Comprehensive Recovery of Metals in Tailings Utilization with Mechanochemical Activation
Abstract
:1. Introduction
- Land rents or environmental fees are commensurate with and often below the profits obtained from the additional production;
- Damage to the health of employees or residents of the mining region is borne by the state (unemployment benefits; chronic diseases, disability, etc.).
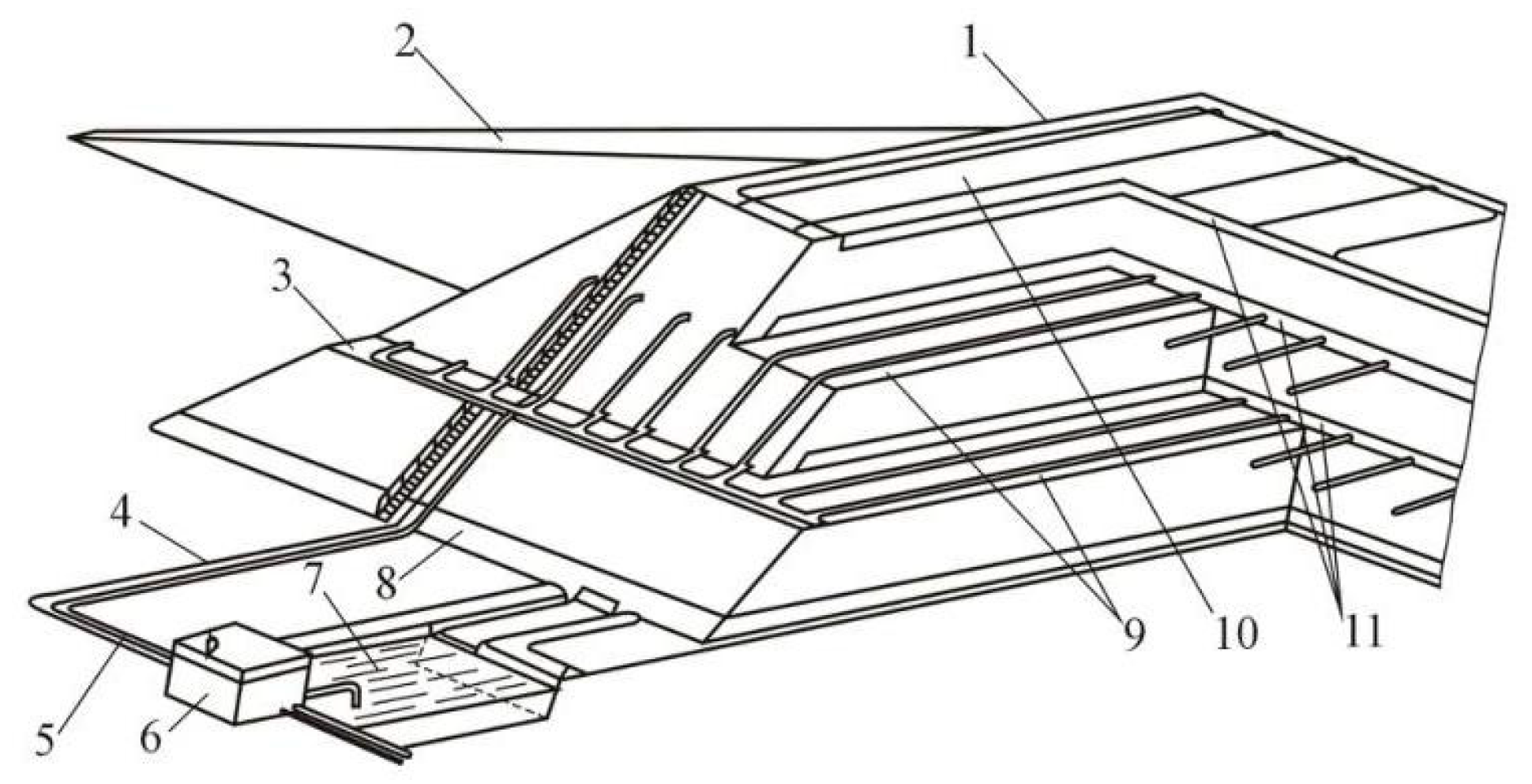
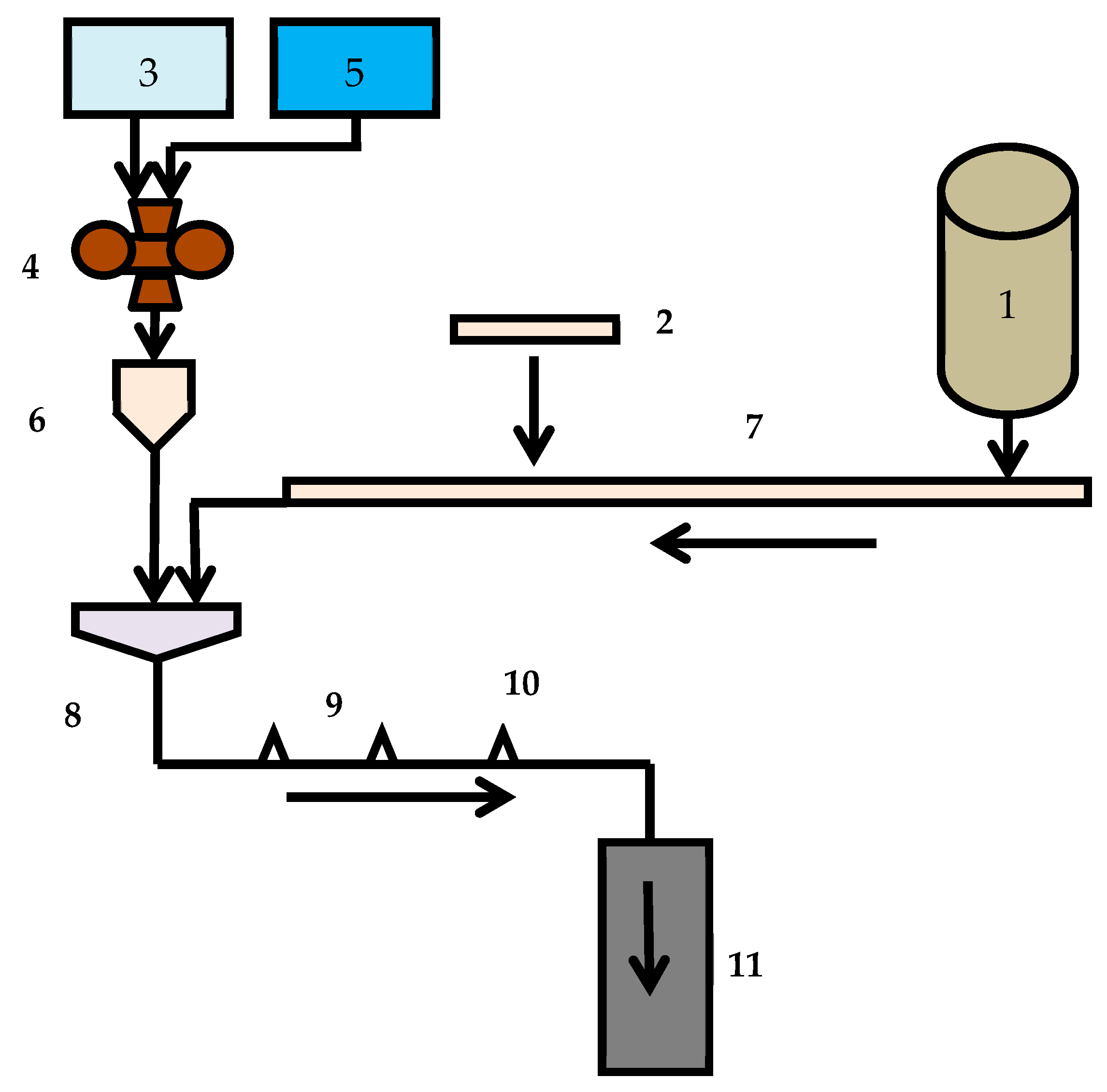
2. Objects and Methods
- Agitation leaching;
- Agitation leaching after activation in a disintegrator;
- Leaching in a disintegrator;
- Agitation leaching and leaching in a disintegrator;
- Repeatedly leaching in the disintegrator.
