The Effect of Bacterial Endotoxin LPS on Serotonergic Modulation of Glutamatergic Synaptic Transmission
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Dissection
2.2. Physiological Recordings
2.3. Statistics
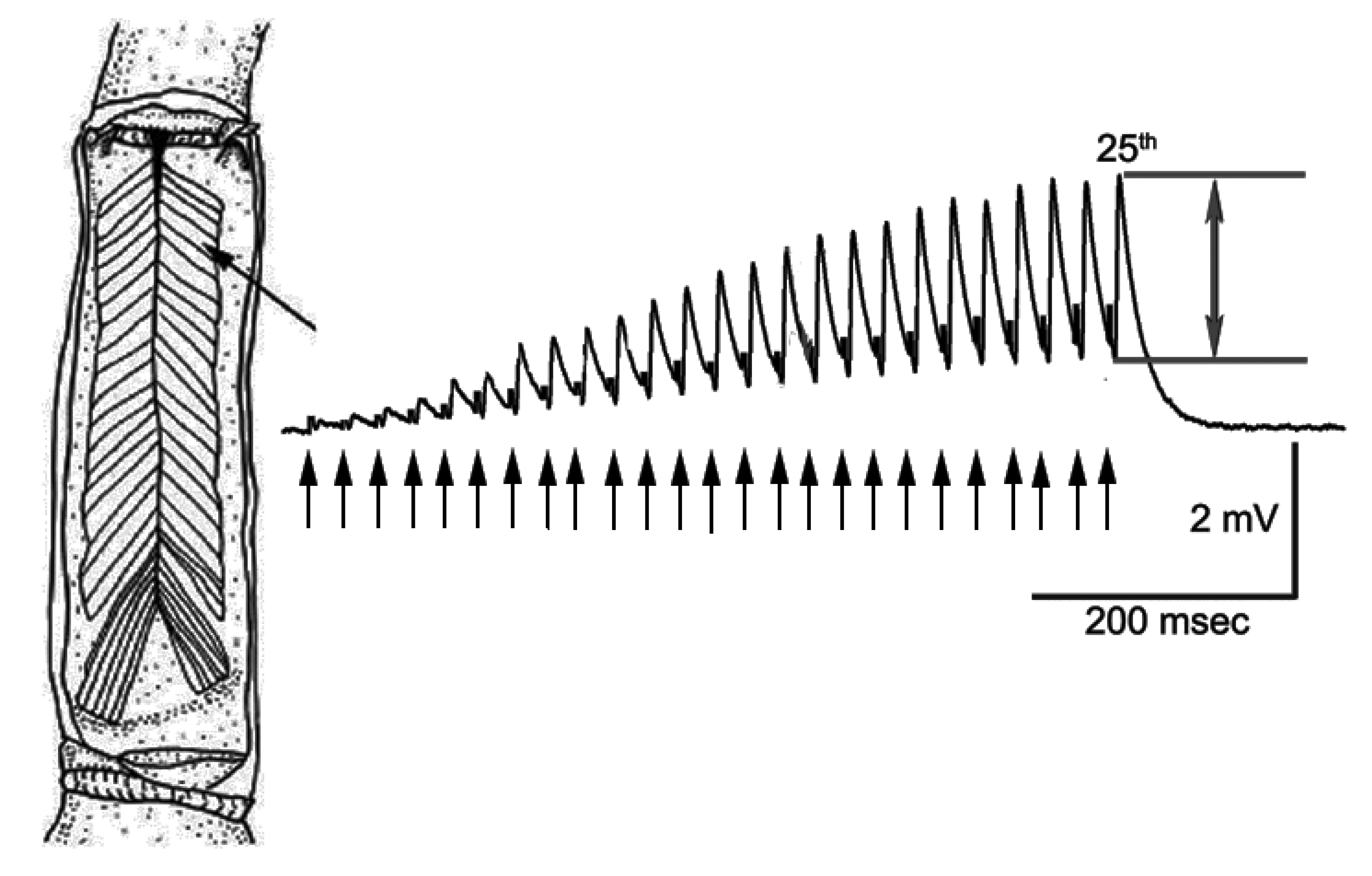
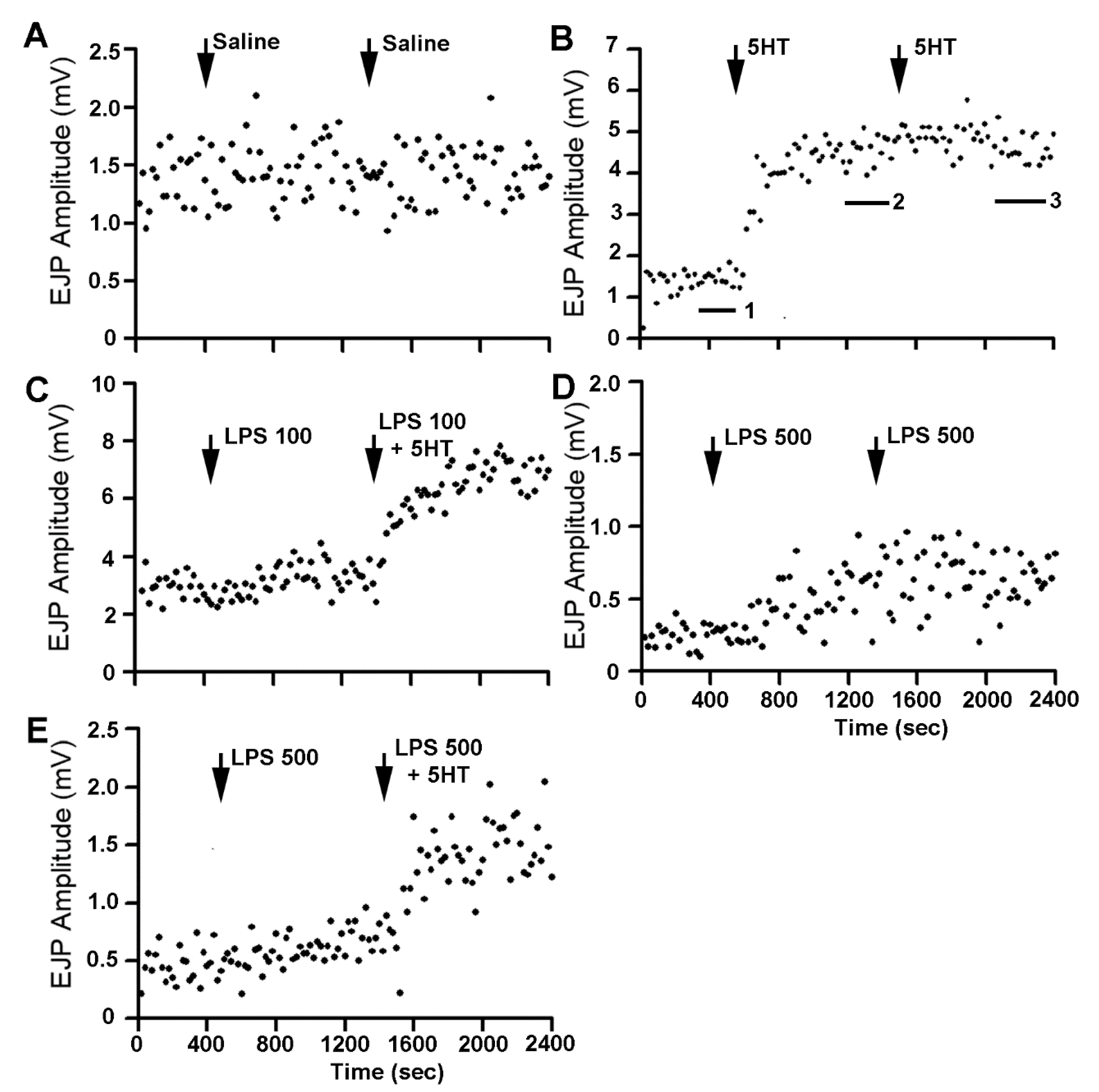
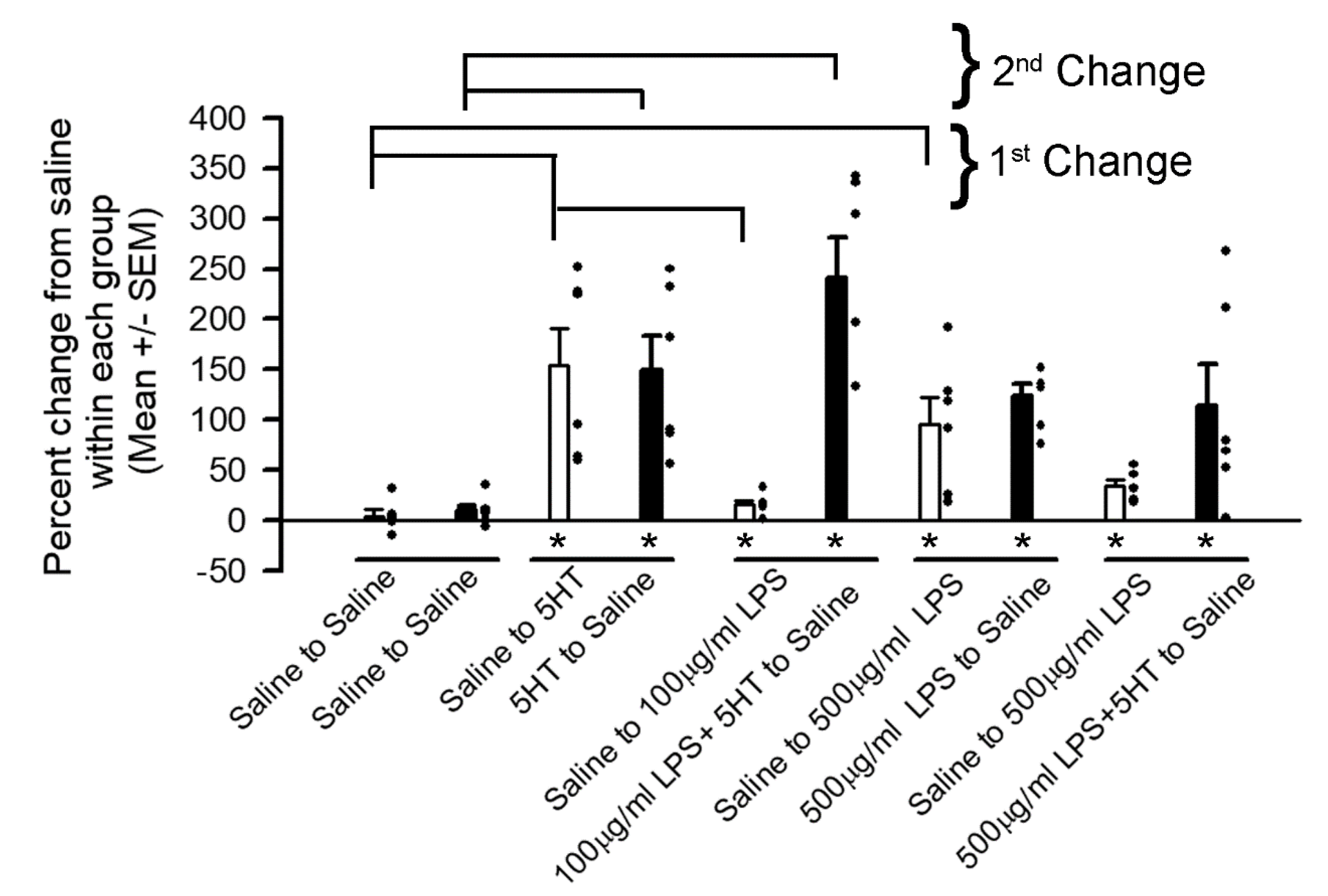
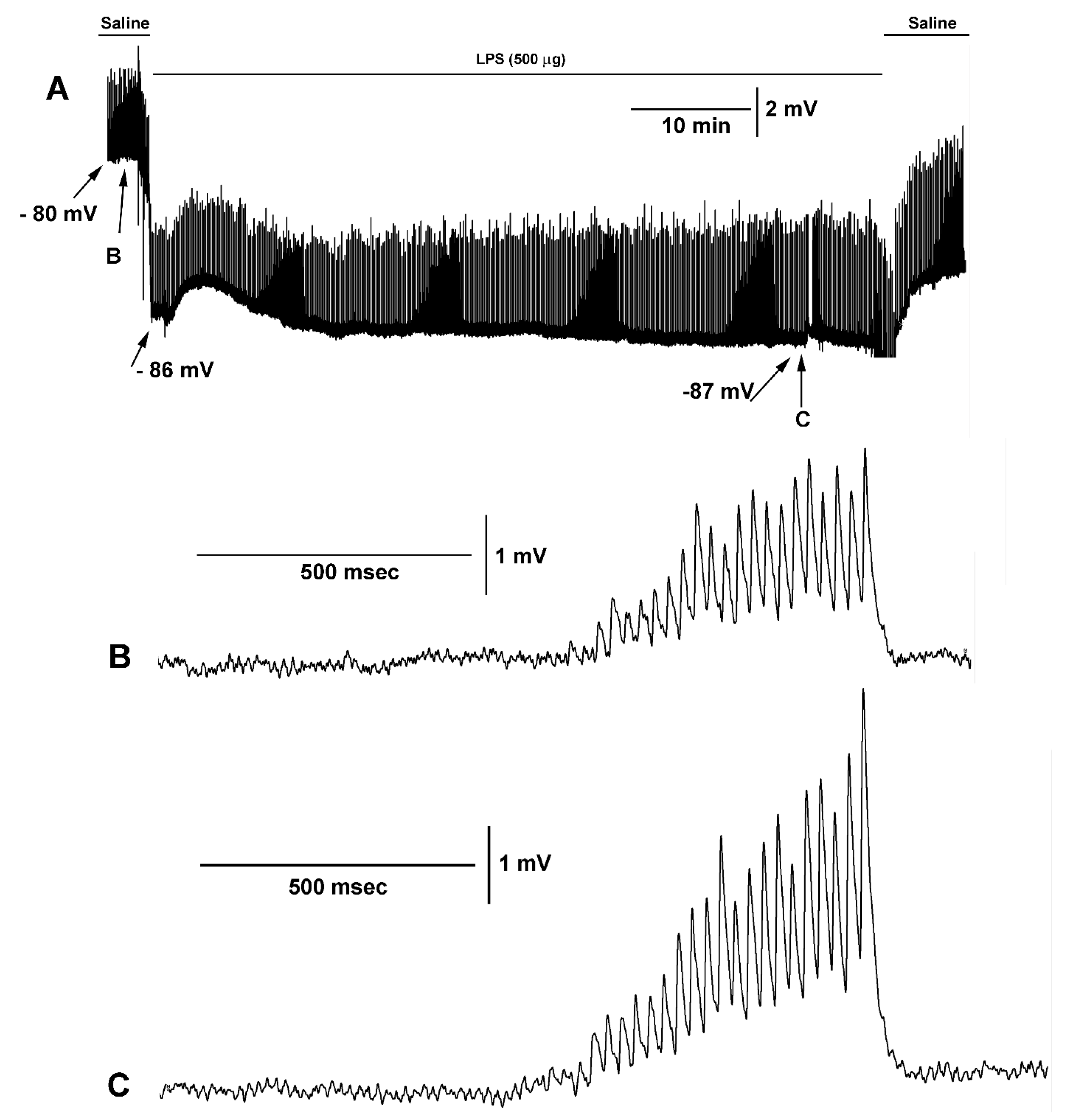
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, M.; Cai, S.; Su, J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, R.V.; Harrison, K.; Oyston, P.C.; Lukaszewski, R.A.; Clark, G.C. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 2013, 20, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tynan, R.J.; Weidenhofer, J.; Hinwood, M.; Cairns, M.J.; Day, T.A.; Walker, F.R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun. 