Abstract
Korea initiated a new experiment, called a dynamic response system for open democratic societies as a principle to respond to the novel coronavirus (COVID-19). The global pandemic of COVID-19 led to a surge in demand for healthcare medical masks and respirators, and strained the global supply chain of mask production and distribution systems. This study provides a systemic view of critical personal protective equipment for both healthcare staff and the public to stop the spread of COVID-19. This study investigates the dynamic response system of healthcare mask production to the coronavirus and discusses lessons learned in view of systems thinking. The study shows that it is critical to developing a quick and dynamic response system to the evolving market conditions with flexible and agile operations. Visibility with transparency with information sharing with the public is also critical under global pandemic. Due to the shortage of mask supply, smart consumption is required along with collaboration with public and private sectors, as well as global organizations. Democratic leadership and a well-prepared strategic plan for long-term period are essential to the open society to prepare the global pandemic in the future. This study serves as a benchmark for dynamic and timely responses to the global pandemic.
1. Introduction
The first novel coronavirus (COVID-19) case was reported in South Korea on 20 January 2020. The Korea Center for Disease Control and Prevention (KCDC) was ready to fight the new pandemic with lessons learned from the painful experience of failing to respond to the 2015 MERS (Middle East Respiratory Syndrome) outbreak. The authority promptly convened an emergency committee meeting to discuss dynamic and timely responses on 22 January 2020 [1]. Korea initiated a new experiment, called a dynamic response system for open democratic societies as a principle to respond to the coronavirus, COVID-19 [2]. Rather than rigid ways of closing the border or deterring the flow of people and products crossing the borders around the world, the government began to build continuous sensing and response capabilities in addition to the contingency plans for responding to pandemics in the complex and uncertain crisis [3].
The KCDC has been trying to persuade and encourage people to wash their hands frequently for hand hygiene, to wear masks, and to practice social distancing in order to sustain global and local social and economic flows in an open society. In general, three common pathways of viruses are reported—droplets, aerosol, and fomites [4,5]. Although people practice social distancing, a campaign considering the cases of direct transmission through droplets promotes wearing a high-grade healthcare mask, such as a KF94 mask, to prevent aerosol infection in certain situations (as seen in China, Hong Kong, Korea, Singapore, and Taiwan). By doing so, people can continue to retain economic and social activities.
In addition, COVID-19 may be active for about 5 to 10 days without any symptoms [6], and can spread to others during this non-symptomatic period. The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) did not recommend that the public wear medical masks or respirators, except for infected patients and medical personnel [7,8]. However, the WHO and CDC recently started to recommend that the general public wear cloth masks to lower the likelihood of transmission, particularly as infected persons may be asymptomatic. Moreover, even though a person has no symptoms, wearing a mask is necessary to protect others from infection in case the person is infected without presenting any symptoms. Thus, the KCDC recommends wearing a healthcare mask if people with underlying medical condition contact others within 2 m of poorly ventilated space. The U.S. CDC also have changed their guidelines for the public, advising them to wear cloth masks in public settings, such as grocery stores and pharmacies, where significant community-based transmission is possible [9,10].
This has led to a surge in demand for healthcare, medical, and respiratory masks. Sudden spikes in demand have not only constrained production, supply of raw materials, and distribution systems from meeting demand, but also put a strain on the social systems. In general, the market size of masks is estimated by subtracting export from the sum of domestic production and import.
Recently, as the inhalation of fine dust has become a frequent cause of serious public health problems, Korea’s mask market has grown rapidly, and thus, its imports and production of masks increased exponentially—until the outbreak of the novel coronavirus in 2020. Exports have decreased, as domestic demand increases (Figure 1). While the capacity of domestic mask production in 2019 reached up to average three million masks per day, it was not enough for the market to respond to the coronavirus outbreak.
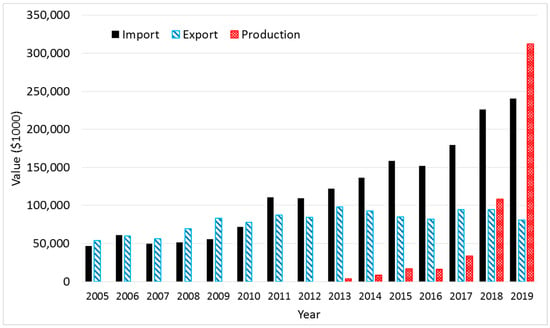
Figure 1.
Import and export data of the category of mask products (HS Code: 6307.90-9000) from 2005 to 2019 [11]. Korean won (KW) was converted into the U.S. Dollar based on the average exchange rate from Reference [12]. Production in 2019 was estimated by 3 million masks × 311 days per year × 390 KW per mask.
The COVID-19 global outbreak is an emergency, which in turn produces a range of consequences that require urgent and coordinated action to produce personal protection equipment (PPE), including face masks [13]. Due to the outbreak, the resources and capacity of production and distribution systems are constrained. To continue the systems operational and sustainable, system resilience plays an important role in the accomplishment of the system’s goals in the face of the global pandemic and limited resources [14]. The resilience system characterizes as adaptive capacity addresses a systems’ capability to dynamically extend capacity on the surge of mask demands to counter the global pandemic [14].
For that reason, the Task Force of Mask Supply Stabilization Measures was established on 26 February 2020 with the Ministry of Economy and Finance. The Task Force determined specific fair distribution and supply expansion measures, in order to address the difficulties of supply of raw materials appealed by manufacturers. This was also done while balancing demand and supply by simultaneously promoting cooperation and collaboration, and controlling mask demand in the market, from 6 March 2020 onwards.
To develop an appropriate resilience strategy, the magnitude and duration of the surge should be evaluated and determined to effectively respond to the disruption. However, the novel coronavirus is new to the world. Thus, organizational learning is central to managing the learning requirement to fight against the tricky and smart invisible enemy in interconnected dynamic systems [15]. This study extends the concept of organizational learning to societal learning—utilizing a system of a multi-organizational approach. The organizational learning is the collective learning to increase capacity, create new knowledge, advance technologies, and innovative process, and respond to unexpected market changes and demand.