3. Results
3.1. For Polymetallic Ores at the Sadonskoye Deposit (North Caucasus, Russia)
3.2. For Ferruginous Quartzites of the Kursk Magnetic Anomaly
3.3. For Coal in the Regions of the Russian Federation
3.4. Particle Size Distribution of Tailings
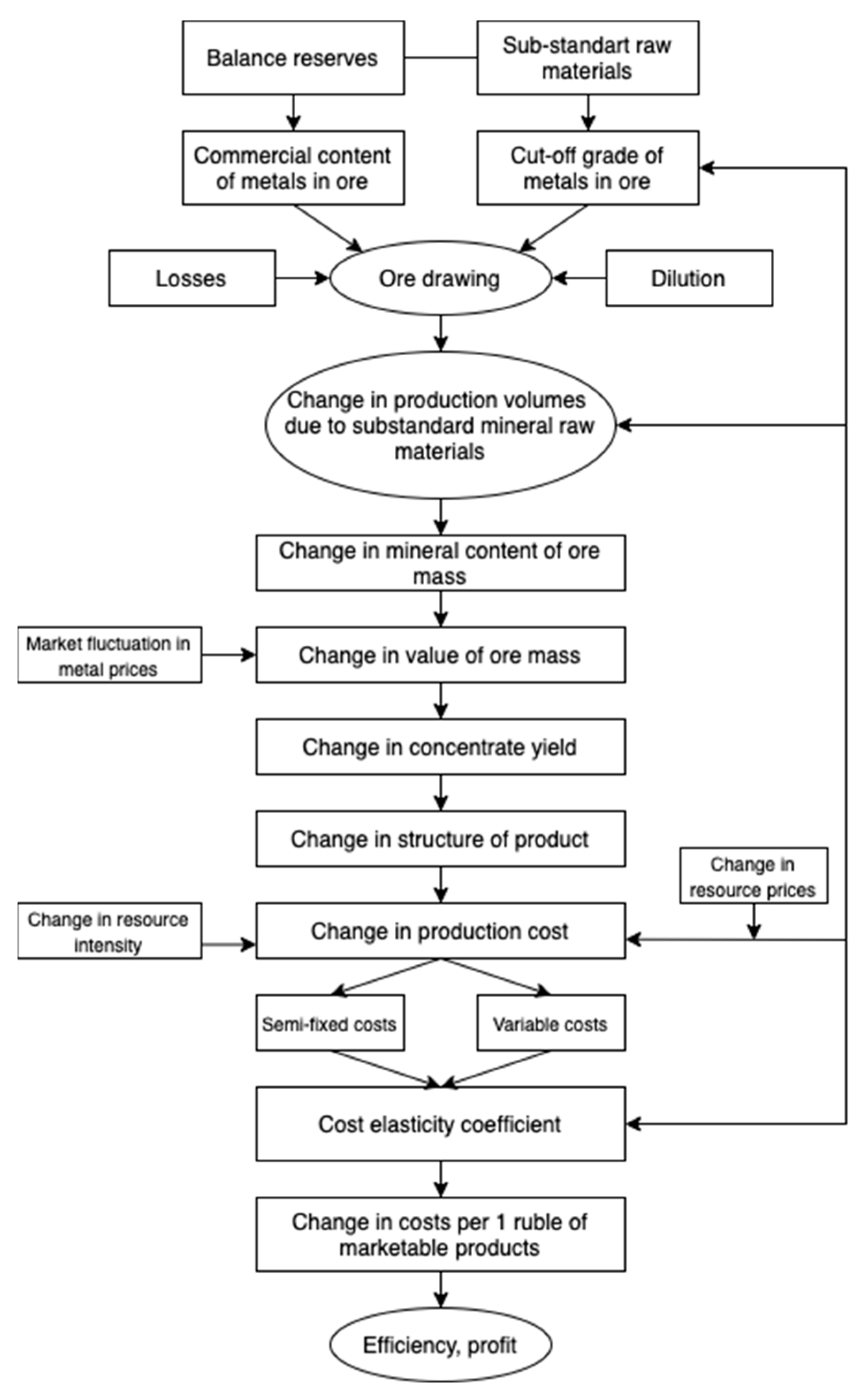
3.5. Efficiency of Tailings Utilization
- Study of the raw material base for the application of techniques for the neutralization of processed products;
- Analysis of market conditions in relation to new products;
- Study of the quality of traditional and newly developed products;
- Analysis of financial flows on means of production during tailings disposal;
- Comparison of the value of mineral raw materials and the products obtained from their processing.
4. Conclusions
- Leaching in a disintegrator in comparison with other options provides an approximately equal extraction of metals, but it is faster by two orders of magnitude (from 15–60 min to several seconds).
- The leaching of metals in a disintegrator increases the recovery by 10–25% compared to the agitation leaching method. Increasing the cycles of processing will make it possible to achieve the environmentally safe presence of metals in tailings.
- Factors influencing the process in decreasing order of influence: the proportion of agent in the leaching solution, the disintegrator rotation frequency and the ratio of liquid to solid in the slurry.
- The economic effect of mechanoactivation in a disintegrator is a greater extraction of metals from mineral raw materials at almost the same cost. This effect is defined as the difference between the damage from environmental pollution and the cost of tailings neutralization. An assessment of the threat from tailings in the natural environment shows that the technology of tailings neutralization provides a profit to the national economy, even if commercial products are not produced from the processing of ores.
- Methods of mechanochemical activation of tailings make it possible to extract a sufficient proportion of metals in accordance with environmental standards. This allows us to use processed materials without limitations, and therefore, to present mechanoactivation as an almost waste-free technology.
- The conditions for the applicability of a compromise optimal criterion for the extraction of metals from tailings are formulated on the basis of mathematical experiment planning, methods of regression analysis and considering sanitary standards for the neutralization of tailings.
