Southern African Soap Plants and Screening of Selected Phytochemicals and Quantitative Analysis of Saponin Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Qualitative Phytochemical Analysis
2.1.1. Detection of Saponins
2.1.2. Detection of Alkaloids
2.1.3. Detection of Terpenoids
2.2. Quantitative Analysis: Saponins
2.2.1. Plant Extraction
2.2.2. Preparation of Calibration Curve
3. Results
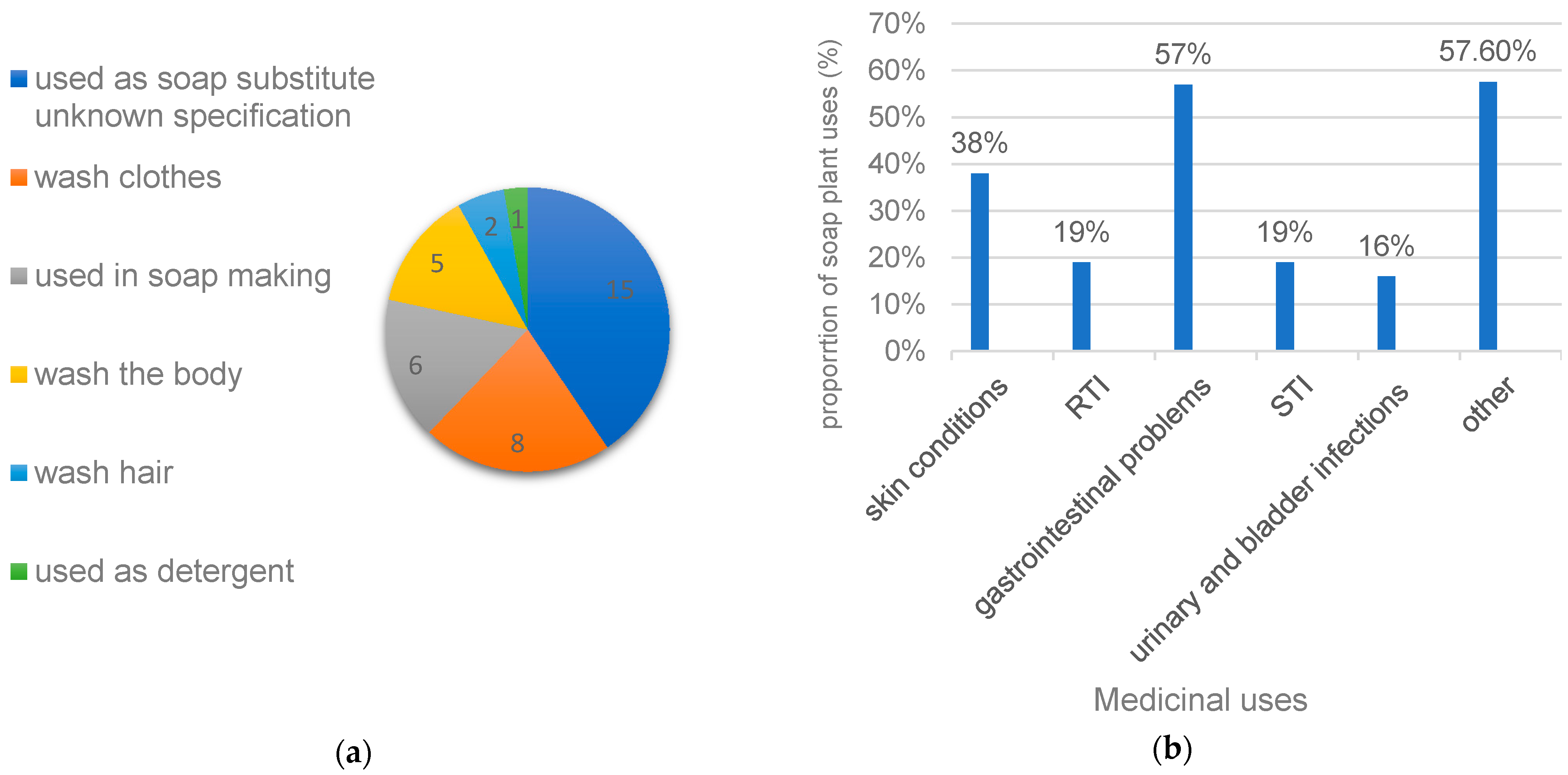
3.1. Ethnobotanical Information
3.2. Medical Uses of Soap Plants
3.3. Study of Selected Bioactive Compounds Found in Soap Plants
3.4. Phytochemical Screening
4. Discussion
4.1. Ethnobotanical Information
4.2. Soap Plant Names and Description
4.3. Saponin Concentrations in the Selected Plants
4.4. Saponins and Environmental Factors
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Plant Species | Weight of Dry Extract per mL m(gm) | Sample Solution (µg/mL) | Absorbance Values | DE Conc. C (µg/mL) | DE Conc. C(mg/mL) | TSC as DE A = CXV/m (mg/g) | Mean (mg/g) | STDev |
|---|---|---|---|---|---|---|---|---|
| Calodendrum capense | 0.001 | 0.25 | 1.927 | 458.78 | 0.45878 | 114.695 | 107.8893 | 4.88689 |
| 0.001 | 0.25 | 1.738 | 413.78 | 0.41378 | 103.445 | |||
| 0.001 | 0.25 | 1.773 | 422.112 | 0.422112 | 105.528 | |||
| Carica papaya | 0.001 | 0.25 | 0.123 | 29.2555 | 0.029256 | 7.313875 | 6.321808 | 1.198715 |
| 0.001 | 0.25 | 0.078 | 18.5412 | 0.018541 | 4.6353 | |||
| 0.001 | 0.25 | 0.188 | 28.065 | 0.028065 | 7.01625 | |||
| Crinum bulbispermum | 0.001 | 0.25 | 0.677 | 161.1603 | 0.16116 | 40.29007 | 35.42896 | 4.252054 |
| 0.001 | 0.25 | 0.503 | 119.7317 | 0.119732 | 29.93293 | |||
| 0.001 | 0.25 | 0.606 | 144.2555 | 0.144256 | 36.06388 | |||
| Cyathula uncinulata | 0.001 | 0.25 | 0.379 | 90.2079 | 0.090208 | 22.55198 | 20.17102 | 1.701038 |
| 0.001 | 0.25 | 0.324 | 77.1126 | 0.077113 | 19.27815 | |||
| 0.001 | 0.25 | 0.314 | 74.7317 | 0.074732 | 18.68293 | |||
| Deinbollia oblogifolia | 0.001 | 0.25 | 0.322 | 76.636 | 0.076636 | 19.159 | 16.02415 | 2.259419 |
| 0.001 | 0.25 | 0.234 | 55.684 | 0.055684 | 13.921 | |||
| 0.001 | 0.25 | 0.252 | 59.9698 | 0.05997 | 14.99245 | |||
| Ilex mitis | 0.001 | 0.25 | 0.145 | 34.4936 | 0.034494 | 8.6234 | 8.365458 | 0.775076 |
| 0.001 | 0.25 | 0.123 | 29.2555 | 0.029256 | 7.313875 | |||
| 0.001 | 0.25 | 0.154 | 36.6364 | 0.036636 | 9.1591 | |||
| Merwilla plumbea | 0.001 | 0.25 | 0.447 | 106.3983 | 0.106398 | 26.59958 | 25.58768 | 0.826213 |
| 0.001 | 0.25 | 0.43 | 102.3508 | 0.102351 | 25.58769 | |||
| 0.001 | 0.25 | 0.413 | 98.3031 | 0.098303 | 24.57578 | |||
| Noltea africana | 0.001 | 0.25 | 0.981 | 233.5412 | 0.233541 | 58.3853 | 52.65117 | 6.806973 |
| 0.001 | 0.25 | 0.949 | 225.9221 | 0.225922 | 56.48053 | |||
| 0.001 | 0.25 | 0.724 | 172.3507 | 0.172351 | 43.08768 | |||
| Plectranthus ciliatus | 0.001 | 0.25 | 0.29 | 69.017 | 0.069017 | 17.25425 | 18.88131 | 1.362031 |
