Socioecological Resilience: Quantitative Assessment of the Impact of an Invasive Species Assemblage on a Lake Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site
2.2. Information Collection
2.3. Information Analysis
3. Results and Discussion
3.1. Socioecological Resilience Variables
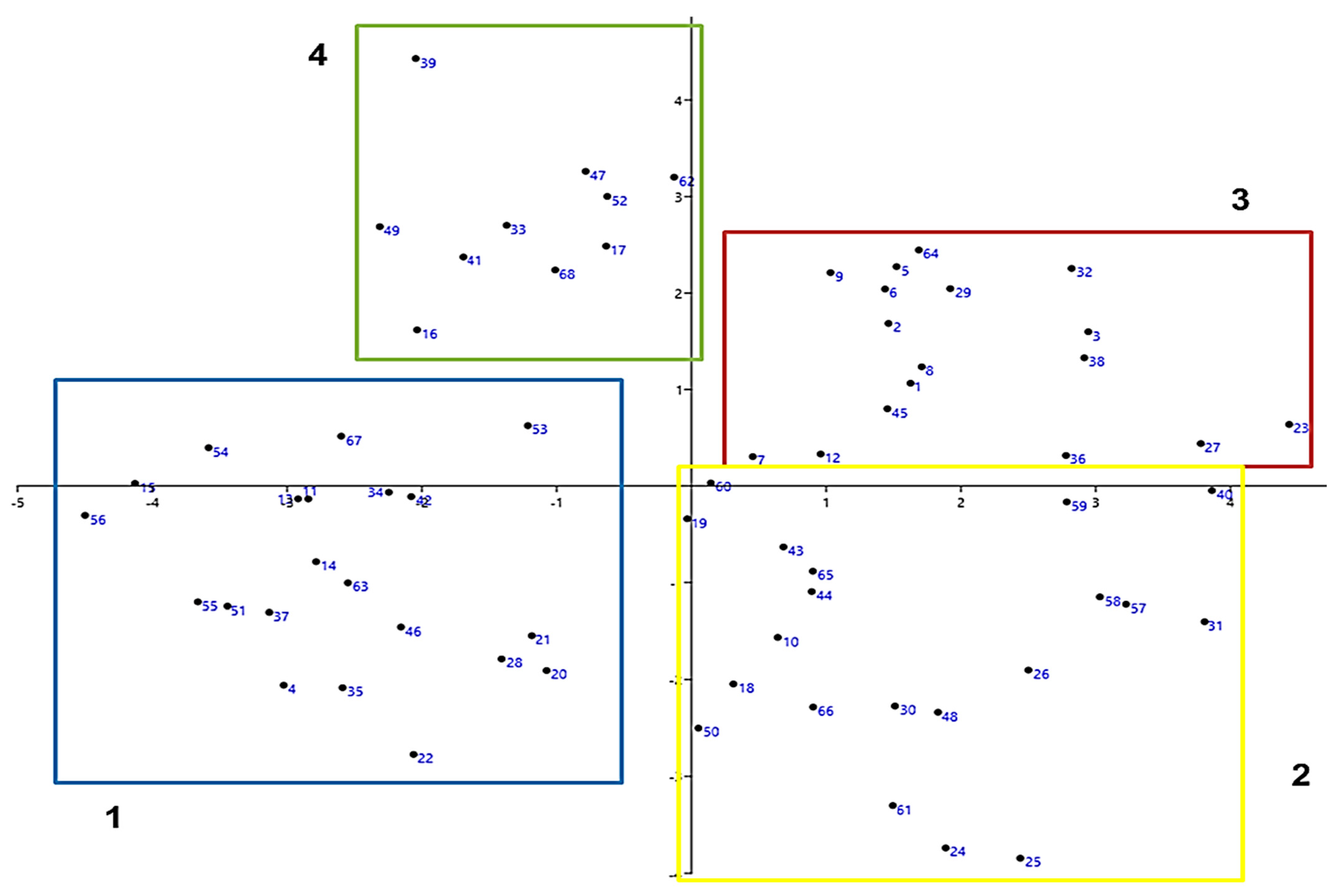
3.2. Perception of Socioecological Resilience
3.3. Socioecological Resilience Assessment
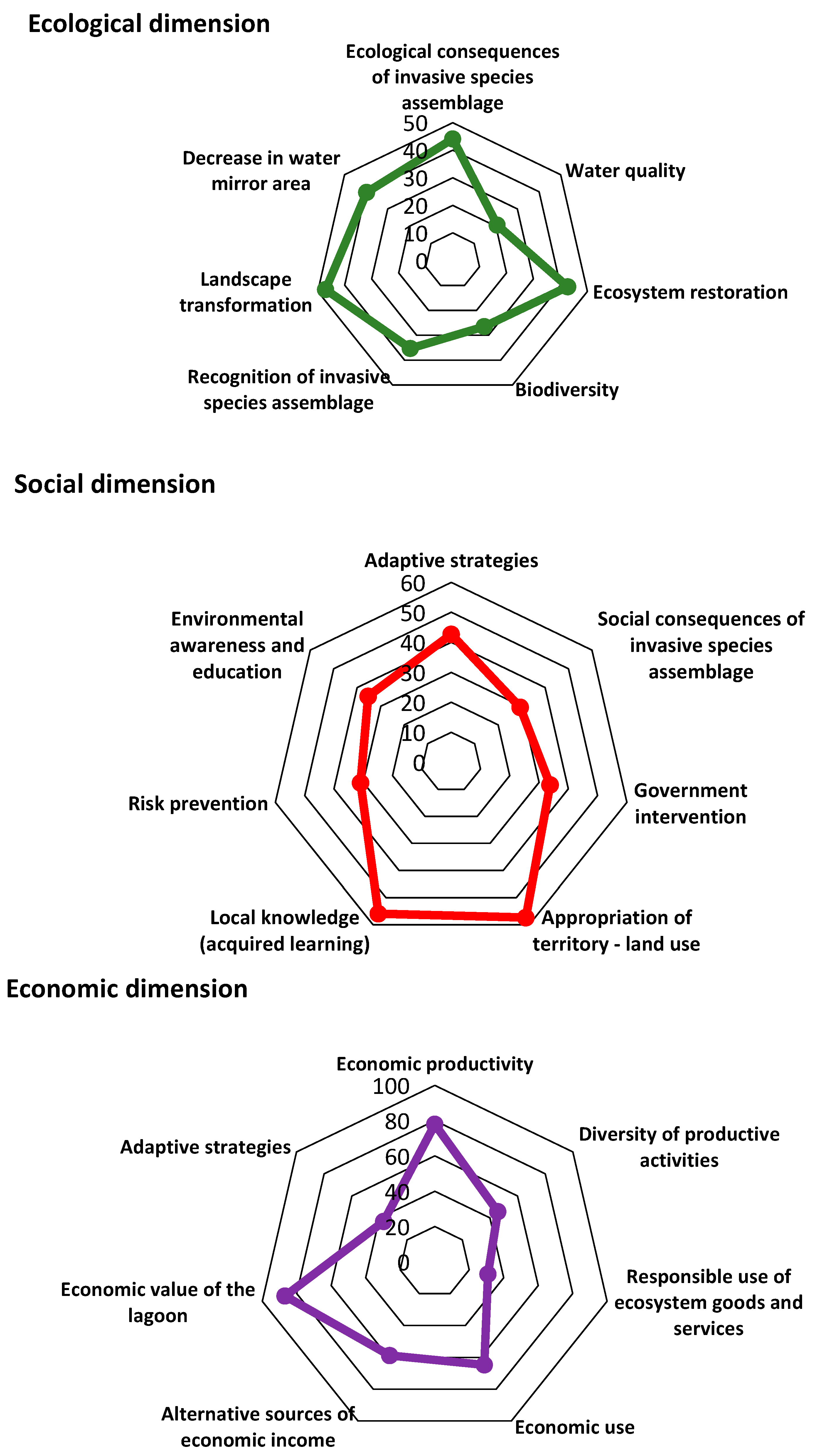
3.3.1. Ecological Dimension
3.3.2. Social Dimension
3.3.3. Economic Dimension
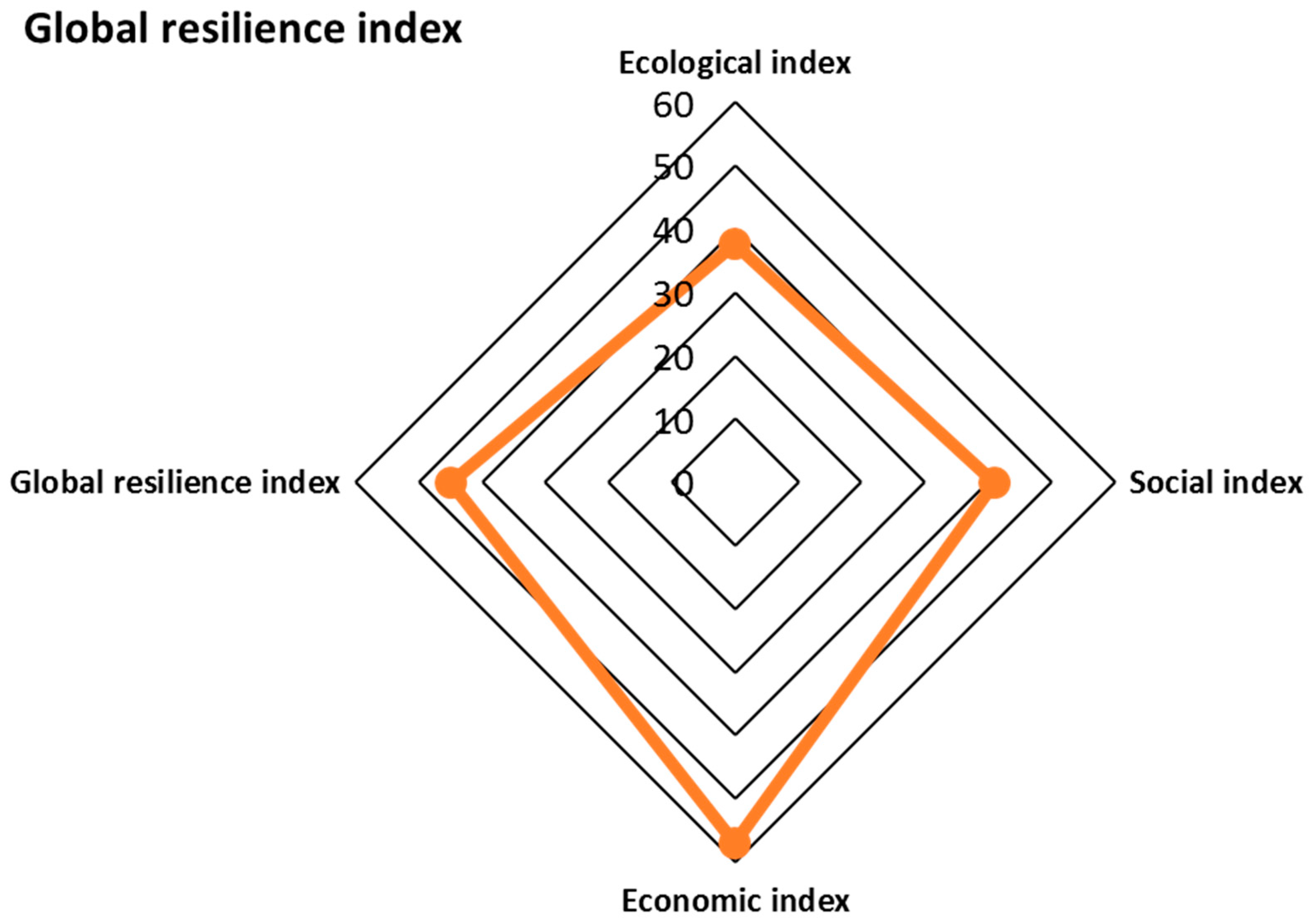
3.4. Indicator and Index Analysis
3.5. Local Management Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cretney, R. Resilience for Whom? Emerging Critical Geographies of Socio-Ecological Resilience. Geogr. Compass 2014, 8, 627–640. [Google Scholar] [CrossRef]
- Phillips, M.A.; Ritala, P. A Complex Adaptive Systems Agenda for Ecosystem Research Methodology. Technol. Forecast. Soc. Change 2019, 148, 119739. [Google Scholar] [CrossRef]
- Domptail, S.; Easdale, M.H. Yuerlita. Managing Socio-Ecological Systems to Achieve Sustainability: A Study of Resilience and Robustness. Environ. Policy Gov. 2013, 23, 30–45. [Google Scholar] [CrossRef]
- Folke, C.; Colding, J.; Berkes, F. Synthesis: Building Resilience and Adaptive Capacity in Social–Ecological Systems. In Navigating Social-Ecological Systems: Building Resilience for Complexity and Change; Folke, C., Berkes, F., Colding, J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 352–387. ISBN 978-0-521-06184-1. [Google Scholar]
- Tran, H.T.; Balchanos, M.; Domerçant, J.C.; Mavris, D.N. A Framework for the Quantitative Assessment of Performance-Based System Resilience. Reliab. Eng. Syst. Saf. 2017, 158, 73–84. [Google Scholar] [CrossRef]
- Quinlan, A.E.; Berbés-Blázquez, M.; Haider, L.J.; Peterson, G.D. Measuring and Assessing Resilience: Broadening Understanding through Multiple Disciplinary Perspectives. J. Appl. Ecol. 2016, 53, 677–687. [Google Scholar] [CrossRef]
- van Strien, M.J.; Huber, S.H.; Anderies, J.M.; Grêt-Regamey, A. Resilience in Social-Ecological Systems: Identifying Stable and Unstable Equilibria with Agent-Based Models. Ecol. Soc. 2019, 24, 8. [Google Scholar] [CrossRef]
- Arias Pineda, J.Y.; Pedroza Martínez, D.R. Presencia del cangrejo rojo Procambarus clarkii (Girard, 1852) en la sabana de Bogotá, Colombia. Boletín SEA 2018, 62, 283–286. [Google Scholar]
- Álvarez-León, R. Nuevas localidades de la langostilla roja introducida (Procambarus clarkii, Girard, 1852) (Decapoda: Cambaridae) en su dispersión y conquista de los humedales dulceacuícolas de Colombia. Boletín Científico. Cent. Museos. Mus. Hist. Nat. 2023, 27, 207–225. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Anastácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The Red Swamp Crayfish Procambarus clarkii in Europe: Impacts on Aquatic Ecosystems and Human Well-Being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
- Mwamburi, J. Spatial Variations in Sedimentary Organic Matter in Surficial Lake Sediments of Nyanza Gulf (Lake Victoria, Kenya) after Invasion of Water Hyacinth. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2016, 21, 94–113. [Google Scholar] [CrossRef]
- Martínez-Nieto, P.; García-Gómez, G.; Mora-Ortiz, L.; Robles-Camargo, G. Polluting Macrophytes Colombian Lake Fúquene Used as Substrate by Edible Fungus Pleurotus Ostreatus. World J. Microbiol. Biotechnol. 2014, 30, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Pegg, J.; South, J.; Hill, J.E.; Durland-Donahou, A.; Weyl, O.L.F. Chapter 16—Impacts of Alien Invasive Species on Large Wetlands. In Fundamentals of Tropical Freshwater Wetlands; Dalu, T., Wasserman, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-822362-8. [Google Scholar]
- Watanabe, R.; Ohba, S. Comparison of the Community Composition of Aquatic Insects between Wetlands with and without the Presence of Procambarus clarkii: A Case Study from Japanese Wetlands. Biol. Invasions 2022, 24, 1033–1047. [Google Scholar] [CrossRef]
