1. Introduction
The continuous monitoring of the dynamics of the spread of infectious diseases such as influenza and COVID-19 is an important investigation, implementing the use of mathematical models to lay the foundations of proposed control strategies and then to evaluate whether the contagion curve can be flattened [
1,
2,
3]. Within control systems, we aim to drive the states of a given system to a desired point. The techniques are very varied as in [
4], and the one to be used depends specifically on the type of problem to be addressed. With the use of impulsive inverse optimal control, we can distribute resources for the treatment of diseases [
5].
By using neural networks as state identifiers, we can replicate the system behavior without completely knowing its parameters [
6,
7] because it does not require a precise model; it is adaptable to both influenza type A and COVID-19. The training approach proposed for the neural network is based on the algorithm of the extended Kalman filter [
8], as it has proven to be a reliable method with a fast learning speed [
9] compared to other methods, such as backpropagation, which requires calculating the gradient descent, and therefore its learning speed is affected [
10].
Epidemiological models based on differential equations allow for a realistic representation of the transmission dynamics of some diseases. Therefore, they become an excellent tool to generate control theory methods that make it possible to stop or eliminate the spread of diseases such as influenza type A and COVID-19, which are the cases of study in this paper. Complex networks describe interactions between different entities that produce a certain state for the whole network in general [
11]. This same interaction occurs in populations when a pandemic occurs.
In the case of seasonal influenza in the United States, it is estimated that 5% to 20% of the population is infected each year [
12]; considered in this range are those infected who are symptomatic and asymptomatic. In the case of COVID-19, there is a record of countries that report up to 68% of their infected population [
13].
Undoubtedly, the adequate distribution of medicines in the event of an epidemic outbreak carries a role of great importance for fighting infections and to control shortages or oversupply in health systems. We seek a proposal that simulates the spread of COVID-19 in a population susceptible to the disease. We consider the nodes of a complex network as dynamic systems represented by a variation of the SIR model. This model is considered to be the representation of various towns’ or cities’ populations interacting with each other during a pandemic.
In this research we use complex networks to simulate the interaction between some towns’ or cities’ populations that are affected by an epidemic outbreak of influenza type A and COVID-19. The use of a neuronal identifier allows us to use this scheme for both influenza type A and COVID-19, or any disease caused by a similar virus. The objective of using a pinning control scheme is to seek an effective distribution of treatments in the populations affected by the spread of these diseases.
2. Mathematical Background
In this section, we present the theoretical framework and mathematical models on which the proposal is based.
2.1. Susceptible-Infective-Removed Model
The complexity of pandemics is a subject of great interest for many areas, from those that are in charge of their surveillance, such as the medical and epidemiological perspective [
14], to other kinds of disciplines originating in engineering, such as control theory [
15]. In recent years, contagious diseases have appeared that require our attention and supervision. Influenza type A and COVID-19, for example, affect the respiratory tract [
16], spread rapidly, and, if not treated, can cause irreversible damage to the human body. Performing numerical simulations of the spread of the disease is a useful tool in the estimation of the scope that this type of phenomena can have [
17].
In 1927, Kermack and McKendrick proposed the Susceptible-Infective-Removed (SIR) compartmental model [
18], in which a susceptible individual in class
who is in contact with an infected individual in class
has a certain probability of moving to class
. In this model, the ability to infect susceptible individuals does not always remain latent, but rather disappears after some time. This situation is covered by individuals in the class
and can occur due to the natural course of the disease and the acquisition of immunity or due to death [
19].
The SIR model is represented mathematically in the following system of differential equations [
20]:
where
and
are the recuperation and transmission rate of the disease, respectively. The sum
, with
as the total population, which is considered to be constant. The conditions in which the set of Equation (1) is solved are:
,
,
and
.
2.1.1. Seasonality in the SIR Model
There exist many variants of the SIR model that accommodate the different behavior of different viruses. The new variants of COVID-19, for example, are considered a new contagion capable of reinfecting an individual who has not been exposed to this evolution of the virus [
21], which is also similar to what happens with influenza type A.
The work in [
22] proposes a variant of the SIR model that adds seasonality and an impulsive control scheme, in which birth and death rates are considered. The model with seasonality is shown in Equation (2):
where
is both the birth and death rate considered to be the same, and
represents the variable transmission rate that changes according to the season. The impulsive control dynamics are represented in Equation (3):
where
represents the portion of individuals who will receive treatment or vaccination against the disease. This type of control allows for establishing adequate measures for the application of treatments.