| 0.001 | 0.25 | 0.316 | 75.2079 | 0.075208 | 18.80198 | |||
| 0.001 | 0.25 | 0.346 | 82.3508 | 0.082351 | 20.5877 | |||
| Thunbergia dregeana | 0.001 | 0.25 | 0.181 | 43.065 | 0.043065 | 10.76625 | 10.09165 | 2.524122 |
| 0.001 | 0.25 | 0.215 | 51.1602 | 0.05116 | 12.79005 | |||
| 0.001 | 0.25 | 0.113 | 26.8746 | 0.026875 | 6.71865 |
References
- Levey, M. The early history of detergent substances. J. Chem. Educ. 1954, 31, 521–524. [Google Scholar] [CrossRef]
- Oleszek, W.; Hamed, A. Saponin-based surfactants. In Surfactants from Renewable Resources; Kjellin, M., Johansson, I., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 244–246. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; E. & S. Livingstone LTD: Edinburgh, UK, 1962. [Google Scholar]
- Routh, H.B.; Bhowmikk, R.; Parishl, C.; Witkowski, J.A. Soaps: From the Phoenicians to the 20th century—A historical review. Clin. Dermatol. 1996, 1, 3–6. [Google Scholar] [CrossRef]
- Bossard, E. Angolan medicinal plants used also as piscicides and/or soaps. J. Ethnopharmacol. 1993, 40, 1–19. [Google Scholar] [CrossRef]
- Mehta, P.S.; Bhatt, K.C. Traditional soap and detergent yielding plants of Uttaranchal. Indian J. Tradit. Knowl. 2005, 6, 279–284. [Google Scholar]
- Wisetkomolmat, J.; Suppakittpaisarn, P.; Sommano, S.R. Detergent plants of northern Thailand: Potential sources of natural saponins. Resources 2019, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, B.; Budzynska, A.; Wieckowska-Szakiel, M.; Paszkiewicz, M.; Stochmal, A.; Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Sarma, B.K.; et al. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food Chem. Toxicol. 2009, 47, 778–786. [Google Scholar]
- Osbourn, A. Saponins and plant defence—A soap story. Trends Plant Sci. 1996, 1, 4–9. [Google Scholar] [CrossRef]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleszek, W.; Kapusta, I.; Stochmal, A. TLC of triterpenes (including saponins). In Thin Layer Chromatography in Phytochemistry; Waksmundzka-Hajnos, M., Sherma, J., Kowalska, T., Eds.; CRC Press, Taylor & Francis Group, LLC: New York, NY, USA, 2008; pp. 519–541. [Google Scholar]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Bart, H.J. Extraction of natural products from plants: An introduction. In Industrial Scale Natural Products Extraction, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1–26. [Google Scholar]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Sidhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press Inc.: Totova, NJ, USA, 2007; pp. 93–101. [Google Scholar]
- Odeh, A.O.; Alshammari, G.A.A.; Almulgabsagher, K.S.R.; Andrew, P.A. Effect of solute polarity on extraction efficiency using deep eutectic solvents. Green Chem. 2021, 23, 5097–5105. [Google Scholar]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/plant-extracts-market-942.html (accessed on 10 July 2021).
