Abstract
Cadmium (Cd) is one of the most toxic metals in the environment, and has noxious effects on plant growth and production. Cd-accumulating plants showed reduced growth and productivity. Therefore, remediation of this non-essential and toxic pollutant is a prerequisite. Plant-based phytoremediation methodology is considered as one a secure, environmentally friendly, and cost-effective approach for toxic metal remediation. Phytoremediating plants transport and accumulate Cd inside their roots, shoots, leaves, and vacuoles. Phytoremediation of Cd-contaminated sites through hyperaccumulator plants proves a ground-breaking and profitable choice to combat the contaminants. Moreover, the efficiency of Cd phytoremediation and Cd bioavailability can be improved by using plant growth-promoting bacteria (PGPB). Emerging modern molecular technologies have augmented our insight into the metabolic processes involved in Cd tolerance in regular cultivated crops and hyperaccumulator plants. Plants’ development via genetic engineering tools, like enhanced metal uptake, metal transport, Cd accumulation, and the overall Cd tolerance, unlocks new directions for phytoremediation. In this review, we outline the physiological, biochemical, and molecular mechanisms involved in Cd phytoremediation. Further, a focus on the potential of omics and genetic engineering strategies has been documented for the efficient remediation of a Cd-contaminated environment.
1. Introduction
Cadmium (Cd) is a non-essential element for plants and humans but is present in many soils in excessive amounts [1,2]. When it enters into the food chain, it poses a major threat to the living biota. The control of Cd accumulation in plants is complicated by the fact that most of the essential nutrient transporters, such as copper (Cu), manganese (Mn), iron (Fe), and zinc (Zn), also facilitate Cd uptake [2]. Cd stress alters plant growth, as evident from a reduced dry matter yield and leaf area, and stunted growth [3,4,5,6]. Cd affects plant growth at both the morphological and physiological level [7]. At the whole plant level, Cd toxicity includes leaf chlorosis, a delay in the growth rate, and inhibition of respiration and photosynthesis [8], increased oxidative damage, and decreased nutrient uptake ability [9].
Generally, Cd occurs in sedimentary rocks (0.3 mg kg−1), lithosphere (0.2 mg kg−1), and soil (0.53 mg kg−1) [10]. Cd enrichment in soil occurs from both anthropogenic and natural sources [11]. Geologically weathering of rocks is the major natural source of Cd contaminants [12,13], while primary anthropogenic sources of Cd include agrochemicals, manufacturing, vehicular emission, irrigation wastewater, smelting, and mining [14,15]. Moreover, improper and uncontrolled waste disposable practices, sea spry, windblown dust, forest fires, and volcanic eruption also increase the Cd level in soil [12,13,14].
Apart from this, Cd toxicity has been reported to damage human physiology by various means, such as Cd-contaminated water and food. For example, Cd exposure influences the human male reproductive organs/system and deteriorates spermatogenesis and semen quality, especially sperm motility and hormonal synthesis/release. Based on experimental and human studies, it also impairs female reproduction and the reproductive hormonal balance and affects menstrual cycles [16,17]. In animals, experimental studies revealed that Cd and Cd compounds (referred to as Cd) by multiple routes of exposure prompt benign and malignant tumor formation at various sites in many species of experimental animals [18]. Besides, environmental Cd contact can cause pancreatic cancer in animals [19].
Efficient and economical remediation of contaminated urban and agriculture land is a pressing need for sustainable agriculture development prospects. Different methods, like biological, chemical, and physical, have been used for the remediation of heavy metal contaminants from soil. Some of them face limitations due to mechanical limitations, logistical problems, time, and cost. Soil remediation techniques include physical, chemical, and biological remediation; electrokinetics; and phytoremediation. Physical remediation includes both the soil high replacement method and thermal desorption method. In the soil replacement method, clean soil is used for partial and full replacement of contaminated soil [20,21]. Importing new soil dilutes the contaminated soil; however, this practice is only useful for small-scale severely contaminated soil.
