Radiation-Induced Senescence Bystander Effect: The Role of Exosomes
Abstract
1. Introduction
1.1. Ageing and Senescence
1.2. IR and Senescence
1.3. Non-Targeted Effects of Radiation
1.4. Secretion of, and Response to, Molecular Signals
2. Materials and Methods
2.1. Cell Culture and Irradiation
2.2. Population Doublings
2.3. Exosome Isolation
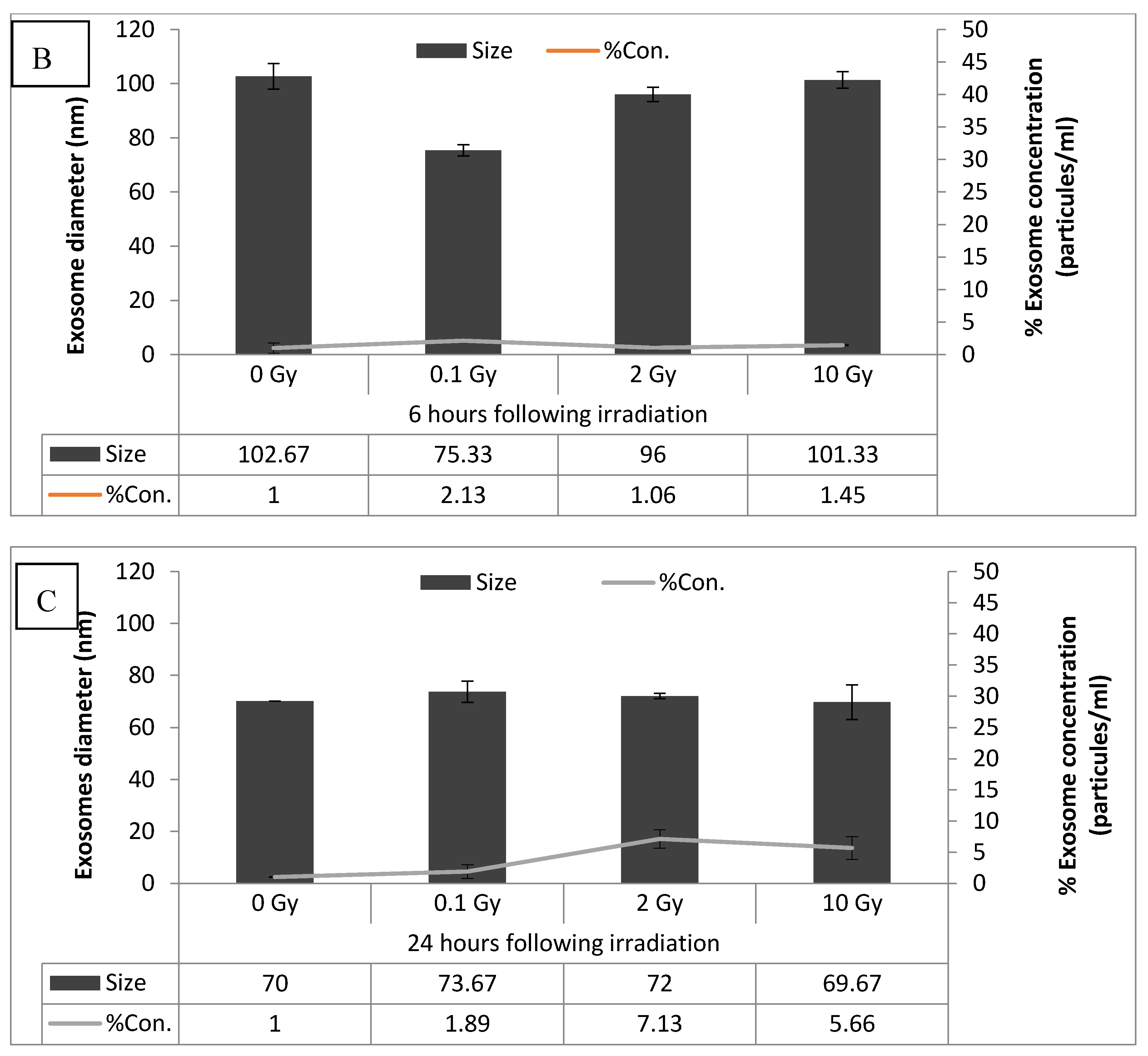
2.4. Exosome Characterization
2.5. Exosome Transfer for Bystander Experiments (Exosome Bystander)
2.6. Media Transfer for Bystander Experiments (Media Bystander)
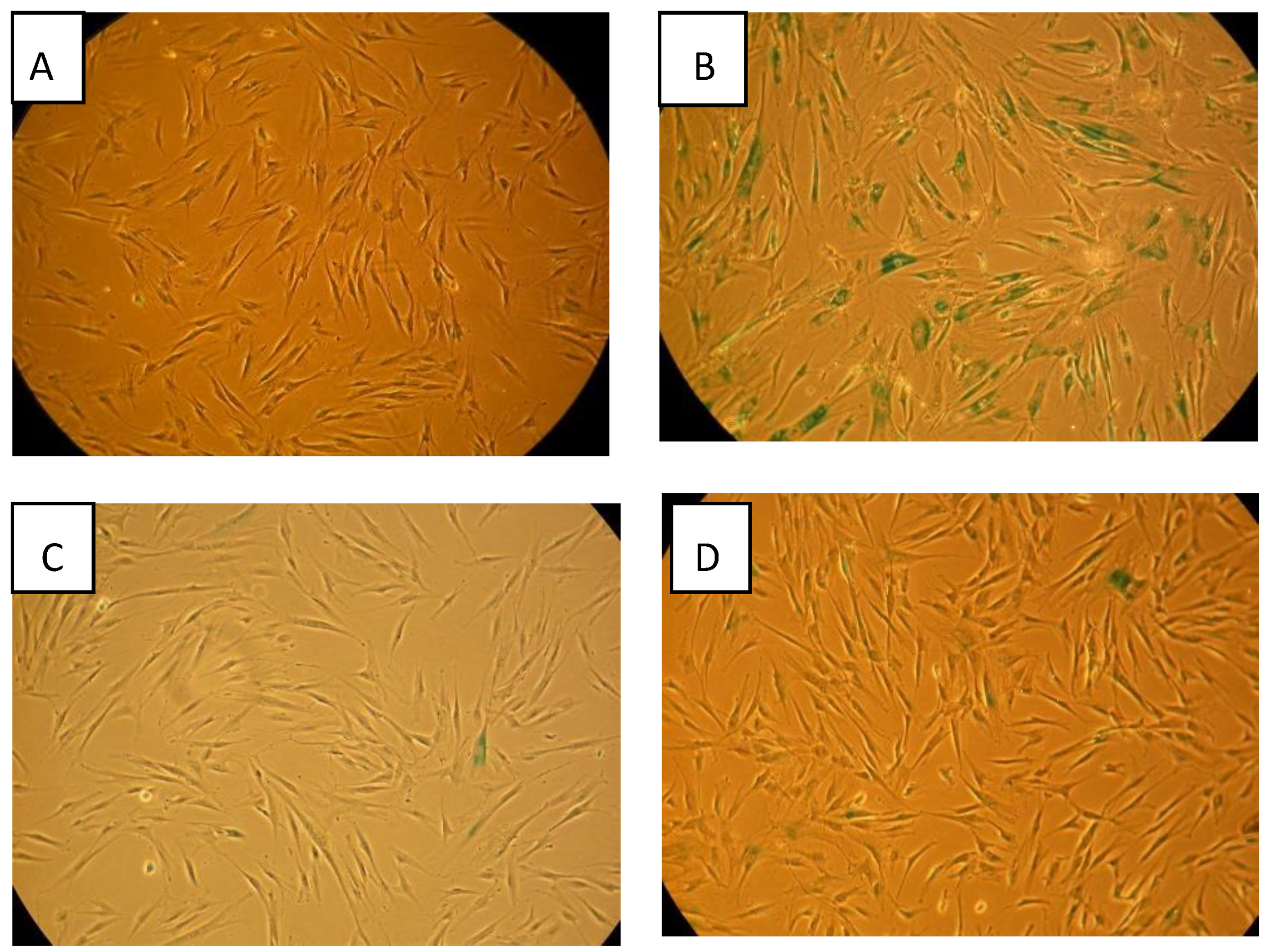
2.7. Beta-Gal Senescence Staining
2.8. Statistical Analysis
3. Results
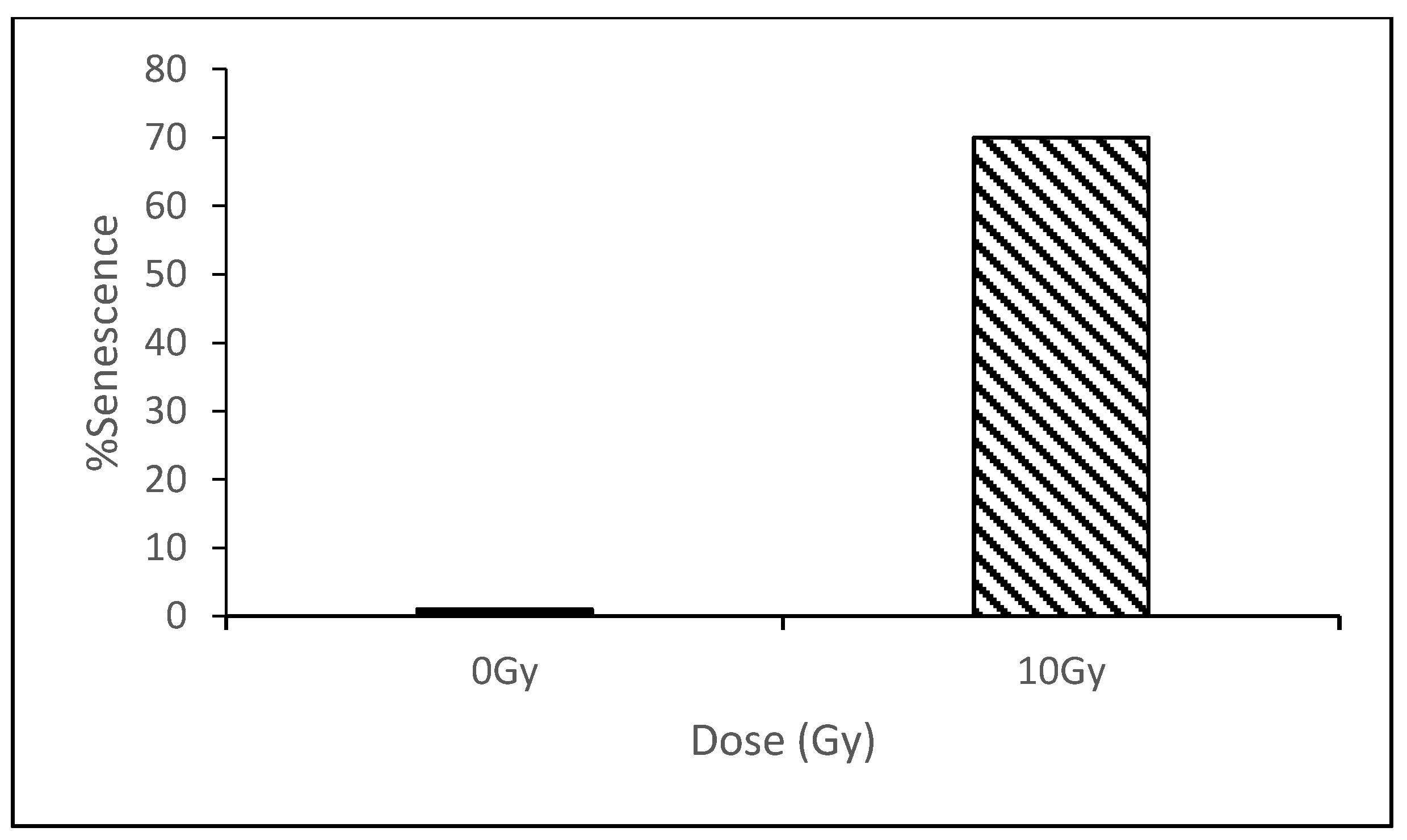
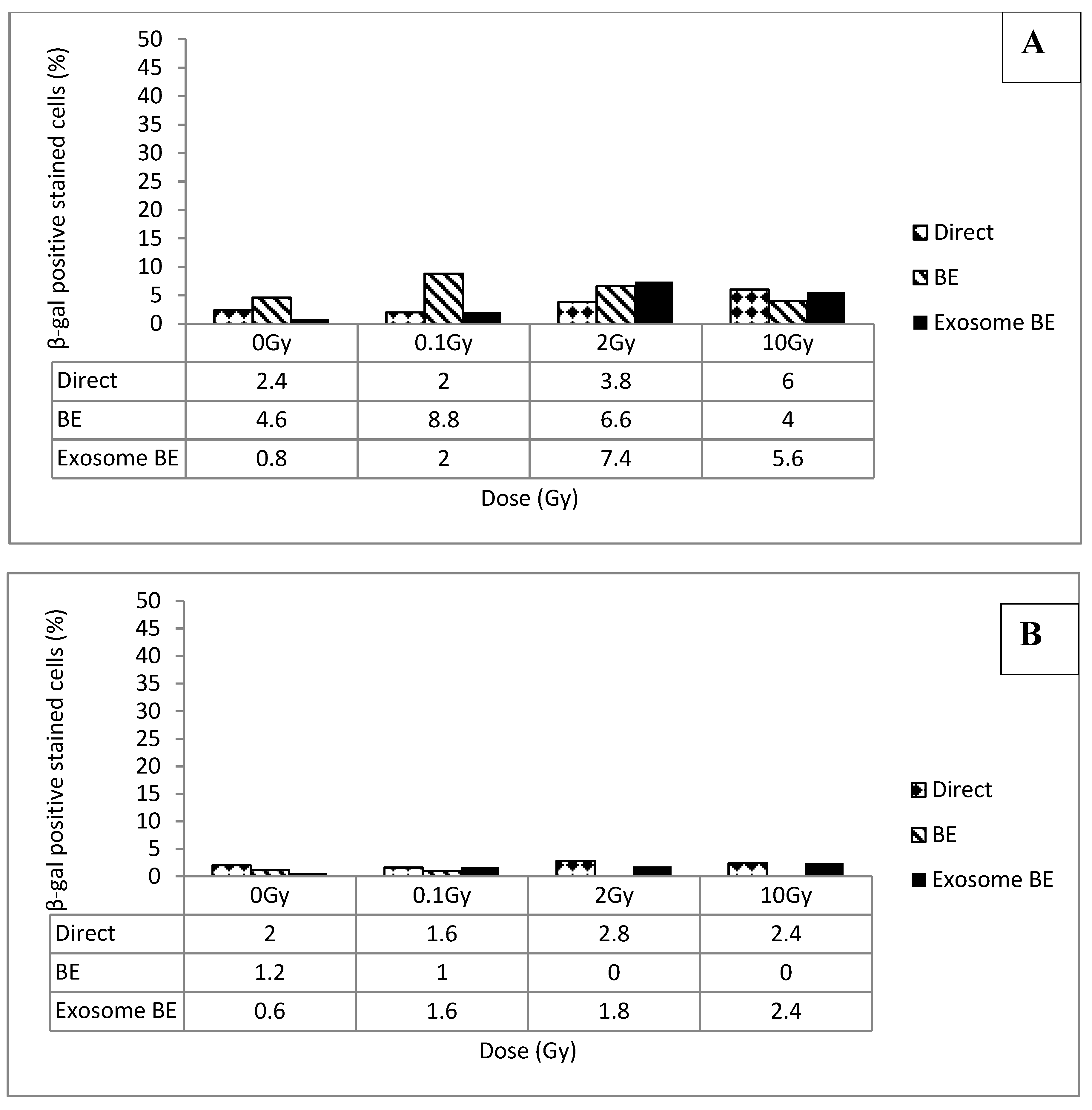
3.1. Senescence Induced by Direct Exposure to Radiation
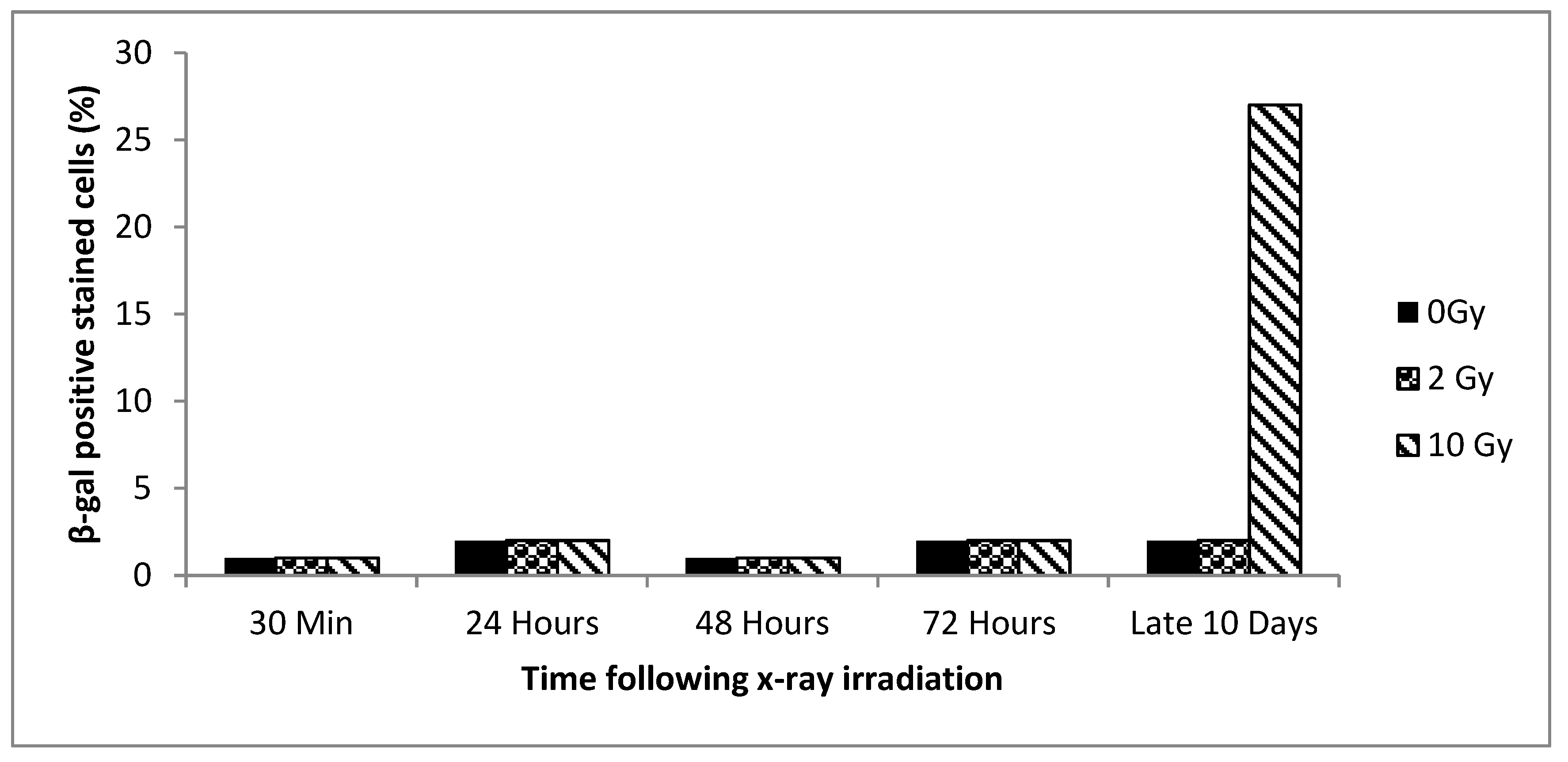
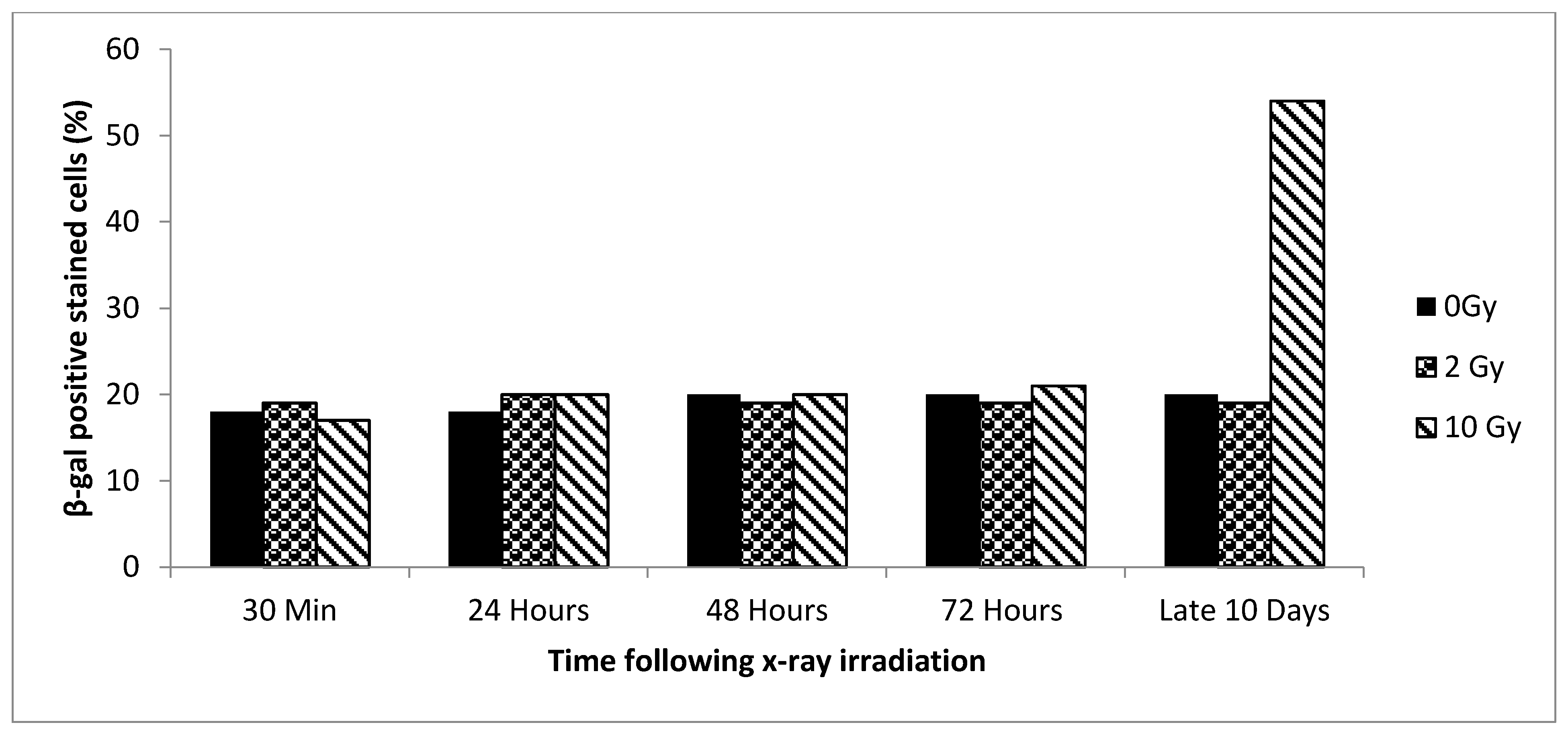
3.2. Senescence vs. Dose and Time
3.3. Senescence Bystander Effect
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BE | bystander effect |
| GI | genomic instability |
References