- Hedberg, I.; Staugaurd, F. Traditional Medicinal Plants: Traditional Medicine in Botswana; Ipeleng Publishers: Gaborone, Botswana, 1989. [Google Scholar]
- Motlhanka, D.M.T. Free radical scavenging activity of selected medicinal plants of Eastern Botswana. Pak. J. Biol. Sci. 2008, 11, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Amusan, O.O.; Dlamini, P.S.; Msonthi, J.D.; Makhubu, L.P. Some herbal remedies from the Manzini region of Swaziland. J. Ethnopharmacol. 2002, 79, 109–112. [Google Scholar] [CrossRef]
- Amusan, O.O.; Sukati, N.A.; Dlamini, P.S.; Sibandze, F.G. Some Swazi phytomedicine and their constituents. Afr. J. Biothechnol. 2007, 6, 267–272. [Google Scholar]
- Amusan, O.O.G. Herbal medicine in Swaziland: An overview. ACS Symp. Ser. 2009, 1021, 31–49. [Google Scholar]
- Seleteng Kose, L.E.M.; Moteetee, A.; van Vuuren, S. Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J. Ethnopharmacol. 2015, 170, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Seleteng Kose, L.E.M. Evaluation of Commonly Used Medicinal Plants of Maseru District in Lesotho for Their Ethnobotanical Uses, Antimicrobial Properties and Phytochemical Composition. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2017. [Google Scholar]
- Koenan, E.V. Medicinal, Poisonous and Edible Plants in Namibia; Klaus Hess Verlag: Windhoek, Namibia, 1996; Volume 2. [Google Scholar]
- Chinsembu, K.C.; Hedimbi, M.; Mukaru, W.C. Putative medicinal properties of plants from the Kavango region, Namibia. J. Med. Plant Res. 2011, 5, 6787–6797. [Google Scholar]
- Hutchings, A.; Scott, A.H.; Lewis, G.; Cunningham, A. Zulu Medicinal Plants. An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996; pp. 21–311. [Google Scholar]
- Van Wyk, B.E. A review of ethnobotanical research in southern Africa. S. Afr. J. Bot. 2002, 68, 1–13. [Google Scholar] [CrossRef]
- Van Wyk, B.E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef] [Green Version]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Intern. Pharm. Sci. 2011, 1, 99–106. [Google Scholar]
- Zohra, S.F.; Meriem, B.; Samira, S.; Muneer, A.M.S. Phytochemical screening and identification of some compounds from Mallow. J. Nat. Prod. Plant Resour. 2012, 2, 512–516. [Google Scholar]
- Kaur, R.; Arora, S.; Thukral, A.K. Quantitative and qualitative analysis of saponins in different plant parts of Chlorophytum borivilianum. Intern. J. Pharma. Bio Sci. 2015, 6, 826–835. [Google Scholar]
- Ncube, B.; Finnie, J.F.; van Staden, J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous plants from South Africa. S. Afr. J. Bot. 2011, 77, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Ndamba, J.; Lemmichi, E.; Molgaard, P. Release of molluscicidal saponins from Phytolacca dodecandra aqueous berry extracts as influenced by the male plant and the extraction rocedure. Biochem. Syst. Ecol. 1994, 22, 249–257. [Google Scholar] [CrossRef]
- Ighodaro, M.O.; Omole, J.O.; Fadipe, S.O. Effects of Piliostigma thonningii ethyl acetate leaf extract on aluminium-cum extract treated wistar rats. Anim. Res. Int. 2012, 9, 1579–1584. [Google Scholar]
- Onyeike, E.N.; Omubo-Dede, T.T. Effect of heat treatment on the proximate composition, energy values, and levels of some toxicants in African yam bean (Sphenostylis stenocarpa) seed varieties. Plant Foods Hum. Nutr. 2002, 57, 223–231. [Google Scholar] [CrossRef]
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Zaman, S.; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plant Res. 2012, 6, 1492–1496. [Google Scholar]
- Street, R.A.; Prinsloo, G. Commercially important medicinal plants of South Africa: A review. J. Chem. 2012, 2013, 1–16. [Google Scholar] [CrossRef]
- Williams, V.L.; Cunningham, A.B.; Raimondo, D. Merwilla Plumbea (Lindl.) Septa. Available online: http//redlist.sanbi.org/species.php?species=7485-2 (accessed on 19 December 2019).