Chemical remediation is a mechanical process used for leaching the contaminated soil by using liquids enriched with solvents, freshwater, and chelating agents [20]. Researchers found that ethylenediaminetetraacetic acid (EDTA) is an effective chelating agent for soil washing. Recent studies have shown that biosurfactants, such as sophorolipids, saponin, and rhamnolipids, can efficiently remove Cd from contaminated soils [22]. According to Juwarkar et al. [23], more than 92% of Cd was removed with 0.1% di-rhamnolipid. In chemical oxidation, oxidants, such as Fe2+-activated peroxymonosulfate, are used to degrade and oxidized the contaminant particles [24]. Bioremediation is the use of microorganisms, plants, and microbial or plant enzymes to treat the contaminated soil through natural biodegradation. Some Cd-removing microorganisms include Aspergillus niger (fungus) [25], Pleurotus ostreatus [26], Spergilus versicolor [27], Fomitopsis pinicola, Pseudomonas aeruginosa, Streptomyces, and Bacillus [28]. Electrokinetics is another technique to remediate heavy metals from soil by using an electrical current [29,30]. In this context, Shen et al. [31] reported an accumulation of 99% of total Cd at the cathode after remediating soil with approaching anode electro-kinetics. Similarly, Li et al. [32] reported a removal of 97.32% of the total Cd through electroosmotic and electromigration, which was positively correlated with the electric voltage. Recently, it has become the best technique and seems to be a promising alternative to conventional approaches.
Phytoremediation is a cost-effective and eco-friendly technique for remediating soils. Notably, phytoremediating plants uptake and accumulate Cd inside their roots, shoots, leaves, and vacuoles. Still, it takes a long time to provide fruitful results because phytoremediation is still under the investigation and progress phase, and several technical barriers have to be overcome. In the present review, we illustrate the recent advancements in the physiological, biochemical, and molecular mechanisms associated with Cd phytoremediation. Additionally, the potential of omics and genetic engineering approaches are outlined for the efficient remediation of a Cd-contaminated environment.
2. Plant Responses to Cadmium Toxicity
The toxic effects of Cd on plant growth and metabolism differ among plant species [3]. The Cd concentration in plants is a direct function of its presence in the soil. An increase in Cd concentration in the growth medium led to a subsequent rise in its accumulation in different parts of the plants [33,34]. It might alter the plant growth and metabolism even if present in a minute amount [33,35]. Cd application in basil seeds delayed the germination period from 4.66 to around 7–10 days [5]. Several other studies have reported high Cd accumulation in wheat seedling roots compared to shoots [36,37].
In some studies, Cd toxicity is linked with the low dry matter accumulation in roots [4], turning them black [38], and leads to a reduction in lateral root growth [39]. It has further been associated with root development with the mature apoplastic pathway, enhanced porosity, and few root tips per surface area in rice plants [40]. In the genus Citrus, seedlings wilted and turned yellow, followed by eventual death under cadmium chloride (CdCl2) treatment [41]. In contrast, tomato plants were reported to endure short-term exposure to high (250 µM) CdCl2 concentrations [42]. Increased exposure to Cd in carrots and radish significantly inhibited the development of radicals due to increased Cd accumulation in roots [43]. Moreover, a considerably high metal concentration was found in parsley seedlings under Cd stress, but the plants did not show any visual stress symptoms [44].
The morphological, biochemical, and physiological effects on plant growth are more pronounced with a high concentration of Cd [39]. An evaluation of the morpho-physiological growth parameters of tomatoes showed that at high Cd concentrations, the root-shoot growth decreased at a relative rate, which could be attributed to the lesser water content in the seedlings due to reduced imbibition. The literature suggests that Cd stress decreased the root-shoot length in wheat [45], peas [46], Corchorus capsularis [47], and Suaeda glauca [48]. Further, it has been documented to reduce the dry weight in wheat [49], maize [50], and tomatoes [51].
Wu et al. [52] described an increase in the net photosynthesis rate by Cd application, which translated to an increase in the net biomass. On the contrary, Cd toxicity has been reported to inhibit plant growth by decreasing the water-use efficiency (WUE) and the net rate of photosynthesis [53]. A significant reduction in the leaf area and dry mass in female Populus cathayana under Cd stress has been observed [54]. In contrast, growth inhibition and a disturbance in photosynthetic performance has been reported in Cd-stressed tomato [55] and cucumber [56]. Cd exposure has further been reported to decrease the stomatal conductance and net rate of photosynthesis in rapeseed [57].
Cd toxicity also affects the plasma membrane of plants, which could be attributed to electrolyte leakage [58], and membrane proteins like H-ATPase inhibition [42]. It has been known to affect the DNA repair system [59]; thus, the stability of the genomic template was distinctly reduced in Phaseolus vulgaris [60] and peas [61] in response to the direct application of Cd.
