Micro and Macroelements in Honey and Atmospheric Pollution (NW and Central Poland)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Contamination of Air (PM and HMs)
2.3. Honey Samples
2.4. Selected Predictors of Climatic Conditions
2.5. Chemical Studies of Elements
2.6. Dataset on MMs Concentration in Honey Samples from EU Countries (Metadata)
2.7. Statistical Analysis
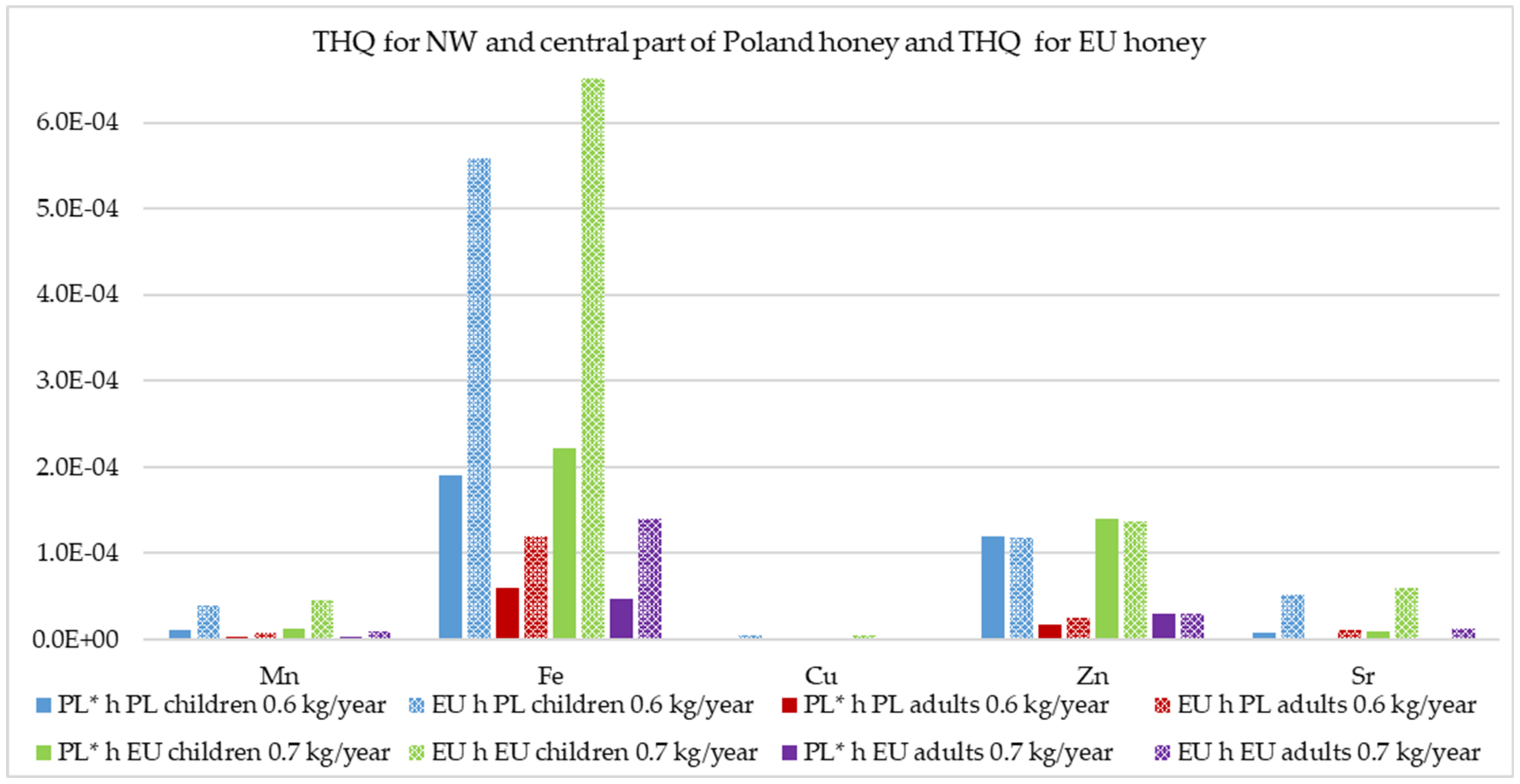
2.8. Non-Carcinogenic Risk Assessment
- C: the concentration of MMs in honey was according to mg/kg dry weight (DW). Hence, their unit was converted to mg/kg-wet weight via the following equation:where
- WW: concentration according to wet weight; DW: concentration according to dry weight and % moisture (M) is content in honey, i.e., 17.9% [28].
- EF: exposure frequency is 350 days/year;
- ED: exposure duration is 6 years for children and 30 years for adults;
- BW: body weight is 15 kg for children and 70 kg for adults;
- AT (days), mean lifetime: AT for estimation non-carcinogenic risk in the children and adults is 2190 and 10,950 days, respectively.
