef]
- Chakhchar, A.; Haworth, M.; El Modafar, C.; Lauteri, M.; Mattioni, C.; Wahbi, S.; Centritto, M. An Assessment of genetic diversity and drought tolerance in argan tree (Argania spinosa) populations: Potential for the development of improved drought tolerance. Front. Plant Sci. 2017, 8, 276. [Google Scholar] [CrossRef]
- Hakam, O.; Ongoma, V.; Beniaich, A.; Meskour, B.; El Kadi, M.A.; Brouziyne, Y.; Hssaisoune, M.; Tairi, A.; Labbaci, A.; Bouchaou, L. Assessment of the impact of climate change on Argan tree in the Mediterranean GIAHS site, Morocco: Current and future distributions. Model. Earth Syst. Environ. 2024, 10, 5529–5552. [Google Scholar] [CrossRef]
- Msanda, F.; Mayad, E.H.; Furze, J.N. Floristic biodiversity, biogeographical significance, and importance of Morocco’s Arganeraie Biosphere Reserve. Environ. Sci. Pollut. Res. 2021, 28, 64156–64165. [Google Scholar] [CrossRef]
- Nait Douch, A.; Boukhalef, L.; El Asbahani, A.; Al-Namazi, A.A.; El Mehrach, K.; Bouqbis, L.; Touaf, M.; Ain-Lhout, F. Photosynthetic Behavior of Argania spinosa (L.) Skeels Induced under Grazed and Ungrazed Conditions. Sustainability 2022, 14, 12081. [Google Scholar] [CrossRef]
- Sinsin, T.; Mounir, F.; El Aboudi, A. Conservation, valuation and sustainable development issues of the Argan Tree Biosphere Reserve in Morocco. Environ. Socio-Econ. Stud. 2020, 8, 28–35. [Google Scholar] [CrossRef]
- Le Polain de Waroux, Y. Dégradation environnementale et développement économique dans l’arganeraie d’Aoulouz (Maroc). Sci. Chang. Planetaires-Secher. 2013, 24, 29–38. [Google Scholar]
- Kirchhoff, M.; Marzolff, I.; Stephan, R.; Seeger, M.; Aït Hssaine, A.; Ries, J.B. Monitoring dryland trees with remote sensing. Part B: Combining tree cover and plant architecture data to assess degradation and recovery of Argania spinosa woodlands of South Morocco. Front. Environ. Sci. 2022, 10, 896703. [Google Scholar] [CrossRef]
- ILarioni, L.; Nasini, N.; Brunori, A.; Proietti, P. Experimental measurement of the biomass of Olea europaea L. Afr. J. Biotechnol. 2013, 12, 1216–1222. [Google Scholar]
- Laaribya, S. Multiple spatial changes in the Argan ecosystem—(Argania spinosa (L.) skeels)—Case study (Morocco). Her. Sci. S. Seifullin Kazakh Agro. Tech. Univ. 2021, 2, 4–12. [Google Scholar] [CrossRef]
- Labarca-Rojas, Y.; Hernández-Bermejo, J.E.; Herrera-Molina, F.; Hernández-Clemente, M.; Quero, J.L. Assessing argan tree (Argania spinosa (L.) skeels) ex-situ collections as a complementary tool to in-situ conservation and crop introduction in the Mediterranean basin. Trees-Struct. Funct. 2023, 37, 567–581. [Google Scholar] [CrossRef]
- Ait Bihi, M.; Ain-Lhout, F.; Hatimi, A.; Fahmi, F.; Tahrouch, S. Ecophysiological response and morphological adjustment of Argania spinosa L. Skeels under contrasting climates: Case study of marginal populations. Int. J. Plant Biol. 2021, 12, 9404. [Google Scholar] [CrossRef]
- GCF. FP022: Development of Argan Orchards in Degraded Environment-DARED; GCF: Songdo, Japan, 2016. [Google Scholar]
- Fadma, F. Impact de L’aridité sur la Phytochimie et le Comportement Écophysiologique de L’arganier, Argania spinosa, (L.) Skeels; Ibnou Zohr University: Agadir, Morocco, 2013; p. 140. [Google Scholar]
- Fahmi, F.; Tahrouch, S.; Amri, O.; El Mehrach, K.; Hatimi, A. Assessment of aridity effects on phytochemistry and ecophysiology of Argania spinosa (L.). Agric. Nat. Resour. 2020, 54, 397–404. [Google Scholar]
- Weatherley, P.E. Studies in the Water Relations of I. the Field Measurement of Water Deficits in Leaves. New Phytol. 1949, 49, 81–86. [Google Scholar] [CrossRef]
- Lfitat, A.; Zejli, H.; Bousraf, F.Z.; Bousselham, A.; El Atki, Y.; Gourch, A.; Lyoussi, B.; Abdellaoui, A. Comparative assessment of total phenolics content and in vitro antioxidant capacity variations of macerated leaf extracts of Olea europaea L. and Argania spinosa (L.) Skeels. Mater. Today Proc. 2021, 45, 7271–7277. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ionac, N.; Grigore, E.; Constantin, D.M. Evaluation des phénomènes de déssechement et sécheresse dans la zone continentale du Plateau de la Dobroudja du sud. In XXVIIIe Colloque de l’Association Internationale de Climatologie; Université De Liège: Liège, Belgium, 2015; pp. 269–274. [Google Scholar]