Reactive oxygen species (ROS) generation in response to Cd-induced oxidative stress affects the electron transport and leaks electrons to molecular oxygen [62]. Cd was found to be toxic for peanuts at a higher dosage, marked by the production and accumulation of ROS in the cytosol. Moreover, it damaged the integrity and selective transport system of plasma membranes, leading to metal transport in cells [63]. Furthermore, the overproduction of ROS in wheat seedlings upon Cd exposure, marked by an increase in the hydrogen peroxide (H2O2) content and malondialdehyde (MDA) level, was linked to genotoxicity [64]. Being sessile, plants try to elude its harmful effects by adopting various defense mechanisms, which include antioxidant activation and other mechanisms of metal homeostasis [65]. In response, plants have developed enzymatic and non-enzymatic antioxidant mechanisms. Increased activities of catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and peroxidase (POD) were found against increased Cd stress in Brassica juncea [66]. In another study, the glutamate-mediated alleviation of Cd toxicity reduced ROS-induced membrane lipid peroxidation, metal uptake, and translocation to rice shoots, and improved the chlorophyll biosynthesis [67].
3. Phytoremediation Processes and Their Salient Features
Phytoremediation refers to the biological cleaning of the environment (soil, water, and air) by plants. Plants make a symbiotic association with microorganisms, which helps in the remediation of the soil, particularly from heavy metals and organic pollutants. Phytoremediation is generally considered as a green technology because of its excellent decontamination ability of heavy metals with a minimum influx of secondary waste to the environment. Alternatively, phytoremediation is highly acceptable among the general public due to its ease of application, low cost, and environmentally friendly nature [1,2]. However, hampered growth activities, such as reduced biomass and increased sensitivity to Cd, were observed in the plants involved in phytoremediation processes [6].
Phytoremediation involves various processes, such as phytoextraction, phytoaccumulation, phytovolatilization, phytostabilization, and phytotransformation. The phytoextraction and phytoaccumulation processes work in association. For instance, during phytoextraction, plants uptake heavy metals, such as Cd, Zn, nickel (Ni), chromium (Cr), and other minerals and nutrients from the soil. After this, these elements accumulate in the shoots and leaves with the help of the phytoaccumulation mechanism [6]. Many plants species have been reported previously for their high accumulation capacity; these are potential candidates for phytoremediation.
In Cd phytoremediation, plants are often used to absorb or translocate Cd into harvestable plant parts. Plants have evolved many diverse adaptations to maintain normal growth even under high Cd-contaminated soils, which also includes detoxification mechanisms [68]. The Cd concentration in plant parts shows the following trend: root > stem > leaves [69]. Many techniques are being used to increase the efficiency of Cd phytoremediation (Table 1).

Table 1.
Types of phytoremediation approaches and their specific methods. Abbreviations are explained in the text.
3.1. Phytoextraction
This technique is used to absorb inorganic and organic contaminants through the stem and roots. Plants that are already growing in the ecosystem should be chosen for this technique. After harvest, they are exposed to another method known as composition, or burned in an incinerator [70]. Hyperaccumulator families, such as Scrophulariaceae, Lamiaceae, Asteraceae, Euphorbiaceae, and Brassicaceae, are essential for this technique. Moreover, some particular plant species, like Celosia argentea [71], Salix mucronata [72], Cassia alata [73], Vigna unguiculata, Solanum melonaena, Momordica charantia [74], Nicotiana tabacum, Kummerowia striata [75], and Swietenia macrophylla [76], may be used as potential plant choices to increase the process of Cd phytoextraction. Moreover, a sub-division of phytoextraction, known as chelate-assisted phytoextraction, is also used as a possible solution for metals that have no hyperaccumulator species. Several amino polycarboxylic acid and chelating agents have been applied to soil to increase the solubility of trace elements. For instance, EDTA-assisted phytoextraction of Cd was preferred by Farid et al. [77]. Similarly, citric acid was used as a chelating agent to increase the Cd uptake ability of jute mallow (Corchorus olitorius) [78].