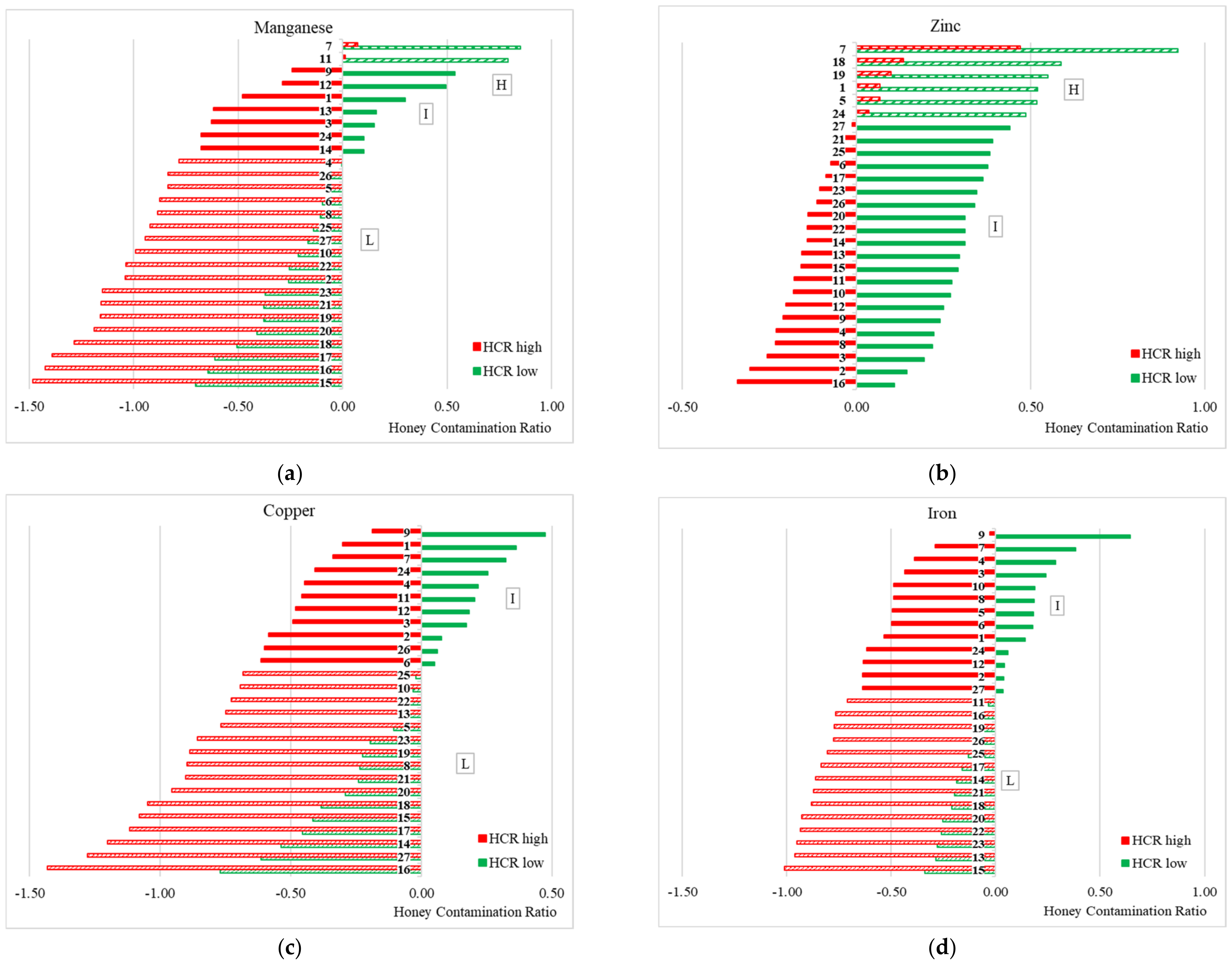
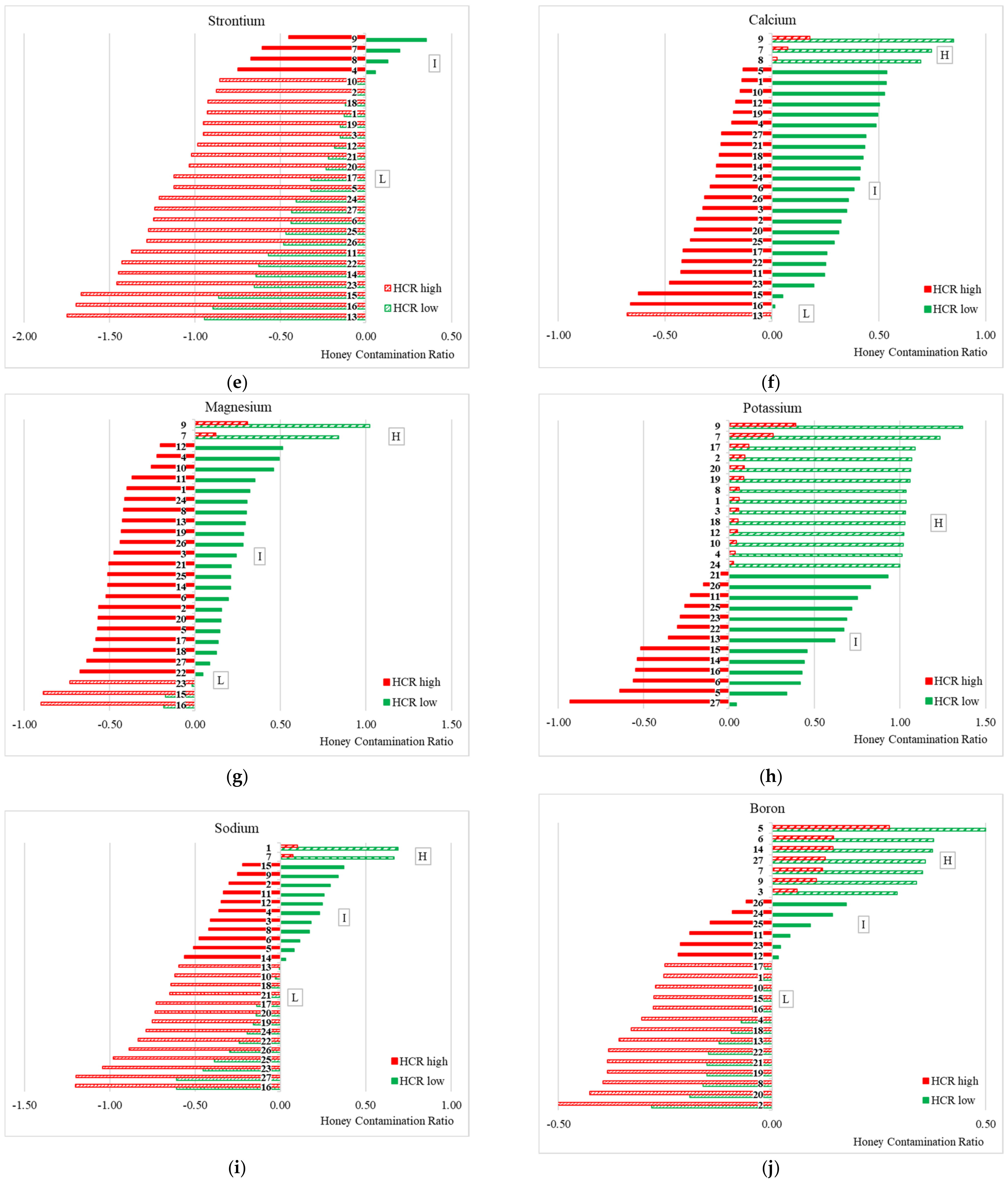
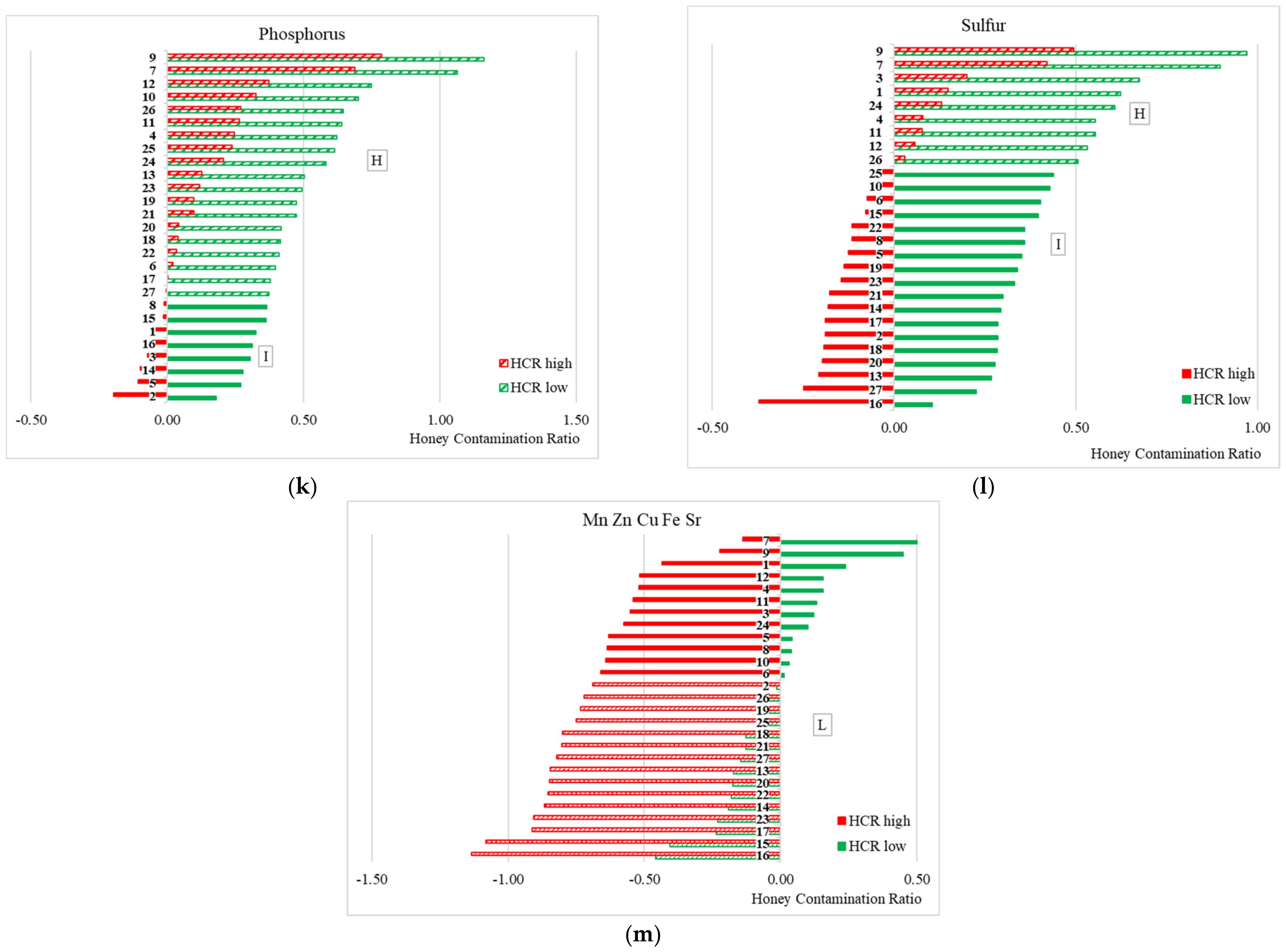
2.9. Honey Contamination Ratio
- H, a high contamination level when HCRhigh is positive;
- L, a low contamination level when HCRlow is negative;
- I, an intermediate contamination level when HCRhigh is negative and HCRlow is positive.