- Marchi, M.; Jørgensen, S.E.; Bécares, E.; Corsi, I.; Marchettini, N.; Bastianoni, S. Dynamic Model of Lake Chozas (León, NW Spain)—Decrease in Eco-Exergy from Clear to Turbid Phase Due to Introduction of Exotic Crayfish. Ecol. Model. 2011, 222, 3002–3010. [Google Scholar] [CrossRef]
- Nyström, P. Ecological Impact of Introduced and Native Crayfish on Freshwater Communities: European Perspectives. In Crayfish in Europe as Alien Species; Routledge: London, UK, 1999; ISBN 978-1-315-14046-9. [Google Scholar]
- Yan, S.-H.; Song, W.; Guo, J.-Y. Advances in Management and Utilization of Invasive Water Hyacinth (Eichhornia Crassipes) in Aquatic Ecosystems—A Review. Crit. Rev. Biotechnol. 2017, 37, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Pragasan, L.A.; Vijayakumar, M. Ecological Dynamics of Water Hyacinth in Coimbatore Lakes: Implications for Biomass, Carbon Stock, and Nutrient Management. Asian J. Environ. Ecol. 2024, 23, 240–251. [Google Scholar] [CrossRef]
- Gherardi, F.; Robert Britton, J.; Mavuti, K.M.; Pacini, N.; Grey, J.; Tricarico, E.; Harper, D.M. A Review of Allodiversity in Lake Naivasha, Kenya: Developing Conservation Actions to Protect East African Lakes from the Negative Impacts of Alien Species. Biol. Conserv. 2011, 144, 2585–2596. [Google Scholar] [CrossRef]
- Godoy, O. Coexistence Theory as a Tool to Understand Biological Invasions in Species Interaction Networks: Implications for the Study of Novel Ecosystems. Funct. Ecol. 2019, 33, 1190–1201. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global Ecological Impacts of Invasive Species in Aquatic Ecosystems. Glob. Change Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Leffler, A.J.; Leonard, E.D.; James, J.J.; Monaco, T.A. Invasion Is Contingent on Species Assemblage and Invasive Species Identity in Experimental Rehabilitation Plots. Rangel. Ecol. Manag. 2014, 67, 657–666. [Google Scholar] [CrossRef]
- Maguire, K.C.; Nieto-Lugilde, D.; Fitzpatrick, M.C.; Williams, J.W.; Blois, J.L. Modeling Species and Community Responses to Past, Present, and Future Episodes of Climatic and Ecological Change. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 343–368. [Google Scholar] [CrossRef]
- Thomson-Laing, G.; Schallenberg, L.; Kelly, D.; Howarth, J.D.; Wood, S.A. An Integrative Approach to Assess the Impact of Disturbance on Native Fish in Lakes. Biol. Rev. 2024, 99, 85–109. [Google Scholar] [CrossRef]
- Domaizon, I.; Winegardner, A.; Capo, E.; Gauthier, J.; Gregory-Eaves, I. DNA-Based Methods in Paleolimnology: New Opportunities for Investigating Long-Term Dynamics of Lacustrine Biodiversity. J. Paleolimnol. 2017, 58, 1–21. [Google Scholar] [CrossRef]
- Hobæk, A.; Manca, M.; Andersen, T. Factors Influencing Species Richness in Lacustrine Zooplankton. Acta Oecologica 2002, 23, 155–163. [Google Scholar] [CrossRef]
- Han, J.-H.; An, K.-G. The Applications of a Multi-metric LEHA Model for an Environmental Impact Assessments of Lake Ecosystems and the Ecological Health Assessments. J. Environ. Impact Assess. 2012, 21, 483–501. [Google Scholar] [CrossRef]
- De Santos, G.; Silva, E.E.C.; Diniz, L.P.; Calvi, R.X.; de Oliveira, D.M.; Delfim, B.L.; De Paula, T.L.T.; Eskinazi-Sant’Anna, E.M. Deep Lakes Support Higher Zooplankton Functional Diversity than Shallow Lakes: A Case Study in Lacustrine Environments Affected by Mining Tailings (Lower Doce River Basin, Brazil). Freshw. Biol. 2024, 69, 945–958. [Google Scholar] [CrossRef]
- Dearing, J.; Acma, B.; Bub, S.; Chambers, F.; Chen, X.; Cooper, J.; Crook, D.; Dong, X.; Dotterweich, M.; Edwards, M.; et al. Social-Ecological Systems in the Anthropocene: The Need for Integrating Social and Biophysical Records at Regional Scales. Anthr. Rev. 2015, 2, 220–246. [Google Scholar] [CrossRef]
- Jiménez, M.; Pérez-Belmont, P.; Schewenius, M.; Lerner, A.M.; Mazari-Hiriart, M. Assessing the Historical Adaptive Cycles of an Urban Social-Ecological System and Its Potential Future Resilience: The Case of Xochimilco, Mexico City. Reg. Environ. Change 2020, 20, 7. [Google Scholar] [CrossRef]