Phytoextraction helps to reduce metalloid toxicity by improving substrate geochemistry for future colonization of native plants [79]. It is an effective, affordable, environmentally friendly, and potentially cost-effective technique for remediating soils [80]. Despite the generally agreed advantages of phytoextraction, there are some disadvantages, such as the time required for the remediation of highly contaminated soils may be decades [81], and a limitation for mine waste applications [82]. Mostly hyperaccumulator plants have developed the capacity to accumulate only one metal and may be sensitive to the presence of other elements [81].
3.2. Phytostabilization
There has been a progressing shift from phytoextraction to phytostabilization. Phytostabilization is the ability of plants to store and immobilized heavy metals by binding with biomolecules; this process prevents metal transport, and converts them into less toxic substances [83]. Most of the plants growing on contaminated soils are not hyperaccumulators but work as excluders. An excluder transforms the metals and metalloids into a less toxic mobile form without extracting them from the soil and accumulates these compounds in roots by absorption or precipitation within the rhizosphere [84]. Recently, promising results of Virola surinamensis for Cd phytostabilization have been documented [85]. Likewise, Miscanthus x giganteus [86], and oats and white mustard [87] also have phytostabilization potential for Cd. In another example, the putative role of Fe-Si-Ca, organic fertilizers, and coconut shell biochar has been reported to enhance the phytostabilization ability of Boehmeria nivea L. for Cd [88].
Phytostabilization is one emerging ecofriendly phytotechnology, which immobilizes the environmental toxins [89]. Roots take part in phytostabilization, so the metal availability is reduced to the plants, thus reducing the exposure to the other tropic level of the environment [90]. At the same time, the major disadvantage is the fact that pollutant remains in the soil or in the root system, generally in the rhizosphere [91].
3.3. Phytofiltration
Phytofiltration is categorized as rhizofiltration that includes blastofiltration (use seedlings) and caulofilteration (use of excised plant) (Table 1) [92]. Rhizofiltration is the remediation of water in which roots effectively absorb contaminates [93]. In rhizofiltration, contaminant clings or assimilates to the roots, and can be transported to the plants. This method is mostly used to sterilize underground wastes or polluted water. Mostly radioactive substances or metals are removed by this method. Abhilash et al. [94] used the phytofiltration technique to increase the Cd uptake from water by using Limnicharis flava as an experimental plant. Islam et al. [95] reported the phytofiltration capability of Micranthemum umbrosum to remove Cd and arsenic (As) from a hydroponic system. In another experiment, the rhizofiltration potential of Arunda donax for Zn and Cd removal, it and recommended the use of the rhizofilteration technique for Cd elimination [96].
It is a cost-effective technique, and plants act as solar-driven pumps to extract the contaminants from the environment [93,95]. However, any contaminant below the rooting depth is not extracted. It is a time-consuming technique and will not suffice for the extraction of both organic and metal contaminants [95,97].
3.4. Phtytostimulation
Phytostimulation is a technique used to boost the process of phytoremediation by stimulating the root-released compounds to enhance microbial activities. These exudates enhance microbial growth by fulfilling their nutrient requirements. This process is being used in rhizoremediation technologies. It is a low-cost technique for Cd removal and other organic compounds [98]. Another method is the addition of resistant microbial inoculants into the soil, which can cause the accumulation of heavy metals, including Cd [99].
It is a more effective technique for converting toxic contaminants into non-toxic chemicals. Both in situ and ex situ practices can be done with low-cost treatments [100]. Microbes are able to help limit the growth of plant pathogens and increase nitrogen (N) fixation [101]. However, it is a more time-consuming technique, and the use of volatile and biodegradable compounds ex situ is not an easy practice. The process is sensitive to the level of toxicity in soil, and in some cases, incomplete breakdown of the organic compounds is observed. Moreover, well-controlled monitoring is required for this technique [100].
4. Effect of Phytoremediation on Cd Removal from Soils
The phytoremediation of soil contaminated with Cd has been a serious issue worldwide. In phytoremediation, hyperaccumulator plants are of particular importance as they are mainly involved in the uptake of Cd from soil [111]. Different hyperaccumulators vary in their capacity for Cd extraction from soil. This is because of the low affinity of Cd and its mobile nature [111]. The mobility of Cd in soil makes it easily available for the plant to extract it from the soil, which is later transported from the root to the aerial parts [112]. Some of the factors that facilitate the remediation of Cd process are pH, temperature, and the presence of other heavy metals in the soil [113]. For example, Chromolaena odorata, Gynura pseudochina, Conyza sumatrensis, and Nicotiana tabacum were tested in field conditions. The field soil was heavily contaminated with Cd; however, all the tested hyperaccumulator plants significantly reduced the Cd concentration in soil [114]. Various mechanisms of Cd phytoremediation are discussed comprehensively below.