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Rivera, R.; Arellanes-Robledo, J.; García de León, M.C.; Shibayama, M.; Serrano-Luna, J. The Role of Senescence in Hepatic Diseases. In Liver Pathophysiology: Therapies and Antioxidants; Academic Press: Cambridge, MA, USA, 2017; Chapter 23; pp. 295–308. [Google Scholar]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Martínez-Zamudio, R.I.; Robinson, L.; Roux, P.F.; Bischof, O. SnapShot: Cellular Senescence Pathways. Cell 2017, 170, 816. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Liu, S.; Limbad, C.; Zawadzka, A.M.; Beck, J.; Demaria, M.; Artwood, R.; Alimirah, F.; Lopez-Dominguez, J.A.; Kuehnemann, C.; et al. SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep. 2019, 28, 3329–3337. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Sabin, R.J.; Anderson, R.M. Cellular Senescence—Its role in cancer and the response to ionizing radiation. Genome Integr. 2011, 2, 7. [Google Scholar] [CrossRef]
- Day, R.M.; Snow, A.L.; Panganiban, R.A.M. Radiation-induced accelerated senescence: A fate worse than death? Cell Cycle 2014, 13, 2011–2012. [Google Scholar] [CrossRef]
- Morgan, W.F. Non-targeted and Delayed Effects of Exposure to Ionizing Radiation: I. Radiation-Induced Genomic Instability and Bystander Effects In Vitro. Radiat. Res. 2012, 178, AV223–AV236. [Google Scholar] [CrossRef]
- Kadhim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.; Prise, K.; Little, M. Non-targeted effects of ionising radiation-Implications for low dose risk. Mutat. Res. 2013, 752, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, M.A.; Hill, M.A. Non-targeted effects of radiation exposure: Recent advances and implications. Radiat. Prot. Dosim. 2015, 166, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Prise, K.M.; Folkard, M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat. Res. 2008, 638, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hamada, N.; Takahashi, A.; Kobayashi, Y.; Ohnishi, T. Vanguards of paradigm shift in radiation biology: Radiation-induced adaptive and bystander responses. Radiat. Res. 2007, 48, 97–106. [Google Scholar] [CrossRef]