2012, 26, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Ballinger-Boone, C.; Anyagaligbo, O.; Bernard, J.; Bierbower, S.M.; Dupont-Versteegden, E.E.; Ghoweri, A.; Greenhalgh, A.; Harrison, D.; Istas, O.; McNabb, M.; et al. The effects of bacterial endotoxin (LPS) on cardiac and synaptic function in various animal models: Larval Drosophila, crayfish, crab, and rodent. Int. J. Zool. Res. 2020, 16, 33–62. [Google Scholar] [CrossRef]
- Bécamel, C.; Berthoux, C.; Barre, A.; Marin, P. Growing Evidence for Heterogeneous Synaptic Localization of 5-HT2A Receptors. ACS Chem. Neurosci. 2017, 8, 897–899. [Google Scholar] [CrossRef]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Brill, J.; Shao, Z.; Puche, A.C.; Wachowiak, M.; Shipley, M.T. Serotonin increases synaptic activity in olfactory bulb glomeruli. J. Neurophysiol. 2016, 115, 1208–1219. [Google Scholar] [CrossRef]
- Wu, W.-H.; Cooper, R.L. Serotonin and synaptic transmission at invertebrate neuromuscular junctions. Exp. Neurobiol. 2012, 21, 101–112. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Del Colle, A.; Israelyan, N.; Margolis, K.G. Novel aspects of enteric serotonergic signaling in health and brain-gut disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G130–G143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sha, H.; Zhou, L.; Chen, Y.; Zhou, Q.; Dong, H.; Qian, Y. The mast cell is an early activator of lipopolysaccharide-induced neuroinflammation and blood-brain barrier dysfunction in the hippocampus. Mediat. Inflamm. 2020, 8098439. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kinoshita, K.; Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996, 271, 13854–13860. [Google Scholar] [CrossRef]
- Steiner, H. Peptidoglycan recognition proteins: On and off switches for innate immunity. Immunol. Rev. 2004, 198, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Levin, T.C.; Malik, H.S. Rapidly evolving Toll-3/4 genes encode male-specific Toll-like receptors in Drosophila. Mol. Biol. Evol. 2017, 34, 2307–2323. [Google Scholar] [CrossRef]
- Lorenzon, S.; de Guarrini, S.; Smith, V.; Ferrero, E. Effects of LPS injection on circulating haemocytes in crustaceans in vivo. Fish Shellfish Immunol. 1999, 9, 31–50. [Google Scholar] [CrossRef]
- Andrä, J.; Garidel, P.; Majerle, A.; Jerala, R.; Ridge, R.; Paus, E.; Novitsky, T.; Koch, M.H.J.; Brandendurg, K. Biophysical characterization of the interaction of Limulus polyphemus endotoxin neutralizing protein with lipopolysaccharide. Eur. J. Biochem. 2004, 271, 2037–2046. [Google Scholar] [CrossRef]
- Novitsky, T.J. Limulus amebocyte lysate (LAL) detection of endotoxin in human blood. J. Endotoxin Res. 1994, 1, 253–263. [Google Scholar] [CrossRef]
- US Department Health and Human Services. Guideline on the Validation of the Limulus Amebocyte Lysate Test as an End-Product Endotoxin Test for Human and Animal Parenteral Drugs, Biological Products, and Medical Devices. 1987. Available online: http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=3A346C6757AD03CB41B6DA54067C9648?doi=10.1.1.178.656&rep=rep1&type=pdf (accessed on 26 December 2019).