This study investigates the dynamic response system of Korea to the COVID-19 pandemic and discusses lessons learned as part of a societal learning. It is too early to say whether the response is complete or successful, because the system is still evolving and adapting to address the dynamic nature of outbreaks globally. Therefore, the dynamic system response approach needs flexible systems with national and global collaboration and cooperation [13]. This study will be beneficial to the societies who experience similar cases of a dearth of healthcare and medical masks for now and for the future.
To investigate Korea’s dynamic response systems, this study adopts an exploratory and anecdotal analysis. In Section 2, types of masks are classified, and mask demand is analyzed regarding three scenarios, including lockdown and open democratic approaches. Demand management is also discussed while considering a rotation system to deal with the shortage of masks in the market and maintain a healthy society. Section 3 examines the ways to increase production capacity, design a supply chain network, and maintain resources of staff, finance, facility, as well as, governance and regulation. Section 4 discusses a production strategy to prepare for unexpected events in the future. Then finally, Section 5 concludes the study with a discussion of lessons learned from Korea’s dynamic response systems, and states the contribution of the study to the literature and the body of knowledge.
2. Product and Demand
2.1. Types of Products
Three categories are known in the face mask market in Korea. It is divided into ’industrial products’, ’non-drug, medical products’, and ’personal products’ (Table 1). Industrial products include respirators that protect wearers from airborne particles, such as dust. Non-drug, medical products, such as medical masks, are products authorized by a health authority for treating, reducing, treating or preventing diseases, and are subdivided into surgical, dental, veterinary, and procedure masks based on practices. Personal products like face masks are used for personal healthcare and safety protection.

Table 1.
Classification of masks.
This study classifies the masks based on the applications (i.e., industrial, medical, and personal products) and fabric types (i.e., woven and non-woven fabric). In general, respirators, known as an industrial product, are made of non-woven fabrics for industries such as construction and hazard areas. These masks are made of four protective layers: one carbon filter and one electrostatic melt-blown non-woven fabric between two spun-bonded non-woven fabrics. Medical products such as surgical, dental, and procedure masks and medical respirators are designed for the medical industry. Medical masks are mainly used in hospitals and clinics to prevent infections caused by blood or saliva during treatment or surgery due to their liquid resistance. The masks are generally three layers with three-ply materials of a melt-blown non-woven fabric between two spun-bonded non-woven fabrics. Woven fabric is also being used for healthcare professionals.
Healthcare masks are personal products used in daily life to protect respiratory organs from harmful particulate impurities or infectious sources due to their particle blocking performance. Sometimes, single-use, disposable respiratory and surgical masks are being used for personal healthcare; however, this study focuses on healthcare masks as a personal product to comply with commodity strategies to respond to the pandemic.
In the case of healthcare masks, the correction filter is used as the principle of applying high-pressure current to non-woven fabric to make the micro-organisms static, adsorbing, and filtering fine dust. For example, KF94 healthcare masks in Korea are prevalent to keep people from virus penetration. KF94 is a Korea quality certification provided from the Ministry of Korean Food and Drug Safety (MFDS), formerly know KFDA, that verifies that the masks can block more than 94% of particles with an average size of 0.4 μm (micrometer) and is resistant to oily particles [16,17]. The diameter of a hair is about 50–70 μm on average. This function similarly to the respirators of the N95/R95 in the U.S., the KN 95 in China, and the FFP2 in the European Union (see Table 2).

Table 2.
Types of certified healthcare, medical, and respiratory masks.
The letter of “N” in N95 stands for non-oily particles, while the nomenclature of “R” in R95 is resistant to oil particle. The standards of respirators N95 and R95 are approved and recommended by the U.S. Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH) [22]. A surgical N95 mask consists of disposable facepiece respirators (FFR) for filtering viral particles and fine dust. The surgical N95 is certified and recommended by both NIOSH and the U.S. Food and Drug Administration (FDA). While surgical masks are not required for seal-check, the respirators are required to be sealed to prevent contamination. Since KF80, KF94, and KF99 have functions combined with the surgical masks and respirators, this study will call KF80, KF94, and KF99 masks as healthcare masks afterwards to differentiate from surgical masks and general respirators.
The production of healthcare masks involves the raw materials of spunbond (SB) non-woven fabric, melt-blown (MB) non-woven fabric filter, earrings or hair ties, and nose adhesion wires. Moreover, compared to other products, it goes through a production process of cutting-folding-inspection-earring attachment-inspection-packaging.
2.2. Demand Planning and Management
Mask market size can be predicted by subtracting exports from the sum of domestic production and imports. However, mask production data is not well published as it is perceived less in value and importance than other personal protective equipment (PPE). International transactions of masks can be queried through the UN Comtrade international trade statistics database. However, due to the nature of the various types of masks, various harmonized system (HS) codes can be used for references as follows [23]:
- HS 6307.90: Textiles, made up articles; sets; worn clothing and worn textile articles; rags (no. 63)/Textiles; made up articles (including dress patterns), n.e.s. in chapter 63, n.e.s. in heading (no. 6307)/non-woven disposal mask, hood mask, face mask, cotton mask, etc./Others (No. 630790)
- HS 3005.90: Wadding, gauze, bandages, and similar articles; (excluding adhesive dressings), impregnated or coated with pharmaceutical substances, packaged for retail sale
- HS 9018.90: Medical, surgical, or dental instruments and appliances; n.e.c. in heading no. 9018
HS 6307.90 is the most common for KF94/N95/KN95 types of masks. Table 3 shows the top 20 countries importing masks, and its volume is reported in U.S. dollars from 2010 to 2018. The data for 2019 is not complete yet at the moment. The United States is the leading country, followed by Japan and Germany. Korea ranked 13th in terms of import volume. The values reported in Table 3 could vary from Figure 1 because of the unit conversion, different sources of data, and the level of granularity in the trade data. While Figure 1 used 12 digits of code, Table 3 queried information using six digits of HS code. Nevertheless, the table tells us that the mask market is growing fast globally. Korea also imports the masks over $200 million per year in recent years.