- Burr, K.L.; Robinson, J.I.; Rastogi, S.; Boylan, M.T.; Coates, P.J.; Lorimore, S.A.; Wright, E.G. Radiation-induced delayed bystander-type effects mediated by hemopoietic cells. Radiat. Res. 2011, 173, 760–768. [Google Scholar] [CrossRef]
- Iyer, R.; Lehnert, B.E.; Svensson, R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000, 60, 1290–1298. [Google Scholar]
- Facoetti, A.; Ballarini, F.; Cherubini, R.; Gerardi, S.; Nano, R.; Ottolenghi, A.; Prise, K.M.; Trott, K.R.; Zilio, C. Gamma ray-induced bystander effectin tumour glioblastoma cells: A specific study on cell survival, cytokine release and cytokine receptors. Radiat. Prot. Dosim. 2006, 122, 271–274. [Google Scholar] [CrossRef]
- Narayanan, K.; Williamson, R.; Zhang, Y.; Stewart, A.F.; Ioannou, P.A. Efficient and precise engineering of a 200 kb beta-globin human/bacterial artificial chromosome in E. coli DH10B using an inducible homologous recombination system. Gene Ther. 1999, 6, 442–447. [Google Scholar] [CrossRef]
- Moore, S.R.; Marsden, S.; Macdonald, D.; Mitchell, S.; Folkard, M.; Michael, B.; Goodhead, D.T.; Prise, K.M.; Kadhim, M.A. Genomic instability in human lymphocytes irradiated with individual charged particles: Involvement of tumor necrosis factor alpha in irradiated cells but not bystander cells. Radiat. Res. 2005, 163, 183–190. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Hill, M.A.; Moore, S.R. Genomic instability and the role of radiation quality. Radiat. Prot. Dosim. 2006, 122, 221–227. [Google Scholar] [CrossRef]
- Al-Mayah, A.H.J.; Irons, S.L.; Pink, R.C.; Carter, D.R.F.; Kadhim, M.A. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat. Res. 2012, 177, 539–545. [Google Scholar] [CrossRef]
- Jella, K.K.; Rani, S.; O’Driscoll, L.; McClean, B.; Byrne, H.J.; Lyng, F.M. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 2014, 181, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Calveley, V.L.; Khan, M.A.; Yeung, I.W.; Vandyk, J.; Hill, R.P. Partial volume rat lung irradiation: Temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int. J. Radiat. Biol. 2005, 81, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayah, A.; Bright, S.; Chapman, K.; Irons, S.; Luo, P.; Carter, D.; Goodwin, E.; Kadhim, M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. 2015, 772, 38–45. [Google Scholar] [CrossRef]