5. Role of Transporters in Cadmium Accumulation on Hyperaccumulator Plants
5.1. Long-Distance Cd Transport
Accumulation of Cd is regulated by several processes, including vacuolar sequestration, xylem loading, cytoplasm across the membrane, energy-driven transport, cell wall adsorption, and Cd apoplastic influx into root tissues (Figure 1 and Figure 2) [115,116]. One of the proposed prerequisites for bioremediation is that heavy metals are transported to and sequestered in aerial parts. Long-distance transport contributes substantially to maintain a low Cd concentration in roots, and takes part in overflow protection machinery [117,118]. These processes are mediated by several families related to metal and metalloid transport, such as P1B-ATPase [(enzymes that catalyze the hydrolysis of a phosphate (P) bond in adenosine triphosphate (ATP) to form adenosine diphosphate (ADP)] [118,119]. When present in ionic form, Cd transport from root to other tissues is mediated by three major transport system [120,121,122], such as low-affinity calcium (Ca) transporter 1 (TaLCT1) ZIP [(Zn transporter proteins (ZRT)- and Fe-regulated transporter (IRT)-like protein)] transporters, TcZNT1/TcZIP4 and Zn/Fe-regulated transporter-like protein (AtRT), and natural resistance-associated macrophage protein (NRAMP) [123], which includes OsNARMP1, 5, and 6. Moreover, the transport system of Fe uptake is also involved in Cd uptake. Takahashi et al. [123] and Milner et al. [124] also observed that OsNramp1 enhanced Cd accumulation in the shoot. Furthermore, yellow strip-like 1 (YS1/YSL1) Fe transporters transport Fe in its chelating form. Murata et al. [125] also identified Fe phytosiderophore transporters (HvYS1) in barley, which showed strict specificity for both metals and ligands. In addition, Sasaki et al. [122] and Ishimaru et al. [126] deduced that OsNRAMP that plays a key role in Mn2+ transport and also showed a major route for Cd transport in rice. From all the above-reported transporters, NRAMP may be involved in several functions, such as metal detoxification, uptake, intracellular transport, and translocation, in many plants [126,127,128,129]. Moreover, the Ca2+ blocker also inhibits Cd transport in Suada salsa, suggesting their contribution to Cd transport [130]. Collectively, Cd is transported through Zn, Fe, and Ca transporters in plants that include LCT1 and ZIP family (Zn/Fe transporters), especially ZIP-IRT [124], and macrophage protein Nramp channels [42]. Therefore, Table 2 shows the summaries of Cd transporters, their function, and location in plants.
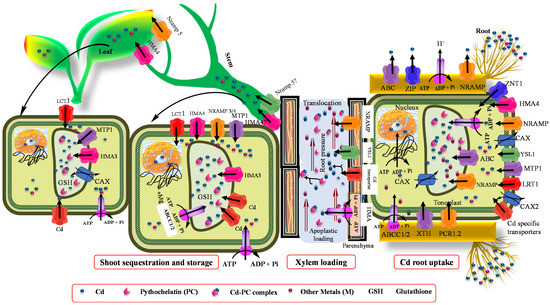
Figure 1.
Depiction of major transporters present on the root, shoot, and leaves for Cd sequestration and storage (these processes are related to phytoremediation). Read text and Table 2 for more information.
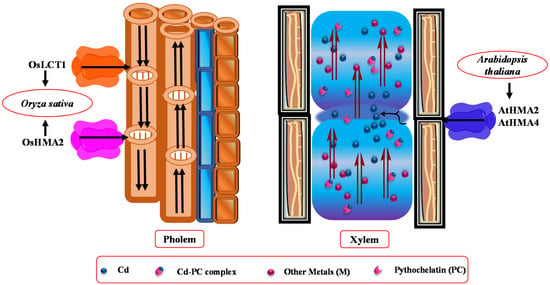
Figure 2.