- Tailings after the extraction of valuable components from them will become raw materials for new products;
- The use of tailings as hardening mixtures improves the properties of materials and reduces the costs of ore processing;
- The use of mechanical activation will contribute to the development of the mining industry, as strategically important mineral ores will be rationally used.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Drobe, M.; Haubrich, F.; Gajardo, M.; Marbler, H. Processing Tests, Adjusted Cost Models and the Economies of Reprocessing Copper Mine Tailings in Chile. Metals 2021, 11, 103. [Google Scholar] [CrossRef]
- Golik, V.I.; Klyuev, R.V.; Martyushev, N.V.; Brigida, V.; Efremenkov, E.A.; Sorokova, S.N.; Mengxu, Q. Tailings Utilization and Zinc Extraction Based on Mechanochemical Activation. Materials 2023, 16, 726. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, M.; Friedmann, D.; Kuhn, T.; Friedrich, B. “Zero-Waste”: A Sustainable Approach on Pyrometallurgical Processing of Manganese Nodule Slags. Minerals 2018, 8, 544. [Google Scholar] [CrossRef]
- Šajn, R.; Ristović, I.; Čeplak, B. Mining and Metallurgical Waste as Potential Secondary Sources of Metals—A Case Study for the West Balkan Region. Minerals 2022, 12, 547. [Google Scholar] [CrossRef]
- Vollprecht, D.; Plessl, K.; Neuhold, S.; Kittinger, F.; Öfner, W.; Müller, P.; Mischitz, R.; Sedlazeck, K.P. Recovery of Molybdenum, Chromium, Tungsten, Copper, Silver, and Zinc from Industrial Waste Waters Using Zero-Valent Iron and Tailored Beneficiation Processes. Processes 2020, 8, 279. [Google Scholar] [CrossRef]
- Zglinicki, K.; Małek, R.; Szamałek, K.; Wołkowicz, S. Mining Waste as a Potential Additional Source of HREE and U for the European Green Deal: A Case Study of Bangka Island (Indonesia). Minerals 2022, 12, 44. [Google Scholar] [CrossRef]
- Mohamadi Nasab, S.; Shafiei Bafti, B.; Yarahmadi, M.R.; Mahmoudi Maymand, M.; Kamalabadi Khorasani, J. Mineralogical Properties of the Copper Slags from the SarCheshmeh Smelter Plant, Iran, in View of Value Recovery. Minerals 2022, 12, 1153. [Google Scholar] [CrossRef]
- Žibret, G.; Lemiere, B.; Mendez, A.-M.; Cormio, C.; Sinnett, D.; Cleall, P.; Szabó, K.; Carvalho, M.T. National Mineral Waste Databases as an Information Source for Assessing Material Recovery Potential from Mine Waste, Tailings and Metallurgical Waste. Minerals 2020, 10, 446. [Google Scholar] [CrossRef]
- Ligotsky, D.N.; Argimbaeva, K.V. Effect of Grain Size distribution of Tailings During the Formation of Technogenic Deposit on the Fragmentation Index. Sustain. Dev. Mt. Territ. 2023, 2, 275–282. [Google Scholar] [CrossRef]
- Rybak, Y.; Khayrutdinov, M.; Kongar-Syuryun, C.; Tyulyayeva, Y. Resource-saving technologies for development of mineral deposits. Sustain. Dev. Mt. Territ. 2021, 13, 405–415. [Google Scholar] [CrossRef]
- Łupieżowiec, M.; Rybak, J.; Różański, Z.; Dobrzycki, P.; Jędrzejczyk, W. Design and Construction of Foundations for In-dustrial Facilities in the Areas of Former Post-Mining Waste Dumps. Energies 2022, 15, 5766. [Google Scholar] [CrossRef]
- Khayrutdinov, M.M.; Kongar-Syuryun, C.B.; Khayrutdinov, A.M.; Tyulyaeva, Y.S. Improving safety when extracting water-soluble ores by optimizing the parameters of the backfill mass. Bezop. Tr. V Promyshlennosti 2021, 2021, 53–59. [Google Scholar] [CrossRef]
- Bahn-Walkowiak, B.; Steger, S. Resource Targets in Europe and Worldwide: An Overview. Resources 2015, 4, 597–620. [Google Scholar] [CrossRef]
- Kongar-Syuryun, C.B.; Aleksakhin, A.V.; Eliseeva, E.N.; Zhaglovskaya, A.V.; Klyuev, R.V.; Petrusevich, D.A. Modern Technologies Providing a Full Cycle of Geo-Resources Development. Resources 2023, 12, 50. [Google Scholar] [CrossRef]
- Gugunskiy, D.; Chernykh, I.; Khairutdinov, A. Legal Models for Activities on the Exploration and Utilization of Space Resources: Towards the “Space-2030” Agenda. Adv. Intell. Syst. Comput. 2020, 1100, 657–664. [Google Scholar] [CrossRef]
- Khayrutdinov, A.M. Current issues of mining activities on celestial bodies: International law aspects. The Advances in the astronautical sciences. In Proceedings of the First IAA/AAS SciTech Forum on Space Flight Mechanics and Space Structures and Materials, Moscow, Russia, 13–15 October 2018; Volume 170, pp. 895–902. [Google Scholar]
- Wu, C.; Zhang, Y.; Zhang, J.; Chen, Y.; Duan, C.; Qi, J.; Cheng, Z.; Pan, Z. Comprehensive Evaluation of the Eco-Geological Environment in the Concentrated Mining Area of Mineral Resources. Sustainability 2022, 14, 6808. [Google Scholar] [CrossRef]
- Golik, V.I.; Razorenov, Y.I.; Lukyanov, V.G. Environmental and economic aspects of resource saving in mining. Bull. Tomsk. Polytech. Univ. Geo. Assets Eng. 2017, 328, 18–27. (In Russian) [Google Scholar]
- Khayrutdinov, A.; Paleev, I.; Artemov, S. Replacement of traditional components of the backfill mixture with man-made waste. IOP Conf. Ser. Earth Environ. Sci. 2021, 942, 012005. [Google Scholar] [CrossRef]
- Kovalski, E.R.; Kongar-Syuryun, C.B.; Petrov, D.N. Challenges and prospects for several-stage stoping in potash minining. Sustain. Dev. Mt. Territ. 2023, 15, 349–364. [Google Scholar] [CrossRef]
- Volohov, E.M.; Kozhukharova, V.K.; Britvin, I.A.; Savkov, B.M.; Zherlygina, E.S. Assessment of impact of mining operations on surface infrastructure. MIAB. Mining Inf. Anal. Bull. 2023, 8, 72–93. [Google Scholar] [CrossRef]
- Cansado, I.P.D.P.; Mourão, P.A.M.; Nabais, J.M.V.; Borges, C.; Matos, G. Use of dirty plastic waste as precursors for activated carbon production—A contribution to the circular economy. Water Environ. J. 2022, 36, 96–104. [Google Scholar] [CrossRef]
- Ya, K.Z.; Goryachev, B.; Adigamov, A.; Nurgalieva, K.; Narozhnyy, I. Thermodynamics and Electrochemistry of the Interaction of Sphalerite with Iron (II)-Bearing Compounds in Relation to Flotation. Resources 2022, 11, 108. [Google Scholar] [CrossRef]
- Kopylov, A.S.; Dzhioeva, A.K.; Kondratyev, Y.I. An integrated approach to the development of the raw material base of the mining region with the use of resource-reproducing technologies. Sustain. Dev. Mt. Territ. 2022, 14, 228–239. [Google Scholar] [CrossRef]
- Golik, V.I. Extraction of metals from tailings by combined activation methods. Obogashchenie Rud. 2010, 5, 38–40. [Google Scholar]
- Golik, V.I.; Komashchenko, V.I.; Stradanchenko, S.G.; Maslennikov, S.A. Mechanical-chemical activation technology of extraction of iu-metals from the rock ore. MIAB. Mining Inf. Anal. Bull. 2012, 9, 20–25. [Google Scholar]
- Ren, B.; Zhou, Y.; Ma, H.; Deng, R.; Zhang, P.; Hou, B. Sb release characteristics of the solid waste produced in antimony mining smelting process. J. Mater. Cycles Waste Manag. 2018, 20, 193–200. [Google Scholar] [CrossRef]
- Golik, V.I.; Marinin, M.A. Practice of underground leaching of uranium in blocks. MIAB. Mining Inf. Anal. Bull. 2022, 6-1, 5–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, B.; Hursthouse, A.S.; Zhou, S. Antimony Ore Tailings: Heavy Metals, Chemical Speciation, and Leaching Characteristics. Pol. J. Environ. Stud. 2019, 28, 485–495. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, B.; Hursthouse, A.; Deng, R.; Hou, B. Leaching and Releasing Characteristics and Regularities of Sb and As from Antimony Mining Waste Rocks. Pol. J. Environ. Stud. 2019, 28, 4017–4025. [Google Scholar] [CrossRef]
- Xie, Q.; Ren, B.; Shi, X.; Hursthouse, A. Factors on the distribution, migration, and leaching of potential toxic metals in the soil and risk assessment around the zinc smelter. Ecol. Indic. 2022, 144, 109502. [Google Scholar] [CrossRef]
- Korkmaz, A.; Kayıran, H. Investigation of mechanical activation effect on high-volume natural pozzolanic cements. Open Chem. 2022, 20, 1029–1044. [Google Scholar] [CrossRef]
- Golik, V.I. Conceptual approaches to the creation of low-waste and waste-free mining production based on a combination of physical-technical and physical-chemical geotechnologies. Gornyi Zhurnal 2013, 5, 93–97. [Google Scholar]
- Wu, F.; Li, H.; Yang, K. Effects of mechanical activation on physical and chemical characteristics of coal-gasification slag. Coatings 2021, 11, 902. [Google Scholar] [CrossRef]
- Korkmaz, A.V. Mechanical activation of diabase and its effect on the properties and microstructure of Portland cement. Case Stud. Const. Mater. 2022, 16, e00868. [Google Scholar] [CrossRef]
- Burminskaya, L.N.; Bulgakov, V.G.; Neginskij, I.V.; Smirnov, K.O. Programming complex for X-ray analysis of thin structure metallic alloys. Sci. J. VolSU 2004, 1, 159–162. [Google Scholar]
- All Union State Standard 30416-96; Soils. Laboratory Tests. General provisions; Goskomitet po Delam Stroitel’stva: Moscow, Russia, 1995.
