Valorizations of Marigold Waste for High-Value Products and Their Industrial Importance: A Comprehensive Review
Abstract
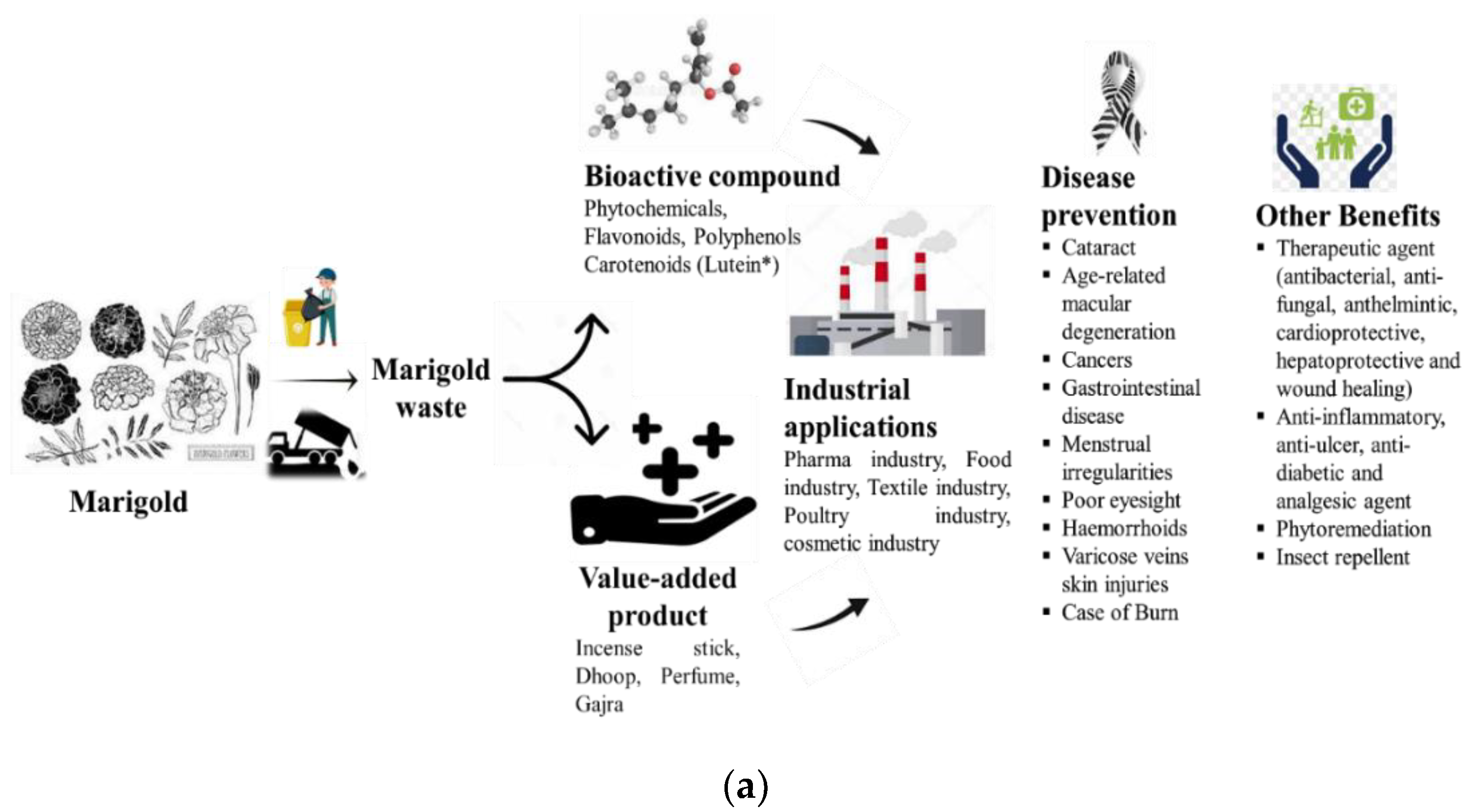
1. Introduction
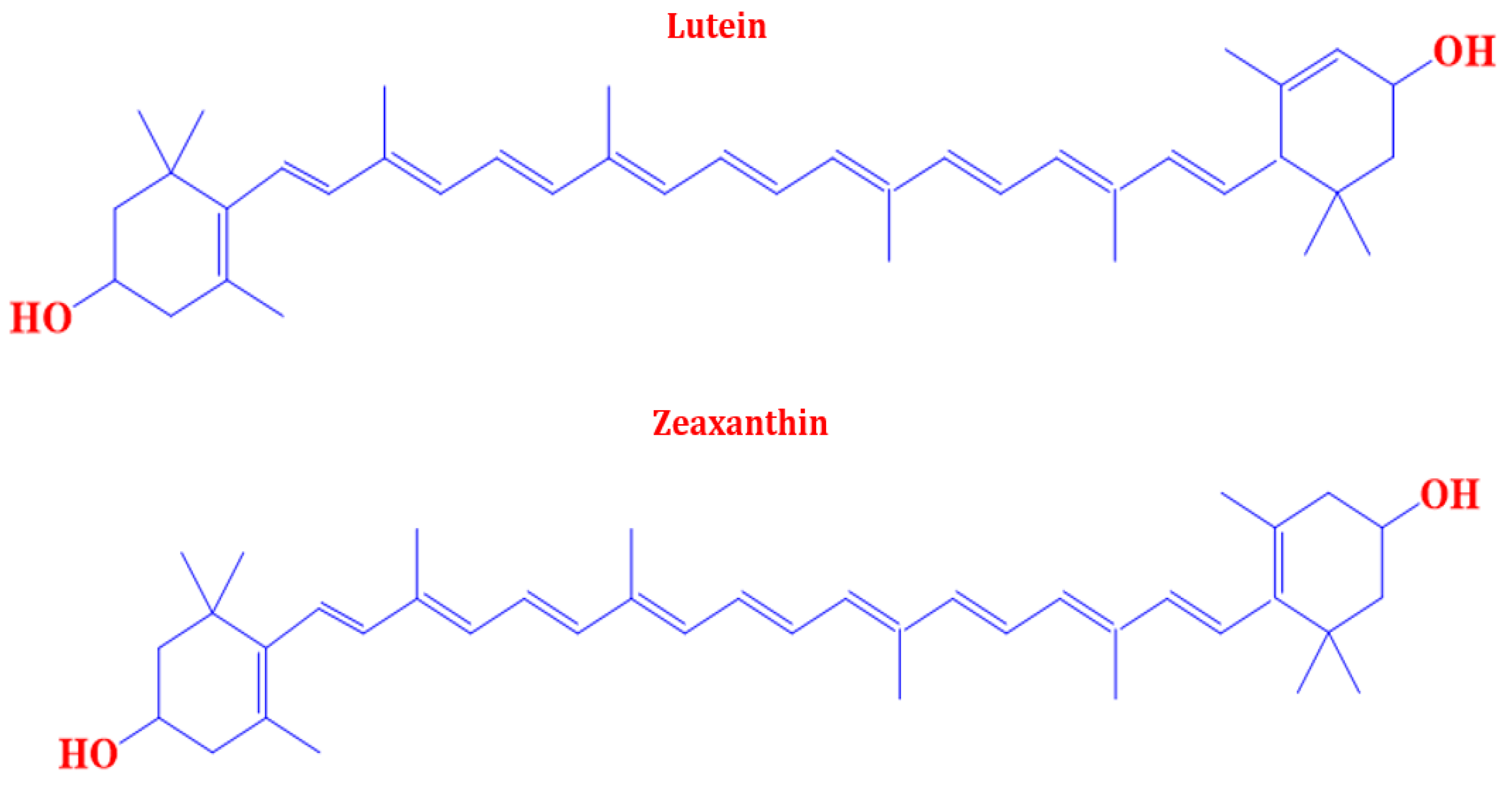
2. Marigold as a Major Source of Commercial Lutein
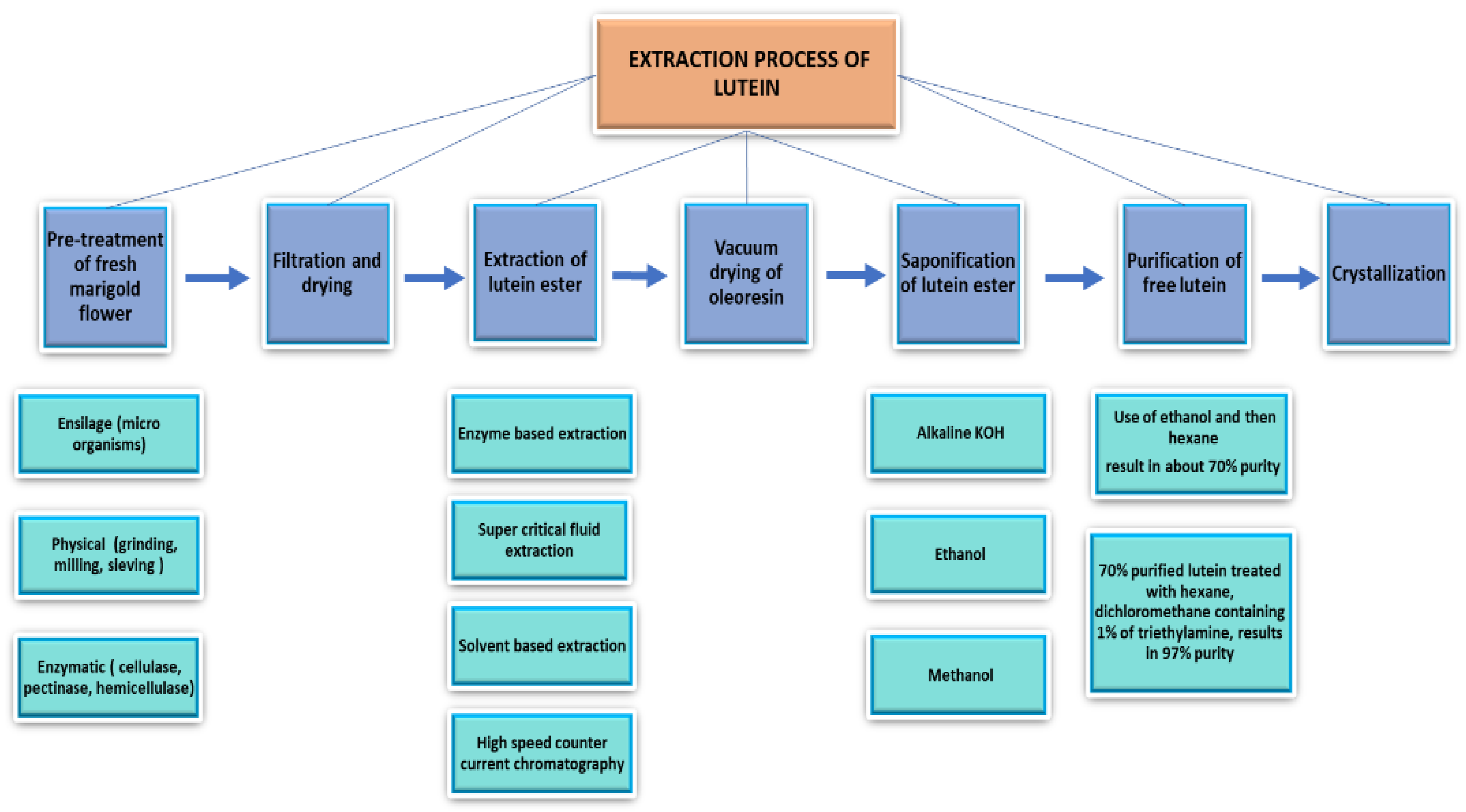
2.1. Methodology for Lutein Extraction
2.1.1. Pretreatment, Filtration, and Drying of Waste Marigold Flowers
2.1.2. Different Approaches for Extraction of Lutein Esters
- (a)
- Solvent-Based Extraction
- (b)
- Enzyme-Assisted Extraction
- (c)
- Super Critical Fluid Extraction
2.1.3. High-Speed Counter-Current Chromatography as a Lutein Separation Method
2.1.4. Saponification
3. Other Value-Added Products from Marigold and Their Roles
3.1. Incense Stick Production from Marigold Waste
3.2. Marigolds to Biogas
3.3. Marigold as a Therapeutic Agent
3.4. Marigold Essential Oil and Its Advantages
4. Ecological Significance of the Marigold Plant in Phytoremediation
5. Industrial Applications of Lutein
6. Economic Importance of Marigold
7. Future Prospective and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shivangi, S.C. Review Paper on-Ecofriendly Practice in Temple to Make Sustainable Approach toward Social and Environment. Int. J. Res. Sci. 2021, 6, 2024–2454. [Google Scholar] [CrossRef]
- Singh, P.; Borthakur, A.; Singh, R.; Awasthi, S.; Pal, D.B.; Srivastava, P.; Mishra, P.K. Utilization of temple floral waste for extraction of valuable products: A close loop approach towards environmental sustainability and waste management. Pollution 2017, 3, 39–45. [Google Scholar]
- Ashritha, D.; Rawat, V.; Lakshmi, V.; Devi, R.H.; Kumar, R.; Sah, S. Post harvesting and value addition in marigold. Pharma Innov. J. 2022, 11, 1295–1299. [Google Scholar]
- Saeed, S.T.; Samad, A. Emerging threats of begomoviruses to the cultivation of medicinal and aromatic crops and their management strategies. VirusDisease 2017, 28, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Casella, P.; Marino, T.; Iovine, A.; Larocca, V.; Balducchi, R.; Musmarra, D.; Molino, A. Optimization of lutein extraction from scenedesmus almeriensis using pressurized liquid extraction. Chem. Eng. Trans. 2021, 87, 475–480. [Google Scholar]
- Siriamornpun, S.; Kaisoon, O.; Meeso, N. Changes in colour, antioxidant activities and carotenoids (lycopene, β-carotene, lutein) of marigold flower (Tagetes erecta L.) resulting from different drying processes. J. Funct. Foods. 2012, 4, 757–766. [Google Scholar] [CrossRef]
- Boonnoun, P.; Tunyasitikun, P.; Clowutimon, W.; Shotipruk, A. Production of free lutein by simultaneous extraction and de-esterification of marigold flowers in liquefied dimethyl ether (DME)–KOH–EtOH mixture. Food Bioprod. Process. 2017, 106, 193–200. [Google Scholar] [CrossRef]
- Manzoor, S.; Rashid, R.; Panda, B.P.; Sharma, V.; Azhar, M. Green extraction of lutein from marigold flower petals, process optimization and its potential to improve the oxidative stability of sunflower oil. Ultrason Sonochem. 2022, 85, 105994. [Google Scholar] [CrossRef]
- Khachik, F.; Goli, M.B.; Beecher, G.R.; Holden, J.; Lusby, W.R.; Tenorio, M.D.; Barrera, M.R. Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetables. J. Agric. Food Chem. 1992, 40, 390–398. [Google Scholar] [CrossRef]
