Evaluating Public Health Efforts to Prevent and Control Chronic Disease: A Systems Modeling Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Development
2.2. Model Overview
2.3. Model Inputs
2.4. Model Testing and Analysis
3. Results
3.1. Base Run
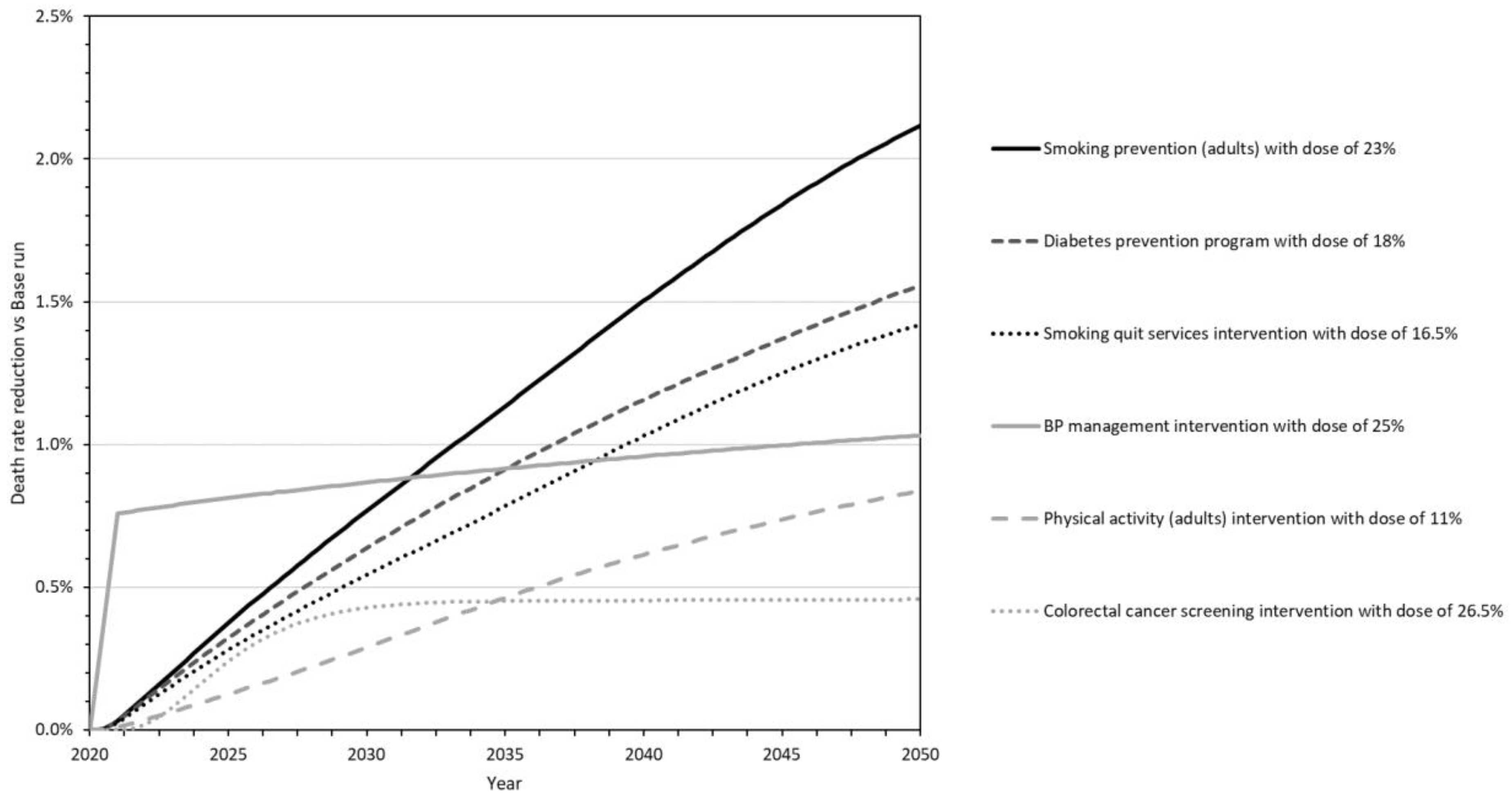
3.2. Individual Intervention Testing Results
- Smoking prevention for adults (Smoking Prevention_23) is a population health intervention that averts eight of the Combo10 causes of death (see Figure 1 to trace this and other interventions to impacted deaths). The dose was estimated to be 23% based on HP2030 goal TU-02. The death reduction is rapid for CVD but delayed for other causes of death as the intervention effects traverse changes in the incidence and prevalence of diabetes, cancer, asthma, and COPD before gaining strength throughout the simulation. It is the second-most impactful intervention through 2030, and the single most impactful by 2040 and 2050.