- Shiade, S.R.G.; Zand-Silakhoor, A.; Fathi, A.; Rahimi, R.; Minkina, T.; Rajput, V.D.; Zulfiqar, U.; Chaudhary, T. Plant metabolites and signaling pathways in response to biotic and abiotic stresses: Exploring bio stimulant applications. Plant Stress 2024, 12, 100454. [Google Scholar] [CrossRef]
- Kara, K.; Brinis, L. Réponse physiologique au stress hydrique de variétés de blé tendre (Triticum aestivum L.) cultivées en Algérie. Eur. J. Sci. Res. 2012, 81, 524–532. [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Blonder, B.; Enquist, B.J. Inferring climate from angiosperm leaf venation networks. New Phytol. 2014, 204, 116–126. [Google Scholar] [CrossRef]
- Zahidi, A.; El Mousadik, A. Variability in leaf size and shape in three natural populations of Argania spinosa (L.) Skeels. Int. J. Curr. Res. Acad. Rev. 2013, 1, 13–25. [Google Scholar]
- Berka, S.; Aïd, F. Réponses physiologiques des plants d’Argania spinosa (L.) Skeels soumis à un déficit hydrique édaphique. Sécheresse 2019, 20, 296–302. [Google Scholar] [CrossRef]
- Ksontini, M.; Louguet, P.; Laffray, D.; Rejeb, M.N. Comparaison des effets de la contrainte hydrique sur la croissance, la conductance stomatique et la photosynthese de jeunes plants de chenes mediterraneens (Quercus suber, Q. faginea, Q. coccifera) en Tunisie. Ann. Sci. For. 1998, 55, 477–495. [Google Scholar] [CrossRef]
- Meslem, H.; Djabeur, A.; Kharoubi, O.; Kaid-Harche, M. Effect of water deficit on Argan tree seedlings (Argania spinosa L. Skeels): Morphological and physiological aspect. Afr. J. Biotechnol. 2015, 14, 1020–1028. [Google Scholar] [CrossRef][Green Version]
- Keyvan, S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010, 8, 1051–1060. [Google Scholar]
- Ilkay, Y.; Muhammad Asif, J.; Kaleem, U.D.; Safdar, A.; Saddam, H.; Muhammad, F. Drought-Induced Changes in Leaf Morphology and Anatomy: Overview, Implications and Perspectives. Pol. J. Environ. Stud. 2024, 33, 1517–1530. [Google Scholar]
- Nsemi, F.M. Identification de Polyphénols, Évaluation de leur Activité Antioxydante et étude de leurs Propriétés Biologiques. Biologie Végétale. Université Paul Verlaine-Metz, 2010. Français. NNT: 2010METZ011S. Tel-01752680. Available online: https://hal.univ-lorraine.fr/tel-01752680 (accessed on 11 August 2024).
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas José, A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas Katiuska, E.; Debouba, M.; Sandalio Luisa, M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the Saharan plant Oudeneya africana. Curr. Opin. Virol. 2020, 176, 104099. Available online: https://digital.csic.es/bitstream/10261/216723/1/2020_Talbi_EEB_Preprint.pdf (accessed on 11 August 2024). [CrossRef]
- Mulugeta, S.M.; Radácsi, P. Influence of Drought Stress on Growth and Essential Oil Yield of Ocimum Species. Horticulturae 2022, 8, 175. [Google Scholar] [CrossRef]
- Tahrouch, S.; Rapior, S.; Bessière, J.M.; Andary, C. Les substances volatiles de Argania spinosa (Sapotaceae). Acta Bot. Gall. 1998, 145, 259–263. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Luis de la Fuente, J.; Zunzunegui, M.; Barradas, M.C.D. Physiological responses to water stress and stress memory in Argania spinosa. Plant Stress 2023, 7, 100133. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, U.N.; He, Y.; Shukla, V.K.S. Marine Lipids and Their Stabilization with Green Tea and Catechins. ACS Symp. Ser. 1997, 674, 186–197. [Google Scholar]
- Joguet, N.; Maugard, T. Characterization and quantification of phenolic compounds of Argania spinosa leaves by HPLC-PDA-ESI-MS analyses and their antioxidant activity. Chem. Nat. Compd. 2013, 48, 1069–1071. [Google Scholar] [CrossRef]
- Emami Bistgani, Z.; Barker, A.V.; Hashemi, M. Physiology of medicinal and aromatic plants under drought stress. Crop J. 2024, 12, 330–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).