2.1.2. Impulsive Inverse Optimal Control for the SIR Model with Seasonality
There are several control methods to drive a given system to the desired state; which one to choose depends on the desired application [
4]. In particular, for this research, impulsive control adapts well to this type of biomedical application due to how medicine is usually administrated [
5,
23]. The work in [
5] proposes an impulsive inverse optimal control for the SIR model with the seasonality of Equations (2) and (3), applied to influenza type A. The controller proposed in [
5] is as follows:
where
,
, and
;
and
replace the constant
in Equation (3); thus, we obtain:
The variation in the portion of people treated or vaccinated against the disease is intended to optimize the available resources. The details on the optimality of this controller are discussed in
Section 2.4.
2.2. Complex Networks
A complex network can be considered as a set of dynamic systems that are connected to each other, and each one has its own characteristics [
24]. The mathematical representation of a complex network in which its connections are linear and diffusive is shown in the following model [
25]:
where
is the state vector of node
;
represents the self-dynamics of node
;
is the coupling strength between node
and node
,
when there is a connection between node
and node
,
when there is no connection between nodes
and
,
with
being equal to the number of connections of node
;
is a constant matrix that represents the way the different elements of the state vector are connected.
For a dynamic network to be synchronous, the states of the nodes must be measurable when the time tends to infinity [
26]. If a system is not stable, we can use a controller to correct that behavior. A control technique developed for complex networks pinning control, this technique allows for controlling only a fraction of the nodes and thus brings the system to the desired state [
27].
2.3. Discrete-Time Recurrent High-Order Neural Networks
A disadvantage of mathematical models is that they do not capture the imperfections of the real world. For this reason, we can use recurrent high-order neural networks (RHONNs), and, by configuring them as neural identifiers, we can recreate the dynamics of the real system [
28].
The following model presents the discrete version of the RHONN [
26]:
where
is the state of the
-th neuron at iteration
with
as the state dimension,
is the online adaptable weight vector, and
is defined as:
where
is the number of high-order connections;
is a set of
unordered subsets
; and
is a vector formed by the inputs of each neuron, defined as:
where
is a nonlinear sigmoid function, and
is the input vector to the neural network.
2.4. Impulsive Inverse Optimal Control
The impulsive controller of Equation (4) was developed from the following concepts [
23]. Consider a dynamic impulsive system defined by:
where
,
,
is an open set with
,
, with
,
and
are continuous, and
is the restart set with
,
,
. It is assumed that the dynamics of
are such that the solution to
in (10) is jointly continuous in
and
between resetting events
,
is the solution of
in (10) at time
with initial condition
, the point
is an equilibrium point of (10) if and only if
for all
and
.
is an equilibrium point of (10) if and only if
and
.
Consider the following controlled impulsive dynamical system:
and the hybrid performance functional:
where
and
are restricted to a class of admissible hybrid controls consisting of measurable functions such that
for all
and
,
,
,
satisfying
,
,
,
satisfying
,
, and
.
We assume there exists a continuously differentiable function
, and functions
and
such that
,
,
,
,
where
is admissible, and
as
.
Then, the zero solution
of the closed-loop system in Equation (12) is globally asymptotically stable with the optimal hybrid feedback control law
and performance functional (12), with
is minimized in the sense that
where
,
, and
satisfies Equation (11) for
,
.
3. Simulation Preliminaries
In this section, we detail the actual models used within the numerical simulation, which are derived from the models in
Section 2.
3.1. Complex Networks and Discretization of the SIR Model with Seasonality
Taking the complex network model of (6), we can define
as Equations (2) and (5) when
. Given the nature of the reported data on epidemics, we use a finite difference discretization for the model, which will result in the next set of equations:
where
is the state vector node
,
is the sampling time,
is the constant coupling strength equal for all node connections. From discretization, the impulsive dynamics result as follows:
where
and
are obtained from a discretized version of the controller described in Equation (4), which will be detailed further in another subsection.
The interpretation for this is that the nodes represent towns or cities that interact with each other during an epidemic outbreak through the connections or links between them.
3.2. Neural Identifier
Based on the model (7), we design the next neural identifier:
where
is a logistic function defined as follows:
To obtain the real estimated value of the system, we use the auxiliar variables obtained from Equation (12) and compute as follows:
This is executed so that the obtained values are consistent with the conditions established for the SIR model, specifically, that the states are positive and that their sum equals one.