Table 3.
Top 20 countries that imported the category of disposable masks (HS 6307.90) from 2010–2018 (Unit: $million).
Regardless of the global trend of mask import, the U.S. mask market is stagnant. Due to the global shortage of respiratory masks, the U.S. CDC recommends Contingency Capacity Strategies during expected shortages and Crisis Strategies during known shortages [24]. When N95 mask supplies are running low, the following are recommended to optimize the supply of the respiratory equipment:
- Use respirators as identified by CDC as performing adequately for healthcare delivery beyond the manufacturer-designated shelf life.
- Use respirators approved under standards used in other countries that are similar to NIOSH (CDC’s National Institute of Occupational Safety and Health)-approved respirators.
- Use additional respirators identified by CDC as NOT performing adequately for healthcare delivery beyond the manufacturer-designated shelf life [24].
Given the lockdown and travel ban approach carried out in many countries, the estimates of demand for healthcare KF-masks in Korea are shown in Figure 2 with the area graphs of demand of masks and a stacked bar chart of mask supply until August of 2020. Scenario A presents a lockdown approach with the travel ban, while Scenarios B and C demonstrate an open democratic approach. Scenario B is forecasted with very strong social distancing and minimal economic and social activities with limited travels. Scenario C assumes regular daily life as it was. However, if the government sustains an open democratic society and responds to dynamic emergency situations with uncertainty, the country needs over 200 million masks a week, resulting in demand volume for masks 1.5 times more than Scenario B and four times more than Scenario A.
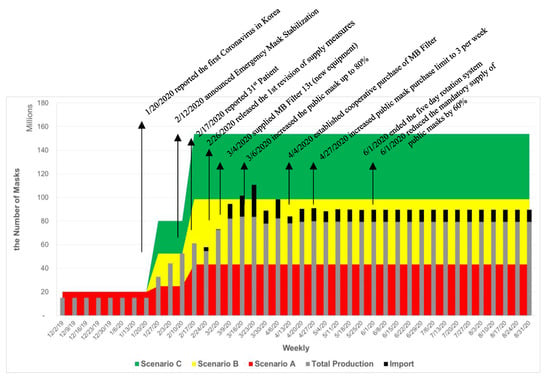
Figure 2.
Timeline of Response of Production System to COVID-19 in Korea. Until February of 2020, the inventory was not reported by the categories of surgical masks and respirators separately.
- Basic assumptions: This study assumes that around 36.8 million masks are estimated for college students, K-12 and kindergarten students, and business population in a day for five days a week. In addition, a medical population of 608,000 requires an average of 1.5 masks per day for seven days [25].
- Scenario A: With a lockdown approach, it is assumed that strong social distancing limits social activities, and that only 20% of the estimated population does basic economic activities five days a week.
- Scenario B: While maintaining strict social distancing and a high level of hygiene management, 50% of the estimated population actively engage in social and economic activities for five days a week.
- Scenario C: While practicing social distancing and a high level of hygiene management, 80% of the population is engaged in daily social and economic activities five days a week.
The normal demand for healthcare masks before COVID-19 was based on the needs of protection from the particle matters, including industrial fine and ultrafine dust and naturally originated yellow dust from the dessert of Mongolia and northern China. Most of the demand was met by the supply from China until January. Once the first coronavirus patient was reported on 20 January 2020, the demand for healthcare masks sharply increased. Since 12 February 2020, the Mask Stabilization Measures has been effective, resulting in double the production capacity. The 31st patient became a super-agent on 17 February 2020, resulting in a surge of mask demand. Even though the national capacity increased, the shortfall of demand for Scenarios B and C was not met without importing masks from other international suppliers or lowering the demand.
In effect on 26 February 2020, mask vendors are banned from exporting healthcare and surgical masks, and producers are limited to export masks less than 10% of the day’s production. Healthcare and surgical mask producers must quickly ship out more than 50% of the day’s production to public sales outlets. As part of Mask Supply Stabilization Measures, the Korea Public Procurement Service (KPPS) contracted with the producers to purchase the public masks.
Based on the revised measures on 6 March 2020, the manufacturers are required to ship out 80% of total production via the public distributors within two days from the production date [26]. Twenty percent of this public volume is allocated for use by medical and healthcare staff and underprivileged people. The masks are being distributed by the four medical associations to ensure fair distribution. The rest of the volume is available to the general public via the public mask distribution system.
On 6 March 2020, 726 million public health masks were distributed via public distribution channels. However, due to the long line to secure masks and limited supply, Korea began implementing the five-day rotation system on 9 March which was successfully implemented in Taiwan to distribute healthcare masks to the public in February 2020 [27]. As the mask production capacity and inventory increase, the mask supply becomes stable, the public has been able to purchase up to three masks per week since 27 April 2020.
To ensure that the general public purchases masks in rural, suburban, and urban areas, the government signed contracts with three wholesalers for distribution, and the final goods are available at around 23,000 local retailers [26]. To effectively control and manage the distribution system and inventory, the information portal of Health Insurance Review and Assessment Service was determined to be used to connect the mask distributors of hospitals, pharmacies, national agricultural cooperatives (Nonghyup marts), and post offices [28].
3. Production Capacity Planning and Expansion
A system is a set of sub-systems, which are inter-related. A system is affected by internal and external environments. Every system has inputs and outputs to achieve its goals. The performance of the system can be measured by the output. In order to respond to the global pandemic, the mask production system is a set of related components that work together in an uncontrollable environment of uncertain demand to sustain an open democratic society in a healthy and safe way. To achieve the goal, the control tower should be able to regulate the inputs to gain the outputs by performing functions of supplying materials, producing high-quality products, and distributing masks to the market in a timely and affordable way.