- Sparg, S.G.; van Staden, J.; Jager, A.K. Pharmacological and phytochemical screening of two Hyacinthaceae species: Scilla natalensis and Ledebouria ovatifolia. J. Ethnopharmacol. 2002, 80, 95–101. [Google Scholar] [CrossRef]
- Mar, W.; Tan, G.T.; Cordele, G.A.; Pezzuto, J.M. Biological activity of novel macrocyclic alkaloids (budmunchiamines) from Albizia amara detected on the basis of interaction with DNA. J. Nat. Prod. 1991, 54, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Thippeswamy, S.; Mohana, D.C.; Manjunath, K. Antimicrobial efficacy and phytochemical analysis of Albizia amara (Roxb.) Boiv. an indigenous medicinal plant against some human and plant pathogenic bacteria and fungi. J. Pharm. Res. 2011, 4, 832–835. [Google Scholar]
- Felhaber, T.; Mayeng, I. South African Traditional Healers’ Primary Health Care Handbook, 1st ed.; Kagiso Publishers: Cape Town, South Africa, 1997; p. 195. [Google Scholar]
- Choi, S.; Supeno, D.; Byun, J.; Kwon, S.; Chung, S.; Kwon, S.; Kwon, D.; Choi, W. The identification of saponin to obtain the maximum benefit from Aloe saponaria. Adv. Sci. Technol. Lett. 2015, 120, 558–563. [Google Scholar]
- Philander, L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Ing’ahu, O.R. Isolation of Metabolites and Screening for Antimicrobial Activity of Calodendrum capense Thunb. (Rutaceae). Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2004; pp. 156–172. [Google Scholar]
- Yushau, M.; Onuorah, F.C.; Murtala, Y. In vitro sensitivity pattern of some urinary tract isolates to Carica papaya extracts. Bayero J. Pure App. Sci. 2009, 2, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M. Indigenous Healing Plants; Southern Book Publishers: Johannesburg, South Africa, 1990. [Google Scholar]
- Elgorashi, E.E.; Taylor, J.L.S.; Verschaeve, L.; Maes, A.; van Staden, J.; de Kimpe, N. Screening of medicinal plants used in South African traditional medicine for genotoxic effects. Toxicol. Lett. 2003, 143, 195–207. [Google Scholar] [CrossRef]
- Glasby, J.S. Dictionary of Plants Containing Secondary Metabolites; Taylor & Francis: London, UK, 1991. [Google Scholar]
- Moffett, R. Sesotho Plant and Animal Names and Plants Used by the Basotho; Sun Press: Bloemfontein, South Africa, 2010. [Google Scholar]
- Ibrahim, B.; Sowemimo, A.; van Rooyen, A.; van de Venter, M. Anti-inflammatory, analgesic, and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae). J. Ethnopharmacol. 2012, 141, 282–289. [Google Scholar] [CrossRef]
- Bisi-Johnson, M.A.; Obi, C.L.; Samuel, B.B.; Eloff, J.N.; Okoh, A.I. Antibacterial activity of crude extracts of some South African medicinal plants against multidrug resistant etiological agents of diarrhoea. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Wurger, G. A Rational in In Vitro Evaluation of 53 Medicinal Plants Used in the Treatment of Diarrhoea and the Potential Use of Deinbolla oblongifolia (Sapindaceae) Extracts. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2010; p. 148. [Google Scholar]
- Du Preez, I.; Mumbengegwi, D. Phytochemical investigation on Namibian plants for anti-malaria compounds. J. Human. Soc. Sci. 2012, 1, 147–158. [Google Scholar]
- Fawole, O.A.; Ndhlala, A.R.; Amoo, S.O.; Finnie, J.F.; van Staden, J. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J. Ethnopharmacol. 2009, 123, 237–243. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Sugimoto, S.; Harinantenaina, L.; Matsunami, K.; Otsuka, H. Entadosides A–D, triterpene saponins and a glucoside of the sulphur-containing amide from the kernel nuts of Entada phaseoloides (L.) Merrill. J. Nat. Med. 2012, 66, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Shai, L.J.; Chauke, M.A.; Magano, S.R.; Mogale, A.M.; Eloff, J.N. Antibacterial activity of sixteen plant species from Phalaborwa, Limpopo Province, South Africa. J. Med. Plants Res. 2013, 7, 1899–1906. [Google Scholar]
- Ntini, V.P. Inhibitory Capabilities of Ten Medicinal Plants Used by Traditional Healers on Mammalian Carbohydrate Digesting Enzymes (Alpha-Amylase and Alpha-Glucosidase). Master’s Thesis, University of Limpopo, Limpopo, South Africa, 2013; pp. 19–23. [Google Scholar]
- Kai, N.S.; Nee, T.A.; Ling, E.L.C.; Ping, T.C.; Kamariah, L.; Lin, N.K. Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague Dawley rats. Asian Pac. J. Trop. Med. 2015, 1, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Forrester, J. Ilex Mitis. Available online: http://pza.sanbi.org/ilex-mitis (accessed on 29 April 2020).