| MMs | Mn | Zn | Cu | Fe | Sr | Mg | Ca | K | Na | B | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 2.63 | 3.73 | 0.99 | 7.60 | 0.81 | 56.42 | 108.62 | 888.21 | 42.91 | 7.63 | 49.96 | 29.39 |
| I quartile | 0.53 | 1.53 | 0.28 | 2.20 | 0.21 | 10.40 | 28.20 | 150.30 | 12.10 | 4.55 | 27.80 | 14.00 |
| III quartile | 3.20 | 4.33 | 1.27 | 10.40 | 1.35 | 54.15 | 133.00 | 1421.00 | 47.12 | 7.80 | 66.10 | 41.88 |
3. Results
3.1. MMs Bioaccumulation in the Analysed Honey Samples
3.2. Honey Contamination Ratio (HCR) Analysis
4. Discussion
4.1. Atmospheric Pollution with Particulate Matter and Their Constituent As, Pb, Ni, Cd in the Analysed Agglomerations
4.2. Comparative Assessment of MM Concentrations in Rural and Urban Honeys from NW and Central Poland
4.3. The Assessment of MMs Concentration in Honey Samples from NW and Central Poland and Other EU Countries
4.4. Non-Carcinogenic Risk Assessment of the Studied Honey Samples Given the Different Consumption Levels in Poland and Other EU Countries
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. Available online: https://www.researchgate.net/publication/23803275 (accessed on 15 August 2020). [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Kapusta, I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of Polish buckwheat honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Herawati, R.; Widiarko, O.P.; Permata, F.S. Preventive potency of sumbawa forest honey on rats exposed by lead acetate based on liver histopathology and AST-ALT level. J. Phys. Conf. Ser. 2020, 1430, 012030. [Google Scholar] [CrossRef]
- Mračević, S.Đ.; Krstić, M.; Lolić, A.; Ražić, S. Comparative study of the chemical composition and biological potential of honey from different regions of Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. Treating severe wounds in pediatrics with medical grade honey: A case series. Clin. Case Rep. 2020, 8, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Jin, R.; Verpoorte, R.; Lam, W.; Chengc, Y.-C.; Xiao, Y.; Xu, J.; Zhang, L.; Qin, X.-M.; Chen, S. Natural deep eutectic characteristics of honey improve the bioactivity and safety of traditional medicines. J. Ethnopharmacol. 2020, 250, 112460. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Azizi-Soleiman, F. Honey and glycemic control: A systematic review. PharmaNutrition 2020, 11, 100–180. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Almaramhy, H.H.; Aljehani, Y.T.; Okashah, A.M.; El-Anzi, M.E.; Al-Harbi, M.B.; El-Tahlawi, R.; Nabo, M.M.H.; Aboonq, M.S.; Hamouda, O.; et al. The evidenced-based Taib UVID nutritional treatment for minimizing COVID-19 fatalities and morbidity and eradicating COVID-19 pandemic: A novel approach for better outcomes (a treatment protocol). Am. J. Public Health 2020, 8, 54–60. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Matin, G.; Kargar, N.; Buyukisik, H.B. Biomonitoring of cadmium, lead, arsenic and mercury in industrial districts of Izmir, Turkey by using honey bees, propolis and pine tree leaves. Ecol. Eng. 2016, 90, 331–335. [Google Scholar] [CrossRef]
- Goretti, E.; Pallottini, M.; Rossi, R.; La Porta, G.; Gardi, T.; Cenci Goga, B.T.; Elia, A.C.; Galletti, M.; Moroni, B.; Petroselli, C.; et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ. Pollut. 2020, 256, 113388. [Google Scholar] [CrossRef]
- Hodel, K.V.S.; Machado, B.A.S.; Santos, N.R.; Costa, R.G.; Menezes-Filho, J.A.; Umsza-Guez, M.A. Metal content of nutritional and toxic value in different types of brazilian propolis. Sci. World J. 2020, 2020, 4395496. [Google Scholar] [CrossRef]
- Bibi, S.; Husain, S.Z.; Malik, R.N. Pollen analysis and heavy metals detection in honey samples from seven selected countries. Pak. J. Bot. 2008, 40, 507–516. [Google Scholar]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Drouza, C.; Papastephanou, C. Characterization and botanical differentiation of monofloral and multifloral honeys produced in Cyprus, Greece, and Egypt using physicochemical parameter analysis and mineral content in conjunction with supervised statistical techniques. J. Anal. Methods Chem. 2018, 2018, 7698251. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Asmaryan, S.; Hovsepyan, A.; Merendino, N. Carcinogenic and non-carcinogenic risk assessment of trace elements and POPs in honey from Shirak and Syunik regions of Armenia. Chemosphere 2020, 239, 124809. [Google Scholar] [CrossRef]
- Kara, M. Assessment of sources and pollution state of trace and toxic elements in street dust in a metropolitan city. Environ. Geochem. Health 2020, 42, 3213–3229. [Google Scholar] [CrossRef] [PubMed]
- Loppi, S.; Corsini, A.; Paoli, L. Estimating environmental contamination and element deposition at an urban area of central Italy. Urban Sci. 2019, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Biba, O.; Hedbavny, J. Content of metals and metabolites in honey originated from the vicinity of industrial town Košice (eastern Slovakia). Environ. Sci. Pollut. Res. Int. 2016, 23, 4531–4540. [Google Scholar] [CrossRef]
- Geslin, B.; Gauzens, B.; Baude, M.; Dajoz, I.; Fontaine, C.; Henry, M.; Ropars, L.; Rollin, O.; Thébault, E.; Vereecken, N.J. Massively introduced managed species and their consequences for plant–pollinator interactions. Adv. Ecol. Res. 2017, 57, 1–53. [Google Scholar] [CrossRef]
- Stange, E.; Zulian, G.; Rusch, G.; Barton, D.; Nowell, M. Ecosystem services mapping for municipal policy: ESTIMAP and zoning for urban beekeeping. One Ecosyst. 2017, 2, e14014. [Google Scholar] [CrossRef] [Green Version]
- Sponsler, D.B.; Bratman, E.Z. Beekeeping in, of, or for the city? A socioecological perspective on urban apiculture. People Nat. 2021, 3, 550–559. [Google Scholar] [CrossRef]
- Lecocq, A.; Kryger, P.; Vejsnæs, F.; Bruun-Jensen, A. Weight watching and the effect of landscape on honeybee colony productivity: Investigating the value of colony weight monitoring for the beekeeping industry. PLoS ONE 2015, 10, e0132473. [Google Scholar] [CrossRef] [PubMed]
- Pouilloux, L. Urban Honey Beekeeping Using Hive-Pollen to Identify Urban Foraging Sites and Preferred Vegetation Communities in Japan. Master’s Thesis, Université de Liège, Liège, Belgium, 2019. Available online: http://hdl.handle.net/2268.2/7769 (accessed on 27 February 2020).
- Peterson Roest, B. Bees in the d: A message of conservation from an urban environment. Challenges 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Madras-Majewska, B.; Jasiński, Z.; Zajdel, B.; Gąbka, J.; Ochnio, M.; Petryka, W.; Kamiński, Z.; Ścięgosz, J. Content of selected toxic elements in bee products. Breed Rev. 2014, 3, 49–51. Available online: http://ph.ptz.icm.edu.pl/wp-content/uploads/2016/12/23-Madras-Majewska.pdf (accessed on 24 April 2020). (In Polish).