- Arnaiz-Schmitz, C.; Aguilera, P.A.; Ropero, R.F.; Schmitz, M.F. Detecting Social-Ecological Resilience Thresholds of Cultural Landscapes along an Urban–Rural Gradient: A Methodological Approach Based on Bayesian Networks. Landsc. Ecol. 2023, 38, 3589–3604. [Google Scholar] [CrossRef]
- Buckman, S.; Arquero de Alarcon, M.; Maigret, J. Tracing Shoreline Flooding: Using Visualization Approaches to Inform Resilience Planning for Small Great Lakes Communities. Appl. Geogr. 2019, 113, 102097. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, X.; Yang, X.; Kattel, G.; Zhao, Y.; Wang, R. Ecological Shift and Resilience in China’s Lake Systems during the Last Two Centuries. Glob. Planet. Change 2018, 165, 147–159. [Google Scholar] [CrossRef]
- Dieperink, C.; Mees, H.; Priest, S.J.; Ek, K.; Bruzzone, S.; Larrue, C.; Matczak, P. Managing Urban Flood Resilience as a Multilevel Governance Challenge: An Analysis of Required Multilevel Coordination Mechanisms. Ecol. Soc. 2018, 23, 31. [Google Scholar] [CrossRef]
- Velez, M.I.; Conde, D.; Lozoya, J.P.; Rusak, J.A.; García-Rodríguez, F.; Seitz, C.; Harmon, T.; Perillo, G.M.E.; Escobar, J.; Vilardy, S.P. Paleoenvironmental Reconstructions Improve Ecosystem Services Risk Assessment: Case Studies from Two Coastal Lagoons in South America. Water 2018, 10, 1350. [Google Scholar] [CrossRef]
- Imbach, P.; Locatelli, B.; Zamora, J.C.; Fung, E.; Calderer, L.; Ciais, L.M.A.P. Impacts of Climate Change on Ecosystem Hydrological Services of Central America: Water Availability. In Climate Change Impacts on Tropical Forests in Central America; Routledge: London, UK, 2015; ISBN 978-1-315-86670-3. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the Design of Climate Change-Resilient Farming Systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Romero-Duque, L.P.; Trilleras, J.M.; Castellarini, F.; Quijas, S. Ecosystem Services in Urban Ecological Infrastructure of Latin America and the Caribbean: How Do They Contribute to Urban Planning? Sci. Total Environ. 2020, 728, 138780. [Google Scholar] [CrossRef] [PubMed]
- Sterling, E.; Ticktin, T.; Morgan, T.K.K.; Cullman, G.; Alvira, D.; Andrade, P.; Bergamini, N.; Betley, E.; Burrows, K.; Caillon, S.; et al. Culturally Grounded Indicators of Resilience in Social-Ecological Systems. Environ. Soc. 2017, 8, 63–95. [Google Scholar] [CrossRef]
- Sherman, K.; Sevilla, N.P.M.; Álvarez Torres, P.; Peterson, B. Sustainable Development of Latin American and the Caribbean Large Marine Ecosystems. Environ. Dev. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Blanco Garrido, F.; Martínez Valdivieso, L.; Polo Ardila, J.; Simanca, F.; Hernandez, M.; Lozano Ayarza, L.P.; Mojica, J. Estrategias para la conservación de la laguna de Fúquene en los departamentos de Cundinamarca y Boyacá. RISTI Rev. Iber. Sist. E Tecnol. Inf. 2020, E36, 440–451. [Google Scholar]
- Guerrero-García, P.K. Dos siglos de desecación en Laguna de Fúquene (Colombia): Impactos en la pesca artesanal. Agua Territ./Water Landsc. 2014, 4, 47–58. [Google Scholar] [CrossRef]
- Baigún, C.; Minotti, P.; Lamizana, B. Wetlands and People at Risk; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2022. [Google Scholar]
- Weichselgartner, J.; Kelman, I. Geographies of Resilience: Challenges and Opportunities of a Descriptive Concept. Prog. Hum. Geogr. 2015, 39, 249–267. [Google Scholar] [CrossRef]
- Olsson, L.; Jerneck, A.; Thoren, H.; Persson, J.; O’Byrne, D. Why Resilience Is Unappealing to Social Science: Theoretical and Empirical Investigations of the Scientific Use of Resilience. Sci. Adv. 2015, 1, e1400217. [Google Scholar] [CrossRef]
- Folke, C.; Biggs, R.; Norström, A.V.; Reyers, B.; Rockström, J. Social-Ecological Resilience and Biosphere-Based Sustainability Science. Ecol. Soc. 2016, 21, 41. [Google Scholar] [CrossRef]
- Meerow, S.; Newell, J.P. Urban Resilience for Whom, What, When, Where, and Why? Urban Geogr. 2019, 40, 309–329. [Google Scholar] [CrossRef]
- Jones, L.; Tanner, T. ‘Subjective Resilience’: Using Perceptions to Quantify Household Resilience to Climate Extremes and Disasters. Reg. Environ. Change 2017, 17, 229–243. [Google Scholar] [CrossRef]