- The National Diabetes Prevention Program (Diabetes Prevention_18) is a clinical prevention intervention that ultimately averts 4 of the 10 specified causes of death. The dose was estimated at 18% based on HP2030 goal D-01. The death reduction is delayed (traversing the incidence and gradual progression of diabetes) but gains strength throughout the simulation to make this the second-most impactful intervention by 2040 and 2050.
- Smoking cessation services and products (Smoke Cessation_16.5) represent a clinical prevention intervention that averts eight causes of death. The dose is estimated at 16.5% based on HP2030 goal TU-14. Its effects are similar to those of the smoking prevention intervention above, only not as strong. It is the third-most impactful intervention by 2040 and 2050.
- Blood pressure management (Blood Pressure Management_25) is a clinical management intervention that averts deaths from hypertension and CVD. The dose is estimated at 25% based on HP2030 goal HDS-05. The death reduction starts quickly and strongly, making this the most impactful of the six interventions through 2030 and the fourth-most impactful in 2040 and 2050.
- Adult physical activity (Physical Activity_11) is a population health intervention that averts seven causes of death. The dose is estimated at 11% based on HP2030 goal PA-02. The death reduction is delayed (traversing changes in the prevalence of obesity, diabetes, hypertension, high cholesterol, and COPD) but grows rapidly after 2030 to make this the fifth-most impactful intervention by 2040 and 2050.
- Colorectal cancer screening (Colorectal Cancer Screening_26.5) is a clinical screening intervention that averts deaths from CRC. The dose is estimated at 26.5% based on HP2030 goal C-07. The death reduction is delayed by several years (traversing the progression of colorectal cancer) but is substantial by 2030, making this the sixth-most impactful intervention by 2040 and 2050.
3.3. Combination Intervention Testing Results
4. Discussion
4.1. Findings
4.2. Next Steps and Future Applications
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Academy of Medical Sciences. Improving the Health of the Public by 2040: Optimizing the Research Environment for a Healthier, Fairer Future; Academy of Medical Sciences: London, UK, 2016. [Google Scholar]
- Gerhardus, A.; Becher, H.; Groenewegen, P.; Mansmann, U.; Meyer, T.; Pfaff, H.; Puhan, M.; Razum, O.; Rehfuess, E.; Sauerborn, R.; et al. Applying for, reviewing and funding public health research in Germany and beyond. Health Res. Policy Syst. 2016, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, H.; Savona, N.; Glonti, K.; Bibby, J.; Cummins, S.; Finegood, D.; Greaves, F.; Harpe, L.; Hawe, P.; Moore, L.; et al. The need for a complex systems model of evidence for public health. Lancet 2017, 390, 2602–2604. [Google Scholar] [CrossRef] [Green Version]
- Diez Roux, A.V. Complex systems thinking and current impasses in health disparities research. Am. J. Public Health. 2011, 101, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.S.; Keyes, K.M. Wrong answers: When simple interpretations create complex problems. In Systems Science and Population Health; El-Sayed, A.M., Galea, S., Eds.; Oxford U Press: New York, NY, USA, 2017; Chapter 3. [Google Scholar]
- Sterman, J.D. Learning from evidence in a complex world. Am. J. Public Health 2006, 96, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Sterman, J.D. Business Dynamics: Systems Thinking and Modeling for a Complex World; Irwin McGraw-Hill: Boston, MA, USA, 2000. [Google Scholar]
- Homer, J. Levels of evidence in system dynamics modeling. Sys. Dyn. Rev. 2014, 30, 75–80. [Google Scholar] [CrossRef]
- Homer, J. Best practices in system dynamics modeling, revisited: A practitioner’s view. Sys. Dyn. Rev. 2019, 35, 177–181. [Google Scholar] [CrossRef]
- Homer, J.; Hirsch, G. System dynamics modeling for public health: Background and opportunities. Am. J. Public Health 2006, 96, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, G.; Homer, J.; Tomoaia-Cotisel, A. (Eds.) System dynamics applications to health and health care. 15 previously published articles with new introduction and extended bibliography. Sys. Dyn. Rev. 2015. Available online: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1099-1727/homepage/VirtualIssuesPage.html (accessed on 1 June 2022).