- Gupta, Y.C.; Panwar, S.; Banyal, N.; Thakur, N.; Dhiman, M.R. Marigold. In Floriculture and Ornamental Plants; Springer: Singapore, 2022; pp. 1–23. [Google Scholar]
- Fernández-Sevilla, J.M.; Fernández, F.A.; Grima, E.M. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Wu, D.; Wu, J.; Cheng, X.; Qian, J.; Du, R.; Tang, S.; Qiao, Y. Safety assessment of marigold flavonoids from marigold inflorescence residue. J. Ethnopharmacol. 2022, 297, 115520. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Vadrale, A.P.; Tseng, Y.S.; Chen, C.W.; Dong, C.D.; Singhania, R.R. Bioprospecting of marine microalgae from Kaohsiung Seacoast for lutein and lipid production. Bioresour. Technol. 2022, 351, 126928. [Google Scholar] [CrossRef] [PubMed]
- Low, K.L.; Idris, A.; Yusof, N.M. An optimized strategy for lutein production via microwave-assisted microalgae wet biomass extraction process. Process Biochem. 2022, 121, 87–99. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Kaushal, J.; Mahajan, P.; Kaur, N. A review on application of phytoremediation technique for eradication of synthetic dyes by using ornamental plants. Environ. Sci. Pollut. Res. 2021, 28, 67970–67989. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O. Phytoremediation: From theory toward practice. In Phytomanagement Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–49. [Google Scholar]
- Kumar, V.; Kumari, S.; Kumar, P. Management and sustainable energy production using flower waste generated from temples. Environ. Degrad. Causes Remediat. Strateg. 2020, 1, 154. [Google Scholar]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Ntan, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., II. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef]
- Bendich, A.; Olson, J.A. Biological actions of carotenoids. FASEB J. 1989, 3, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–203. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di, P.F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye heaoh. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Rabalski, I. Composition of lutein ester regioisomers in marigold flower, dietary supplement, and herbal tea. J. Agric. Food Inf. 2015, 63, 9740–9746. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Schlatterer, J. Lutein and zeaxanthin in new dietary supplements—Analysis and quantification. Eur. Food Res. Technol. 2005, 220, 648–652. [Google Scholar] [CrossRef]
- Tyczkowski, J.K.; Hamilton, P.B. Research Note: Preparation of Purified Lutein and Its Diesters from Extracts of Marigold (Tagetes erecta). Poult. Sci. J. 1991, 70, 651–654. [Google Scholar] [CrossRef]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Bioprocess. 2022, 9, 8. [Google Scholar] [CrossRef]
- Philip, T. Purification of Lutein-Fatty Acid Esters from Plant Materials. U.S. Patent 4,048,203, 13 September 1997. [Google Scholar]
- Khachik, F.; Beecher, G.R.; Smith, J.C., Jr. Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J. Cell. Biochem. 1995, 59, 236–246. [Google Scholar] [CrossRef]
- Ausich, R.L.; Sanders, D.J. Process for the Formation, Isolation and Purification of Comestible Xanthophyll Crystals from Plants. U.S. Patent 5,648,564, 15 July 1997. [Google Scholar]