- Reuter, C.; Spielhofer, T. Towards Social Resilience: A Quantitative and Qualitative Survey on Citizens’ Perception of Social Media in Emergencies in Europe. Technol. Forecast. Soc. Change 2017, 121, 168–180. [Google Scholar] [CrossRef]
- Jain, P.; Mentzer, R.; Mannan, M.S. Resilience Metrics for Improved Process-Risk Decision Making: Survey, Analysis and Application. Saf. Sci. 2018, 108, 13–28. [Google Scholar] [CrossRef]
- Khan, M.G.M.; Reddy, K.G.; Rao, D.K. Designing Stratified Sampling in Economic and Business Surveys. J. Appl. Stat. 2015, 42, 2080–2099. [Google Scholar] [CrossRef]
- Heo, M.; Kim, N.; Faith, M.S. Statistical Power as a Function of Cronbach Alpha of Instrument Questionnaire Items. BMC Med. Res. Methodol. 2015, 15, 86. [Google Scholar] [CrossRef]
- Rodríguez-Santamaría, K.; Zafra-Mejía, C.A.; Rondón-Quintana, H.A. Macro-Morphological Traits of Leaves for Urban Tree Selection for Air Pollution Biomonitoring: A Review. Biosensors 2022, 12, 812. [Google Scholar] [CrossRef]
- Proietti, T.; Frale, C. New Proposals for the Quantification of Qualitative Survey Data. J. Forecast. 2011, 30, 393–408. [Google Scholar] [CrossRef]
- Claveria, O.; Monte, E.; Torra, S. A New Approach for the Quantification of Qualitative Measures of Economic Expectations. Qual. Quant. 2017, 51, 2685–2706. [Google Scholar] [CrossRef]
- Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. (Colombia); Fundación Humedales. Caracterización Biofísica, Ecológica y Sociocultural del Complejo de Humedales del Valle de Ubaté: Fúquene, Cucunubá y Palacio: Una Contribución a la Definición de Escenarios y Objetivos de Manejo para la Conservación de Biodiversidad. Informe Final; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogota, Colombia, 2009. [Google Scholar]
- Salazar Noguera, A. Ordenación de la Cuenca del río Ubaté—Laguna de Fúquene en Colombia; Universitat Politecnica de Valencia: Valencia, Spain, 2016. [Google Scholar]
- Corporación Autónoma Regional de Cundinamarca POMCA Ríos Ubaté y Suárez. Available online: https://www.car.gov.co/vercontenido/84 (accessed on 31 July 2024).
- Hox, J.J.; Kreft, I.G.G.; Hermkens, P.L.J. The Analysis of Factorial Surveys. Sociol. Methods Res. 1991, 19, 493–510. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. Paleontological Statistics Software Package for Education and Data Analsis. Palaeontol. Electron. 2001, 4, 9–18. [Google Scholar]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal Component Analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Bécue-Bertaut, M.; Pagès, J. Multiple Factor Analysis and Clustering of a Mixture of Quantitative, Categorical and Frequency Data. Comput. Stat. Data Anal. 2008, 52, 3255–3268. [Google Scholar] [CrossRef]
- Ríos Atehortúa, G.P. Propuesta Para Generar Indicadores de Sostenibilidad en Sistemas de Producción Agropecuaria, para la Toma de Decisiones Caso: Lechería Especializada. 2010. Available online: https://repositorio.unal.edu.co/handle/unal/3392 (accessed on 31 June 2024).
- Langebeck Cuéllar, E.; Beltrán Vargas, J. Tipologías de percepción, bajo criterios de sustentabilidad territorial, del proceso de ocupación urbano-rural: Localidad Quinta de Bogotá. Rev. Luna Azul 2016, 43, 415–447. [Google Scholar] [CrossRef]
- Casimiro Rodríguez, L. Bases metodológicas para la resiliencia socioecológica de fincas familiares en Cuba. Ph.D. Thesis, Universidad de Antioquia, Medellín, Colombia, 2016. [Google Scholar]
- Browne, M.W. Cross-Validation Methods. J. Math. Psychol. 2000, 44, 108–132. [Google Scholar] [CrossRef]
- Link, W.A.; Sauer, J.R. Bayesian Cross-Validation for Model Evaluation and Selection, with Application to the North American Breeding Bird Survey. Ecology 2016, 97, 1746–1758. [Google Scholar] [CrossRef]
- Dunbar, H.; Theron, C.; Spangenberg, H. A Cross-Validation Study of the Performance Index. Manag. Dyn. J. S. Afr. Inst. Manag. Sci. 2011, 20, 2–24. [Google Scholar]