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- Fakhri, Y.; Abtah, M.; Atamaleki, A.; Raoofi, A.; Atabati, H.; Asadi, A.; Miri, A.; Shamloo, E.; Alinejad, A.; Keramati, H.; et al. The concentration of potentially toxic elements (PTEs) in honey: A global systematic review and meta-analysis and risk assessment. Trends Food Sci. Technol. 2019, 91, 498–506. [Google Scholar] [CrossRef]
- Bartha, S.; Taut, I.; Goji, G.; Vlad, J.A.; Dinulică, F. Heavy metal content in polyfloralhoney and potential health risk. a case study of Copșa Mică, Romania. Int. J. Environ. Res. Public Health 2020, 17, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inspectorate of Environmental Protection (IEP). Annual Air Quality Assessment in the Mazowieckie Voivodeship, Voivodeship Report for 2018; Chief Inspectorate of Environmental Protection, Department of Monitoring and Information on Environment: Warsaw, Poland, 2019; pp. 1–166. Available online: http://powietrze.gios.gov.pl/pjp/publications/card/14050 (accessed on 15 April 2020). (In Polish)
- Inspectorate of Environmental Protection (IEP). Annual Air Quality Assessment in Lubuskie Voivodeship, Voivodeship Report for 2018; Chief Inspectorate of Environmental Protection, Department of Monitoring and Information on Environment: Zielona Góra, Poland, 2019; pp. 1–108. Available online: http://www.zgora.pios.gov.pl/wp-content/uploads/2019/05/RAPORT_OR_2018_LUBUSKIE-ostat.pdf (accessed on 8 June 2020). (In Polish)
- Inspectorate of Environmental Protection (IEP). Annual Air Quality Assessment in the West Pomeranian Voivodeship, Voivodeship Report for 2018; Chief Inspectorate of Environmental Protection, Department of Monitoring and Information on Environment: Szczecin, Poland, 2019; pp. 1–94. Available online: http://powietrze.gios.gov.pl/pjp/publications/card/14064 (accessed on 6 May 2020). (In Polish)
- Jabłoński, B.; Kołtowski, Z.; Marcinkowski, J.; Rybak-Chmielewska, H.; Szczęsna, T.; Warakomska, Z. The content of heavy metals [Pb, Cd, and Cu] in nectar, honey and pollen from plants growing along communication routes. Apic. Sci. Exerc. Books 1995, 39, 129–144. Available online: http://miesiecznik-pszczelarstwo.pl/pzn/sites/default/files/pzn1995_129-144_0.pdf (accessed on 28 September 2020). (In Polish).
- Sustainable Development in the European Union (SDEU). Monitoring Report on Progress towards the SDGs in an EU Context, 2019 ed.; Publications Office of the European Union: Luxembourg, 2019; pp. 1–372. Available online: https://ec.europa.eu/eurostat/documents/3217494/9940483/KS-02-19-165-EN-N.pdf/1965d8f5-4532-49f9-98ca-5334b0652820 (accessed on 10 April 2020). [CrossRef]
- Lawson, D.; Rands, S. The effects of rainfall on plant-pollinator interactions. Arthropod-Plant Interact. 2019, 13, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Semkiw, P.; Skubida, P.; Jeziorski, K.; Pioś, A. Support for the Beekeeping Sector in Poland. The Apiculture Division in Puławy, 2018, 1–12. Available online: http://www.inhort.pl/files/program_wieloletni/PW_2015_2020_IO/spr_2018/Semkiw_2018_Sektor_pszczelarski_zadanie_4.3.pdf (accessed on 1 August 2020). (In Polish).
- Popp, J.; Kiss, A.; Oláh, J.; Máté, D.; Bai, A.; Lakner, Z. Network analysis for the improvement of food safety in the international honey. Amfiteatru Econ. 2018, 20, 84–98. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency (USEPA). United Quantitative Risk Assessment Calculations. 2015. Available online: https://www.epa.gov/sites/production/files/2015-05/documents/13.pdf (accessed on 8 December 2019).
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and classification of commercial thyme honeys produced in specific Mediterranean countries according to geographical origin, us-ing physicochemical parameter values and mineral content in combination with chemometrics. Eur. Food Res. Technol. 2016, 243, 889–900. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Air Quality in Europe 2019, Report No 10/2019; European Environment Agency, Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- European Environment Agency (EEA). Report European Union Emission Inventory Report 1990–2018 under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP), No 05/2020; European Environment Agency, Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Central Statistical Office (CSO). Environment 2018: Warsaw; Central Statistical Office, Statistical Publishing Establishment: Warsaw, Poland, 2018.
- Cincinelli, A.; Guerranti, C.; Martellini, T.; Scodellini, R. Residential wood combustion and its impact on urban air quality in Europe. Curr. Opin. Environ. Sci. Health 2019, 8, 10–14. [Google Scholar] [CrossRef]
- REPORT REP-0641, Austria’s Informative Inventory Report (IIR) 2018, Submission Under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP) and Directive (EU) 2016/2284 on the Reduction of National Emissions of Certain Atmospheric Pollutants: Vienna. Umweltbundesamt GmbH: Vienna, Austria, 2018; p. 485. ISBN 978-3-99004-459-9.
- Czarnowska, K.; Kozanecka, T. Soluble forms of heavy metals in anthropogenic soils of Warsaw area. Soil Sci. Ann. 2001, 52, 45–51. Available online: http://ssa.ptg.sggw.pl/files/artykuly/2001_52/2001_tom_52_nr_3-4/tom_52_nr_3-4_45-51.pdf (accessed on 19 June 2020). (In Polish).