Representation of phloem and xylem transporters. Low-affinity calcium transporter 1 (LCT1) and metal-transporting ATPases (HMA2) that function in the translocation of Cd into the phloem. The major transporters of Cd loading into the xylem are HMA2 and HMA4. However, the role of transporters between the xylem and phloem is unclear.

Table 2.
Summary of Cd transporters; their localization and function in plants.
AtPDR8 is an ABC transporter that is involved in metal homeostasis and Cd tolerance, and is mainly localized on the epidermis and membrane of root hairs [131]. Once taken by the roots, Cd is then transported into the xylem and other shoot parts. The transport differences in Cd transport in the xylem and shoot are due to genetic variations [132]. Metal tolerance proteins (MTPs) and cation diffusion facilitators (CDFs) can be involved in this whole process [133,134]. Moreover, the P18-type metal transporter ATPase (HMAs) also takes part in Cd transport across the membrane, which is required for metal homeostasis. While working on an Arabidopsis hma2/hma4 mutant, it was observed that OsHMA (pericycle transporter) also transports Cd [135]. OsHMA2 has been described as a key transporter of vascular Cd [136]. The knockout mutant of OsHMA2 resulted in the reduction of Cd in the shoot and grain, which has been confirmed in several studies [118,119,137,138].
Furthermore, low-affinity cation transporters OsLCT1 load Cd metal into the phloem sap [120]. Glutathione (GSH) and its derivatives, phytochelatins (PCs), showed strong bounding with As, Hg, and Cd (Figure 1) [139]. A Cd complex with PCs was also seen in rapeseed phloem sap. According to Mendoza-Cozatl et al. [139] and Kato et al. [140], GSH-Cd (reduced GSH-Cd complex) contributes to long-distance transport of Cd in the phloem. However, PCs appeared in phloem sap after the application of Cd, and they showed a strong affinity for Zn [141]. Mendoza-Cozatl et al. [139] observed thiol-conjugates in phloem that were transported in different sinks after uptake from ATP-binding cassette subfamily C proteins (ABCC1 and ABCC2). PCs-Cd conjugates were involved in root vacuole Cd sequestration, while the GSH-Cd complex was only detected in the seed source. Moreover, the LCT1 transporters also mediates phloem-based Cd distribution (Figure 2) [120,142].
5.2. Vacuolar Storage and Sequestration
Several families of transporters, such as ABCCs, NRAMPs, Ca2+ exchanger (CAXs), and HMAs, have been investigated in vacuolar sequestration of Cd [143,144]. Among these, ABCC transport PCs (PCs-Cd conjugated). Similarly, Park et al. [143] reported that ABCC1 and ABCC2 are important vacuolar transporters that confer tolerance to Cd, mainly AtAbCC3 plays a role in PC-mediated Cd tolerance [145]. The NRAMPs transport various divalent metals, such as Zn, Mn, Fe, and Cd. NRAMP3 and NRAMP4 are located on the tonoplast and play an important role in the remobilization of essential metals from the vacuole to the cytosol [146]. The CAXs are tonoplast-localized transporters that have specific transportability of Ca2+. However, Korenkov et al. [147] reported that AtCAX2 and AtCAX4 transporters are not only specific to Ca2+ but also transport other metals, including Cd. In this consistency, the Cd hyperaccumulator Arabidopsis halleri showed Cd tolerance with higher expression of AhCAXI [3]. In low-Cd-accumulating rice cultivars, OsHMA3 is functional and able to sequester Cd into vacuoles; however, in high-Cd-accumulating cultivars, it is present in an inactive form due to a single amino acid mutation (Table 2) [148,149]. Moreover, higher NcHMA3 expression also plays a role in Cd hyperaccumulation in Noccaea caerulescens [150]. Furthermore, the Cd hyperaccumulator Sedum alfredii also showed a higher expression of SaHMA3 [151]. Currently, Liu et al. [152] has discovered that SpHMA3 is critical for Cd detoxification and vacuolar sequestration in young leaf cells of the Sedum plumbizincicola plant. They found elevated expression of SpHMA3 in shoots, while Ueno et al. [150] observed the same expression level of HMA3 in both the shoot and roots. The CDF transporter family is also involved in vacuolar sequestration and storage and transport of metal ions [153].