To meet emergency needs in a short time period, the mask production should respond to the needs quickly. The production activity is a set of processes relying on direct input such as raw materials and indirect inputs of capital investment in facilities and equipment and staff (human resources). For transforming inputs to outputs, technology availability, regulation, market condition, a quality standard is one of the essential factors affecting the process as a whole system. The outputs can be measured by the number of high-grade masks, unit cost, and profit of a product (Figure 3).

Figure 3.
Generalized process diagram of medical mask production.
3.1. Global Production Trends
Fifty percent of the world’s production volume of about 10 million has been produced in China. Mask production volume from the five provinces of Zhejiang, Shandong, Hebei, Beijing, and Henan is 61.38% of China’s production volume [29]. To meet the dearth of masks, China has undertaken a mobilization of wartime proportions for the production of surgical masks. Since then, daily production increased sharply from about 10 million to 115 million in February of 2020.
However, according to the World Health Organization (WHO), demand for masks has increased more than 100 times due to the COVID-19 pandemic [30], and in January and February 2020, most of the world’s production was exported to China, ironically, where explosive demand was generated. Other countries (who were exporting masks to China) were unable to purchase raw materials supplied from China, eventually resulting in disrupting the global supply chain. Therefore, it has become impossible to meet the demand for mask products and to export finished goods to China. According to a model presented by the World Health Organization, 89 million masks will be needed every month in response to COVID-19 [31]. However, this number is still uncertain and not reliable for future demand.
Based on the UN Comtrade database, China led the mask related products export by over $5 billion in 2018 (Table 4). This explains more than 45% of the world export in value. Korea’s exporting activity was decreased by the increased domestic demand due to the MERS (Middle East respiratory syndrome) outbreak in 2015 and the increased frequency of severe fine dust alarms in the Korean peninsula. The export market is led by China, Germany, USA, Vietnam, Mexico, and India by 71.5% of total export in 2018.

Table 4.
Top 20 countries that exported the category of disposal masks (HS 6307.90) from 2010–2018 (Unit: $million).
3.2. Dynamic Response
Until the first case of the infected by coronavirus on 20 January 2020 in Korea [32], the healthcare masks including KF80, KF90, and KF94 were used to protect from the seasonal yellow sand and particle-laden smog, which are fine dust of PM2.5 particulate matter (a diameter less than 2.5 micrometers). The demand exceeding the domestic production was filled by imported masks from China. After the first case of coronavirus, the KCDC recommended people wear one of KF healthcare masks in public places or in situations where practicing social distancing is difficult.
The local production capacity cannot meet the surge of demand. Furthermore, the Chinese government banned exporting medical masks and respirators produced in China on 19 February 2020 until lifting the ban on 5 March 2020 [33], and the price of masks doubled in February 2020 [34]. Nevertheless, the mask demand is also extremely high in China, and China plans to export masks one-on-one to each country under control along with mask-diplomacy. Thus, it is expected that it will be difficult to expand the supply of high-grade masks in Korea. While the United States and Japan were believed to have their own capacity to supply the masks and its raw materials by mobilizing their industries, Korea heavily relied on the Chinese suppliers of the mask raw materials by almost 90% [35,36].
The healthcare mask producers in Korea started to produce more masks by utilizing the full capacity of all production lines. In addition, as 12 new manufacturers of healthcare masks have been approved since 4 February 2020, if the company starts production in earnest, it is expected that the capacity of mask production will increase and contribute to supply and demand stability [37].
On 12 February 2020, the Korea Ministry of Food and Drug Safety announced Emergency Demand and Supply Stability Measures for Masks and Hand Sanitizer in response to the Price Stabilization Act (Article 2 and 6) to fill the gap of the imbalance of healthcare mask and surgical mask supply and demand in the COVID-19 emergency situations. The act was implemented for the purpose of stipulating matters concerning the highest price designation and emergency supply adjustment measures for healthcare and surgical masks and hand sanitizers (Report 2020-9 on the Ministry of Food and Drug Safety) [38]. This emergency implementation allows the director of the Ministry of Food and Drug Safety to order mask producers to expand healthcare mask production beyond a certain quantity considering the status of production facilities and the quantity produced in the past, if necessary, for the supply and demand for healthcare masks. The government can provide physical, human and administrative support manufacturing workforce, if it is deemed necessary for mask producers to fulfill the above orders. In principle, the export of masks is prohibited except for the export of masks for humanitarian purposes until 30 April 2020 [38].
On 26 February 2020, the above measures deleted the temporary schedule (30 June 2020) specified by the revision and added a surgical mask to the listed items (the Ministry of Food and Drug Safety Notice 2020-13). Manufacturers will be allowed to export up to 10% of the masks they produce each day.
On 6 March 2020, the measures for supplying public masks was revised, resulting in banning the export of healthcare and medical masks. It was decided that up to 80% of domestic production would be allocated by the government to the market [26].
3.3. Investing in Facility and Equipment
The government encouraged the manufacturers to produce more masks to fill the gap between demand and supply. Facilities and equipment are capital intensive inputs for the production process. For the producers expanding their capacity by upgrading existing equipment and installing new production lines and purchasing equipment, the government provided financial supports and incentives [26]. New production lines require capital cost and time to install. Accordingly, the government decided to use existing production lines and equipment for short-term strategies. The maintenance and upgrading costs are also subsidized.
3.4. Mobilizing Industry
If companies switch similar products, such as clothing and household goods, to masks and mask filters, the companies are eligible to receive special funds, and their licensing process will be expedited. The government reserved a special fund of $3.5 million (4200 million Korean won) for procurement of a high performance packaging machine to raise the existing production capacity by 30%, which is equivalent to 700,000 high-grade healthcare masks per day.
Although mask production is less valuable than other products, other manufacturers are urged to focus on mask production to respond to the pandemic. If these investments in production and equipment result in low demand in the future, the company will hesitate to invest, so the government decided to purchase all of its excessive production for national safety stock until the production lines return to its original production line.