- Niedźwiecki, E.; Protasowicki, M.; Wojcieszczuk, T.; Sammel, A.; Dembińska, K.; Jaruta, G. Morphological features and chemical composition of soils in northwestern part of Szczecin. Probl. J. Adv. Agric. Sci. 2009, 542, 797–808. (In Polish) [Google Scholar]
- Bilandžić, N.; Tlak-Gajger, I.; Kosanović, M.; Čalopek, B.; Sedak, M.; Kolanović, B.S.; Varenina, I.; Luburić, D.B.; Varga, I.; Đokić, M. Essential and toxic element concentrations in monofloral honeys from southern Croatia. Food Chem. 2017, 234, 245–253. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Iijima, A.; Cao, J. Hazard Quotients, Hazard Indexes, and Cancer Risks of Toxic Metals in PM10 during Firework Displays. Atmosphere 2018, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Wu, Z.; Han, P.; Taira, Y.; Wang, H.; Meng, Q.; Feng, Z.; Zhai, S.; Yu, J.; Zhu, W.; et al. Chemical content and source apportionment of 36 heavy metal analysis and health risk assessment in aerosol of Beijing. Environ. Sci. Pollut. Res. Int. 2019, 27, 7005–7014. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Chao, S.; Cao, H.; Zhang, A. Levels and health risks of PM2.5-bound toxic metals from firework/firecracker burning during festival periods in response to management strategies. Ecotoxicol. Environ. Saf. 2019, 171, 406–413. [Google Scholar] [CrossRef]
- Hickey, C.; Gordon, C.; Galdanes, K.; Blaustein, M.; Horton, L.; Chillrud, S.; Ross, J.; Yinon, L.; Chen, L.C.; Gordon, T. Toxicity of particles emitted by fireworks. Part. Fibre Toxicol. 2020, 17, 17–28. [Google Scholar] [CrossRef]
- Formicki, G.; Greń, A.; Stawarz, R.; Zyśk, B.; Gał, A. Metal content in honey, propolis, wax, and bee pollen and implications for metal pollution monitoring. Pol. J. Environ. Stud. 2013, 22, 99–106. Available online: http://www.pjoes.com/Metal-Content-in-Honey-Propolis-Wax-r-nand-Bee-Pollen-and-Implications-for-Metal,88957,0,2.html (accessed on 2 February 2019).
- Madejczyk, M.; Baralkiewicz, D. Characterization of polish rape and honeydew honey according to their mineral contents using ICP-MS and F-Aas/AES. Anal. Chim. Acta 2008, 617, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P. Determination of metal content in honey by atomic absorption and emission spectrometry’s. Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Matusevicius, P.; Staniskiene, B.; Budreckiene, R. Metals and organochlorine compounds in Lithuanian honey. Pol. J. Food Nutr. Sci. 2010, 60, 159–163. [Google Scholar]
- Kacaniová, M.; Knazovicka, V.; Melich, M.; Fikselova, M.; Massanyi, P.; Stawarz, R.; Hascik, P.; Pechociak, T.; Kuczkowska, A.; Putała, A. Environmental concentration of selected elements and relation to physicochemical parameters in honey. J. Environ. Sci. Health Part A 2009, 44, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 1–21. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR) 2015, Division of Toxicology and Human Health, ATSDR’s Substance Priority List. 2020. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 16 June 2020).
- Murashova, E.A.; Tunikov, G.M.; Nefedova, S.A.; Karelina, O.A.; Byshova, N.G.; Serebryakova, O.V. Major factors determining accumulation of toxic elements by bees and honey products. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2020, 11, 3. [Google Scholar] [CrossRef]
- Zambelli, B.; Uversky, V.N.; Ciurli, S. Nickel impact on human health: An intrinsic disorder perspective. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1714–1731. [Google Scholar] [CrossRef]
- Maroney, M.J.; Ciurli, S. Bioinorganic chemistry of nickel. Inorganics 2019, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Mae, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Des Marais, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ. Geochem. Health 2014, 36, 797–814. [Google Scholar] [CrossRef]
- PN-88/A-77626. Bee Honey Polish Committee for Standardization, Measures and Quality; Wydawnictwa Normalizacyjne Alfa: Warsaw, Poland, 1988. [Google Scholar]
- Höllriegl, V.; München, H.Z. Strontium in the Environment and Possible Human Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 268–275. [Google Scholar] [CrossRef]
- Hassan, A.S.M.; El Rahman, T.A.A.; Eissa, A.A. A evaluation and comparison of some trace elements in bee honey from eleven countries. Egy. Sci. J. Pestic. 2015, 1, 39–44. [Google Scholar]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese Metabolism in Humans; University of Potsdam Mathematical and Natural Science Series—Bioscience: Potsdam, Germany, 2018; Volume 711, pp. 1655–1679. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The impact of trace minerals on bone metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Sousa, C.; Moutinho, C.; Vinha, A.F.; Matos, C. Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. Int. J. Sci. Res. Methodol. 2019, 13, 57–80. Available online: http://ijsrm.humanjournals.com/wp-content/uploads/2019/10/5.Carla-Sousa-Carla-Moutinho-Ana-F.-Vinha-Carla-Matos.pdf (accessed on 19 August 2020).
- Jiménez, M.; Abradelo, C.; Román, J.S.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef]
- Umair, M.; Alfadhel, M. Genetic disorders associated with metal metabolism. Cells 2019, 8, 1598. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Czauderna, M.; Krajewska, K.A.; Przybylski, W. Selected physiological effects of boron compounds for animals and humans. A review. J. Anim. Feed Sci. 2019, 28, 307–320. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Waxman, S.G. Sodium channels in human pain disorders: Genetics and pharmacogenomics. Annu. Rev. Neurosci. 2019, 42, 87–106. [Google Scholar] [CrossRef]
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fundam. Clin. Pharmacol. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Sulfur in human health. EC Nutr. 2019, 14, 785–791. Available online: https://www.researchgate.net/publication/335653705_Sulfur_and_Human_Health (accessed on 20 August 2020).