- Levi, L.W. Trans-Xanthophyll Ester Concentrates of Enhanced Purity and Methods of Making Same. U.S. Patent 6,191,293 B1, 20 February 2001. [Google Scholar]
- Khachik, F. Process for Extraction and Purification of Lutein, Zeaxanthin and Rare Carotenoids from Marigold Flowers and Plants. U.S. Patent 6,262,284 B1, 17 July 2001. [Google Scholar]
- Mora-Pale, J.M.; Pérez-Munguía, S.; González-Mejía, J.C.; Dordick, J.S.; Bárzana, E. The lipase-catalyzed hydrolysis of lutein diesters in non-aqueous media is favored at extremely low water activities. Biotechnol. Bioeng. 2007, 98, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, B.K.; Kumar, A.; Nanda, A.K.; Chakraborty, R. A study on optimization of marigold petal yield, pure lutein, and formulation of free-flowing lutein esters. J. Crop. Sci. Biotechnol. 2014, 17, 175–181. [Google Scholar] [CrossRef]
- Jalali-Jivan, M.; Abbasi, S.; Scanlon, M.G. Microemulsion as nanoreactor for lutein extraction: Optimization for ultrasound pretreatment. J. Food Biochem. 2019, 43, e12929. [Google Scholar] [CrossRef] [PubMed]
- Hojnik, M.; Škerget, M.; Knez, Ž. Extraction of lutein from Marigold flower petals—Experimental kinetics and modelling. LWT-Food. Sci. Technol. 2008, 41, 2008–2016. [Google Scholar] [CrossRef]
- Pratheesh, V.B.; Benny, N.; Sujatha, C.H. Isolation, stabilization and characterization of xanthophyll from marigold flower-Tagetes erecta-L. Mod. Appl. Sci. 2009, 3, 19–28. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Purnima, K.T.; Florence, S.P.; Rao, A.A.; Srinivas, P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem. 2009, 113, 1234–1238. [Google Scholar] [CrossRef]
- Navarrete-Bolaños, J.L.; Jiménez-Islas, H.; Botello-Alvarez, E.; Rico-Martínez, R.; Paredes-López, O. Improving xanthophyll extraction from marigold flower using cellulolytic enzymes. J. Agric. Food Chem. 2004, 52, 3394–3398. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Surendranath, R.; Ganga, M.; Jawaharlal, M.; Anitha, K. Extraction and quantification of marigold lutein using different solvent systems. Culture 2016, 19, 24. [Google Scholar]
- Piccaglia, R.; Marotti, M.; Grandi, S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998, 8, 45–51. [Google Scholar] [CrossRef]
- Alotaibi, H.N.; Anderson, A.K.; Sidhu, J.S. Influence of lutein content of marigold flowers on functional properties of baked pan bread. Ann. Agric. Sci. 2021, 66, 162–168. [Google Scholar] [CrossRef]
- Munhoz, V.M.; Longhini, R.; Souza, J.R.; Zequi, J.A.; Mello, E.V.; Lopes, G.C.; Mello, J.C. Extraction of flavonoids from Tagetes patula: Process optimization and screening for biological activity. Rev. Bras. Farmacogn. 2014, 24, 576–583. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Nesterovitsch, J.; Maidla, K. Analysis of carotenoids, flavonoids and essential oil of Calendula officinalis cultivars growing in Estonia. Nat. Prod. Commun. 2016, 11, 831. [Google Scholar] [CrossRef]
- Vechpanich, J.; Shotipruk, A. Recovery of free lutein from Tagetes erecta: Determination of suitable saponification and crystallization conditions. Sep. Sci. Technol. 2010, 46, 265–271. [Google Scholar] [CrossRef]
- Palumpitag, W.; Prasitchoke, P.; Goto, M.; Shotipruk, A. Supercritical carbon dioxide extraction of marigold lutein fatty acid esters: Effects of cosolvents and saponification conditions. Sep. Sci. Technol. 2011, 46, 605–610. [Google Scholar] [CrossRef]