3.3. Training with the Extended Kalman Filter Algorithm
We use the extended Kalman filter algorithm to train the neural network shown in (19). The equation for the adaptable weights is [
15]:
where
is the error signal between the system output
and the neural network output
, and
is the Kalman gain matrix defined as:
where
is defined as
in (10),
, and
is computed as:
where
is a
diagonal matrix with the number
in its diagonal elements,
, and all initial weights are random.
3.4. Control Law Synthesis
From the values obtained in (21), we use the controller shown in (4) to compute the impulsive control input. Then, Equation (4) turns into:
where the values for
,
, and
are heuristically determined as:
The values shown in (26) are used for both study cases. The general form of the controller has been used in [
29] in a single-system case with fairly good results.
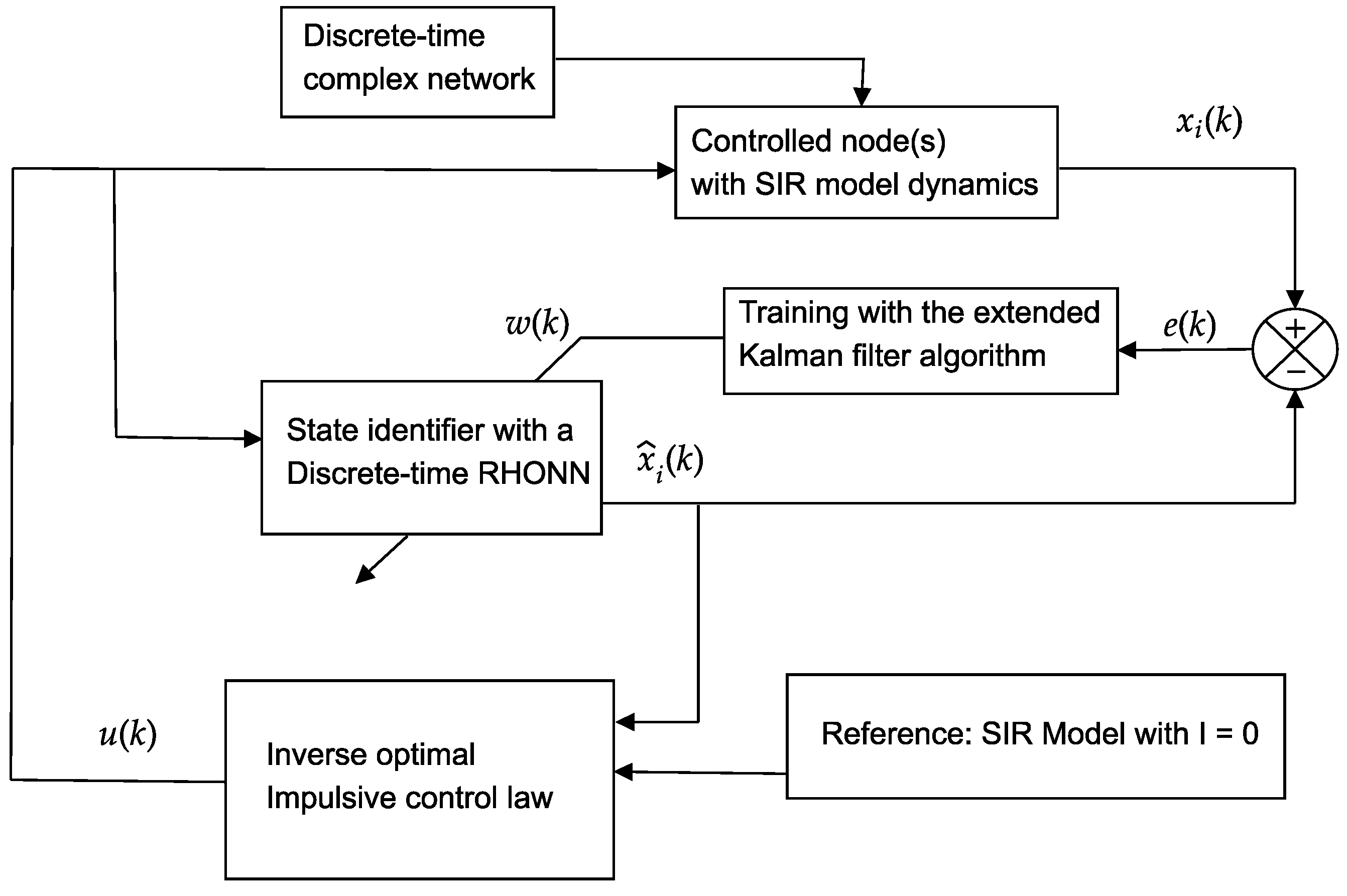
The control scheme used is illustrated in
Figure 1.