- Pasek, M. A role for phosphorus redox in emerging and modern biochemistry. Curr. Opin. Chem. Biol. 2019, 49, 53–58. [Google Scholar] [CrossRef]
- Smith, G.L.; Eisner, D.A. Calcium buffering in the heart in health and disease. Circulation 2019, 139, 2358–2371. [Google Scholar] [CrossRef]
- Borgi, L. Inclusion of phosphorus in the nutrition facts label. Clin. J. Am. Soc. Nephrol. 2018, 14, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The physiological role of boron on health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Farapti, F.; Sulistyowati, M.; Artanti, K.D.; Setyaningtyas, S.W.; Sumarmi, S.; Mulyana, B. Highlighting of urinary sodium and potassium among indonesian schoolchildren aged 9–12 years: The contribution of school food. J. Nutr. Metab. 2019, 2019, 1028672. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Rahman, S.; Ojeh, N.; Grant, W.B.; Kaur, H.; Haq, A.; Razzaque, M.S. Oral manifestations of magnesium and vitamin D inadequacy. J. Steroid Biochem. Mol. Biol. 2020, 200, 105636. [Google Scholar] [CrossRef] [PubMed]
- Clase, C.M.; Carrero, J.-J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020, 97, 42–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogut, I.; Oglakci, A.; Kartkaya, K.; Ol, K.K.; Sogut, M.S.; Kanbak, G.; Inal, M.E. Effect of boric acid on oxidative stress in rats with fetal alcohol syndrome. Exp. Ther. Med. 2014, 9, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Sakuma, M.; Narishima, Y.; Yoshida, T.; Kumagai, H.; Arai, H. Habitual confectionery intake is associated with serum phosphorus levels: A cross-sectional study on healthy subjects. J. Med. Investig. 2019, 66, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Louppis, A.P.; Badeka, A.; Papastephanou, C.; Kontominas, M.G. Nutritional aspects and botanical origin recognition of Mediterranean honeys based on the “mineral imprint” with the application of supervised and non-supervised statistical techniques. Eur. Food Res. Technol. 2019, 245, 1939–1949. [Google Scholar] [CrossRef]
- Kuras, M.; Zielińska-Pisklak, M.; Perz, K.; Szeleszczuk, Ł. Iron and zinc—The main micronutrients necessary for the proper functioning of the body. Pharmacotherapy 2015, 25, 6–13. Available online: https://www.researchgate.net/publication/280598065_Zelazo_i_cynk-glowne_mikroelementy_niezbedne_do_prawidlowego_funkcjonowania_organizmu (accessed on 10 May 2020). (In Polish).
- Available online: www.statista.com (accessed on 17 April 2020).
- P8_TA. 0057 Prospects and challenges for the EU apiculture sector, 2018, European Parliament resolution of 1 March 2018 on prospects and challenges for the EU apiculture sector (2017/2115(INI)), (2019/C 129/05) C 129/30 EN. Off. J. Eur. Union 2019, C129, 30. Available online: https://www.europarl.europa.eu/doceo/document/TA-8-2018-0057_EN.pdf (accessed on 17 April 2020).
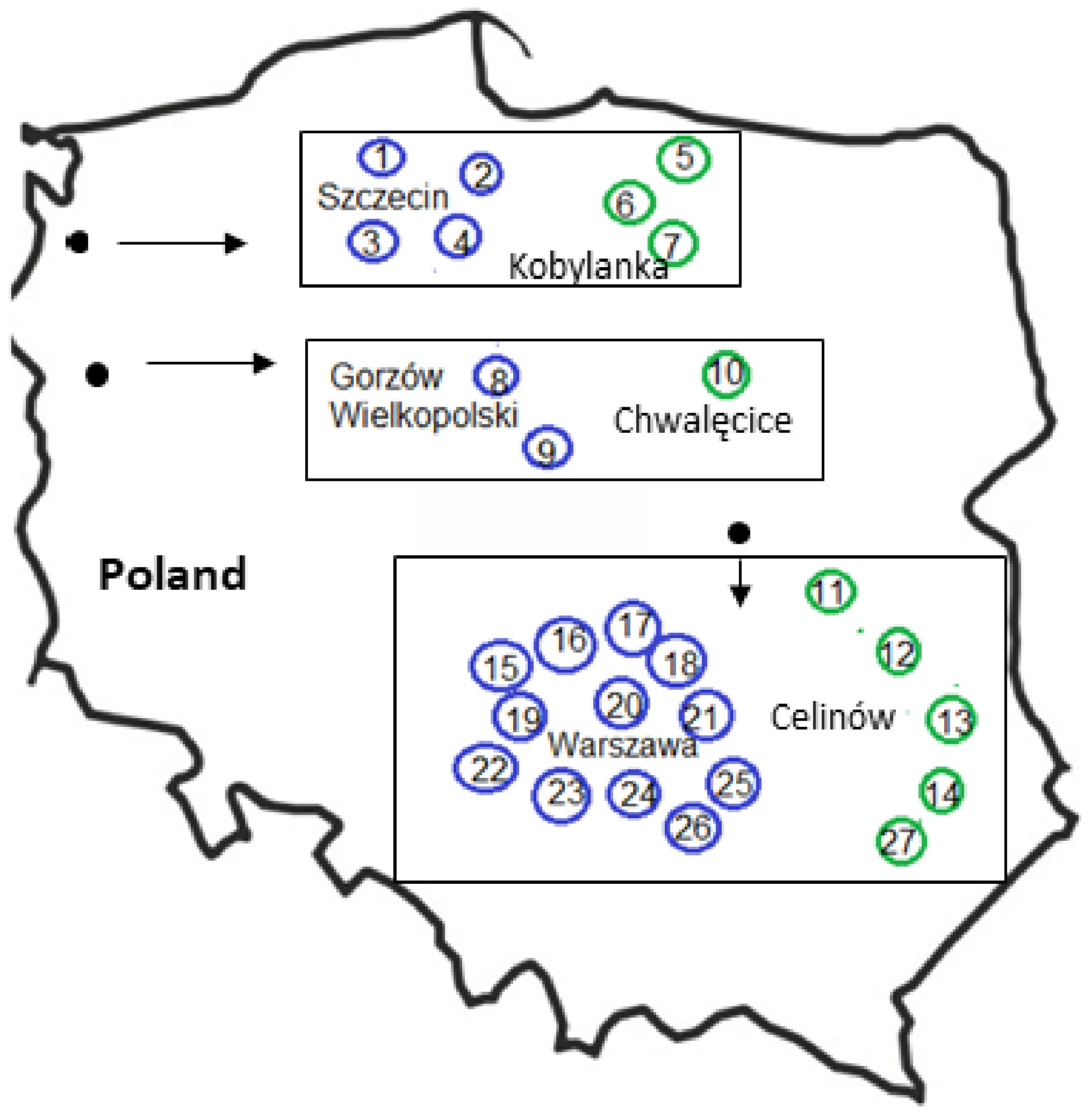


| Features of Cities | City | ||
|---|---|---|---|
| Warsaw | Szczecin | Gorzów Wlkp. | |
| * PM10 dust average annual value [ug/m3] | 30; 30; 32; 44 | 24; 24; 26 | 24; 27 |
| * PM2.5 dust average annual value [ug/m3] | 20; 21; 23; 25 | 19; 20 | 17 |
| * Pb(PM10) average annual value [ng/m3] | 10; 10 | 10 | 10; 10 |
| * Ni(PM10) average annual value [ng/m3] | 0.6; 2.1 | 0.9 | 1.9; 2.7 |