- Sabela, M.I.; Makhanya, T.; Kanchi, S.; Shahbaaz, M.; Idress, D.; Bisetty, K. One-pot biosynthesis of silver nanoparticles using Iboza Riparia and Ilex Mitis for cytotoxicity on human embryonic kidney cells. J. Photochem. Photobiol. B Biol. 2018, 178, 500–567. [Google Scholar] [CrossRef]
- Van den Bergh, C.K. The healing practices of Bishop Joseph Matuwane: Example of healing practices. Res. Natl. Mus. 1995, 11, 386–394. [Google Scholar]
- Bouftira, I.; Abdelly, C.; Sfar, S. Characterization of cosmetic cream with Mesembryanthemum crystallinum plant extract: Influence of formulation composition on physical stability and antioxidant activity. Int. J. Cosmet. Sci. 2008, 30, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hanen, F.; Riadh, K.; Samia, O.; Sylvain, G.; Christian, M.; Chedly, A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem. Toxicol. 2009, 47, 2308–2313. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Abd-Ellatif, S.A.; Deraz, S.F.; Khalil, A.A. Antibacterial activity of some wild medicinal plants collected from western Mediterranean coast, Egypt: Natural alternatives for infectious disease treatment. Afr. J. Biotechnol. 2011, 10, 10733–10743. [Google Scholar]
- Moteetee, A.; van Wyk, B.-E. The medical ethnobotany of Lesotho: A review. Bothalia 2011, 41, 209–228. [Google Scholar] [CrossRef]
- Ashafa, A.O.T. Medicinal potential of Morella serrata (Lam.) Killick (Myricaceae) root extracts: Biological and pharmacological activities. BMC Complement. Altern. Med. 2013, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sabiu, S.; Ashafa, O.T.A. Toxicological implications and laxative potential of ethanol root extract of Morella serrata in loperamide-induced constipated Wistar rats. Pharm. Biol. 2016, 54, 2901–2908. [Google Scholar] [CrossRef]
- Parkhurst, R.M.; Mthupha, B.M.; Liang, Y.S.; Bruce, J.I.; Lambert, J.D.H.; Collier, T.L.; ApSimon, J.W.; Wolde-Yohannes, L.; Heath, G.E.; Jones, W.O.; et al. The molluscicidal activity of Phytolacca dodecandra I. Location of the activating esterase. Biochem. Biophys. Res. Commun. 1989, 158, 436–439. [Google Scholar] [CrossRef]
- Nostro, A.; Germano, M.P.; D’Angelo, V.; Marino, A.; Cannatelli, M.A. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 2000, 30, 379–384. [Google Scholar] [CrossRef]
- Dluya, T.; Dahiru, D. Antibacterial activity of Piliostigma thonningii methanol stem bark extract. Int. J. Res. Pharm. Biosci. 2018, 5, 15–20. [Google Scholar]
- Phillips, W.C. The Treatment of Labyrinthine Affections; Medical Society: New York, NY, USA, 1916; pp. 191–195. [Google Scholar]
- Viljoen, A.M.; Demirci, B.; Başer, K.H.C.; Potgieter, C.J.; Edwards, T.J. Microdistillation and essential oil chemistry—A useful tool for detecting hybridisation in Plectranthus (Lamiaceae). S. Afr. J. Bot. 2006, 72, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Sewani-Rusike, C.R. Antifertility effects of Pouzolzia mixta in female wistar rats. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimington, C. Psilocaulon absimile N.E.Br. as a stock poison. Onderstepoort J. Vet. Sci. Anim. Ind. 1933, 1, 439–455. [Google Scholar]
- Hutchings, A.; van Staden, J. Plants used for stress-related ailments in traditional Zulu, Xhosa and Sotho medicine. J. Ethnopharmacol. 1994, 43, 89–124. [Google Scholar] [CrossRef]
- Moteetee, A.; van Wyk, B.-E. The concept of ’Musa-pelo and the medicinal use of shrubby legumes (Fabaceae) in Lesotho. Bothalia 2007, 37, 75–77. [Google Scholar] [CrossRef]
- Meiring, I. Notes on some experiments with the active principle of Mesembryanthemum tortousum L. Trans. S. Afr. Phi. Soc. 1896, 9, 48–50. [Google Scholar]
- Welman, M. Solanum Aculeastrum. Available online: http://pza.sanbi.org/solanum-aculeastrum (accessed on 29 April 2020).
- Wanyonyia, A.W.; Chhabraa, S.C.; Mkojib, G.; Udo Eilertc, U.; Njuea, W.M. Bioactive steroidal alkaloid glycosides from Solanum aculeastrum. Phytochemistry 2002, 59, 79–84. [Google Scholar] [CrossRef]
- Onyeke, C.C.; Akueshi, C.O. Pathogenicity and reproduction of Meloidogyne incognita (Kofoid and White) chitwood on African yam bean, Sphenostylis stenocarpa (Hochst Ex. A. Rich) Harms accessions. Afr. J. Biotechnol. 2012, 11, 1607–1616. [Google Scholar] [CrossRef]
- Nyananyo, B.L.; Nyingifa, A.L. Phytochemical investigation on the seed of Sphenostylis stenocarpa (Hochst ex A. Rich.) Harms (Family Fabaceae). J. Appl. Sci. Environ. Manag. 2011, 15, 419–423. [Google Scholar]
- Kanyua, J.F. The Potential of Telfairia Pedata for Liquid Biofuel and Soap Production. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2011; pp. 1–119. [Google Scholar]
- Ajayi, S.A.; Dulloo, M.E.; Vodouhe, R.S.; Berjak, P.; Kioko, J.I. Conservation status of Telfairia spp. in sub-Saharan Africa. In Plant Genetic Resources and Food Security in West and Central Africa, Proceedings of the Regional Conference, Ibadan, Nigeria, 26–30 April 2004; Vodouhe, R., Atta-Krah, K., Achigan-Dako, G.E., Eyog-Matig, O., Avohou, H., Eds.; Bioversity International: Rome, Italy, 2007. [Google Scholar]
- Kaur, K.; Michael, H.; Arora, S.; Harkonen, P.; Kumar, S. In vitro bioactivity-guided fractionation and characterization of polyphenolic inhibitory fractions from Acacia nilotica (L.) Willd. ex Del. J. Ethnopharmacol. 2005, 99, 353–630. [Google Scholar] [CrossRef] [PubMed]
- Singh., R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. In Vitro 2008, 22, 1965–1970. [Google Scholar] [CrossRef]
- Agrawal, S.; Kulkarni, G.T.; Sharma, V.N. A comparative study on the antioxidant activity of methanol extracts of Acacia. Adv. Nat. Appl. Sci. 2010, 4, 78–84. [Google Scholar]
- Kalaivani, T.; Mathew, L. Free radical scavenging activity from leaves of Acacia nilotica (L.) Wil.ex Delile, an Indian medicinal tree. Food Chem. Toxicol. 2010, 48, 298–305. [Google Scholar] [CrossRef]
- Hassan, K.J.; Zubairu, M.S.; Olayemi, O.R. Production of soap from neem seed oil and Acacia nilotica seed oil. Int. J. Mod. Org. Chem. 2015, 4, 70–84. [Google Scholar]
- Abdirahman, Y.A.; Juma, K.K.; Mukundi, M.J.; Gitahi, S.M.; Agyirifo, D.S.; Ngugi, P.M.; Gathumbi, P.K.; Ngeranwa, J.J.N.; Njagi, E.N.M. The hypoglycemic activity and safety of aqueous stem bark extracts of Acacia nilotica. Drug Metab. Toxicol. 2015, 6, 1–9. [Google Scholar]
- Banso, A. Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica. J. Med. Plant Res. 2009, 3, 082–085. [Google Scholar]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. In Application and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen: London, UK, 2017; pp. 183–205. [Google Scholar]
- Acevedo-Rodríguez, P.; van Welzen, P.; Adema, F.; van der Ham, R.W.J.M. Sapindaceae. In Flowering Plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 357–407. [Google Scholar]
- Moteetee, A. A review of plants used for magic by Basotho people in comparison with other cultural groups in southern Africa. Indian J. Tradit. Knowl. 2017, 16, 229–234. [Google Scholar]
- Hoveka, L. Dicerocaryum Eriocarpum. Available online: http://pza.sanbi.org/dicerocaryum-eriocarpum (accessed on 29 April 2020).