- All Union State Standard 5180-84; Soils. Methods for Determining Physical Characteristics. USSR State Committee for Construction: Moscow, Russia, 1984.
- Zadkov, D.A.; Gabov, V.V.; Babyr, N.V.; Stebnev, A.V.; Teremetskaya, V.A. Adaptable and energy-efficient powered roof support unit. MIAB. Mining Inf. Anal. Bull. 2022, 6, 46–61. [Google Scholar] [CrossRef]
- Didmanidze, O.N.; Afanasev, A.S.; Hakimov, R.T. Natural gas methane number and its influence on the gas engine working process efficiency. J. Min. Inst. 2021, 251, 730–737. [Google Scholar] [CrossRef]
- Didmanidze, O.N.; Afanasev, A.S.; Khakimov, R.T. Mathematical model of the liquefied methane phase transition in the cryogenic tank of a vehicle. J. Min. Inst. 2020, 243, 337–347. [Google Scholar] [CrossRef]


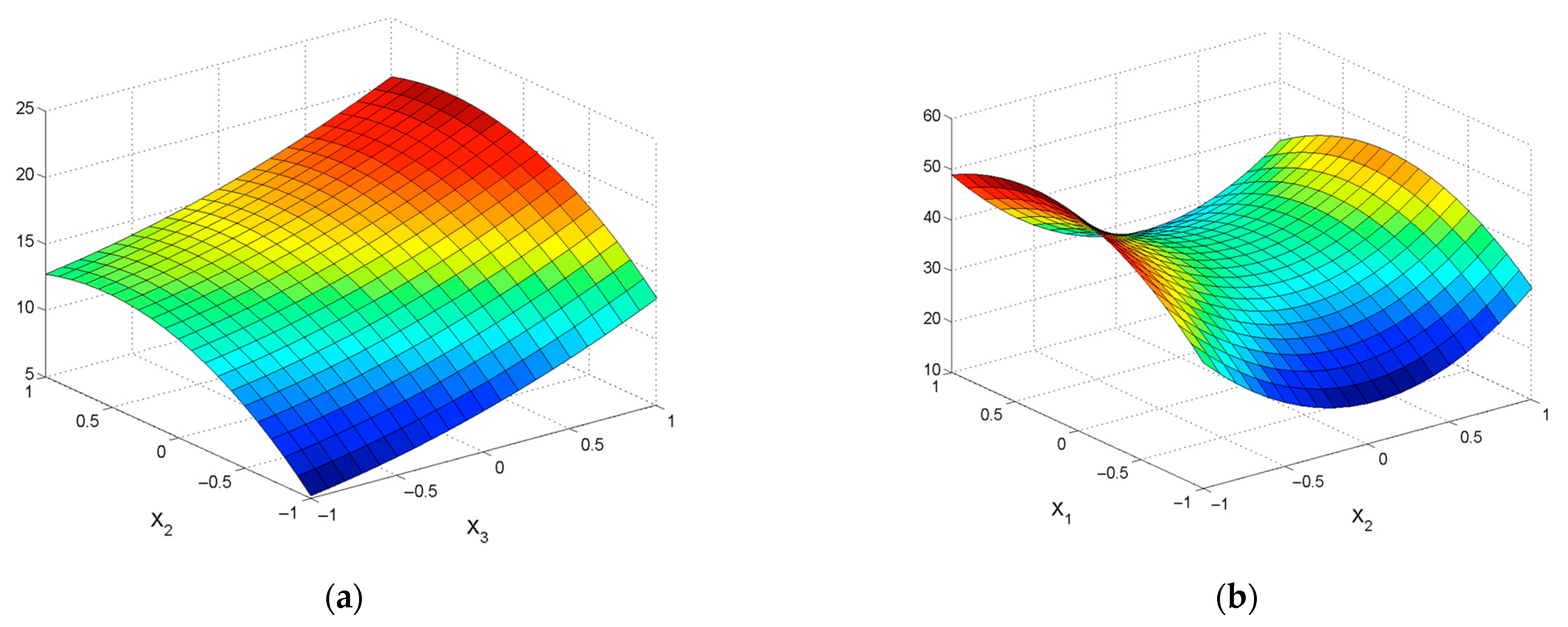

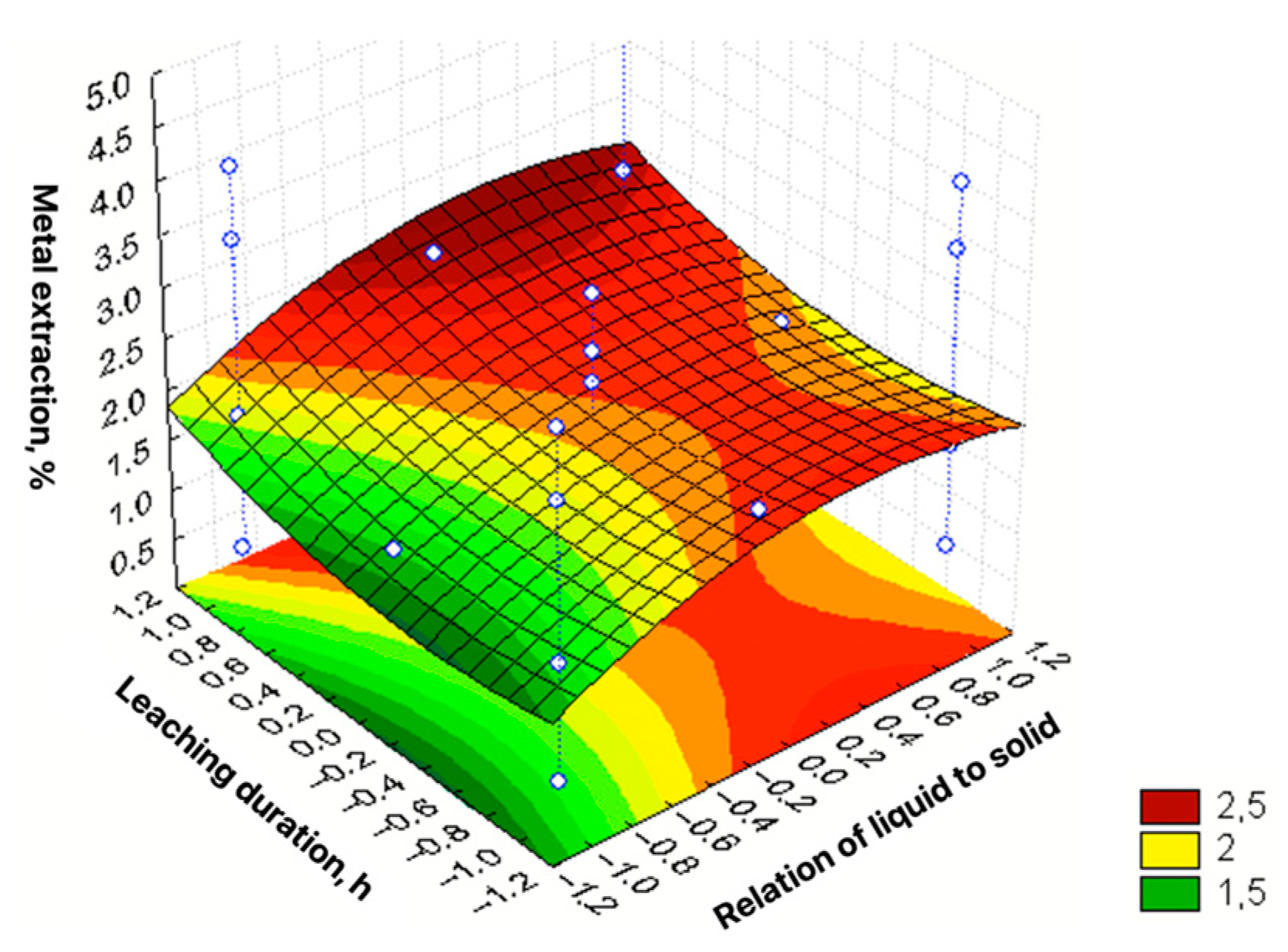

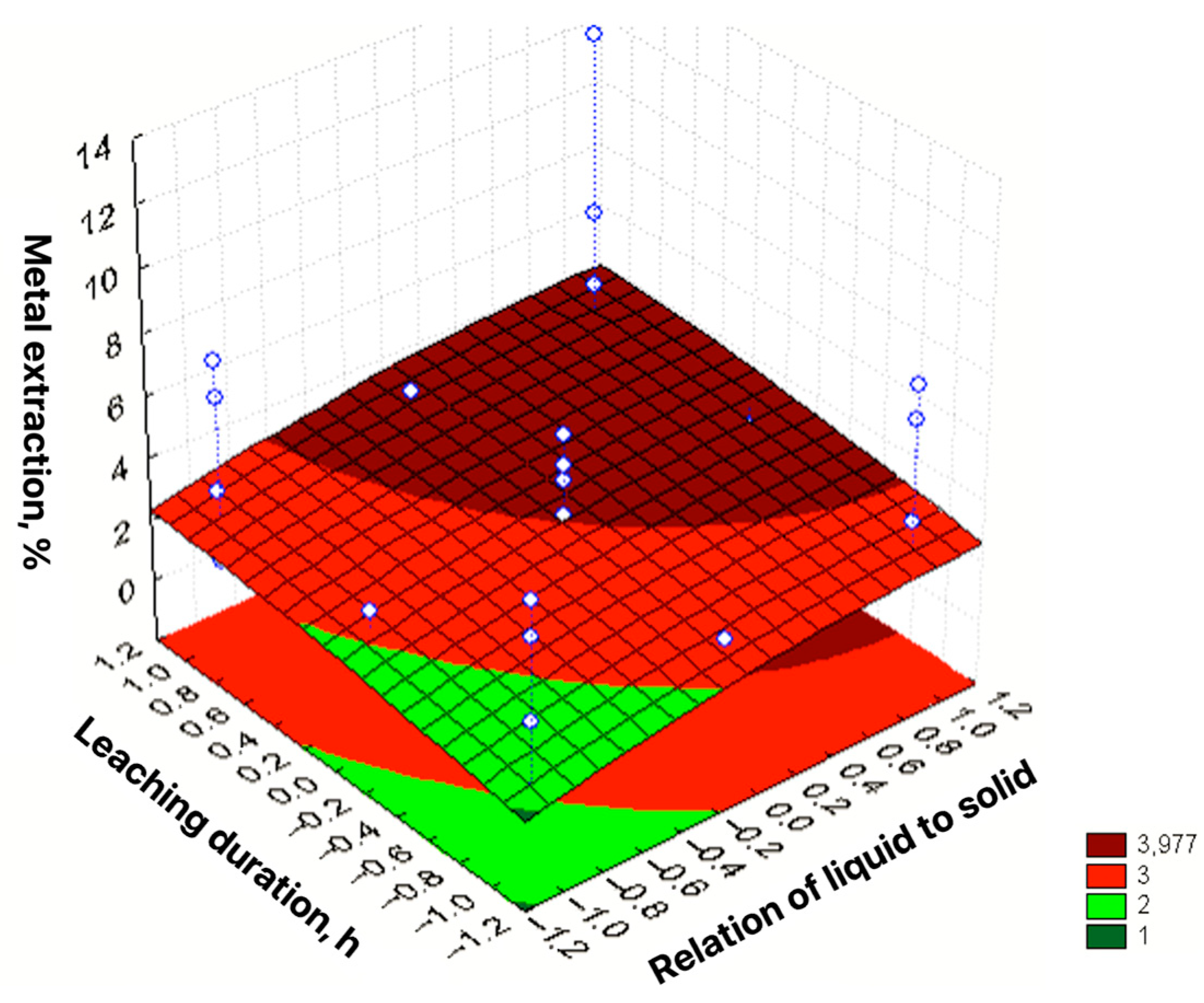
| No | Agent Content, g/L | Ratio of Liquid to Solid | Agitation Leaching Duration, h | Extraction into Solution, % | ||
|---|---|---|---|---|---|---|
| H2SO4 | NaCl | |||||
| Zn | Pb | |||||
| 1 | 2 [−1] | 20 [−1] | 4 [−1] | 0.25 [−1] | 41.25 | 1.42 |
| 2 | 10 [1] | 20 [−1] | 4 [−1] | 0.25 [−1] | 57.75 | 0.49 |
| 3 | 2 [−1] | 160 [1] | 4 [−1] | 0.25 [−1] | 18.12 | 36.18 |
| 4 | 10 [1] | 160 [1] | 4 [−1] | 0.25 [−1] | 24.01 | 38.11 |
| 5 | 2 [−1] | 20 [−1] | 10 [1] | 0.25 [−1] | 48.43 | 3.58 |
| 6 | 10 [1] | 20 [−1] | 10 [1] | 0.25 [−1] | 82.12 | 4.77 |
| 7 | 2 [−1] | 160 [1] | 10 [1] | 0.25 [−1] | 12.64 | 30.94 |
| 8 | 10 [1] | 160 [1] | 10 [1] | 0.25 [−1] | 17.90 | 35.72 |
| 9 | 2 [−1] | 20 [−1] | 4 [−1] | 1 [1] | 44.59 | 17.15 |
| 10 | 10 [1] | 20 [−1] | 4 [−1] | 1 [1] | 70.27 | 1.42 |
| 11 | 2 [−1] | 160 [1] | 4 [−1] | 1 [1] | 10.94 | 24.77 |
| 12 | 10 [1] | 160 [1] | 4 [−1] | 1 [1] | 28.22 | 37.15 |
| 13 | 2 [−1] | 20 [−1] | 10 [1] | 1 [1] | 49.48 | 3.58 |
| 14 | 10 [1] | 20 [−1] | 10 [1] | 1 [1] | 50.54 | 1.78 |
| 15 | 2 [−1] | 160 [1] | 10 [1] | 1 [1] | 15.78 | 46.44 |
| 16 | 10 [1] | 160 [1] | 10 [1] | 1 [1] | 18.94 | 44.07 |
| 17 | 2 [−1] | 90 [0] | 7 [0] | 0.625 [0] | 21.38 | 35.82 |
| 18 | 10 [1] | 90 [0] | 7 [0] | 0.625 [0] | 34.64 | 41.68 |
| 19 | 6 [0] | 20 [−1] | 7[0] | 0.625 [0] | 67.78 | 1.65 |
| 20 | 6 [0] | 160 [1] | 7 [0] | 0.625 [0] | 25.78 | 45.01 |
| 21 | 6 [0] | 90 [0] | 4 [−1] | 0.625 [0] | 40.85 | 21.28 |
| 22 | 6 [0] | 90 [0] | 10 [1] | 0.625 [0] | 36.85 | 58.34 |
| 23 | 6 [0] | 90 [0] | 7 [0] | 0.25 [−1] | 40.54 | 49.18 |
| 24 | 6 [0] | 90 [0] | 7 [0] | 1 [1] | 42.75 | 40.84 |
| Regression Equations | Significance Indicators |
|---|---|
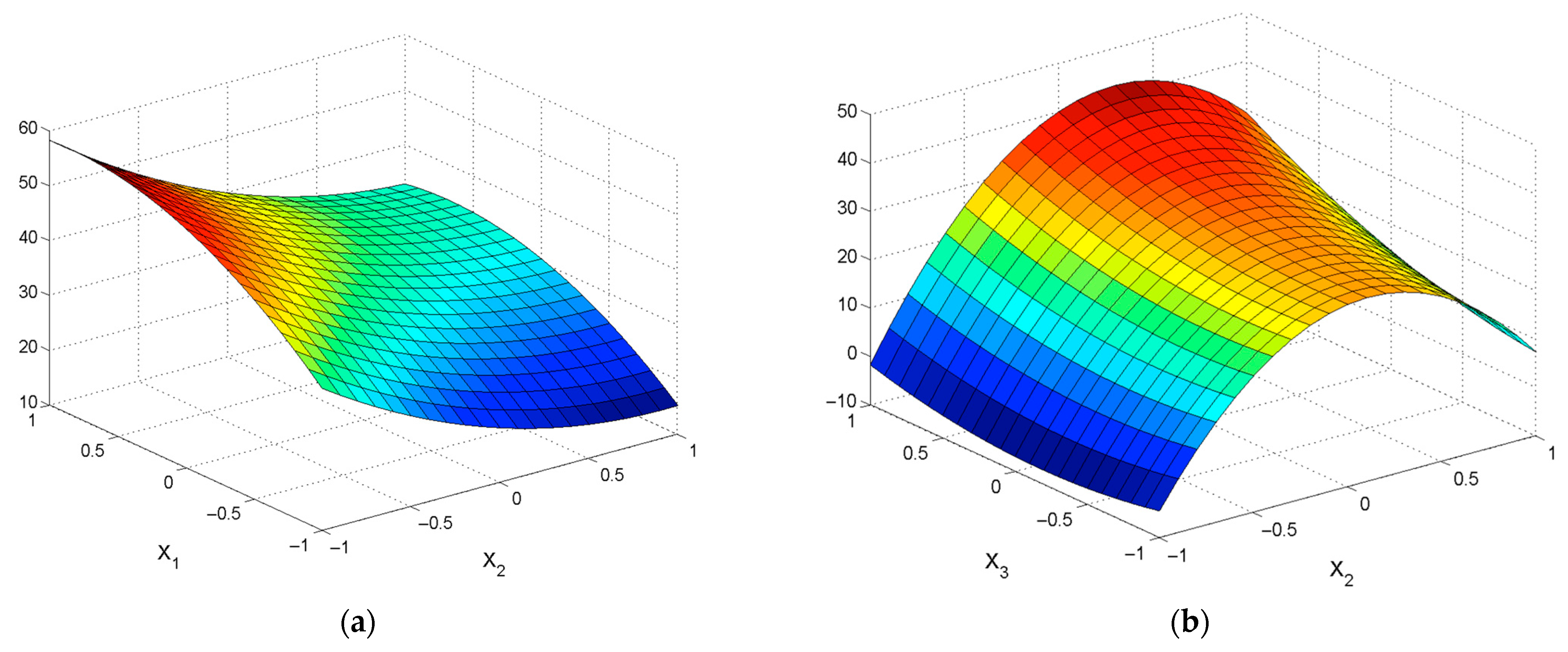
| εZn = 39.35 + 6.76X1 − 18.88X2 − 0.62X4 − 11.6X12 + 7.19X22 + 2.03X42 −2.84X1X2 − 1.39X1X3 − 0.89X1X4 − 2.04X2X3 + 1.00X2X4 − 2.45X3X4 | R2 = 0.9393; Sad = 46.93; F = 68.59 |