- Pant, S.; Hilton, H.; Burczynski, M.E. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 2012, 83, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Subra, C.; Silvente-Poirot, S.; Poirot, M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 2011, 81, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Maser, R.S.; DePinho, R.A. Telomeres and the DNA damage response: Why the fox is guarding the henhouse. DNA Repair 2004, 3, 979–988. [Google Scholar] [CrossRef]
- Borghesan, M.; Fafián-Labora, J.; Eleftheriadou, O.; Carpintero-Fernández, P.; Paez-Ribes, M.; Vizcay-Barrena, G.; Swisa, A.; Kolodkin-Gal, D.; Ximénez-Embún, P.; Lowe, R.; et al. Small Extracellular Vesicles Are Key Regulators of Non-Cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Rep. 2019, 27, 3956–3971. [Google Scholar] [CrossRef]
- Slijepcevic, P. Telomere length measurement by Q-FISH. Methods Cell Sci. 2011, 32, 17–22. [Google Scholar]
- Finnon, P.; Wong, H.P.; Silver, A.R.; Slijepcevic, P.; Bouffler, S.D. Long but dysfunctional telomeres correlate with chromosomal radiosensitivity in a mouse AML cell line. Int. J. Radiat. Biol. 2001, 77, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Holliman, G.; Lowe, D.; Cohen, H.; Felton, S.; Raj, K. Ultraviolet Radiation-Induced Production of Nitric Oxide:A multi-cell and multi-donor analysis. Sci. Rep. 2017, 7, 11105. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.; Broekman, M.L.; de Vrij, J. Tunable Resistive Pulse Sensing for the Characterization of Extracellular Vesicles. Methods Mol. Biol. 2017, 1545, 21–33. [Google Scholar]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; von Zglinicki, T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef]
- Cheong, N.; Zeng, Z.C.; Wang, Y.; Iliakis, G. Evidence for factors modulating radiation-induced G2-delay: Potential application as radioprotectors. Phys. Med. 2001, 1, 205–209. [Google Scholar]