- Budan, A.; Bellenot, D.; Freuze, I.; Gillmann, L.; Chicoteau, P.; Richomme, P.; Guilet, D. Potential of extracts from Saponaria officinalis and Calendula officinalis to modulate in vitro rumen fermentation with respect to their content in saponins. Biosci. Biotechnol. Biochem. 2014, 78, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.Y.; Dong, X.; Yang, J.; Hu, Y.H.; Peng, L.Q.; Zheng, H.; Cao, J. Vesicle based ultrasonic-assisted extraction of saponins in Panax notoginseng. Food Chem. 2020, 303, 125394. [Google Scholar] [CrossRef]
- Setiadi, S.; Putri.; Anindia, F. Manufacture of solid soap based on crude papain enzyme and antioxidant from papaya. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; Volume 105, pp. 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kunatsa, Y.; Katerere, D.R. Checklist of African soapy saponin-rich plants for possible use in communities’ response to global pandemics. Plants 2021, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.; Martins, A.P.J.; Gallardo, E.; Silvestre, S. Diosgenin: Recent highlights on pharmacology and analytical methodology. J. Anal. Methods Chem. 2016, 2016, 4156293. [Google Scholar] [CrossRef]
- Szakiel, A.; Paczkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Majinda, R.R.T. Extraction and Isolation of Saponins: Natural Products Isolation. Methods Mol. Biol. 2012, 864, 415–425. [Google Scholar]
- Chaudhary, N.K.; Bhattarai, A.; Guragain, B.; Bhattarai, A. Conductivity, Surface Tension, and Comparative Antibacterial Efficacy Study of Different Brands of Soaps of Nepal. Available online: https://www.hindawi.com/journals/jchem/2020/6989312/ (accessed on 2 June 2020).
- Fisher Science. Available online: https://www.google.com/url?sa=t&source=web&rct=j&url=http://fscimage.fishersci.com/cmsassets/downloads/segment/ScienceEducation/pdf/green_ChemicalCrossRef.pdf&ved=2ahUKEwif4vLb8rXyAhWDiVwKHbtXB1QQFnoECAMQBg&usg=AOvVaw2jK50D97qyy8edv04GO95d (accessed on 10 August 2021).
- Rankin, G.O.; Valentovic, M.A. Chloroform. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier Inc.: Cambridge, ME, USA, 2014. [Google Scholar]
- Saleh, H.E.M.; Koller, M. Introductory chapter: Principles of green chemistry. In Green Chemistry; Saleh, H., Ed.; IntechOpen Limited: London, UK; Available online: https://www.intechopen.com/chapters/57200 (accessed on 10 August 2021).
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
| Species Name and Common Name | Family Name | Uses | Plant Part | Quantitative Saponin Content | Qualitative Saponin Content | Alkaloids | Terpenoids |
|---|---|---|---|---|---|---|---|
| Adenodolichos rhomboideus (O. Hoffm.) Harms Pumbulu | Fabaceae | Powder of the leaves heals severe wounds, used as a detergent [5]. | Unknown | No records | No records | No records | No records |
| Albizia amara (Roxb.) B. Boivin Bitter Albizia | Fabaceae | Used as an astringent, treating piles, diarrhoea, gonorrhoea, leprosy, leucoderma, erysipelas and abscesses. The leaves and flowers have been applied to boils, eruptions, and swellings; used as an emetic and a remedy for coughs, ulcer, dandruff and malaria, used as soap [42]. | Root | No records | Present [43] | Present [43] | Present [43] |
| Aloe maculata All. Soap aloe | Asphodelaceae | Used for ‘blood scours’ in calves and enteritis and indigestion in poultry [24]; crushed leaves used as an enema and ingested for fevers and colds; roots used to treat skin disorders, used as soap [44]. | Leaves | Yield = 1.712 ± 0.051 mg/g, [45] | Present [45] | No records | Present [45] |
| Calodendrum capense (L.f.) Thunb. Cape chestnut | Rutaceae | Bark used externally to lighten skin, used as a moisturizer and to treat pimples, used for building material, used in soap, tied around wrists for skill and luck in hunting [46]. | Seeds | Yield = 107.89 ± 4.89 Current study | Present [47], 2004 | Present [47] | Present [47] |
| Carica papaya L. Pawpaw | Caricaceae | Root used as an anthelmintic and an infusion as a remedy for syphilis, used as a soap substitute [3]. | Leaves | Yield = 6.32 ± 1.20 Current study | Present [48] | Present [48] | Current study |
| Crinum bulbispermum (Burm.f) Milne- Redh. and Schweick Orange river lily | Amaryllidaceae | To treat rheumatism, septic sores, varicose veins and kidney and bladder infections [49]; used as a soap substitute [44]; applied to sores, haemorrhoids, and abscesses [3]. | Bulb | Yield = 35.43 ± 4.25 Current study | Present (current study) | Present [50] | Present [51]), |
| Cyathula uncinulata (Schrad.) Schinz Burweed | Amaranthaceae | Cleanses stomach and intestines by enabling voluntary vomiting, unguent, love charm, syphilis, urinary tract infection [3,52,53] rheumatism, aching joints, sceptic wounds, varicose veins, kidney, and bladder infections [50]; used as a soap substitute [3]. | Root | Yield = 20.17 ± 1.70 Current study | Present (current study) | Absent (current study) | Present [54] |