| εPb = 42.43 + 16.8X2 + 2.68X3 + 0.93X4 − 3.89X12 − 19.31X22 + 2.36X42 + 2.12X1X2 − 0.9X1X4 + 1.73X2X3 + 1.04X3X4 | R2 = 0.8888; Sad = 71.17; F = 30.19 |
| No | Agent Content, g/L | Ratio of Liquid to Solid | Rotational Rate, Hz | Extraction into Solution, ε, % | ||
|---|---|---|---|---|---|---|
| H2SO4 | NaCl | |||||
| Zn | Pb | |||||
| 1 | 2 [−1] | 20 [−1] | 4 [−1] | 50 [−1] | 26.95 | 0.33 |
| 2 | 10 [1] | 20 [−1] | 4 [−1] | 50 [−1] | 78.74 | 0.95 |
| 3 | 2 [−1] | 160 [1] | 4 [−1] | 50 [−1] | 10.95 | 27.14 |
| 4 | 10 [1] | 160 [1] | 4 [−1] | 50 [−1] | 27.37 | 40.50 |
| 5 | 2 [−1] | 20 [−1] | 10 [1] | 50 [−1] | 47.37 | 4.76 |
| 6 | 10 [1] | 20 [−1] | 10 [1] | 50 [−1] | 54.74 | 1.79 |
| 7 | 2 [−1] | 160 [1] | 10 [1] | 50 [−1] | 6.32 | 40.48 |
| 8 | 10 [1] | 160 [1] | 10 [1] | 50 [−1] | 15.79 | 35.71 |
| 9 | 2 [−1] | 20 [−1] | 4 [−1] | 200 [1] | 32.42 | 0.71 |
| 10 | 10 [1] | 20 [−1] | 4 [−1] | 200 [1] | 61.47 | 1.43 |
| 11 | 2 [−1] | 160 [1] | 4 [−1] | 200 [1] | 13.47 | 27.14 |
| 12 | 10 [1] | 160 [1] | 4 [−1] | 200 [1] | 27.37 | 40.00 |
| 13 | 2 [−1] | 20 [−1] | 10 [1] | 200 [1] | 42.11 | 5.95 |
| 14 | 10 [1] | 20 [−1] | 10 [1] | 200 [1] | 52.63 | 1.55 |
| 15 | 2 [−1] | 160 (1) | 10 [1] | 200 [1] | 12.63 | 44.05 |
| 16 | 10 (1) | 160 (1) | 10 [1] | 200 [1] | 65.26 | 18.38 |
| 17 | 2 [−1] | 90 [0] | 7 [0] | 125 [0] | 22.11 | 39.17 |
| 18 | 10 [1] | 90 [0] | 7 [0] | 125 [0] | 36.11 | 28.33 |
| 19 | 6 [0] | 20 [−1] | 7 [0] | 125 [0] | 58.95 | 1.67 |
| 20 | 6 [0] | 160 [1] | 7 [0] | 125 [0] | 23.58 | 47.50 |
| 21 | 6 [0] | 90 [0] | 4 (−1) | 125 [0] | 35.37 | 34.29 |
| 22 | 6 [0] | 90 [0] | 10 [1] | 125 [0] | 29.47 | 42.86 |
| 23 | 6 [0] | 90 [0] | 7 [0] | 50 (−1) | 32.42 | 38.33 |
| 24 | 6 [0] | 90 [0] | 7 [0] | 200 (1) | 26.53 | 40.83 |
| Regression Equations | Significance Indicators |
|---|---|
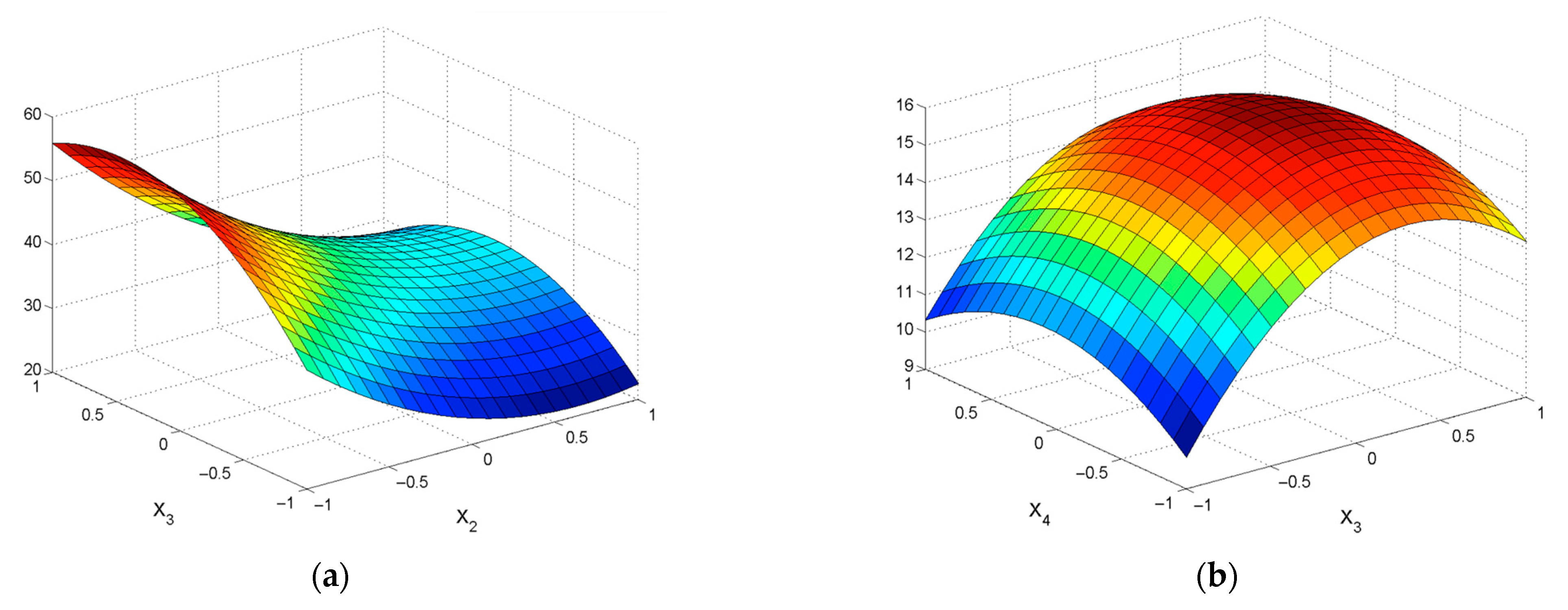
| εZn = 32.15 + 11.4X1 − 14.04X2 +0.68X3 + 1.85X4 − 2.90X12 + 9.25X22 – 2.53X42 − 0.39X1X2 − 1.95X1X3 + 1.32X1X4 + 1.47X2X3 + 4.84X2X4 + 3.61X3X4 | R2 = 0.8277; Sad = 143.62; F = 18.06 |
| εPb = 39.44 − 1.17X1 + 16.76 X2 + 1.28X3 − 0.55X4 − 5.64X12 − 14.81X22 – 0.86X32 − 4.09X1X3 − 1.42X1X4 − 0.42X2X3 − 1.00X2X4 − 0.82X3X4 | R2 = 0.9483; Sad = 35.09; F = 44.58 |
| No | Agent Content, g/L | Rotational Rate, Hz | Number Of Activation Cycles | Extraction into Solution, ε,% | ||
|---|---|---|---|---|---|---|
| H2SO4 | NaCl | |||||
| Zn | Pb | |||||
| 1 | 2 [−1] | 20 [−1] | 50 [−1] | 7 [1] | 39.58 | 1.24 |
| 2 | 10 [1] | 20 [−1] | 50 [−1] | 3 [−1] | 64.42 | 1.19 |
| 3 | 2 [−1] | 160 [1] | 50 [−1] | 3 [−1] | 8.84 | 21.43 |
| 4 | 10 [1] | 160 [1] | 50 [−1] | 7 [1] | 26.52 | 40.48 |
| 5 | 2 [−1] | 20 [−1] | 50 [−1] | 3 [−1] | 53.68 | 3.57 |
| 6 | 10 [1] | 20 [−1] | 50 [−1] | 7 [1] | 70.53 | 2.38 |
| 7 | 2 [−1] | 160 [1] | 50 [−1] | 7 [1] | 1.05 | 39.29 |