- Darabi, N.; Hosseinichimeh, N. System dynamics modeling in health and medicine: A systematic literature review. Sys. Dyn. Rev. 2020, 36, 29–73. [Google Scholar] [CrossRef] [Green Version]
- Yarnoff, B.; Honeycutt, A.; Khavjou, O.; Bradley, C.; Bates, L.; Homer, J. PRISM: The Prevention Impacts Simulation Model; Reference Guide for Model Version 3s; RTI International: Research Triangle Park, NC, USA, 2020; Available online: https://prism-simulation.cdc.gov/app/cdc/prism/#/ (accessed on 1 June 2022).
- Yarnoff, B.; Honeycutt, A.; Bradley, C.; Khavjou, O.; Bates, L.; Bass, S.; Kaufmann, R.; Barker, L.; Briss, P. Validation of the Prevention Impacts Simulation Model (PRISM). Prev. Chron. Dis. 2021, 18, E09. Available online: www.cdc.gov/pcd/issues/2021/20_0225.htm (accessed on 1 June 2022).
- Homer, J.; Milstein, B.; Wile, K.; Trogdon, J.; Huang, P.; Labarthe, D.; Orenstein, D. Simulating and evaluating local interventions to improve cardiovascular health. Prev. Chron. Dis. 2010, 7, A18. Available online: http://www.cdc.gov/pcd/issues/2010/jan/08_0231.htm (accessed on 1 June 2022).
- Hirsch, G.; Homer, J.; Wile, K.; Trogdon, J.G.; Orenstein, D. Using simulation to compare 4 categories of intervention for reducing cardiovascular risks. Am. J. Public Health 2014, 104, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Homer, J.; Wile, K.; Yarnoff, B.; Trogdon, J.G.; Hirsch, G.; Cooper, L.; Soler, R.; Orenstein, D. Using simulation to compare established and emerging interventions to reduce cardiovascular disease risks in the United States. Prev. Chron. Dis. 2014, 11, E195. Available online: http://www.cdc.gov/pcd/issues/2014/14_0130.htm (accessed on 1 June 2022).
- Honeycutt, A.A.; Wile, K.; Dove, C.; Hawkins, J.; Orenstein, D. Strategic planning for chronic disease prevention in rural America: Looking through a PRISM lens. J. Public Health Mgmt. Pract. 2015, 21, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Orenstein, D.; Honeycutt, A.; Bradley, C.; Trogdon, J.; Kent, C.K.; Wile, K.; Haddix, A.; O’Neil, D.; Bunnell, R. Community-based interventions to decrease obesity and tobacco exposure and reduce health care costs: Outcome estimates from Communities Putting Prevention to Work for 2010–2020. Prev. Chron. Dis. 2016, 13, e47. Available online: http:///www.cdc.gov/pcd/issues/2016/15_0272.htm (accessed on 1 June 2022).
- Colorado Department of Public Health and Environment. CoHID: Colorado Health Information Dataset. 2021. Available online: https://cdphe.colorado.gov/cohid (accessed on 1 June 2022).