- Costanza, R. A Theory of Socio-Ecological System Change. J. Bioecon. 2014, 16, 39–44. [Google Scholar] [CrossRef]
- Ungar, M. Resilience and Culture: The Diversity of Protective Processes and Positive Adaptation. In Youth Resilience and Culture: Commonalities and Complexities; Theron, L.C., Liebenberg, L., Ungar, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 37–48. ISBN 978-94-017-9415-2. [Google Scholar]
- Kertész, Á.; Nagy, L.A.; Balázs, B. Effect of Land Use Change on Ecosystem Services in Lake Balaton Catchment. Land Use Policy 2019, 80, 430–438. [Google Scholar] [CrossRef]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of Agricultural Drainage on Aquatic Ecosystems: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 909–1001. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic Invasive Species: Challenges for the Future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Baho, D.L.; Allen, C.R.; Garmestani, A.; Fried-Petersen, H.; Renes, S.E.; Gunderson, L.; Angeler, D.G. A Quantitative Framework for Assessing Ecological Resilience. Ecol. Soc. 2017, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shalloof, K.; El-Ganiny, A.; El-Far, A.; Fetouh, M.; Aly, W.; Amin, A. Catch Composition and Species Diversity during Dredging Operations of Mediterranean Coastal Lagoon, Lake Manzala, Egypt. Egypt. J. Aquat. Res. 2023, 49, 347–352. [Google Scholar] [CrossRef]
- Escobar, L.E.; Mallez, S.; McCartney, M.; Lee, C.; Zielinski, D.P.; Ghosal, R.; Bajer, P.G.; Wagner, C.; Nash, B.; Tomamichel, M.; et al. Aquatic Invasive Species in the Great Lakes Region: An Overview. Rev. Fish. Sci. Aquac. 2018, 26, 121–138. [Google Scholar] [CrossRef]
- Stebbing, P. The Management of Invasive Crayfish. In Biology and Ecology of Crayfish; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-08391-4. [Google Scholar]
- Momot, W.T. Redefining the Role of Crayfish in Aquatic Ecosystems. Rev. Fish. Sci. 1995, 3, 33–63. [Google Scholar] [CrossRef]
- Cordeiro, P.F.; Goulart, F.F.; Macedo, D.R.; de Campos, M.C.S.; Castro, S.R. Modeling of the Potential Distribution of Eichhornia Crassipes on a Global Scale: Risks and Threats to Water Ecosystems. Rev. Ambient. Água 2020, 15, e2421. [Google Scholar] [CrossRef]
- Degaga, A.H. Water Hyacinth (Eichhornia crassipes) Biology and Its Impacts on Ecosystem, Biodiversity, Economy and Human Well-Being. J. Nat. Sci. Res. 2019, 9, 24–30. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Bini, L.M.; Cai, Y.; Heiskanen, A.-S.; Hellsten, S.; Kortelainen, P.; Kotamäki, N.; Tolonen, K.T.; Vihervaara, P.; et al. Lakes in the Era of Global Change: Moving beyond Single-Lake Thinking in Maintaining Biodiversity and Ecosystem Services. Biol. Rev. 2021, 96, 89–106. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive Species, Ecosystem Services and Human Well-Being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, H.; Zhang, H.; Liao, M.; Wang, H.; Kang, Y.; Liu, Z.; Jeppesen, E. Effects of the Invasive Red Swamp Crayfish (Procambarus clarkii) on Eelgrass-Dominated Clearwater Lakes: The Role of Bioturbation. Hydrobiologia 2024, 851, 1993–2005. [Google Scholar] [CrossRef]
- Budnick, W.R.; Roth, B.; Nathan, L.R.; Thomas, S.M.; Smith, K.; Walker, S.N.; Herbst, S. Evaluation of Five Trap Designs for Removal of Invasive Red Swamp Crayfish (Procambarus clarkii Girard, 1852) in Southern Michigan: Catch per Unit Effort, Body Size, and Sex Biases. Manag. Biol. Invasions 2022, 13, 369–390. [Google Scholar] [CrossRef]
- El Mahrad, B.; Abalansa, S.; Newton, A.; Icely, J.D.; Snoussi, M.; Kacimi, I. Social-Environmental Analysis for the Management of Coastal Lagoons in North Africa. Front. Environ. Sci. 2020, 8, 37. [Google Scholar] [CrossRef]
- Ehalt Macedo, H.; Lehner, B.; Nicell, J.; Grill, G.; Li, J.; Limtong, A.; Shakya, R. Distribution and Characteristics of Wastewater Treatment Plants within the Global River Network. Earth Syst. Sci. Data 2022, 14, 559–577. [Google Scholar] [CrossRef]