- Boonnoun, P.; Opaskonkun, T.; Prasitchoke, P.; Goto, M.; Shotipruk, A. Purification of free lutein from marigold flowers by liquid chromatography. Eng. J. 2012, 16, 145–156. [Google Scholar] [CrossRef][Green Version]
- Kashyap, P.K.; Singh, S.; Singh, M.K.; Gupta, A.; Tandon, S.; Shanker, K.; Verma, R.S. An efficient process for the extraction of lutein and chemical characterization of other organic volatiles from marigold (Tagetes erecta L.) flower. Food Chem. 2022, 396, 133647. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.M.; Yusuf, M.M.; Azmi, S.S.; Salim, K.P.; Prihastyanti, M.N.U.; Indrawati, R.; Brotosudarmo, T.H.P. Effect of drying treatments on the contents of lutein and zeaxanthin in orange-and yellow-cultivars of marigold flower and its application for lutein ester encapsulation. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 509, p. 012060. [Google Scholar]
- Yara-Varon, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high-pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Barzana, E.; Rubio, D.; Santamaria, R.I.; Garcia-Correa, O.; Garcia, F.; Ridaura, S.V.E.; López-Munguía, A. Enzyme-mediated solvent extraction of carotenoids from marigold flower (Tagetes erecta). J. Agric. Food Chem. 2002, 50, 4491–4496. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Paredes-López, O. Effects of enzymatic treatments of marigold flowers on lutein isomeric profiles. J. Agric. Food Chem. 1997, 45, 1097–1102. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids-a review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Gao, Y.; Nagy, B.; Liu, X.; Simándi, B.; Wang, Q. Supercritical CO2 extraction of lutein esters from marigold (Tagetes erecta L.) enhanced by ultrasound. J. Supercrit. Fluids 2009, 49, 345–350. [Google Scholar] [CrossRef]
- Aman, R.; Carle, R.; Conrad, J.; Beifuss, U.; Schieber, A. Isolation of carotenoids from plant materials and dietary supplements by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 99–105. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, T.; Xu, G.; Ito, Y. Application of CCC for the separation of lutein from a crude extract of marigold flower petals. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 1659–1669. [Google Scholar] [CrossRef]
- Sarkar, C.R.; Bhagawati, B.; Das, L.; Goswami, B.C. An efficient condition of saponification of lutein ester from marigold flower. Ann. Biol. Res. 2012, 3, 1461–1466. [Google Scholar]
- Armas, K.; Rojas, J.; Rojas, L.; Morales, A. Comparative study of the chemical composition of essential oils of five Tagetes species collected in Venezuela. Nat. Prod. Commun. 2012, 7, 932. [Google Scholar] [CrossRef]
- Jan, N.; Andrabi, K.I.; John, R. Calendula officinalis—An important medicinal plant with potential biological properties. Proc. Indian Natl. Sci. Acad. 2017, 83, 769–787. [Google Scholar]
- Chamorro, E.R.; Ballerini, G.; Sequeira, A.F.; Velasco, G.A.; Zalazar, M.F. Chemical composition of essential oil from Tagetes minuta L. leaves and flowers. J. Argent. Chem. Soc. 2008, 96, 80–86. [Google Scholar]
- Cruz Flores, O.; Espinoza Ruiz, M.; Santiesteban Hernández, A.; Cruz-López, L. Chemical characterization of the volatiles of Tagetes nelsonii. Polibotánica 2021, 203–211. [Google Scholar] [CrossRef]