4. Numerical Simulations
Based on the encouraging results we obtained when applying this control scheme to the problem of influenza type A, we now apply it to a complex network case, including a scenario with COVID-19, which has a higher contagion rate.
In the work in [
30], it was shown that by using an impulsive pinning controller with a neural identifier, we can obtain good results for lowering the contagion curve. We now consider the nodes of a complex network as towns’ or cities’ populations described by the SIR model, which is represented by Equations (17) and (18).
The networks of
Figure 2 were designed for simulation tests in both study cases.
The parameters for the five-node network for both study cases are:
The only parameter that changes for the ten-node network is matrix
which is given by:
Next, we present other parameters specific to each study case and the results of the simulations.
4.1. Influenza Type A
Using models (17) and (18), the parameters used are
and
for the winter months and
for the rest of the year.
, and
which is roughly equivalent to 3 days of simulation time. These values were taken from [
31].
In the five-node network simulation, control was placed at node 3. In the ten-node network simulation, control was placed at nodes 1 and 2. Initial conditions for the controlled nodes are assigned at random. Both simulation scenarios start with the assumption that all nodes are unconnected to each other. After a month of simulation time, nodes in the network are connected according to matrix of Equations (27) and (28) for the network with five and ten nodes, respectively. The start of the connections simulates the transmission of the infection to other nodes. Vaccination and application of treatments occur from the beginning of the simulation and are repeated every year in the winter season.
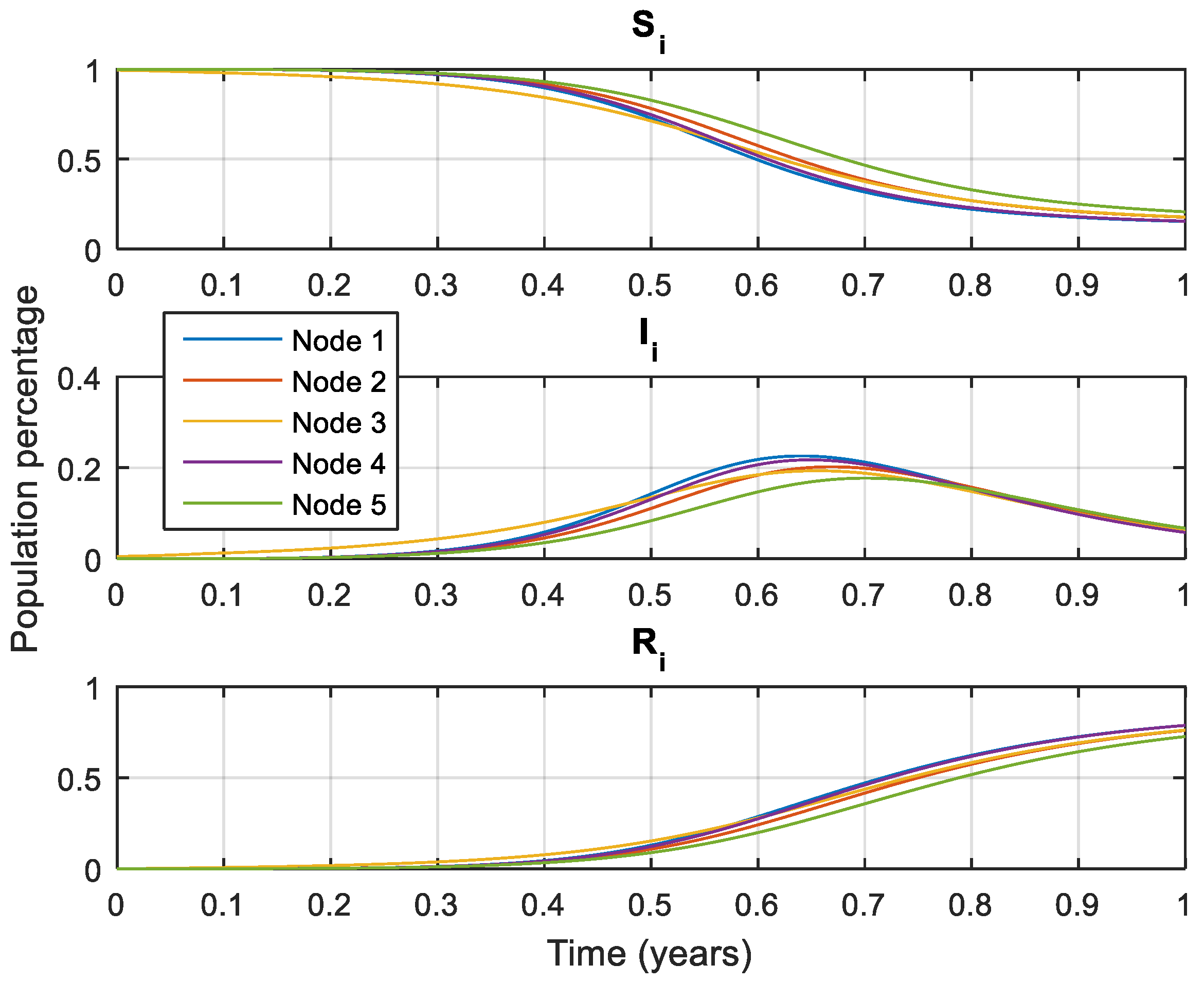
Figure 3 shows the dynamics of the five-node network for the case of influenza type A.
Figure 4 shows the suggested treatment for node 3 obtained by the control law shown in (25) for the five-node network and influenza type A disease.
Figure 5 shows the state of the network for the ten-node network and influenza type A.
Figure 6 and
Figure 7 show the suggested treatment for nodes 1 and 2 for the ten-node network and influenza type A disease.
4.2. COVID-19
The changes in parameters used for this study case are the following. The recuperation rate
, and the transmission rate is now invariant through time, which means there is no seasonality in this case, but the transmission rate varies between the different nodes with
,
,
,
, and
, for nodes
,
,
,
, and
respectively. These values were estimated according to the basic reproduction number of COVID-19 found in page 10 of [