3.5. Staffing
The Korea Labor Standards Act (KLSA) stated that work hours per week should not exceed 40 h a week and 8 h a day (Labor Standards Act, Article 50); however, the maximum number of hours an employee can be extended up to is 52 h upon an agreement between the employer and employee (Labor Standards Act, Article 53). This is effective from 1 July 2018 in workplaces with 300 or more employees and 1 January 2020 in smaller workplaces with 50–300 employees [39]. Where the Minister of Employment and Labor deems that the extension of work hours may order the employer to give employees recess hours or leaves of absence, corresponding to the extended work hours (Labor Standards Act, Article 53). Referring to KLSA Article 52, the approval process of the special extension of work hours was expedited. Workplaces with 5–50 employees will be given one more year—until 1 July 2021—to conform to the law (Act No. 15513, Article 1). Workplaces with fewer than 30 employees will be allowed from 1 July 2021 to 31 December 2022, to have up to eight hours of special overtime if such an agreement is made between the employer and employees (Labor Standards Act, No. 15513, Article 53).
The government also subsidized the labor cost for overtime and weekend. The production line has been running 24 h for seven days (three shifts a day) like during a war. The capacity expansion should receive machinery to produce mask and raw materials and adequate staff level. Overtime working hour regulations were released, and the overtime labor cost was subsidized by the government. Referring to the law, the overtime wages are as follows:
“An employer shall, in addition to the ordinary wages, pay employees at least 50/100 thereof for extended work and pay employees who perform work on a holiday an amount the same as or more than the following amounts:
An employer shall, in addition to the ordinary wages, pay at least 50/100 thereof to employees who perform night work (referring to the work performed between 10:00 p.m. and 6:00 a.m. of the next day). (Labor Standards Act, Article 56)”
Even if the overtime and weekend work of the producers is carried out, the difficulties of operations are aggravated, due to the safety and health issues caused by the working hours of the maximum hyperactive capacity, and the burden of childcare, due to the postponement of beginning for the spring semester. Therefore, flexible working hours and additional staffing may be considered in addition to the overtime.
The mask manufacturers will first arrange the human resource of mask producers through employment centers. For the mask producers that hire additional workers to expand mask production capacity, the government temporarily support the monthly labor costs of up to 800,000 Korean won (KW) per person to secure the necessary workforce until the end of June, which is the deadline of public mask production plan [26].
3.6. Regulations
Healthcare and surgical masks were classified as a “quasi-drug”, due to the fiber products used for the purpose of alleviating or preventing human diseases. Non-medical sanitary aids, such as a mask that offers protection from cold weather, are classified as industrial consumer goods (Pharmaceutical Affairs Act, No. 14328, Article 2, 2 December 2016). Healthcare masks, as non-medical sanitary aids, should be sealed properly for each product and include essential information legally required to label and package for protecting consumers under the pharmacist’s law. Unpackaged bulk products have no way to be checked (for example, if they are masks that cheat on the expiration date or fail to meet the performance criteria). For faster processing and lowering the packaging cost, regulations on mask packaging may be mitigated from individual packages to an economy pack or bulk at the production stage. If doing so, public sales outlets designated by the government temporarily allow the sale of individual masks by unbulking a box of hundreds of masks and repackaging them. However, mitigation measures should not be compromised with product safety. The package should indicate the product, manufacturer or importer, quantity, serial number, and expiration date, etc. By eliminating non-value-added activities of repackaging and splitting bulk at distribution centers and retailers, the distribution channels and pharmacies can improve quality and focus on customer service. The Korea Pharmaceutical Association argued that in order to ensure consumers’ right to know and secure the safety of the public masks, one or two masks should be packed.
Under the current law, masks can only be licensed as non-medical (healthcare mask) if the dust collection efficiency is over 94% based on a certain standard (the Ministry of Food and Drug Safety Notice 2017-40). Pre-shipping and post-inspection are applied to relieve the burden of mask inspection and shorten the lead time. In addition, if the government plans to change the grade of the public masks from KF94 to KF80, the production capacity will increase by 1.5 times and decrease the requirement of raw materials of the mask filter fabric.
3.7. Research and Development (R&D)
Research on nano filter masks, which are masks that can be washed and reused several times by replacing disposable MB filters that lack supply and demand, is being actively carried out. However, no product has yet been officially approved by passing safety tests. According to the Ministry of Food and Drug Safety, nanofabric should meet the criteria of the high standards. In order to manufacture and sell nanofabric for medical and health masks, the product must be approved after reviewing the safety, effectiveness, and quality standards of the product with the guidelines of the manufacturing industry. However, since nanofilters are new materials that are not used for medical supplies in Korea, the authorized test center need to prepare new safety and effectiveness criteria [40]. Several R&D centers and companies have been developing functional filter materials with a 99.9% sterilization rate in recent years using electrospun copper sulfide (CuS) nano materials that are harmless to the human body [41].
3.8. Cost and Profit
In the market, a KF94 mask is being sold at the range of 730 to 6900 KW in the middle of February 2020 [42]. As per the Price Stabilization Act 10,623 (5/2/2011), the government may, when deemed necessary, to stabilize the people’s livelihood and the national economy, set price ceilings on particularly important commodities. Price ceilings may be set for each region and for each trading stage, such as the production stage, the wholesale stage, and the retail stage [43].
Due to the increase of the price of raw materials, especially with MB unwoven fabric, and adding incentives for quick production expansion, the unit cost of a product increased as well to 1000 KW per mask (Figure 4). The average labor cost per mask is 80 KW during the week, and 140 KW for the night and weekend working hours. To expand the capacity, the government increased the unit price of a mask by 50 KW for overproduction compared to the regular production capacity during a week and all products produced during the weekend. The production cost of the public masks is around 600–700 KW.