- Ruiz, C.; Cachay, M.; Domínguez, M.; Velásquez, C.; Espinoza, G.; Ventosilla, P.; Rojas, R. Chemical composition, antioxidant and mosquito larvicidal activities of essential oils from Tagetes filifolia, Tagetes minuta and Tagetes elliptica from Perú. Planta Med. 2011, 77, PE30. [Google Scholar] [CrossRef]
- Bennurmath, P.; Bhatt, D.S.; Gurung, A.; Singh, A.; Bhatt, S.T. Novel green approaches towards utilization of flower waste: A review. Environ. Conserv. J. 2021, 22, 225–230. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Singh, A.K.; Bansal, R.P.; Pal, A.; Khare, P.; Sharma, R.S.; Kalra, A. Innovative technique for management of offered floral bio-resources for protecting the environment and generating additional livelihood opportunities for women. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Yadav, I.; Juneja, S.K.; Chauhan, S. Temple waste utilization and management: A review. Int. J. Eng. Sci. Technol. 2015, 2, 14–19. [Google Scholar]
- Saoji, R.Y.; Zalte, A.; Guleccha, V. Preparation of Incense Stick using Marigold floral waste from Nasik region. J. Pharm. Sci. Res. 2021, 13, 635–637. [Google Scholar]
- Sharma, D.; Yadav, K.D. Vermicomposting of flower waste: Optimization of maturity parameter by response surface methodology. Malays. J. Sustain. Agric. 2017, 1, 15–18. [Google Scholar] [CrossRef]
- Bisoyi, L.K.; Swain, R.; BhimaRao, R. Computer Applications to Assess City Municipal Solid Waste for Better Utilization: A Case Study on Internationally Recognized Pilgrimage City, Puri, Odisha, India. Int. J. Eng. Res. Technol. 2013, 2. [Google Scholar] [CrossRef]
- Ponkiya, N.; Desai, S.; Mistry, J.; Patel, S.; Ingalhalli, R. Development of economical mosquito repellent using marigold plant. Int. J. Res. Trends Innov. 2018, 3, 47–54. [Google Scholar]
- Jørgensen, P.J. Biogas-Green Energy; Faculty of Agricultural Sciences, Aarhus University: Aarhus, Denmark, 2009. [Google Scholar]
- Kulkarni, M.B.; Ghanegaonkar, P.M. Biogas generation from floral waste using different techniques. Glob. J. Environ. Sci. Manag. 2019, 5, 17–30. [Google Scholar]
- Poveda-Giraldo, J.A.; Alzate, C.C. A biorefinery for the valorization of marigold (Calendula officinalis) residues to produce biogas and phenolic compounds. Food Bioprod. Process. 2021, 125, 91–104. [Google Scholar] [CrossRef]
- Scano, E.A.; Asquer, C.; Pistis, A.; Ortu, L.; Demontis, V.; Cocco, D. Biogas from anaerobic digestion of fruit and vegetable wastes: Experimental results on pilot-scale and preliminary performance evaluation of a full-scale power plant. Energy Convers. Manag. 2014, 7, 22–30. [Google Scholar] [CrossRef]
- Yazdani, R.; Barlaz, M.A.; Augenstein, D.; Kayhanian, M.; Tchobanoglous, G. Performance evaluation of an anaerobic/aerobic landfill-based digester using yard waste for energy and compost production. J. Waste Manag. 2012, 32, 912–919. [Google Scholar] [CrossRef]
- Jin, G.; Bierma, T.; Walker, P.M. Low-heat, mild alkaline pretreatment of switchgrass for anaerobic digestion. J. Environ. Sci. Health A 2014, 49, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Birhan, Y.S.; Kitaw, S.L.; Alemayehu, Y.A.; Mengesha, N.M. Medicinal plants with traditional healthcare importance to manage human and livestock ailments in Enemay District, Amhara Region, Ethiopia. Acta Ecol. Sin. 2022. [Google Scholar] [CrossRef]