- Harner, L.T.; Kuo, E.S.; Cheadle, A.; Rauzon, S.; Schwartz, P.M.; Parnell, B.; Kelly, C.; Solomon, L. Using population dose to evaluate community-level health initiatives. Am. J. Prev. Med. 2018, 54, S117–S123. [Google Scholar] [CrossRef] [PubMed]
- Office of Disease Prevention and Health Promotion. Healthy People 2030. U.S. Department of Health and Human Services. 2020. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives (accessed on 1 June 2022).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. In National Center for Health Statistics Data Brief; No. 360; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020; p. 7. [Google Scholar]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Newman, M.E.J. Power laws, Pareto distributions and Zipf’s law. Contemp. Phys. 2005, 46, 323–351. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, A.B.; Zauber, A.G.; Rutter, C.M.; Naber, S.K.; Doria-Rose, V.P.; Pabiniak, C.; Johanson, C.; Fischer, S.E.; Lansdorp-Vogelaar, I.; Kuntz, K.M.; et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: Modeling study for the US Preventive Services Task Force. JAMA 2016, 315, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Moolgavkar, S.H.; Holford, T.R.; Levy, D.T.; Long, C.Y.; Foy, M.; Clarke, L.; Jeon, J.; Hazelton, W.D.; Meza, R.; Schultz, F.; et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J. Natl. Cancer Inst. 2012, 104, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Plevritis, S.K.; Munoz, D.; Kurian, A.W.; Stout, N.K.; Alagoz, O.; Near, A.M.; Lee, S.J.; van den Broek, J.J.; Huang, X.; Schechter, C.B.; et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA 2018, 319, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Coxson, P.; Pletcher, M.J.; Lightwood, J.; Goldman, L. Adolescent overweight and future adult coronary heart disease. New Eng. J. Med. 2007, 357, 2371–2379. [Google Scholar] [CrossRef] [Green Version]
- Najafzadeh, M.; Marra, C.A.; Lynd, L.D.; Sadatsafavi, M.; FitzGerald, J.M.; McManus, B.; Sin, D. Future impact of various interventions on the burden of COPD in Canada: A dynamic population model. PLoS ONE 2012, 7, e46746. [Google Scholar] [CrossRef] [Green Version]

| Variable | Colorado | US Overall | Ratios 1 |
|---|---|---|---|
| Adult obesity | BR 1999–2018 | BR 1999–2016, NH 1999–2008 | CO vs. US: 0.73; BR vs. NH: 0.76 |
| Youth obesity | NSCH 2003–2011 (age 10–17) | NH 1999–2008 (age 12–17) | CO vs. US: 0.72 |
| Adult healthy diet | BR 1999–2009 (fruit-veg 5×day) | BR 1999–2009 (fruit-veg 5×/day) | CO vs. US: 1.05 |
| Youth healthy diet (high school) | HKCS 2015 (veg 2×/day) | (n/a) | (n/a) |
| Adult healthy drinks | BR 2009–2017 (sugary < 1/day) | (n/a) | (n/a) |
| Youth healthy drinks (age 1–14) | CCHS 2004–2014 (sugary < 1/day) | (n/a) | (n/a) |
| Adult physical activity | BR 2001–2009 (per guideline) | BR 2001–2009 (per guideline) | CO vs. US: 1.13 |
| Youth physical activity (high school) | HKCS 2013–2015 (per guideline) | (n/a) | (n/a) |
| Breastfeeding (6 months+) | NIS 2001–2016 | NIS 2001–2015 | CO vs. US: 1.17 |
| Adult current smoking | BR 1999–2019 | BR 1999–2019, NH 1999–2008 | CO vs. US: 0.91; BR vs. NH: 0.935 |
| Adult former smoking | BR 2011–2019 | BR 2011–2019, NH 1999–2008 | CO vs. US: 1.04; BR vs. NH: 1.09 |
| Youth smoking (high school) | YR 2005–2019 | YR 1999–2019 | CO vs. US: 0.85 |
| Adult prediabetes | (n/a) | NH 1999–2008 | (n/a) |
| Adult diabetes | BR 1999–2018 | BR 1999–2016, NH 1999–2008 | CO vs. US: 0.70; BR vs. NH: 0.74 |
| Diabetes self-management education (DSME) or control | BR 2000–2017 (DSME) | BR 2011–2015 (DSME), NH 2005–2008 (control) | CO vs. US: 1.08; BR vs. NH: 0.98 |