- Rodrigues-Filho, J.L.; Macêdo, R.L.; Sarmento, H.; Pimenta, V.R.A.; Alonso, C.; Teixeira, C.R.; Pagliosa, P.R.; Netto, S.A.; Santos, N.C.L.; Daura-Jorge, F.G.; et al. From Ecological Functions to Ecosystem Services: Linking Coastal Lagoons Biodiversity with Human Well-Being. Hydrobiologia 2023, 850, 2611–2653. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Atisa, G. Environmental Education and Awareness: The Present and Future Key to the Sustainable Management of Ramsar Convention Sites in Kenya. Int. Environ. Agreem. 2021, 21, 611–630. [Google Scholar] [CrossRef]
- Bell, K.P.; Lindenfeld, L.; Speers, A.E.; Teisl, M.F.; Leahy, J.E. Creating Opportunities for Improving Lake-Focused Stakeholder Engagement: Knowledge–Action Systems, pro-Environment Behaviour and Sustainable Lake Management. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2013, 18, 5–14. [Google Scholar] [CrossRef]
- Dann, S.L.; Schroeder, B. Developing Great Lakes Literacy and Stewardship through a Nonformal Science Education Camp. J. Contemp. Water Res. Educ. 2015, 156, 21–36. [Google Scholar] [CrossRef]
- Williamson, M. Biological Invasions; Springer: Dordrecht, UK, 1997; ISBN 978-0-412-59190-7. [Google Scholar]
- Marbuah, G.; Gren, I.-M.; McKie, B. Economics of Harmful Invasive Species: A Review. Diversity 2014, 6, 500–523. [Google Scholar] [CrossRef]
- Jetoo, S. Multi-Level Governance Innovations of the Baltic Sea and the North American Great Lakes: New Actors and Their Roles in Building Adaptive Capacity for Eutrophication Governance. Mar. Policy 2018, 98, 237–245. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Reyes-García, V.; Olsson, P.; Montes, C. Traditional Ecological Knowledge and Community Resilience to Environmental Extremes: A Case Study in Doñana, SW Spain. Glob. Environ. Change 2012, 22, 640–650. [Google Scholar] [CrossRef]
- Roy, E.D.; Martin, J.F.; Irwin, E.G.; Conroy, J.D.; Culver, D.A. Transient Social–Ecological Stability: The Effects of Invasive Species and Ecosystem Restoration on Nutrient Management Compromise in Lake Erie. Ecol. Soc. 2010, 15, 20. [Google Scholar] [CrossRef]
- Nanayakkara, L.; Jurdi-Hage, R.; Leavitt, P.R.; Wissel, B. In Lakes but Not in Minds: Stakeholder Knowledge of Invasive Species in Prairie Lakes. Biol. Invasions 2018, 20, 633–652. [Google Scholar] [CrossRef]
- Kurtul, I.; Haubrock, P.J. The Need of Centralized Coordination to Counter Biological Invasions in the European Union. Environ. Sci. Eur. 2024, 36, 129. [Google Scholar] [CrossRef]
- Adams, C.R.; Hovick, S.M.; Anderson, N.O.; Kettenring, K.M. We Can Better Manage Ecosystems by Connecting Solutions to Constraints: Learning from Wetland Plant Invasions. Front. Environ. Sci. 2021, 9, 715350. [Google Scholar] [CrossRef]
- Angeler, D.G.; Fried-Petersen, H.B.; Allen, C.R.; Garmestani, A.; Twidwell, D.; Chuang, W.-C.; Donovan, V.M.; Eason, T.; Roberts, C.P.; Sundstrom, S.M.; et al. Chapter One—Adaptive Capacity in Ecosystems. In Advances in Ecological Research; Bohan, D.A., Dumbrell, A.J., Eds.; Resilience in Complex Socio-Ecological Systems; Academic Press: Cambridge, MA, USA, 2019; Volume 60, pp. 1–24. [Google Scholar]
- Lodge, D.M.; Simonin, P.W.; Burgiel, S.W.; Keller, R.P.; Bossenbroek, J.M.; Jerde, C.L.; Kramer, A.M.; Rutherford, E.S.; Barnes, M.A.; Wittmann, M.E.; et al. Risk Analysis and Bioeconomics of Invasive Species to Inform Policy and Management. Annu. Rev. Environ. Resour. 2016, 41, 453–488. [Google Scholar] [CrossRef]
- Sharp, R.L.; Cleckner, L.B.; DePillo, S. The Impact of On-Site Educational Outreach on Recreational Users’ Perceptions of Aquatic Invasive Species and Their Management. Environ. Educ. Res. 2017, 23, 1200–1210. [Google Scholar] [CrossRef]
- Goulden, M.C.; Adger, W.N.; Allison, E.H.; Conway, D. Limits to Resilience from Livelihood Diversification and Social Capital in Lake Social–Ecological Systems. Ann. Assoc. Am. Geogr. 2013, 103, 906–924. [Google Scholar] [CrossRef]
- Cochrane, L.; Cafer, A. Does Diversification Enhance Community Resilience? A Critical Perspective. Resilience 2018, 6, 129–143. [Google Scholar] [CrossRef]