5.3. Mechanism of Cd Crossing the Plasma Membrane of Root
At the root plasma membrane, H2CO3 dissociates into HCO3 and H+ trough root respiration, so absorbed H+ rapidly exchanges with Cd+, and then Cd absorbed on the surface of the root epidermis cells, and its exchange into root epidermis cells layers occurs trough the apoplastic pathway [154]. Roots hairs provide a large surface area for Cd absorption from the soil through diffusion [155]. Plant roots also secrete certain organic compounds, such as chelates, that complex with Cd ions to form ligands, allowing its entry into the root epidermis [156]. Moreover, Cd is also taken up by non-selective cation channels, Zn/Fe-regulated transporters [157], and MTPI [158]. Additionally, certain protein transports, such as NRAMPs [159], P-type ATPase (AtHMA4 and AtHMA9) [160,161], ABC transporters (OsPDR9 and AtPDR8 [131,162], and the CAX family (AtCAX2 and AtCAX5) [163,164], impart a critical role in Cd transport across the root plasma membrane. In general, after Cd uptake by plant roots, the maximum portion of Cd gets fractionated into the roots, and only a small portion gets fractionalized to the upper areal parts [160].
6. Antioxidant Defense: A Key Mechanism of Cadmium Tolerance and Phytoremediation
Cadmium inhibits the activity of various metabolic cycles and a non-redox active metal that induces many ROS, including H2O2, superoxide radicals (O2•−), and hydroxyl radical (OH•). Plants have an established mechanism to eradicate oxidative impairment through the protective antioxidant defense system that includes enzymatic antioxidants (SOD, CAT, APX, glutathione reductase (GR), glutathione peroxidase (GPX), glutathione S-transferase (GST)) and non-enzymatic antioxidants (GSH, carotenoids, ascorbic acids (AsA), and tocopherol) [179,180]. Antioxidants’ response against Cd toxicity varies amongst different plant species and experimental conditions [180]. The modulation of antioxidant machinery during the phytoremediation process is important because it prevents the plant from Cd toxicity. For example, some hyperaccumulators have the tendency to uptake/remove an abundance of Cd from the soil, but their physiological and biochemical processes still function properly. This could be because of the high antioxidant enzyme activities, which keeps these hyperaccumulators alive even in the most unfriendly environment. Table 3 documents the experiments showing the potential of the antioxidant defense system as a key mechanism of Cd tolerance and phytoremediation.

Table 3.
Summary of experiments showing the potential of the antioxidant defense system as a key mechanism of cadmium tolerance and phytoremediation. Abbreviations are explained in the text.
In wheat, Cd tolerance is linked with high activity of antioxidant enzymes, photosynthetic rate, and hormone concentrations [181]. Cd stress increases the activity of SOD, CAT, POD, and MDA, and reduces the photosynthetic rate, transpiration rate, stomatal conductance, and auxin, gibberellin, and zeatin nucleoside concentrations in wheat leaves. Shah and Nahakpam [182] found six SOD isozymes in a Cd-tolerant rice cultivar (Bh-1) while only three were found in sensitive (DR-92) rice cultivars, suggesting the SOD improves the tolerance capability of rice against Cd stress. Different plant nutrients, such as silicon, sulfur, and iron, reduce the Cd toxicity in higher plants by affecting its accumulation. Glutamate (Glu; 3 mM) is also involved in the abiotic stress response and has been found to significantly elevate Cd accumulation in rice by up to 44% (root) and 66% (shoot) [169,183]. Macleaya cordata has shown a high Cd phytoremediation ability (393 μg plant−1) with increased biomass. The high Cd concentration, showing high SOD and MDA activity required, and increased capacity of ROS, proves M. cordata’s efficiency in Cd phytoremediation [184]. Phosphorus with 100 µM Cd enhances the activity of SOD, POT, CAT, AsA, and α-tocopherol in wheat and decreases the Cd accumulation in shoots [185]. Sunflower seeds under Cd stress showed reduced biomass, carotenoid, and low chlorophyll concentration with an increase Cd in the shoot and root. Pan et al. [186] examined the genetic insight of high Cd-tolerant Kandelia obovata, a mangrove plant, and reported two genes KoFSD2 and KoCSD3 that showed greater SOD levels and differentially maintained the reactive oxygen mechanism when overexpressed in Nicotiana benthamiana under Cd toxicity.