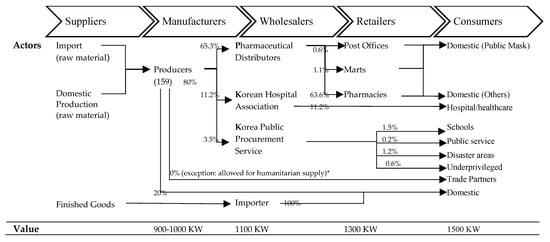
Figure 4.
Actors in the value chain of mask products and value chain mapping [26]. The value is based on the public mask measures [44]. The volume is the mean value between 17 April and 15 May 2020 [45]. (*) From 1 June 2020, 10% of health mask production is allowed to export.
On 6 March 2020, the government contracted with healthcare mask manufacturers for 900–1000 KW per mask, and pharmaceutical distributors supply them to the pharmacies for 1100 KW [46]. Some pharmacies pay 1300 KW for each unit to the pharmaceutical distributors. With the value-added tax (VAT) of 150 KW, the card membership fee of 30 KW, and labor cost of pharmacists, the price of a mask is 1500 KW to the consumers [47]. First of all, in order to provide incentives for increased mask production, the standard purchase price will be raised by more than 100 KW, and the purchase price will be raised further, especially depending on weekend and night production performance [48].
4. Strategic Production Plan for Emergency
Table 5 summarizes production strategies for emergency preparedness, response, and recovery phases. If the production level is high, and the demand becomes slow in the near future after flattening the pandemic curve, the inventory level will go high with high holding costs. Thus, the Korean government guarantees to purchase the surplus inventory and production from the manufacturers. However, the demand level and the duration of the pandemic is unknown and cannot be predicted with high uncertainty. Thus, once production systems are under control, and the demand is met, chase strategy may be implemented [49]. Ontario, Canada has stockpiled around 55 million N95 face masks after the outbreak of Severe Acute Respiratory Syndrome (SARS) in 2002 and 2003, and 80% of them expired in 2017 based upon the manufacturer’s determination [50]. They can be used only under special shortage situations such as COVID-19 based on U.S. CDC guidelines. Given this lesson, the level of inventory should be tracked, and a replacement plan should be included with the production strategy for long-term prediction.

Table 5.
Production strategies for an emergency.
Once the supply exceeds the demand with approaching the end of the pandemic, the production strategy will be switched to make-to-stock producing. The excess production will be purchased by the government for the strategic national stockpile (SNS) in response to unpredictable public health emergencies [51,52,53]. The emergency supply chain plays a critical role in the public health to assist the control of epidemic outbreaks [54]. For long-term strategic logistics and supply chain plan and short-term operational logistics management, the program should review the following functions: Collaborating and coordinating information sharing with private sectors, national agencies, and local government, managing quality and controlling inventory, and designing supply chain network and forecasting demand [55].
In short, the strategic objectives should achieve a resilient system with the flexibility, whole-of-government, whole-of-society, approach, and operational readiness [13]. The strategic approaches can be accomplished using a plan-do-check-act (PDCA) cycle. The cycle requires to plan operational readiness with stakeholder at regional, national, and international level, successfully implement (do) timely, cost-effective action plan by coordinating stakeholders and multi-sectors, evaluating and taking corrective action by monitoring and evaluating in line with standardized performance measures, tools, and process based on social and/or organizational agreement, and take action based on the lessons learned from the previous steps. This cycle will be reciprocal to improve the response systems in response to dynamic environments and changes of demand.
5. Conclusions
This study investigated how Korea responded to the challenging expansion of facemask production. This is terms of dealing with the high public demand and high-quality standards to meet the sudden increase of demand during the COVID-19 pandemic. The case study was conducted using an anecdotal analysis and exploratory analysis with publicly available data.
The study found that transparency, openness, and cooperation are essential to get through these challenging times. Because the global supply chain system is critical for prosperity, and supply chain systems all rely on each other, collaboration is essential to overcome global health crises together in open democratic systems [13]. Since the coronavirus evolves and the spread of the virus is dynamic, borderless, and can be very rapid; the pandemic production systems needs an all-inclusive approach to management systems to meet the shortfall of PPE, including mask needs [14]. Due to the novelty of COVID-19, the world is still learning. Thus, system thinking is crucial to public health disaster management for adaptation and learning [15], thereby allowing the world to respond to the virus in a manner that is timely, effective, and efficient [13].
This study contributes to the existing literature and body of knowledge by providing lessons from the case of Korea’s dynamic response systems of mask production. This study also provides a systemic view of a critical piece of PPE—both for health staff and the public—to help prevent the spread of COVID-19. This study can serve as a benchmark for dynamic and timely response to the global pandemic and health issues.
In conclusion, the authors recommend the following:
- To develop a dynamic and timely response system to the changing market with flexible and agile operations.
- To increase visibility of inventory, distribution, and production systems.
- To collaborate with public and private sectors along with global organizations (such as WHO) for the global supply chain to overcome global challenges.
- To secure multiple separate channels for healthcare staff, public service providers, the underprivileged, and general consumers.
- To increase awareness of smart consumption with effective demand management.
- To develop management systems that respond to the dynamic, uncertain situations.
- To develop a strategic plan for long-term, and efficient operations for short-term.
The study was limited by the shortfall of collected data, since the period of study was shortly after the outbreak. The mask production data is also limited because the product has been managed as several categories of general masks, medical masks, and industrial respirators. PPE, such as healthcare masks, is essential to the outbreaks; therefore, masks should be maintained and controlled in effective ways.
Author Contributions
Conceptualization, E.L. and Y.-Y.C.; investigation, E.L., Y.-Y.C. and M.M.; resources, Y.-Y.C., M.M. and E.O.; data curation, E.L. and Y.-Y.C.; writing—original draft preparation, E.L. and Y.-Y.C.; writing—review and editing, M.M. and E.O.; visualization, E.L.; project administration, E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the reviewers for valuable comments. The authors also would like to thank Hoobum Yoon, a student research assistant, for collecting data of public mask production in Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Novel Coronavirus—Republic of Korea (ex-China). Available online: https://www.who.int/csr/don/21-january-2020-novel-coronavirus-republic-of-korea-ex-china/en/ (accessed on 21 January 2020).