- Lorenzon, S.; Pasqual, P.; Ferrero, E.A. Different bacterial lipopolysaccharides as toxicants and stressors in the shrimp Palaemon elegans. Fish Shellfish Immunol. 2002, 13, 27–45. [Google Scholar] [CrossRef]
- Pien, B.C.; Sundaram, P.; Raoof, N.; Costa, S.F.; Mirrett, S.; Woods, C.W.; Reller, L.B.; Weinstein, M.P. The clinical and prognostic importance of positive blood cultures in adults. Am. J. Med. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- Carl, M.A.; Ndao, I.M.; Springman, A.C.; Manning, S.D.; Johnson, J.R.; Johnston, B.D.; Burnham, C.A.; Weinstock, E.S.; Weinstock, G.M.; Wylie, T.N.; et al. Sepsis from the gut: The enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin. Infect. Dis. 2014, 58, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Jeon, Y.D.; Kim, J.H.; Kim, J.K.; Ann, H.W.; Choi, H.; Kim, M.H.; Song, J.E.; Ahn, J.Y.; Jeong, S.J.; et al. Risk factors for mortality in patients with Serratia marcescens bacteremia. Yonsei Med. J. 2015, 56, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Palavutitotai, N.; Jitmuang, A.; Tongsai, S.; Kiratisin, P.; Angkasekwinai, N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS ONE 2018, 13, e0193431. [Google Scholar] [CrossRef] [PubMed]
- Saelinger, C.M.; McNabb, M.C.; McNair, R.; Bierbower, S.; Cooper, R.L. Effects of bacterial endotoxin on regulation of the heart, a sensory-CNS-motor nerve circuit and neuromuscular junctions: Crustacean model. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 237, 110557. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.; Istas, O.; Cooper, R.L. Bacterial endotoxin lipopolysaccharide enhances synaptic transmission at low-output glutamatergic synapses. Neurosci. Res. 2020. in review. [Google Scholar]
- Delaney, K.; Tank, D.W.; Zucker, R.S. Presynaptic calcium and serotonin-mediated enhancement of transmitter release at crayfish neuromuscular junction. J. Neurosci. 1991, 11, 2631–2643. [Google Scholar] [CrossRef]
- Dixon, D.; Atwood, H.L. Crayfish motor nerve terminal’s response to serotonin examined by intracellular microelectrode. J. Neurobiol. 1985, 16, 409–424. [Google Scholar] [CrossRef]
- Southard, R.C.; Haggard, J.; Crider, M.; Whiteheart, S.; Cooper, R.L. Influence of serotonin on the kinetics of vesicular release. Brain Res. 2000, 871, 16–28. [Google Scholar] [CrossRef]
- Sparks, G.M.; Cooper, R.L. 5-HT offsets homeostasis of synaptic transmission during short-term facilitation. J. Appl. Physiol. 2004, 96, 1681–1690. [Google Scholar] [CrossRef]
- Dudel, J. Modulation of quantal synaptic releasee by serotonin and forskolin in crayfish motor nerve terminals. In Modulation of Synaptic Transmission and Plasticity in Nervous Systems; Hertting, G., Spatz, H.C., Eds.; Springer: Berlin, Germany, 1988; Volume 19, pp. 259–270. [Google Scholar]
- Dixon, D.; Atwood, H.L. Conjoint action of phosphoinositol and adenylate cyclase systems in serotonin-induced facilitation at the crayfish neuromuscular junction. J. Neurophysiol. 1989, 62, 1251–1259. [Google Scholar] [CrossRef]
- Logsdon, S.; Johnstone, A.F.M.; Viele, K.; Cooper, R.L. The regulation of synaptic vesicles pools within motor nerve terminals during short-term facilitation and neuromodulation. J. Appl. Physiol. 2006, 100, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.S.; Cooper, R.L. Historical view and demonstration of physiology at the NMJ at the crayfish opener muscle. J. Vis. Exp. 2009, 33. [Google Scholar] [CrossRef]
- Cooper, R.L.; Marin, L.; Atwood, H.L. Synaptic differentiation of a single motor neuron: Conjoint definition of transmitter release, presynaptic calcium signals, and ultrastructure. J. Neurosci. 1995, 15, 4209–4222. [Google Scholar] [CrossRef]
- Crider, M.E.; Cooper, R.L. The importance of the stimulation paradigm in determining facilitation and effects of neuromodulation. Brain Res. 1999, 842, 324–331. [Google Scholar] [CrossRef]