| Deinbollia oblongifolia (E. Mey.ex Arn.) Radlk. Dune soapberry | Sapindaceae | Treatment of diarrhoea, used as a soap substitute [55]. | Seed, fruit (berry) and leaves | Yield = 16.02 ± 2.26 Current study | Present [55] | Present (current study) | Present [55] |
| Dianthus crenatus Thunb | Caryophyllaceae | The root infusion with Tephrosia lurida Sond. Is used as an emetic, used to wash the face [3]. | Root | No records | No records | No records | No records |
| Dicerocaryum eriocarpum (Decne.) Abels Devil’s thorn (Eng) seepbos (Afr.) | Pedaliaceae | Southern and Eastern Africans, used as a soap substitute [3] used for treating measles, treats STIs [56]. | Leaves | No records | No records | Present [56] | Present [56] |
| Dissotis princeps (Kunth) Triana Purple dissotis | Melastomataceae | Leaf infusions are administered as enemas for dysentery and diarrhoea [27]. Used as a soap substitute [3]. | Root | No records | Present [57] | Present [57] | No records |
| Entada phaseoloides Merr. Box bean | Fabaceae | Used for its anti-inflammatory activity, used as a soap substitute [58]. | Bark | No records | Present [58] | Present [58] | No records |
| Helinus integrifolius (Lam.) KuntzeSoap bush | Rhamnaceae | Treats leg pains and stroke [59,60], treats hysteria, soothes irritation of the sand worm, used as an emetic and a soap substitute [3]. | Root | No records | No records | No records | Present [59] |
| Hibiscus cannabinus L. Lu | Malvaceae | Remedy for eye diseases and dysenteries, used as a soap substitute [5]. | Root | No records | Present [61] | Present [61] | Present [61] |
| Ilex mitis (L.) Radlk. African holly | Aquifoliaceae | An enema for colic in children. The leaves and the bark are grounded in water whereby a lather is formed to wash the body [62]. | Leaves and Bark | 8.37 ± 0.78 Current study | Present (current study) | Present (current study) | Present [63] |
| Kedrostis capensis (Sond.) A. Meeuse sesepa-sa-linoha | Cucurbitaceae | The Southern Sotho use the root as a purgative for the relief of constipation, used as a remedy for colic, and as a soap substitute [3,64]. | Root | No records | No records | No records | No records |
| Merwilla plumbea (Lindl.) Wild squill | Hyacinthaceae | Extracts of the bulb are known for their antibacterial, anthelmintic, anti-inflammatory, medicinal activity and used as ointment for wounds, scarification, as a laxative, and as an enema; it is also considered as a soap plant [39]. | Bulb | 20 mg/g [34] | Present [39] | Absent [41] | Present [55] |
| Mesembryanthemum crystallinum L. Crystalline ice-plant | Mesembrythamaceae | Leaf juice used to soothe inflammation of the mucous membranes of the respiratory or urinary system. In Europe, the fresh juice has been used to treat water retention, painful urination and to soothe lung inflammation [65]; antiseptic for burns sores and mouth infections [66]; used as a soap substitute [3]. | Stem bark | No records | Present [67] | Present [67] | No records |
| Morella serrata (Lam.) Killick Lance-leaved waxberry | Myricaceae | Used for headaches, TB, mental illness. Powdered roots sniffed to cause sneezing to get rid of headache [68,69]; used to treat chest-related problems such as asthma, coughing and shortness of breath [70]; used as a soap substitute [3]. | Fruit (berry) | No records | Present [69] | Absent [69] | Present [69] |
| Noltea africana (L.) Endl. Soap glossy-leaf | Rhamnaceae | Xhosa people use for washing clothes, used to treat “quarter-evil “(sponssiekte) in livestock [3]. | Leaves and twigs | 52.65 ± 6.81 Current study | Present (current study) | Present (current study) | Present (current study) |
| Phytolacca dodecandra L’ Herit African soapberry | Phytolaccaceae | Emetic in febrile conditions, treatment of genito-urinary conditions, purgative, herpes, and epilepsy remedy [3] used as a soap to wash clothes [71]. | Leaves | 120 mg/g [71] | Present [71] | Present [72] | Present [71] |
| Piliostigma thonningii (Schumach.) Milne- Redh. Camel’s foot | Fabaceae | Treats of dysentery, wounds, respiratory ailments, snake bites, hookworms and skin diseases, chronic ulcers, diarrhoea, toothache, gingivitis, cough and bronchitis, used as soap [73]. | Stem bark | 2.1 mg/g [36] | Present [73] | Present [73] | Absent [73] |
| Plectranthus ciliatus E.Mey. Speckled spur-flower | Lamiaceae | Medicinal (for general pain); in the olden days used as a substitute for soap to wash sheepskin garments [3,52,74]. | Whole plant | 18.88 ± 1.36 Current study | Present (current study) | Present (current study) | Present [75] |