- KTV. South Korean Government Joint-Briefing on COVID-19; Korea TV: Daejeon, Korea, 9 March 2020; Available online: http://www.ktv.go.kr/content/view?content_id=594852 (accessed on 18 April 2020).
- Nohria, N. What Organizations Need to Survive a Pandemic; Harvard Business Review: Boston, MA, USA, 2020; Volume 30. [Google Scholar]
- Mubareka, S.; Lowen, A.C.; Steel, J.; Coates, A.L.; García-Sastre, A.; Palese, P. Transmission of Influenza Virus via Aerosols and Fomites in the Guinea Pig Model. J. Infect. Dis. 2009, 199, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.D.; Loutfy, M.; McDonald, L.C.; Martinez, K.F.; Ofner, M.; Wong, T.; Wallington, T.; Gold, W.L.; Mederski, B.; Green, K.; et al. Possible SARS Coronavirus Transmission during Cardiopulmonary Resuscitation. Emerg. Infect. Dis. 2004, 10, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Harvard Medical School, Coronavirus Resource Center. 2020. Available online: https://www.health.harvard.edu/diseases-and-conditions/coronavirus-resource-center (accessed on 14 April 2020).
- Guzman, J. WHO: No Evidence Wearing a Mask Can Protect Healthy People from Coronavirus. Available online: https://thehill.com/changing-america/well-being/prevention-cures/491725-who-no-evidence-wearing-a-mask-can-protect (accessed on 25 April 2020).
- World Health Organization. Advice on the Use of Masks the Community, during Home Care and in Health Care Settings in the Context of the Novel Coronavirus (2019-nCoV) Outbreak. Available online: https://www.who.int/docs/default-source/documents/advice-on-the-use-of-masks-2019-ncov.pdf (accessed on 29 January 2020).
- U.S. Center for Disease Control and Prevention. Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html (accessed on 3 April 2020).
- World Health Organization. Advice on the Use of Masks in the Context of COVID-19. Available online: https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1&isAllowed=y (accessed on 6 April 2020).
- Korea Customs Service. Trade Statistics. Available online: https://unipass.customs.go.kr/ets/index_eng.do (accessed on 13 May 2020).
- Bank of Korea. Economic Statistics System. Available online: https://ecos.bok.or.kr/flex/EasySearch.jsp (accessed on 13 May 2020).
- World Health Organization. A Strategic Framework for Emergency Preparedness; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Madni, A.M.; Erwin, D.; Sievers, M. Constructing Models for Systems Resilience: Challenges, Concepts, and Formal Methods. Systems 2020, 8, 3. [Google Scholar] [CrossRef]
- Ratnapalan, S.; Uleryk, E. Organizational Learning in Health Care Organization. Systems 2014, 2, 24–33. [Google Scholar] [CrossRef]
- Ham, S.; Choi, W.-J.; Lee, W.; Kang, S.-K. Characteristics of Health Masks Certified by the Ministry of Food and Drug Safety. J. Environ. Health Sci. 2019, 45, 134–141. [Google Scholar] [CrossRef]
- Kyung, S.Y.; Jeong, S.H. Particulate-Matter Related Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- U.S. Center for Disease Control and Prevention. NIOSH-Approved Particulate Filtering Facepiece Respirators. 2020. Available online: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/default.html (accessed on 16 April 2020).
- Dato, V.M.; Hostler, D.; Hahn, M.E. Simple Respiratory Mask. Emerg. Infect. Dis. 2006, 12, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, J.; Lee, S.; Lee, J.; Kim, J.; Tsai, P.; Yoon, C. Comparison of Filtration Efficiency and Pressure Drop in Anti-Yellow Sand Masks, Quarantine Masks, Medical Masks, General Masks, and Handkerchiefs. Aerosol Air Q. Res. 2004, 14, 991–1002. [Google Scholar] [CrossRef]
- European Committee on Standardization, Making Standards for Europe, 6 May 2009. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT:32928&cs=1B0AB06FEB70E43960D46D1198C37CC09 (accessed on 15 April 2020).
- U.S. Food and Drug Administration. N95 Respirators and Surgical Masks (Face Masks). Available online: https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-and-surgical-masks-face-masks#s6 (accessed on 20 April 2020).
- UN Comtrade. “UN Comtrade,” United Nations Commodity Trade Statistics Database. 2019. Available online: https://comtrade.un.org/db/default.aspx (accessed on 14 May 2020).
- U.S. Center for Disease Control and Prevention. Summary for Healthcare Facilities: Strategies for Optimizing the Supply of N95 Respirators during the COVID-19 Response. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/checklist-n95-strategy.html (accessed on 6 April 2020).
- KOSIS. Summary of Industry and City & Province. Available online: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1KB9001&vw_cd=MT_ZTITLE&list_id=J_20_6&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE (accessed on 27 March 2020).
- Korea Ministry of Economy and Finance. Mask Supply and Demand Stabilization Measures. Available online: http://www.moef.go.kr/com/synap/synapView.do;jsessionid=7kkTyiOn4kjlqZZHDh1ckLY4.node10?atchFileId=ATCH_000000000013363&fileSn=3 (accessed on 5 March 2020).
- Chuan, K.; Kao, E. Taiwan Approves New Rationing System for Surgical Masks. Available online: https://focustaiwan.tw/society/202002030019 (accessed on 3 February 2020).
- Health Insurance and Review Assessment Service. “HIRA System,” May 2020. Available online: https://www.hira.or.kr/eng/about/08/02/index.html (accessed on 14 May 2020).
- Bradsher, K.; Alderman, L. The World Needs Masks. China Makes Them, but has been Hoarding Them, The New York Times. Available online: https://www.nytimes.com/2020/03/13/business/masks-china-coronavirus.html (accessed on 13 March 2020).