| Adult high blood pressure | BR 1999–2015 | BR 1999–2015, NH 1999–2008 | CO vs. US: 0.81; BR vs. NH: 0.825 |
| Adult high cholesterol | BR 1999–2015 | BR 1999–2015, NH 1999–2008 | CO vs. US: 0.92; BR vs. NH: 0.715 |
| Cardiovasc. disease (ever event) | BR 2005–2018 | BR 2005–2016, NH 1999–2008 | CO vs. US: 0.69; BR vs. NH: 0.99 |
| Adult asthma | BR 2000–2016 | BR 2000–2016, NHIS 2001–2016 | CO vs. US: 0.99; BR vs. NHIS: 1.11 |
| Youth asthma (0–17) | (n/a) | NHIS 2001–2016 | (n/a) |
| Adult COPD | BR 2011–2016, NHIS 1999–2011 | BR 2011–2016, NHIS 1999–2011 | CO vs. US: 0.71 (BR), 0.78 (NHIS) |
| HPV vaccination female (age 13–17) | NIS 2008–2017 (2+ doses) | NIS 2012–2016 (2+ doses) | CO vs. US: 1.05 |
| HPV vaccination male (age 13–17) | NIS 2013–2017 (2+ doses) | NIS 2012–2016 (2+ doses) | CO vs. US: 1.13 |
| Colorectal cancer screen (age 50–85) | BR 2014–2016 | BR 2014–2016 | CO vs. US: 1.00 |
| Mammography past 2 years (age 50–74) | BR 2014–2016 | BR 2014–2016 | CO vs. US: 0.95 |
| Pap test past 3 years (age 21–65) | BR 2014–2016 | BR 2014–2016 | CO vs. US: 1.02 |
| Cancer incidence over 5 years | USCS 2011–2015 | (n/a) | (n/a) |
| Deaths by 5-or-10 year age group | CDPHE VSP 1999–2017 annual | (n/a) | (n/a) |
| Intervention Types | Target Description | Performance Definition (Data Source) | 2018 Value |
|---|---|---|---|
| Population health | |||
| Healthy food—adults | Adults age 18+ | Fruits/vegetables 5× per day (BRFSS) | 25% |
| Healthy food—youth | Youth age 0–17 | Vegetables 2× per day, high school (HKCS) | 30.5% |
| Healthy beverage—adults | Adults age 18+ | Less than 1 sugary drink per day (BRFSS) | 74% |
| Healthy beverage—youth | Youth age 0–17 | Less than 1 sugary drink per day, ages 1–14 (CCHS) | 85% |
| Physical activity—adults | Adults age 18+ | Exercise per national guidelines (BRFSS) | 57% |
| Physical activity—youth | Youth age 0–17 | Exercise per national guidelines, high school (HKCS) | 52% |
| Breastfeeding | New mothers | Breastfeed non-exclusive for 6 months (NIS for CO) | 67% |
| Antismoking—adults | Adults age 18+ | Smoking initiation below 2018 level, ages 18+ (NHIS) | 0% |
| Antismoking—youth | Youth age 0–17 | Smoking rate below 2018 level, high school (YRBSS) | 0% |
| Radon in new homes | New housing units | Radon mitigation beyond 2018 level (CDPHE) | 0% |
| Radon in resales | Housing unit resales | Radon mitigation beyond 2018 level (CDPHE) | 0% |
| Clinical prevention | |||
| Diabetes prevention program | Diagnosed (or high risk for) prediabetes | Completion of NDPP program (CDC for CO) | 0.1% |
| Female HPV vaccination | Females age 13–26 | At least 2 doses (NIS for CO) | 55% |
| Male HPV vaccination | Males age 13–26 | At least 2 doses (NIS for CO) | 55% |
| Smoking quit services | Adults age 18+ | Successful quit rate above 2018 level (NHIS) | 0% |
| Clinical screening | |||
| Blood glucose | Adults age 18+ | Checked past 2 years (BRFSS) | 74% |
| Blood pressure | Adults age 18+ | Checked past 2 years (BRFSS) | 83% |
| Cholesterol | Adults age 18+ | Checked past 2 years (BRFSS) | 81% |
| Lung CT scan | Smokers age 50–80 | Per national guidelines (NHIS for US) | 4.4% |
| Colorectal cancer | Adults age 50–84 | Per national guidelines (BRFSS) | 68% |
| Mammography | Females age 50–74 | Per national guidelines (BRFSS) | 74% |
| Pap test | Females age 21+ | Per national guidelines (BRFSS) | 81% |
| Clinical management | |||
| Diabetes | Diagnosed diabetes | Completion of diabetes self-mgmt class (BRFSS) | 60% |
| Hypertension | Diagnosed hypertension | Control per guidelines (NHANES for US) | 65% |