- Crider, M.E.; Cooper, R.L. Differentially facilitation of high- and low-output nerve terminals from a single motor neuron. J. Appl. Physiol. 2000, 88, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Baierlein, B.; Thurow, A.L.; Atwood, H.L.; Cooper, R.L. Membrane potentials, synaptic responses, neuronal circuitry, neuromodulation and muscle histology using the crayfish: Student laboratory exercises. J. Vis. Exp. 2011, 47, e2322. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, E.M.; Martin, A.R. Non-linear summation of end-plate potentials in the frog and mouse. J. Physiol. 1981, 311, 307–324. [Google Scholar] [CrossRef]
- Istas, O.; Greenhalgh, A.; Cooper, R.L. The effects of a bacterial endotoxin on behavior and sensory-CNS-motor circuits in Drosophila melanogaster. Insects 2019, 10, 115. [Google Scholar] [CrossRef]
- Iwaya, A.; Nakagawa, S.; Iwakura, N.; Taneike, I.; Kurihara, M.; Kuwano, T.; Gondaira, F.; Endo, M.; Hatakeyama, K.; Yamamoto, T. Rapid and quantitative detection of blood Serratia marcescens by a real-time PCR assay: Its clinical application and evaluation in a mouse infection model. FEMS Microbiol. Lett. 2005, 248, 163–170. [Google Scholar] [CrossRef][Green Version]
- Wu, W.-H.; Cooper, R.L. The regulation and packaging of synaptic vesicles as related to recruitment within glutamatergic synapses. Neuroscience 2012, 225, 185–198. [Google Scholar] [CrossRef]
- Holsinger, R.C.; Cooper, R.L. Regional phenotypic differences of the opener muscle in Procambarus clarkii: Sarcomere length, fiber diameter, and force development. Biology 2020, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-H.; Cooper, R.L. Physiological separation of vesicle pools in low- and high-output nerve terminals. Neurosci. Res. 2013, 75, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Dropic, A.J.; Brailoiu, E.; Cooper, R.L. Presynaptic mechanism of action induced by 5-HT in nerve terminals: Possible involvement of ryanodine and IP3 sensitive Ca2+ stores. Comp. Biochem. Physiol. A 2005, 142, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.L.; McNabb, M.; Nadolski, J. The effects of a bacterial endotoxin LPS on synaptic transmission at the neuromuscular junction. Heliyon 2019, 5, e01430. Available online: https://www.heliyon.com/article/e01430 (accessed on 1 January 2020). [CrossRef] [PubMed]
- Dudel, J. Facilitatory effects of 5-Hydroxy-trytamine on the crayfish neuromuscular junction. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1965, 249, 515–528. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Southard, R.C.; Whiteheart, S.W.; Cooper, R.L. Role of alpha-SNAP in promoting efficient neurotransmission at the crayfish neuromus-cular junction. J. Neurophysiol. 1999, 82, 3406–3416. [Google Scholar] [CrossRef]
- Istas, O.; Greenhalgh, A.; Cooper, R.L. Repetitive exposure to bacterial endotoxin LPS alters synaptic transmission. J. Pharmacol. Toxicol. 2020, 15, 65–72. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, J.; Greenhalgh, A.; Istas, O.; Marguerite, N.T.; Cooper, R.L. The Effect of Bacterial Endotoxin LPS on Serotonergic Modulation of Glutamatergic Synaptic Transmission. Biology 2020, 9, 210. https://doi.org/10.3390/biology9080210
Bernard J, Greenhalgh A, Istas O, Marguerite NT, Cooper RL. The Effect of Bacterial Endotoxin LPS on Serotonergic Modulation of Glutamatergic Synaptic Transmission. Biology. 2020; 9(8):210. https://doi.org/10.3390/biology9080210
Chicago/Turabian StyleBernard, Jate, Abigail Greenhalgh, Oscar Istas, Nicole T. Marguerite, and Robin L. Cooper. 2020. "The Effect of Bacterial Endotoxin LPS on Serotonergic Modulation of Glutamatergic Synaptic Transmission" Biology 9, no. 8: 210. https://doi.org/10.3390/biology9080210
APA StyleBernard, J., Greenhalgh, A., Istas, O., Marguerite, N. T., & Cooper, R. L. (2020). The Effect of Bacterial Endotoxin LPS on Serotonergic Modulation of Glutamatergic Synaptic Transmission. Biology, 9(8), 210. https://doi.org/10.3390/biology9080210