- Zhang, L. Coronavirus: Demand for Face Masks Creates Shortfall for Those in Real Need, 7 February 2020. Available online: https://news.un.org/en/story/2020/02/1056942 (accessed on 12 April 2020).
- World Health Organization. Shortage of Personal Protective Equipment Endangering Health Workers Worldwide, 3 March 2020. Available online: https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide (accessed on 12 April 2020).
- Song, J.-Y.; Yun, J.-G.; Noh, J.-Y.; Cheong, H.-J.; Kim, W.-J. Covid-19 in South Korea—Challenges of Subclinical Manifestations. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Villasanta, A. China Hoards PPEs, Blocks Export of Coronavirus Masks: This Is ‘Considered First-Degree Murder’. Available online: https://www.ibtimes.com/china-hoards-ppes-blocks-export-coronavirus-masks-considered-first-degree-murder-2953130 (accessed on 15 April 2020).
- Kwai, I. How a Pharmacy Handles Mask Hoarders and Coronavirus Fears. Available online: https://www.nytimes.com/2020/02/18/world/asia/mask-hoarders-coronavirus-pharmacy.html (accessed on 18 February 2020).
- Kang, S.-Y. China Expands Mask Production and Exports, but...It’s Hard to Meet the Demand in Korea. Available online: http://www.newspim.com/news/view/20200303000784 (accessed on 3 March 2020).
- Jin, Y.Z. Responding to the Global “Mask Shortage”: China, the United States and Japan have Made a Big Hit on Their Capacity. Available online: https://finance.sina.com.cn/chanjing/cyxw/2020-03-03/doc-iimxxstf5939393.shtml (accessed on 3 March 2020).
- Korea Foods and Drug Administration. Announcement of the Current Status of Health Mask Production. Available online: https://www.mfds.go.kr/brd/m_99/view.do?seq=43965&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 18 February 2020).
- Korea Law Information Center. Emergency Demand and Supply Stabilization Measures of Mask and Hand Sanitizer. Available online: http://www.law.go.kr/admRulSc.do?tabMenuId=tab133&eventGubun=060103&query=%EB%A7%88%EC%8A%A4%ED%81%AC+%EB%B0%8F+%EC%86%90%EC%86%8C%EB%8F%85%EC%A0%9C+%EA%B8%B4%EA%B8%89%EC%88%98%EA%B8%89%EC%A1%B0%EC%A0%95%EC%A1%B0%EC%B9%98#J595633 (accessed on 6 March 2020).
- Korea Legislation Research Institute. Labor Standards Act. Available online: https://elaw.klri.re.kr/eng_service/lawView.do?hseq=50313&lang=ENG (accessed on 4 September 2019).
- Yonhap, Scientists Develop Reusable Face Mask Filter. Available online: https://www.koreatimes.co.kr/www/nation/2020/03/119_286296.html (accessed on 17 March 2020).
- Kwon, Y.-T.; Ryu, S.H.; Shin, J.W.; Yeo, W.-H.; Chao, Y.-H. Electrospun CuS/PVP Nanowires and Superior Near-Infrared Filtration Efficiency for Thermal Shielding Applications. ACS Appl. Mater. Interfaces 2019, 11, 6575–6580. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H. Mask Prices are Up To 27.2% Higher Per Unit than They Were Two Weeks Ago. Available online: https://www.khanews.com/news/articleView.html?idxno=200901 (accessed on 18 February 2020).
- Korea Law Translation Center, Korea Price Stabilization Act. Available online: https://elaw.klri.re.kr/kor_service/lawView.do?hseq=22059&lang=ENG (accessed on 27 January 2012).
- Korea Ministry of Finance and Economy. Price Structure of Public Mask; Korea Ministry of Finance and Economy: Seoul, Korea, 2020.
- Korea Ministry of Food and Drug Safety. New and Notice; Korea Ministry of Food and Drug Safety: Seoul, Korea, 2020.
- The Ministry of Economy and Finance, Price Structure of Public Mask Supply Rights. Available online: https://www.mfds.go.kr/brd/m_99/view.do?seq=44004&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1# (accessed on 9 March 2020).
- Yul, L. Mask Distributors Receive 900 to 1000 Won and Supply 1100 Won to Pharmacies. Available online: https://www.yna.co.kr/view/AKR20200309001500002 (accessed on 9 March 2020).
- Public Procurement Service, Policy Briefing. Available online: Pps.go.kr/bbs/selectBoard.do?boardSeqNo=3060&pageIndex=1&boardId=PPS093 (accessed on 6 March 2020).
- Swamidass, P. (Ed.) Chase Strategy for Capacity Planning. In Encyclopedia of Production and Manufacturing Management; Springer: Boston, MA, USA, 2000. [Google Scholar]
- Martell, A.; Warburton, M. Millions of Masks Stockpiled in Canada’s Ontario Expired before Coronavirus Hit; Reuters: London, UK, 2020. [Google Scholar]
- Esbitt, D. The Strategic National Stockpile: Roles and Responsibilities of Health Care Professionals for Receiving the Stockpile Assets. Dis. Manag. Response 2003, 1, 68–70. [Google Scholar] [CrossRef]
- Malatino, E.M. Strategic National Stockpile: Overview and Ventilator Assets. Respir. Care 2008, 53, 91–95. [Google Scholar] [PubMed]
- Dimitrov, N.; Goll, S.; Hupert, N.; Pourbohloul, B.; Meyers, L. Optimizing Tactics for Use of the U.S. Antiviral Strategic National Stockpile for Pandemic Influenza. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Dasaklis, T.; Pappis, C.; Rachaniotis, N. Epidemics Control and Logistics Operations: A Review. Int. J. Prod. Econ. 2012, 393–410. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. About the Strategic National Stockpile. Available online: https://www.phe.gov/about/sns/Pages/about.aspx (accessed on 12 September 2019).
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N. Engl. J. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- National Research Council of the National Academies. Chapter 3: Emergency Management Framework; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).