| High cholesterol | Diagnosed high cholesterol | Control per guidelines (NHANES for US) | 60% |
| Asthma—youth | Diagnosed asthma age 0–17 | No past year attack (NHIS for US) | 47% |
| Asthma—adults | Diagnosed asthma age 18+ | No past year attack (NHIS for US) | 55% |
| COPD | Diagnosed COPD | Daily treatment (BRFSS for selected states) | 50% |
| Intervention_Dose % 1 | Rank 2 | Independent Impact | Cumulative Impact | Proportion of Total Cumulative Impact | ||
|---|---|---|---|---|---|---|
| Death Rate per 100,000 | % Change | Death Rate per 100,000 | % Change | |||
| Base run | 0 | 411.098 | 0.00% | 411.098 | 0.00% | 0.0% |
| Smoking Prevention _23 | 1 | 402.391 | 2.12% | 402.391 | 2.12% | 22.1% |
| Diabetes Prevention_18 | 2 | 404.690 | 1.56% | 396.066 | 3.66% | 38.2% |
| Smoking Cessation_16.5 | 3 | 405.258 | 1.42% | 391.119 | 4.86% | 50.7% |
| Blood Pressure Management_25 | 4 | 406.856 | 1.03% | 387.019 | 5.86% | 61.1% |
| Physical Activity_11 | 5 | 407.652 | 0.84% | 383.876 | 6.62% | 69.1% |
| Colorectal Cancer Screening_26.5 | 6 | 409.220 | 0.46% | 382.029 | 7.07% | 73.8% |
| Diabetes Screening_20 | 7 | 409.494 | 0.39% | 380.604 | 7.42% | 77.4% |
| Asthma Control_16 | 8 | 409.605 | 0.36% | 379.371 | 7.72% | 80.6% |
| Cholesterol Management_18 | 9 | 409.766 | 0.32% | 378.140 | 8.02% | 83.7% |
| Blood Pressure Screening_20 | 10 | 409.806 | 0.31% | 376.764 | 8.35% | 87.2% |
| Fruit and Vegetable Consumption_5 | 11 | 409.934 | 0.28% | 375.771 | 8.59% | 89.7% |
| Diabetes Management_7 | 12 | 410.033 | 0.26% | 374.768 | 8.84% | 92.2% |
| Radon Reduction, Housing Resale_100 | 13 | 410.138 | 0.23% | 374.009 | 9.02% | 94.2% |
| Cholesterol Screening_20 | 14 | 410.577 | 0.13% | 373.480 | 9.15% | 95.5% |
| Radon Reduction, New Construction_100 | 15 | 410.704 | 0.10% | 373.249 | 9.21% | 96.1% |
| Respiratory Cancer Screening_10.5 | 16 | 410.789 | 0.08% | 372.986 | 9.27% | 96.8% |
| Smoking Prevention, Youth_23 | 17 | 410.805 | 0.07% | 372.765 | 9.32% | 97.3% |
| Asthma Control, Youth_16 | 18 | 410.876 | 0.05% | 372.605 | 9.36% | 97.7% |
| COPD Treatment_3.9 | 19 | 410.884 | 0.05% | 372.409 | 9.41% | 98.2% |
| Mammograms_16 | 20 | 410.924 | 0.04% | 372.239 | 9.45% | 98.7% |
| HPV Vaccination, Females_61.5 | 21 | 410.977 | 0.03% | 372.119 | 9.48% | 99.0% |
| Sugar Sweetened Beverage Policy_5 | 22 | 410.997 | 0.02% | 372.036 | 9.50% | 99.2% |
| Physical Activity Youth_6 | 23 | 410.999 | 0.02% | 371.960 | 9.52% | 99.4% |
| Breastfeeding Iniatives_23 | 24 | 411.007 | 0.02% | 371.873 | 9.54% | 99.6% |
| Pap Smears_19.5 | 25 | 411.028 | 0.02% | 371.810 | 9.56% | 99.8% |
| HPV Vaccination, Male_61.5 | 26 | 411.035 | 0.02% | 371.750 | 9.57% | 99.9% |
| Fruit/Vegetable Consumption Youth_5 | 27 | 411.052 | 0.01% | 371.714 | 9.58% | 100.0% |
| Sugar Sweetened Beverage Policy Youth_5 | 28 | 411.096 | 0.00% | 371.712 | 9.58% | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clennin, M.; Homer, J.; Erkenbeck, A.; Kelly, C. Evaluating Public Health Efforts to Prevent and Control Chronic Disease: A Systems Modeling Approach. Systems 2022, 10, 89. https://doi.org/10.3390/systems10040089
Clennin M, Homer J, Erkenbeck A, Kelly C. Evaluating Public Health Efforts to Prevent and Control Chronic Disease: A Systems Modeling Approach. Systems. 2022; 10(4):89. https://doi.org/10.3390/systems10040089
Chicago/Turabian StyleClennin, Morgan, Jack Homer, Alex Erkenbeck, and Cheryl Kelly. 2022. "Evaluating Public Health Efforts to Prevent and Control Chronic Disease: A Systems Modeling Approach" Systems 10, no. 4: 89. https://doi.org/10.3390/systems10040089