- Faizal, S.K.; Sheela, R.R.R.; Nilayangode, P. Status of plant bioresources utilised in herbal industries and the need for conservation in kerala. Int. J. Conserv. Sci. 2022, 13, 267–278. [Google Scholar]
- Sharma, S.; Kumari, K. An overview on Calendula Officinalis Linn.: (Pot Marigold). J. Adv. Sci. Res. 2021, 12 (Suppl. S2), 13–18. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Antioxidant properties of marigold extracts. Int. Food Res. J. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Ashwlayan, V.D.; Kumar, A.; Verma, M. Therapeutic potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar] [CrossRef]
- Sultana, A.; Hasan, M.; Rahman, M.; Alam, M.M. Healing potentials of Marigold flower (Tagetes erecta) on full thickness dermal wound in caprine model. Eur. J. Sci. Res. 2021, 7, 332–339. [Google Scholar] [CrossRef]
- Patil, K.; Sanjay, C.; Doggalli, N.; Devi, K.R.; Harshitha, N. A Review of Calendula Officinalis—Magic in Science. J. Clin. Diagn. Res. 2022, 16. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Chader, H.; Houche, A.; Oudjida, F.; Benkebaili, F.; Hakim, Y. Topical Emulsion containing lavandula stoechas essential oil as a therapeutic agent for cutaneous wound healing. J 2021, 4, 288–307. [Google Scholar] [CrossRef]
- Sharma, M.; Gargi, A.; Borah, A. Rhododendron arboreum and its potential health benefit: A review. Pharma Innov. J. 2022, 11, 926–933. [Google Scholar]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, R.D.; Kumar, A.; Singh, S.; Singh, S. Understanding the Effect of Different Abiotic Stresses on Wild Marigold (Tagetes minuta L.) and Role of Breeding Strategies for Developing Tolerant Lines. Front. Plant Sci. 2022, 12, 754457. [Google Scholar] [CrossRef] [PubMed]
- Taban, A.; Rastegar, S.; Nasirzadeh, M.; Saharkhiz, M.J. Essential oil composition and comparative phytotoxic activity of fennel, summer savory, Mexican marigold and feverfew: A potential bioherbicide. Vegetos 2022, 35, 502–510. [Google Scholar] [CrossRef]
- Arora, K.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Allelopathic potential of the essential oil of wild marigold (Tagetes minuta L.) against some invasive weeds. J. Agric. Environ. Sci. 2015, 3, 56–60. [Google Scholar]
- Chatterjee, S.; Singh, L.; Chattopadhyay, B.; Datta, S.; Mukhopadhyay, S.K. A study on the waste metal remediation using floriculture at East Calcutta Wetlands, a Ramsar site in India. Environ. Monit. Assess. 2012, 184, 5139–5150. [Google Scholar] [CrossRef]
- Coelho, L.C.; Bastos, A.R.R.; Pinho, P.J.; Souza, G.A.; Carvalho, J.G.; Coelho, V.A.T.; Faquin, V. Marigold (Tagetes erecta): The potential value in the phytoremediation of chromium. Pedosphere 2017, 27, 559–568. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Islam, M.S.; Ahmed, Z.U.; Nayar, F. Phytoremediation of heavy metal contaminated buriganga riverbed sediment by Indian mustard and marigold plants. Environ. Prog. Sustain. Energy 2016, 35, 117–124. [Google Scholar] [CrossRef]