32] and the disease recovery rate stated on page 14 of [
32], considering a hypothetical scenario. For the ten-node network, the transmission rate is the same for all nodes with
.
The initial conditions in the five-node network are , , and for node 3; the rest of the nodes start with , , and . The sample time is now which is a day of simulation time. Initial conditions in the ten-node network simulation are , , and for node 1 and , , and for the rest of the nodes. Simulations for this case are performed as before with the assumption that all nodes start unconnected to each other. After a month of simulation time, nodes in the network are connected according to matrix of Equations (27) and (28) for the network with five and ten nodes, respectively. The start of the connections simulates the transmission of the infection to other nodes. In this case, vaccination and application of treatments begin until day 105 of simulation time and are repeated each week of the duration of simulation for the selected nodes.
Figure 8 shows the dynamics of the five-node network for the case of COVID-19.
Figure 9 shows the suggested treatment for node 3 obtained by the control law shown in (25) for the five-node network and COVID-19 disease.
Figure 10 shows the state of the network for the ten-node network and COVID-19.
Figure 11 and
Figure 12 show the suggested treatment for nodes 1 and 2 for the ten-node network and COVID-19 disease.
5. Discussion
The results of
Figure 3 and
Figure 5 show the infected population declining to a low level, and, though is not zero, it is lower than without treatment or vaccination, and this has been accomplished with only one of the nodes for the five-node network and two nodes for the ten-node network. We can conclude the same for the results of the COVID-19 case shown in
Figure 8 and
Figure 10.
Figure 4,
Figure 6 and
Figure 7 show the variation in the suggested treatment and vaccination over time and how it settles as the simulation concludes. The overall population vaccinated and treated for the disease is quite low if one considers that only a certain percentage of the susceptible or infected population classes are receiving said vaccine and treatment. If we compare the vaccinated and treated population against the population for the whole node, the percentage is even lower. Similar conclusions can be made for the results shown in
Figure 9,
Figure 11 and
Figure 12 of the COVID-19 case.
Figure 13 shows an example of the five-node network with no treatment at all for the case of COVID-19. With this, we can compare the effects of the control scheme and how much it lowers the percentage of infected people, which is certainly a good feature of this proposal.