| 8 | 10 [1] | 160 [1] | 50 [−1] | 3 [−1] | 17.89 | 45.00 |
| 9 | 2 [−1] | 20 [−1] | 200 [1] | 7 [1] | 40.58 | 0.95 |
| 10 | 10 [1] | 20 [−1] | 200 [1] | 3 [−1] | 52.26 | 0.33 |
| 11 | 2 [−1] | 160 [1] | 200 [1] | 3 [−1] | 10.11 | 20.95 |
| 12 | 10 [1] | 160 [1] | 200 [1] | 7 [1] | 29.05 | 48.10 |
| 13 | 2 [−1] | 20 [−1] | 200 [1] | 3 [−1] | 56.84 | 4.76 |
| 14 | 10 [1] | 20 [−1] | 200 [1] | 7 [1] | 66.32 | 2.38 |
| 15 | 2 [−1] | 160 [1] | 200 [1] | 7 [1] | 7.37 | 28.57 |
| 16 | 10 [1] | 160 [1] | 200 [1] | 3 [−1] | 22.11 | 48.81 |
| 17 | 2 [−1] | 90 [0] | 125 [0] | 5 [0] | 20.63 | 29.17 |
| 18 | 10 [1] | 90 [0] | 125 [0] | 5 [0] | 40.53 | 26.67 |
| 19 | 6 [0] | 20 [−1] | 125 [0] | 5 [0] | 63.37 | 2.50 |
| 20 | 6 [0] | 160 [1] | 125 [0] | 5 [0] | 24.32 | 28033 |
| 21 | 6 [0] | 90 [0] | 125 [0] | 5 [0] | 20.21 | 19.05 |
| 22 | 6 [0] | 90 [0] | 125 [0] | 5 [0] | 55.79 | 46.43 |
| 23 | 6 [0] | 90 [0] | 50 [−1] | 3 [−1] | 35.37 | 33.33 |
| 24 | 6 [0] | 90 [0] | 200 [1] | 7 [1] | 35.37 | 33.33 |
| Regression Equations | Significance Indicators |
|---|---|
| εZn = 38.15 + 10.66X1 − 15.17X2 +2.42X3 − 1.37X4 − 6.36X12 + 3.92X22 – 2.99X32 – – 1.68X42 − 4.85X1X2 − 4.62X1X3 + 2.1X1X4 – 3.56X2X3 + 1.95X2X4 + 1.6X3X4 | R2 = 0.9206; Sad= 73.40; F = 30.72 |
| εPb = 40.94 + 16.12X2 + 4.13X3 + 0.67X4 − 6.37X12 − 17.44X22 + 3.58X32 + + 1.36X42 + 4.04X1X2 − 1.32X1X3 + 2.47X2X3 − 2.00X2X4 − 0.72X3X4 | R2 = 0.9535; Sad= 29.69; F = 55.26 |
| Extraction of Metals in Agitator | Extraction in Disintegrator for 10 s | ||||||
|---|---|---|---|---|---|---|---|
| concentration in tailings, % | concentration in tailings, % | ||||||
| zinc—0.94 | lead—0.85 | zinc—0.94 | lead—0.85 | ||||
| Extraction for 0.2–1.0 h, % | Extraction for 0.2–1.0 h, % | Extraction for 10 s, % | Extraction for 10 s, % | ||||
| extracted | left | extracted | left | extracted | left | extracted | left |
| 24 | 72 | 17 | 71 | 29 | 68 | 25 | 61 |
| Extraction in Activator | Extraction in the Working Body of Disintegrator for 10 s, % | ||
|---|---|---|---|
| concentration in tailings, % | concentration in tailings, % | ||
| of iron—8 | of iron—8 | ||
| Duration of extraction: 0.2 to1.0 h, % | Duration of extraction: 10 s,% | ||
| extracted | left | extracted | left |
| 0.8 | 7.36 | 1.2 | 7.4 |
| No | Concentration in Solution, C, g/L | Ratio of Liquid to Solid | Leaching Duration, t, h | Fe-Content in the Production Solution, ε, % | |
|---|---|---|---|---|---|
| NaCl | |||||
| 1 | 2 [−1] | 20 [−1] | 4 [−1] | 0.25 [−1] | 0.40 |
| 2 | 10 [1] | 20 [−1] | 4 [−1] | 0.25 [−1] | 3.08 |
| 3 | 2 [−1] | 160 [1] | 4 [−1] | 0.25 [−1] | 1.56 |
| 4 | 10 [1] | 160 [1] | 4 [−1] | 0.25 [−1] | 3.76 |
| 5 | 2 [−1] | 20 [−1] | 10 [1] | 0.25 [−1] | 0.84 |
| 6 | 10 [1] | 20 [−1] | 10 [1] | 0.25 [−1] | 3.72 |