- Sun, R.; Sun, Q.; Wang, R.; Cao, L. Cadmium accumulation and main rhizosphere characteristics of seven French marigold (Tagetes patula L.) cultivars. Int. J. Phytoremediat. 2018, 20, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Singh, S.K.; Patra, A.; Mohapatra, K.K. Evaluation of phytoremediation capability of French marigold (Tagetes patula) and African marigold (Tagetes erecta) under heavy metals contaminated soils. Int. J. Phytoremediat. 2022, 24, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Sathya, V.; Mahimairaja, S.; Bharani, A.; Krishnaveni, A. Influence of soil bioamendments on the Availabilty of nickel and Phytoextraction capability of Marigold from the contaminated soil. Int. J. Plant Soil Sci. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Zaman, Q.; Anwar, S.; Mehmood, F.; Nawaz, R.; Masood, N.; Nazir, A.; Iqbal, M.; Nazir, S.; Sultan, K. Experimental modeling, optimization and comparison of coagulants for removal of metallic pollutants from wastewater. Z. Für Phys. Chem. 2021, 235, 1041–1053. [Google Scholar] [CrossRef]
- Madanan, M.T.; Shah, I.K.; Varghese, G.K.; Kaushal, R.K. Application of Aztec Marigold (Tagetes erecta L.) for phytoremediation of heavy metal polluted lateritic soil. J. Environ. Chem. Ecotoxicol. 2021, 3, 17–22. [Google Scholar] [CrossRef]
- Asgari, L.B.; Khadem, M.N.; Maghsoodi, M.R.; Ghorbanpour, M.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484. [Google Scholar] [CrossRef]
- Fuad, N.I.N.; Sekar, M.; Gan, S.H.; Lum, P.T.; Vaijanathappa, J.; Ravi, S. Lutein: A Comprehensive Review on its Chemical, Biological Activities and Therapeutic Potentials. Pharmacogn. J. 2020, 12, 1769–1778. [Google Scholar] [CrossRef]
- Karmakar, A.; Das, A.K.; Ghosh, S.; Sil, P.C. Carotenoids as Coloring Agents. In Carotenoids: Structure and Function in the Human Body; Springer: Cham, Switzerland, 2021; pp. 189–207. [Google Scholar]
- Titcomb, T.J.; Kaeppler, M.S.; Cook, M.E.; Simon, P.W.; Tanumihardjo, S.A. Carrot leaves improve color and xanthophyll content of egg yolk in laying hens but are not as effective as commercially available marigold fortificant. Poult. Sci. J. 2019, 98, 5208–5213. [Google Scholar] [CrossRef]
- Meurer, M.; de Oliveira, B.M.; Cury, B.J.; Jerônimo, D.T.; Venzon, L.; França, T.C.; da Silva, L. Extract of Tagetes erecta L., a medicinal plant rich in lutein, promotes gastric healing and reduces ulcer recurrence in rodents. J. Ethnopharmacol. 2022, 293, 115258. [Google Scholar] [CrossRef]
- Bahadirli, N.P. Essential Oil Content and Compositions of Naturalized Tagetes minuta L. (Wild marigold). Nat. Volatiles Essent. Oils 2020, 7, 17–21. [Google Scholar] [CrossRef]
- Baig, U.; Khatri, A.; Ali, S.; Sanbhal, N.; Ishaque, F.; Junejo, N. Ultrasound-assisted dyeing of cotton fabric with natural dye extracted from marigold flower. J. Text. Inst. 2021, 112, 801–808. [Google Scholar] [CrossRef]
- Anonymous. Vision 2050; Directorate of Floriculture, ICAR: Maharashtra, India, 2015. [Google Scholar]
- Misra, D.; Ghosh, S. Growth and export status of Indian floriculture: A review. Agric. Rev. 2016, 37, 77–80. [Google Scholar] [CrossRef][Green Version]
- Agricultural and Processed Food Products Export Development Authority (APEDA). 2017. Available online: www.apeda.gov.in (accessed on 16 May 2017).
- Annual Report. ICAI-DFR 2018–19. Available online: https://dfr.icar.gov.in/Content/Pdf/DFR-Annula-Report2018–19.pdf (accessed on 20 August 2022).