Though these results are encouraging, the model still needs to be validated and compared against real data of cities or towns populations. Through a time series of data from different population zones, we can verify the behavior of our model. This work remains in progress.
6. Conclusions
This research addressed an impulsive neural-pinning control model for complex networks based on a SIR model, which was previously applied to the case of influenza type A and is now adapted to the case of COVID-19 and a complex network approach in which nodes are considered to be towns’ or cities’ populations.
The results show that by applying the control to a small percentage of the nodes, the infected class of both diseases is reduced to a level much less than 50%. This represents a great advantage for the better use of resources during pandemics. However, we should not forget that this approach needs more work and validation before it serves as an accurate representation of reality. Some characteristics can be better modeled, such as the way node connections are presented as well as the interaction that nodes have through these nodes. Another important validation is to run tests along with real data to make comparisons. The presented results encourage us to continue this research line, and the missing details remain now as a work in progress.
Another important feature is that the proposed control scheme does not know the dynamic model, and by using the neural identifier, we approximate the necessary values for the controller to work.
Author Contributions
Conceptualization, E.A.H.-V. and A.Y.A.; methodology, A.Y.A., N.F.R. and E.A.H.-V.; formal analysis and investigation, D.R.-R.; writing—original draft preparation, D.R.-R.; writing—review and editing, D.R.-R., A.Y.A., N.F.R. and E.A.H.-V.; funding acquisition, A.Y.A.; resources, D.R.-R. and A.Y.A.; supervision, A.Y.A. and N.F.R.; validation, A.Y.A., N.F.R. and D.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACYT Mexico through Project FOP16-2021-01-375 319619.
Data Availability Statement
Data are available under request to the corresponding author.
Acknowledgments
Authors thank the Universidad de Guadalajara for support in the development of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, Z.; Glasser, J.W.; Hill, A.N. On the benefits of flattening the curve: A perspective. Math. Biosci. 2020, 326, 108389. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.; Mondal, A.; Antonopoulos, C.G. Dynamic tracking with model-based forecasting for the spread of the COVID-19 pandemic. Chaos Solitons Fractals 2020, 139, 110298. [Google Scholar] [CrossRef] [PubMed]
- Hethcote, H.W. The Mathematics of Infectious Diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
- Kovacevic, R.M.; Stilianakis, N.I.; Veliov, V.M. A Distributed Optimal Control Model Applied to COVID-19 Pandemic. SIAM J. Control Optim. 2022, 60, 221–245. [Google Scholar] [CrossRef]
- Sanchez, E.N.; Covarrubias, R.; Alanis, A.Y.; Hernandez-Vargas, E.A. Inverse optimal impulsive control for a SIR epidemic model. In Proceedings of the 2018 15th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE), Mexico City, Mexico, 5–7 September 2018. [Google Scholar] [CrossRef]
- Poznyak, A.S.; Sanchez, E.N.; Yu, W. Differential Neural Networks for Robust Nonlinear Control, 1st ed.; World Scientific Publishing Company: Singapore, 2001. [Google Scholar] [CrossRef]
- Ricalde, L.J.; Sanchez, E.N. Inverse optimal nonlinear recurrent high order neural observer. In Proceedings of the 2005 IEEE International Joint Conference on Neural Networks, Montreal, QC, Canada, 31 July–4 August 2005. [Google Scholar] [CrossRef]
- Singhal, S.; Wu, L. Training multilayer perceptrons with the extended Kalman algorithm. Adv. Neural Inf. Process. Syst. 1988, 1, 133–140. [Google Scholar]
- Williams, R.J.; Zipser, D. A Learning Algorithm for Continually Running Fully Recurrent Neural Networks. Neural Comput. 1989, 1, 270–280. [Google Scholar] [CrossRef]
- Werbos, P.J. Backpropagation through time: What it does and how to do it. Proc. IEEE 1990, 78, 1550–1560. [Google Scholar] [CrossRef]