- Schallenberg, M.; de Winton, M.D.; Kelly, D.J. Indicators of Ecological Integrity. In Lake Restoration Handbook: A New Zealand Perspective; Hamilton, D.P., Collier, K.J., Quinn, J.M., Howard-Williams, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-93043-5. [Google Scholar]
- Spears, B.M.; Hamilton, D.P.; Pan, Y.; Zhaosheng, C.; May, L. Lake Management: Is Prevention Better than Cure? Inland Waters 2022, 12, 173–186. [Google Scholar] [CrossRef]
- Sy, M.; Keenleyside, K.; Adare, K.; Reader, B.; Plante, M.; Deering, P. Protecting Native Biodiversity from High-Impact Invasive Species through the Protected Areas of Parks Canada. Biodiversity 2009, 10, 51–55. [Google Scholar] [CrossRef]
- Reaser, J.K.; Burgiel, S.W.; Kirkey, J.; Brantley, K.A.; Veatch, S.D.; Burgos-Rodríguez, J. The Early Detection of and Rapid Response (EDRR) to Invasive Species: A Conceptual Framework and Federal Capacities Assessment. Biol. Invasions 2020, 22, 1–19. [Google Scholar] [CrossRef]
- Obiero, K.; Lawrence, T.; Ives, J.; Smith, S.; Njaya, F.; Kayanda, R.; Waidbacher, H.; Olago, D.; Miriti, E.; Hecky, R.E. Advancing Africa’s Great Lakes Research and Academic Potential: Answering the Call for Harmonized, Long-Term, Collaborative Networks and Partnerships. J. Great Lakes Res. 2020, 46, 1240–1250. [Google Scholar] [CrossRef]
- Cianci-Gaskill, J.A.; Klug, J.L.; Merrell, K.C.; Millar, E.E.; Wain, D.J.; Kramer, L.; van Wijk, D.; Paule-Mercado, M.C.A.; Finlay, K.; Glines, M.R.; et al. A Lake Management Framework for Global Application: Monitoring, Restoring, and Protecting Lakes through Community Engagement. Lake Reserv. Manag. 2024, 40, 66–92. [Google Scholar] [CrossRef]
- Papeş, M.; Sällström, M.; Asplund, T.R.; Vander Zanden, M.J. Invasive Species Research to Meet the Needs of Resource Management and Planning. Conserv. Biol. 2011, 25, 867–872. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Adriaens, T.; Brundu, G.; Dehnen-Schmutz, K.; Estévez, R.A.; Fried, J.; Larson, B.M.H.; Liu, S.; Marchante, E.; Marchante, H.; et al. Stakeholder Engagement in the Study and Management of Invasive Alien Species. J. Environ. Manag. 2019, 229, 88–101. [Google Scholar] [CrossRef]
- Kumar, A. Ecosystem-Based Adaptation: Approaches to Sustainable Management of Aquatic Resources; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-815691-9. [Google Scholar]
- Larson, D.L.; Phillips-Mao, L.; Quiram, G.; Sharpe, L.; Stark, R.; Sugita, S.; Weiler, A. A Framework for Sustainable Invasive Species Management: Environmental, Social, and Economic Objectives. J. Environ. Manag. 2011, 92, 14–22. [Google Scholar] [CrossRef]
- Agol, D.; Reid, H.; Crick, F.; Wendo, H. Ecosystem-Based Adaptation in Lake Victoria Basin; Synergies and Trade-Offs. R. Soc. Open Sci. 2021, 8, 201847. [Google Scholar] [CrossRef]

| Dimension | Question Focus |
|---|---|
| Basic characterization | Gender, age, visitor or resident, length of residence or number of visits, and occupation. |
| Social | Cause of the social problem, consequence of the invasive species assemblage, degree of ecosystem appropriation (land use, water quality, ecosystem services, and biodiversity), local knowledge (acquired learning and human activity), and development, selection, and implementation of adaptive strategies. |
| Ecological | Cause of the ecological problem, ecological consequences of the invasive species assemblage (biodiversity, water quality, ecosystem services, and land use), adaptive strategies implemented (restoration and conservation), and their contribution to mitigation (adaptation and human activity). |
| Economic | Cause of the economic problem, economic consequences of the invasive species assemblage, economic value of the ecosystem (land use), ecosystem services (water quality and biodiversity), economic productivity, and adaptive alternatives implemented. |
| Ecosystem | Municipality | Population (Inhabitants) | Proportion (%) | Number of Surveys |
|---|---|---|---|---|
| Fúquene Lagoon | Ráquira | 13,588 | 28.6 | 19 |
| Susa | 12,302 | 25.9 | 18 | |
| Guachetá | 11,385 | 23.9 | 16 | |
| Fúquene | 5617 | 11.8 | 8 | |
| San Miguel de Sema | 4556 | 9.60 | 7 | |