| 7 | 2 [−1] | 160 [1] | 10 [1] | 0.25 [−1] | 1.84 |
| 8 | 10 [1] | 160 [1] | 10 [1] | 0.25 [−1] | 4.35 |
| 9 | 2 [−1] | 20 [−1] | 4 [−1] | 1 [1] | 0.45 |
| 10 | 10 [1] | 20 [−1] | 4 [−1] | 1 [1] | 3.48 |
| 11 | 2 [−1] | 160 [1] | 4 [−1] | 1 [1] | 1.77 |
| 12 | 10 [1] | 160 [1] | 4 [−1] | 1 [1] | 4.18 |
| 13 | 2 [−1] | 20 [−1] | 10 [1] | 1 [1] | 1.17 |
| 14 | 10 [1] | 20 [−1] | 10 [1] | 1 [1] | 3.93 |
| 15 | 2 [−1] | 160 [1] | 10 [1] | 1 [1] | 2.55 |
| 16 | 10 [1] | 160 [1] | 10 [1] | 1 [1] | 4.75 |
| 17 | 2 [−1] | 90 [0] | 7 [0] | 0.625 [0] | 1.64 |
| 18 | 10 [1] | 90 [0] | 7 [0] | 0.625 [0] | 3.13 |
| 19 | 6 [0] | 20 [−1] | 7 [0] | 0.625 [0] | 2.27 |
| 20 | 6 [0] | 160 [1] | 7 [0] | 0.625 [0] | 2.58 |
| 21 | 6 [0] | 90 [0] | 4 [−1] | 0.625 [0] | 1.53 |
| 22 | 6 [0] | 90 [0] | 10 [1] | 0.625 [0] | 2.01 |
| 23 | 6 [0] | 90 [0] | 7 [0] | 0.25 [−1] | 2.10 |
| 24 | 6 [0] | 90 [0] | 7 [0] | 1 [1] | 2.54 |
| Regression Equations | Significance Indicators |
|---|---|
| ε = 2.095 + 1.231X1 + 0.444X2 + 0.275X3 + 0.176X4 + 0.29X12 + 0.33X22 + + 0.325X32 + 0.225X42 − 0.127X1X2 + 0.047X2X3 + 0.036X3X4 | R2 = 0.977; Sad = 0.0673; F = 225.99 |
| No | Concentration in Leaching Solution, C, g/l | Rotational Rate, X4, Hz | Leaching Duration, t, h | Fe-Content in the Production Solution, ε,% | |
|---|---|---|---|---|---|
| H2SO4 | NaCl | ||||
| 1 | 2 [−1] | 20 [−1] | 50 [−1] | 0.25 [−1] | 0.5 |
| 2 | 10 [1] | 20 [−1] | 50 [−1] | 0.25 [−1] | 5.33 |
| 3 | 2 [−1] | 160 [1] | 50 [−1] | 0.25 [−1] | 2.67 |
| 4 | 10 [1] | 160 [1] | 50 [−1] | 0.25 [−1] | 6.50 |
| 5 | 2 [−1] | 20 [−1] | 200 [1] | 0.25 [−1] | 1.45 |
| 6 | 10 [1] | 20 [−1] | 200 [1] | 0.25 [−1] | 6.44 |
| 7 | 2 [−1] | 160 [1] | 200 [1] | 0.25 [−1] | 3.18 |
| 8 | 10 [1] | 160 [1] | 200 [1] | 0.25 [−1] | 7.53 |
| 9 | 2 [−1] | 20 [−1] | 50 [−1] | 1 [1] | 0.78 |
| 10 | 10 [1] | 20 [−1] | 50 [−1] | 1 [1] | 6.02 |
| 11 | 2 [−1] | 160 [1] | 50 [−1] | 1 [1] | 3.06 |
| 12 | 10 [1] | 160 [1] | 50 [−1] | 1 [1] | 7.23 |
| 13 | 2 [−1] | 20 [−1] | 200 [1] | 1 [1] | 2.02 |
| 14 | 10 [1] | 20 [−1] | 200 [1] | 1 [1] | 6.80 |
| 15 | 2 [−1] | 160 [1] | 200 [1] | 1 [1] | 4.41 |
| 16 | 10 [1] | 160 [1] | 200 [1] | 1 [1] | 12.50 |
| 17 | 2 [−1] | 90 [0] | 125 [0] | 0.625 [0] | 2.84 |
| 18 | 10 [1] | 90 [0] | 125 [0] | 0.625 [0] | 5.41 |
| 19 | 6 [0] | 20 [−1] | 125 [0] | 0.625 [0] | 3.93 |
| 20 | 6 [0] | 160 [1] | 125 [0] | 0.625 [0] | 4.46 |
| 21 | 6 [0] | 90 [0] | 50 [−1] | 0.625 [0] | 2.65 |
| 22 | 6 [0] | 90 [0] | 200 [1] | 0.625 [0] | 2.98 |
| 23 | 6 [0] | 90 [0] | 125 [0] | 0.25 [−1] | 2.28 |
| 24 | 6 [0] | 90 [0] | 125 [0] | 1 [1] | 3.55 |
| Regression Equation | Significance Indicators |
|---|---|
| ε = 3.091 + 2.381X1 + 1.014X2 + 0.698X3 + 0.583X4 + 1.035X12 + 1.104X22 − 0.276X32 − 0.176X42 + 0.259X1X3 − 0.268X1X4 + 0.255X2X3 + 0.339X2X4 + 0.315X3X4 | R2 = 0.9384; Sad = 1.0111; F = 43.03 |