- Squillaro, T.; Galano, G.; De Rosa, R.; Peluso, G.; Galderisi, U. Concise Review: The Effect of Low-Dose Ionizing Radiation on Stem Cell Biology: A Contribution to Radiation Risk. Stem Cells 2018, 36, 1146–1153. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbakrawy, E.; Kaur Bains, S.; Bright, S.; AL-Abedi, R.; Mayah, A.; Goodwin, E.; Kadhim, M. Radiation-Induced Senescence Bystander Effect: The Role of Exosomes. Biology 2020, 9, 191. https://doi.org/10.3390/biology9080191
Elbakrawy E, Kaur Bains S, Bright S, AL-Abedi R, Mayah A, Goodwin E, Kadhim M. Radiation-Induced Senescence Bystander Effect: The Role of Exosomes. Biology. 2020; 9(8):191. https://doi.org/10.3390/biology9080191
Chicago/Turabian StyleElbakrawy, Eman, Savneet Kaur Bains, Scott Bright, Raheem AL-Abedi, Ammar Mayah, Edwin Goodwin, and Munira Kadhim. 2020. "Radiation-Induced Senescence Bystander Effect: The Role of Exosomes" Biology 9, no. 8: 191. https://doi.org/10.3390/biology9080191
APA StyleElbakrawy, E., Kaur Bains, S., Bright, S., AL-Abedi, R., Mayah, A., Goodwin, E., & Kadhim, M. (2020). Radiation-Induced Senescence Bystander Effect: The Role of Exosomes. Biology, 9(8), 191. https://doi.org/10.3390/biology9080191