| Total | 47,448 | 100 | 68 | |
| Phase | Description | Keywords | Scopus | Google Scholar | Average Frequency (%) | ||
|---|---|---|---|---|---|---|---|
| Documents | Frequency (%) | Documents | Frequency (%) | ||||
| 1 | Universe | Resilience and lake ecosystem | 959 | 100 | 254,000 | 100 | 100 |
| 2 | Dimensions | Ecological | 425 | 44.3 | 220,000 | 86.6 | 65.5 |
| Economic | 98 | 10.2 | 172,000 | 67.7 | 39.0 | ||
| Social | 103 | 10.7 | 165,000 | 65.0 | 37.9 | ||
| 3 | Ecological variables | Land use | 24 | 5.65 | 196,000 | 89.1 | 47.4 |
| Water Quality | 47 | 11.1 | 174,000 | 79.1 | 45.1 | ||
| Biodiversity | 60 | 14.1 | 163,000 | 74.1 | 44.1 | ||
| Conservation | 20 | 4.71 | 179,000 | 81.4 | 43.0 | ||
| Human Activity | 20 | 4.71 | 142,000 | 64.5 | 34.6 | ||
| Ecosystem Service | 35 | 8.24 | 122,000 | 55.5 | 31.8 | ||
| Restoration | 25 | 5.88 | 107,000 | 48.6 | 27.3 | ||
| Adaptive Management | 21 | 4.94 | 89,900 | 40.9 | 22.9 | ||
| Social variables | Human | 9 | 9.18 | 156,000 | 90.7 | 49.9 | |
| Water Quality | 15 | 15.3 | 123,000 | 71.5 | 43.4 | ||
| Ecosystem Service | 15 | 15.3 | 119,000 | 69.2 | 42.2 | ||
| Biodiversity | 12 | 12.2 | 85,200 | 49.5 | 30.9 | ||
| Adaptive Management | 13 | 13.3 | 76,500 | 44.5 | 28.9 | ||
| Economic variables | Land Use | 12 | 11.7 | 156,000 | 94.5 | 53.1 | |
| Water Quality | 17 | 16.5 | 136,000 | 82.4 | 49.5 | ||
| Ecosystem Service | 13 | 12.6 | 124,000 | 75.2 | 43.9 | ||
| Biodiversity | 12 | 11.7 | 92,300 | 55.9 | 33.8 | ||
| Restoration | 9 | 8.74 | 75,800 | 45.9 | 27.3 | ||
| Dimension | Indicators | Index | Frequency (%) |
|---|---|---|---|
| Ecological | Ecological consequences of invasive species assemblages | VEL1 | 44.1 |
| Water quality | VEL2 | 20.6 | |
| Ecosystem restoration | VEL3 | 42.7 | |
| Biodiversity | VEL4 | 26.5 | |
| Recognition of invasive species assemblage | VEL5 | 35.3 | |
| Landscape transformation | VEL6 | 47.1 | |
| Decrease in water mirror area | VEL7 | 39.7 | |
| Social | Adaptive strategies | VS1 | 42.7 |
| Social consequences of invasive species assemblage | VS2 | 29.4 | |
| Government intervention | VS3 | 33.8 | |
| Appropriation of territory—land use | VS4 | 57.4 | |
| Local knowledge (acquired learning) | VS5 | 55.9 | |
| Risk prevention | VS6 | 30.9 | |
| Environmental awareness and education | VS7 | 35.3 | |
| Economic | Economic productivity | VEC1 | 77.9 |
| Diversity of productive activities | VEC2 | 45.6 | |
| Responsible use of ecosystem goods and services | VEC3 | 30.9 | |
| Economic use | VEC4 | 64.7 | |
| Alternative sources of economic income | VEC5 | 58.8 | |
| Economic value of the lagoon | VEC6 | 86.8 | |
| Adaptive strategies | VEC7 | 36.8 |
| Index | Equation | Value |
|---|---|---|
| Ecological index | 37.7% (weakly) | |
| Social index | 40.9% (moderately) | |
| Economic index | 56.9% (moderately) | |
| Global resilience index − GSRI = | 45.0% (moderately) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroza-Martínez, D.R.; Beltrán-Vargas, J.E.; Zafra-Mejía, C.A. Socioecological Resilience: Quantitative Assessment of the Impact of an Invasive Species Assemblage on a Lake Ecosystem. Resources 2024, 13, 132. https://doi.org/10.3390/resources13100132
Pedroza-Martínez DR, Beltrán-Vargas JE, Zafra-Mejía CA. Socioecological Resilience: Quantitative Assessment of the Impact of an Invasive Species Assemblage on a Lake Ecosystem. Resources. 2024; 13(10):132. https://doi.org/10.3390/resources13100132
Chicago/Turabian StylePedroza-Martínez, David Ricardo, Julio Eduardo Beltrán-Vargas, and Carlos Alfonso Zafra-Mejía. 2024. "Socioecological Resilience: Quantitative Assessment of the Impact of an Invasive Species Assemblage on a Lake Ecosystem" Resources 13, no. 10: 132. https://doi.org/10.3390/resources13100132
APA StylePedroza-Martínez, D. R., Beltrán-Vargas, J. E., & Zafra-Mejía, C. A. (2024). Socioecological Resilience: Quantitative Assessment of the Impact of an Invasive Species Assemblage on a Lake Ecosystem. Resources, 13(10), 132. https://doi.org/10.3390/resources13100132