| From the Tails in the Agitator for 0.2–1.0 h, % | From the Tails in Disintegrator for 10 s, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Amount in tailings, g/t | Amount in tailings, g/t | ||||||||
| Manganese | Nickel | Chrome | Lead | Zinc | Manganese | Nickel | Chrome | Lead | Zinc |
| 319 | 24 | 84 | 54 | 51 | 321 | 27 | 87 | 54 | 50 |
| Extraction into concentrate, % | Extraction into concentrate, % | ||||||||
| 1.0 | 0.9 | 14 | 31 | 33 | 1.4 | 1.2 | 18 | 43 | 37 |
| Type of Waste | Residuals on Sieves, % | ||||
|---|---|---|---|---|---|
| 0.2 | 0.14 | 0.071 | <0.071 | Total | |
| Iron quartzites of Lebedinsky Mining and Processing Plant | |||||
| original | 13.7 | 27.6 | 21.4 | 37.3 | 100 |
| after agitation leaching | 15.3 | 26.4 | 21.7 | 38.6 | 100 |
| after leaching in the disintegrator | 9.4 | 20.1 | 25.5 | 55.0 | 100 |
| Polymetallic ores of the Sadon lead-zinc Plant | |||||
| original | 18.6 | 29.3 | 12.9 | 39.2 | 100 |
| after agitation leaching | 16.7 | 28.0 | 13.3 | 42.0 | 100 |
| after leaching in the disintegrator | 13.4 | 24.1 | 20.3 | 52.2 | 100 |
| Hard coal of Russian regions | |||||
| original | 19.5 | 27.2 | 30.5 | 42.8 | 100 |
| after agitation leaching | 11.4 | 16.0 | 29.6 | 43.0 | 100 |
| after leaching in the disintegrator | 8.9 | 15.3 | 33.7 | 57.9 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golik, V.I.; Mitsik, M.F.; Aleksakhina, Y.V.; Alenina, E.E.; Ruban-Lazareva, N.V.; Kruzhkova, G.V.; Kondratyeva, O.A.; Trushina, E.V.; Skryabin, O.O.; Khayrutdinov, M.M. Comprehensive Recovery of Metals in Tailings Utilization with Mechanochemical Activation. Resources 2023, 12, 113. https://doi.org/10.3390/resources12100113
Golik VI, Mitsik MF, Aleksakhina YV, Alenina EE, Ruban-Lazareva NV, Kruzhkova GV, Kondratyeva OA, Trushina EV, Skryabin OO, Khayrutdinov MM. Comprehensive Recovery of Metals in Tailings Utilization with Mechanochemical Activation. Resources. 2023; 12(10):113. https://doi.org/10.3390/resources12100113
Chicago/Turabian StyleGolik, Vladimir I., Mikhail F. Mitsik, Yulia V. Aleksakhina, Elena E. Alenina, Natalia V. Ruban-Lazareva, Galina V. Kruzhkova, Olga A. Kondratyeva, Ekaterina V. Trushina, Oleg O. Skryabin, and Marat M. Khayrutdinov. 2023. "Comprehensive Recovery of Metals in Tailings Utilization with Mechanochemical Activation" Resources 12, no. 10: 113. https://doi.org/10.3390/resources12100113
APA StyleGolik, V. I., Mitsik, M. F., Aleksakhina, Y. V., Alenina, E. E., Ruban-Lazareva, N. V., Kruzhkova, G. V., Kondratyeva, O. A., Trushina, E. V., Skryabin, O. O., & Khayrutdinov, M. M. (2023). Comprehensive Recovery of Metals in Tailings Utilization with Mechanochemical Activation. Resources, 12(10), 113. https://doi.org/10.3390/resources12100113