- Malik, M.; Kumar, T.; Jawla, S.K.; Sahrawat, A. Economic analysis of marigold production under the different applications of organic manures. J. Pharm. Innov. 2021, 10, 155–157. [Google Scholar] [CrossRef]
- BCC Research. The Global Market for Carotenoids 2018. Available online: https://cdn2.hubspot.net/hubfs/308401/FODReportOverviews/FOD025FReportOverview.pdf?t=1540560162021&utm_campaign=FOD025F&utm_source=hs_automation&utm_medium=email&utm_content=62915556&_hsenc=p2ANqtz-_OztnyxUYCQnEJDkpVyJjPscS_yIrM-Vmjts9TCJijoR2hCgyN9-H (accessed on 28 October 2018).
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 1678. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, C.H.; Ng, I.S.; Chang, J.S. Enhancing lutein production with mixotrophic cultivation of Chlorella sorokiniana MB-1-M12 using different bioprocess operation strategies. Bioresour. Technol. 2019, 278, 17–25. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine microalgae for potential lutein production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]

| Marigold | Extraction Method | Organic Solvent Used | Recovery/Efficiency | References |
|---|---|---|---|---|
| Tagetes patula | Solvent-based extraction | Acetone | 78.26 ± 0.66 ppm lutein | [47] |
| Tagetes patula | Solvent-based extraction | Acetone, ethanol, and water | 25.13 ± 1.02 g/100 g flavonoids | [48] |
| Calendula officinalis | Solvent-based extraction | n- hexane and acetone | 0.7 to 2.7% total carotenoids | [49] |
| Calendula officinalis L. | Solvent-based extraction | n- hexane and acetone | 0.8% to 1.7% flavonoids | [49] |
| Tagetes erecta | Solvent-based extraction | Hexane | 99.12% free lutein | [50] |
| Tagetes erecta | Supercritical carbon dioxide extraction method | Hexane, palm oil, and CO2 | 157.2 ± 4.4 mg free lutein/g oleoresin | [51] |
| Tagetes erecta | Solvent-based extraction | Hexane | 65% free lutein | [52] |
| Tagetes erecta | Green method | 2-methyltetrahydrofuran | 97.64% | [53] |
| Tagetes erecta | Dimethyl ether extraction | Liquefied dimethyl ether | 20.71 mg/g | [7] |
| Tagetes erecta | Solvent-based extraction | n-hexane, acetone, and ethanol | 8.95 mg/g (dw) lutein | [54] |
| Tagetes erecta | Solvent-based extraction | n-hexane, acetone, and ethanol | 14.55 mg/g (dw) zeaxanthin | [54] |
| Marigold Species | Chemical/Flavonoid Composition | Value-Added Product | Reference |
|---|---|---|---|
| Tagetes patula | piperitone, trans-β-ocimene, terpinolene, and β-caryophyllene | Essential oil | [65] |
| Calendula officinalis | lupeol, taraxasterol, erythrodiol, calenduloside, quercetin, isorhamnetin, cubenol, α-casino, and oplopanonec | Medicines (treatment for inflammation of the skin and wound healing) | [66] |
| Tagetes minuta L. | β-phelandrene, limonene, β-ocimene, dihydrotagetone, tagetone, and tagetenone | Essential oil | [67] |
| Tagetes erecta | β-caryophyllene, limonene, methyleugenol, E-ocimene, piperetone, piperitenone, and terpinolene | Essential oil | [65] |
| Tagetes nelsonii Greenm. | α and β-pinene, trans-β-ocimene, limonene, linalool, (E) and (Z)-tagetones, dihydrotagetone, and cis- and trans-tagetenone | Perfume and incense sticks for pest control | [68] |
| Tagetes terniflora | cis-tagetone and cis-ocimene | Essential oil | [65] |
| Tagetes laxa | trans-tagetenone, cis-tagetenone, cis-β-ocimene, and trans-β-ocimene | Essential oil | [65] |
| Tagetes filifolia | trans-ocimenone, cis-tagetone, and cis-ocimenone | Native teas of Mexico and insect repellent | [17,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, A.S.; Chen, C.-W.; Singhania, R.R.; Tiwari, M.; Sartale, R.G.; Dong, C.-D.; Patel, A.K. Valorizations of Marigold Waste for High-Value Products and Their Industrial Importance: A Comprehensive Review. Resources 2022, 11, 91. https://doi.org/10.3390/resources11100091
Chauhan AS, Chen C-W, Singhania RR, Tiwari M, Sartale RG, Dong C-D, Patel AK. Valorizations of Marigold Waste for High-Value Products and Their Industrial Importance: A Comprehensive Review. Resources. 2022; 11(10):91. https://doi.org/10.3390/resources11100091
Chicago/Turabian StyleChauhan, Ajeet Singh, Chiu-Wen Chen, Reeta Rani Singhania, Mansi Tiwari, Rijuta Ganesh Sartale, Cheng-Di Dong, and Anil Kumar Patel. 2022. "Valorizations of Marigold Waste for High-Value Products and Their Industrial Importance: A Comprehensive Review" Resources 11, no. 10: 91. https://doi.org/10.3390/resources11100091
APA StyleChauhan, A. S., Chen, C.-W., Singhania, R. R., Tiwari, M., Sartale, R. G., Dong, C.-D., & Patel, A. K. (2022). Valorizations of Marigold Waste for High-Value Products and Their Industrial Importance: A Comprehensive Review. Resources, 11(10), 91. https://doi.org/10.3390/resources11100091