- Wang, Z.; Moreno, Y.; Boccaletti, S.; Perc, M. Vaccination and epidemics in networked populations—An introduction. Chaos Solitons Fractals 2017, 103, 177–183. [Google Scholar] [CrossRef]
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin. Infect. Dis. 2018, 66, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Moreno Ariza, A.; Hernández Barrios, J.; Alfaro Sarmiento, I.; Troncoso Palacio, A. Comportamiento Adoptado en América Latina Debido al covid-19. Bol. Innov. Logíst. Oper. 2020, 2, 107–115. [Google Scholar] [CrossRef]
- Sanz, A.M. Concept and projection of infectiology. Med. Clín. 1995, 105, 534–536. [Google Scholar]
- Sanchez, E.N.; Alanís, A.Y. Neural Networks: Fundamental Concepts and Applications to Automatic Control, 1st ed.; Pearson Education: Madrid, Spain, 2006. [Google Scholar]
- De Haro López, C.; Ferrer Roca, R.; Vallés Daunis, J. Neumonía y síndrome de distrés respiratorio agudo producido por el virus influenza A (H1N1). Med. Intensiva 2009, 33, 455–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broeck, W.V.D.; Gioannini, C.; Gonçalves, B.; Quaggiotto, M.; Colizza, V.; Vespignani, A. The GLEaMviz computational tool, a publicly available software to explore realistic epidemic spreading scenarios at the global scale. BMC Infect Dis. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Kermack, W.O.; McKendrick, A.G. Contributions to the mathematical theory of epidemics. II. —The problem of endemicity. Proc. R. Soc. Lond. A 1932, 138, 55–83. [Google Scholar] [CrossRef]
- Poletto, C.; Gomes, M.F.; Pastore y Piontti, A.; Rossi, L.; Bioglio, L.; Chao, D.L.; Longini, I.M., Jr.; Halloran, M.E.; Colizza, V.; Vespignani, A. Assessing the impact of travel restrictions on international spread of the 2014 West African Ebola epidemic. Euro Surveill 2014, 19, 20936. [Google Scholar] [CrossRef] [PubMed]
- Brauer, F.; Castillo-Chavez, C. Mathematical Models in Population Biology and Epidemiology, 1st ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Organización Mundial de la Salud. Available online: https://www.who.int/es/activities/tracking-SARS-CoV-2-variants (accessed on 10 March 2022).
- Liu, X.; Stechlinski, P. Pulse and constant control schemes for epidemic models with seasonality. Nonlinear Anal. Real World Appl. 2011, 12, 931–946. [Google Scholar] [CrossRef]
- Haddad, W.M.; Chellaboina, V.; Nersesov, S.G. Impulsive and Hybrid Dynamical Systems, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Khalil, H.K. Nonlinear Systems, 3rd ed.; Prentice-Hall: Hoboken, NJ, USA, 2002. [Google Scholar]
- Chen, G.; Wang, X.; Li, X. Fundamentals of Complex Networks: Models, Structures and Dynamics, 1st ed.; John Wiley & Sons: Singapore, 2014. [Google Scholar]
- Wang, X.F. Complex Networks: Topology, Dynamics and Synchronization. Int. J. Bifurc. Chaos 2002, 12, 885–916. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, G. Pinning a complex dynamical network to its equilibrium. IEEE Trans. Circuits Syst. I Regul. Pap. 2004, 51, 2074–2087. [Google Scholar] [CrossRef]
- Alanis, A.Y.; Sanchez, E.N.; Loukianov, A.G.; Hernandez, E.A. Discrete-time recurrent high order neural networks for nonlinear identification. J. Frankl. Inst. 2010, 347, 1253–1265. [Google Scholar] [CrossRef]
- Covarrubias, R.; Alanis, A.Y.; Rios, D.; Sanchez, E.N.; Hernandez-Vargas, E.A. Discrete-time neural identification of a SIR epidemic model. In Proceedings of the 2018 IEEE Latin American Conference on Computational Intelligence (LA-CCI), Guadalajara, Mexico, 7–9 November 2018. [Google Scholar] [CrossRef]
- Ramirez, N.F.; Alanis, A.Y.; Hernandez-Vargas, E.A.; Rios-Rivera, D. Inverse impulsive optimal neural control for complex networks applied to epidemic influenza type A model. In Proceedings of the 2021 IEEE Latin American Conference on Computational Intelligence (LA-CCI), Temuco, Chile, 2–4 November 2021. [Google Scholar] [CrossRef]
- Centros para el Control y la Prevención de Enfermedades. Available online: https://espanol.cdc.gov/flu/professionals/acip/background-epidemiology.htm (accessed on 26 August 2021).
- World Health Organization. Available online: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed on 4 April 2020).
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).