| * As(PM10) average annual value [ng/m3] | 0.6; 0.7 | 0.8 | 1.2; 1.1 |
| * Cd(PM10) average annual value [ng/m3] | 0.3; 0.2 | 0.2 | 0.2; 0.2 |
| Classes for the protection of health | C | A | A |
| Particulate pollutants emission from plants of significant nuisance to air quality [t/y] | 456 | 299 | 28 |
| Contamination | State | ||||||
|---|---|---|---|---|---|---|---|
| Poland | Germany | Czech | Slovakia | Lithuania | EU | ||
| G | PS | Republic | |||||
| PM10 dust [µg/m3] | 32.2 | 29.0 | 17.5 | 23.9 | 24.2 | 22.8 | 21.6 |
| PM2.5 dust [µg/m3] | 23.8 | 20.7 | 12.7 | 18.4 | 17.5 | - | 14.1 |
| MMs | Mn | Zn | Cu | Fe | Sr | Mg | Ca | K | Na | B | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 0.734 | 3.80 | 0.287 | 2.58 | 0.132 | 23.3 | 77.0 | 1255 | 16.43 | 5.90 | 104.1 | 42.6 |
| Median | 0.416 | 3.14 | 0.238 | 2.03 | 0.101 | 16.9 | 72.6 | 1501 | 11.98 | 4.38 | 72.8 | 32.1 |
| I quartile | 0.223 | 2.72 | 0.141 | 1.40 | 0.057 | 14.2 | 51.0 | 625 | 7.72 | 3.42 | 64.1 | 27.1 |
| III quartile | 0.752 | 4.22 | 0.420 | 3.33 | 0.160 | 21.7 | 89.8 | 1627 | 21.26 | 8.94 | 116.4 | 50.1 |
| S.D. | 0.911 | 2.06 | 0.193 | 1.80 | 0.104 | 21.1 | 38.6 | 759 | 13.86 | 3.26 | 80.9 | 25.6 |
| Min. | 0.105 | 1.97 | 0.047 | 1.01 | 0.024 | 6.8 | 28.00 | 166 | 2.98 | 2.38 | 42.1 | 17.8 |
| Max. | 3.764 | 12.8 | 0.826 | 9.73 | 0.477 | 109.3 | 200.2 | 3490 | 59.37 | 14.71 | 405.1 | 130.8 |
| MMs | Kind of Location | MMs | Kind of Location | ||
|---|---|---|---|---|---|
| City | Village | City | Village | ||
| Zn | 3.47 (n = 18) | 4.48 (n = 9) | B | 4.71 (n = 18) | 8.26 (n = 9) |
| Cu | 0.29 (n = 18) | 0.29 (n = 9) | P | 96.9 (n = 18) | 118.6 (n = 9) |
| Mn | 0.45 (n = 18) | 1.30 (n = 9) | S | 42.2 (n = 18) | 43.3 (n = 9) |
| Fe | 2.50 (n = 18) | 2.75 (n = 9) | Pb | 0.07 (n = 11) | 0.07 (n = 7) |
| Ca | 74.6 (n = 18) | 81.8 (n = 9) | Cr | 0.03 (n = 5) | 0.03 (n = 1) |
| Mg | 21.6 (n = 18) | 26.6 (n = 9) | Ba | 0.10 (n = 3) | 0.04 (n = 3) |
| K | 1411 (n = 18) | 942 (n = 9) | Ni | 0.03 (n = 3) | 0.02 (n = 5) |
| Na | 15.3 (n = 18) | 18.7 (n = 9) | Co | 0.03 (n = 3) | below LOD |
| Sr | 0.14 (n = 18) | 0.12 (n = 9) | As, Cd | below LOD | below LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałczyńska, M.; Gamrat, R.; Bosiacki, M.; Sotek, Z.; Stasińska, M.; Ochmian, I. Micro and Macroelements in Honey and Atmospheric Pollution (NW and Central Poland). Resources 2021, 10, 86. https://doi.org/10.3390/resources10080086
Gałczyńska M, Gamrat R, Bosiacki M, Sotek Z, Stasińska M, Ochmian I. Micro and Macroelements in Honey and Atmospheric Pollution (NW and Central Poland). Resources. 2021; 10(8):86. https://doi.org/10.3390/resources10080086
Chicago/Turabian StyleGałczyńska, Małgorzata, Renata Gamrat, Mateusz Bosiacki, Zofia Sotek, Małgorzata Stasińska, and Ireneusz Ochmian. 2021. "Micro and Macroelements in Honey and Atmospheric Pollution (NW and Central Poland)" Resources 10, no. 8: 86. https://doi.org/10.3390/resources10080086
APA StyleGałczyńska, M., Gamrat, R., Bosiacki, M., Sotek, Z., Stasińska, M., & Ochmian, I. (2021). Micro and Macroelements in Honey and Atmospheric Pollution (NW and Central Poland). Resources, 10(8), 86. https://doi.org/10.3390/resources10080086