| Pouzolzia mixta Sohms Soap nettle | Urticaceae | Treats painful uterus, use for expulsion of retained placenta, treat venereal diseases, used as soap [76]. | Leaves | No records | No records | Absent [76] | No records |
| Psilocaulon absimile N.E. Br Asbos | Aizoaceae | Used in rural areas as soap and for crop poisoning due to the alkaloid piperdene [77]. | Leaves | No records | No records | Present [77] | No records |
| Rhynchosia caribaea (Jacq.) DC. Monya-a-mali | Fabaceae | Headaches, rheumatism; soap substitute [52,68,78,79]. | Unknown | No records | No records | No records | No records |
| Salsola aphylla L. f. Asbos | Chenopodiaceae | With high alkali used in making soaps and glass [3]. | Leaves | No records | No records | No records | No records |
| Sceletium tortuosum N. E. Br. Kanna | Aizoaceae | The Hottentot chew the leaf for the relief of toothache, used as soap [3]. | Unknown | No records | No records | Present [80] | No records |
| Sesbania bispinosa Fawc. and Rendle Prickly sesban | Fabaceae | Suitable for rope-making and used as soap [5]. | Unknown | No records | No records | No records | No records |
| Solanum aculeastrum Dunal var. albifolium Bitter-apple | Solanaceae | Fruit used as a remedy for ringworm in cattle and horses, remedy for scarifications over the knees for the relief of rheumatism in that situation [3]; used as a soap [81]. | Unknown | No records | No records | Present [82] | No records |
| Sphenostylis stenocarpa (A. Rich.) Harms | Fabaceae | Used for food and nutrition for both humans and livestock, used as a soap [83]. | Unknown | 5.76 ± 0.30 mg/g [37] | Present [37] | Present [84] | No records |
| Talinum caffrum (Thunb.) Eckl. and Zeyh. Porcupine-root | Anacampserotaceae | Used for nervousness and stomach-aches, also used as a soap substitute [3]. | Leaves | No records | No records | No records | No records |
| Telfairia pedata (Sm. ex Sims) Hook. Oysternut | Cucurbitaceae | Used in soap production, used for biodiesel [85]; used as an ornamental [86]. | Seed oil | No records | Present [85] | Present [86] | No records |
| Thunbergia atriplicifolia E. Mey. Natal primrose | Acanthaceae | Used by the Zulu and by the Natal Indian in making a hair-wash [3]. | Leaves and unripe fruits | No records | No records | No records | No records |
| Thunbergia dregeana Nees Isiphondo | Acanthaceae | Used by the Zulu people as a remedy for venereal diseases, used as a soap [3]. | Fruit and leaves | 10.09 ± 2.52 Current study | Present (current study) | Present (current study) | Present (current study) |
| Vachellia nilotica (L.) P. J. Hurter and Mabb Scented-pod acacia | Fabaceae | Cytotoxic bark is used as an astringent, assists in treating haemorrhaging, wound ulcers, leprosy, leucoderma, skin diseases, burning sensation, assists with seminal weakness. The trunk bark is used for colds, bronchitis, nausea, piles [87,88,89,90], soap making [91]. | Seed oil | 105.600 ± 9.994 mg/g [92] | Present [38] | Present [92] | Present [93] |
| Zygophyllum foetidum Schrad. and J.C.Wendl. Scrambling twinleaf | Zygophyllaceae | Used for washing clothes [3]. | Unknown | No records | No records | No records | No records |
| Plant Species | Alkaloids | Terpenoids | Saponins | Emulsion | Saponin Concentration (mg DE/g DW) |
|---|---|---|---|---|---|
| Calodendrum capense | + | + | + | + | 107.89 ± 4.89 |
| Carica papaya | + | + | + | + | 6.32 ± 1.20 |
| Crinum bulbispermum | + | + | + | + | 35.43 ± 4.25 |
| Cyathula uncinulata | − | + | + | + | 20.17 ± 1.70 |
| Deinbollia oblogifolia | + | + | + | + | 16.02 ± 2.26 |
| Ilex mitis | + | + | + | + | 8.37 ± 0.78 |
| Merwilla plumbea | − | + | + | + | 25.59 ± 0.83 |
| Noltea africana | + | + | + | + | 52.65 ± 6.81 |
| Plectranthus ciliatus | + | + | + | + | 18.88 ± 1.36 |
| Thunbergia dregeana | + | + | + | + | 10.09 ± 2.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohlakoana, M.; Moteetee, A. Southern African Soap Plants and Screening of Selected Phytochemicals and Quantitative Analysis of Saponin Content. Resources 2021, 10, 96. https://doi.org/10.3390/resources10100096
Mohlakoana M, Moteetee A. Southern African Soap Plants and Screening of Selected Phytochemicals and Quantitative Analysis of Saponin Content. Resources. 2021; 10(10):96. https://doi.org/10.3390/resources10100096
Chicago/Turabian StyleMohlakoana, Mpho, and Annah Moteetee. 2021. "Southern African Soap Plants and Screening of Selected Phytochemicals and Quantitative Analysis of Saponin Content" Resources 10, no. 10: 96. https://doi.org/10.3390/resources10100096
APA StyleMohlakoana, M., & Moteetee, A. (2021). Southern African Soap Plants and Screening of Selected Phytochemicals and Quantitative Analysis of Saponin Content. Resources, 10(10), 96. https://doi.org/10.3390/resources10100096