An increased level of H2O2 decreases the efficiency of the plant for Cd tolerance; therefore, its accumulation is avoided properly by the action of the oxidoreductase enzymes CAT and POD [187]. An increased content of CAT in the leaf of Cd-tolerant wheat lines reduced the translocation of Cd from the root to shoot whereas the 50 µM Cd enhanced the MDA and reduced the activity of CAT and SOD in leaf [188]; while in Glycine max, greater Cd accumulation was observed in root with an increased level of GR (up to 370%) followed by CAT (271%) and SOD (193%) in a tolerant cultivar [189].
Gratao et al. [190] applied the grafted technique in Micro-Tom to analyze the antioxidant response to Cd stress. The grafted plant showed a better signaling response to Cd stress from the root to shoot while the non-grafted plants showed decreased levels of CAT, APX, and GR. In rapeseed, high Cd stress decreased the activity of antioxidant enzymes, i.e., SOD, GR, APX, and CAT, while the lipid peroxidation level increased. Likewise, Brassica juncea exposed to Cd stress showed no change in antioxidant activity, with an increased level of NP-SH and PCs, that worked under metal stress [191], or increased GR activity up to 50 µM L−1 with more accumulated Cd in the leaf of the sensitive cultivar [192]. Enzyme activity increased with an increase in the Cd concentration (100 mg kg−1), such as SOD (81%), CAT (36%), and POX (57%), in Brassica juncea as compared to the control [66].
7. Chelate-Assisted Cadmium Phytoremediation
Due to the limitations in the phytoextraction technique, the use of chelating agents is considered as a suitable alternative to other conventional methods to remediate contaminated soils [202]. In general, chelates are known as stimulating chelating agents in the release of divalent and trivalent cationic metals into soil water to enhance their absorption by the roots of plants. They are classified into three groups of (i) synthetic amino-polycarboxylic acids (APCAs), such as EDTA, ethylene-glycol-tetraacetic acid (EGTA) and sodium-dodecyl-sulfate (SDS); (ii) natural amino-polycarboxylic acids, including S,S-ethylene-diamine-disuccinic acid (EDDS) and nitrile-triacetic acid (NTA); and (iii) low molecular weight organic acids (LMWOAs) containing oxalic acid (OA), citric acid (CA), and tartaric acid (TA) [203,204,205].
Several investigations have been carried out on EDTA as a heavy metals chelator from polluted water and soils for effective phytoextraction [204,206,207]. In several studies, it has been reported that the application of EDTA in contaminated soils leads to elevated Cd accumulation in the aerial parts of plants [207,208]. As stable EDTA-metal complexes have a long-term persistence in soil water, concerns have been raised about the leaching of soil-cationic metal ions and contamination of groundwater and adverse impacts on rhizosphere microorganisms. Therefore, natural chelating agents, such as EDDS, were proposed as a biodegradable chelating agent, a substitute for EDTA [202]. Use of EDDS can decrease metal leaching compared to EDTA but not wholly prevent it [203]. For example, Evangelou et al. [209] described that the half-life of EDDS, depending on the dose of EDDS added to the soil, was between 4.2 and 6.6 days, which is less than EDTA; however, there was Cd and Cu leaching. APCA chelates help to increase the solubility of Cd in soils and efficient phytoextraction but have no role in its elimination. Adding low doses of LMWOAs to Cd contaminated-soil has been suggested as a suitable alternative to other chelants for phytoremediation by (i) the formation of soluble organic acid-Cd complexes, (ii) providing a carbon source for the microorganisms present in the rhizosphere and increasing their diversity; (iii) amending soil quality; (iv) a high degree of biodegradability and low leaching risk; (v) and also improving soil acidity, thus elevating the solubility of Cd ions in soil water [181,210]. In a study on biodegradable chelators, effective phytoextraction of Cd, As, and Pb by Pteris vittata in the presence of 1 mM kg−1 EDDS compared to OA and CA was identified. However, adding of 2.5 mM kg−1 OA improved the soil quality and diversity of soil microorganisms [211]. Moreover, the results of the experiments proved that usually LOWMs in low doses had the highest efficiency of phytoextraction, while higher doses of these chelates negatively affect the Cd uptake and plant biomass. For instance, a study using sunflower plants in Cd-polluted soil showed that Cd absorption at the plant’s roots in the existence of higher levels of CA was much lower than in plants exposed to lower doses of CA [212]. A hypothetical model of phytoremediation by adding chelating agents to remediate Cd-contaminated soil is illustrated in Figure 3.
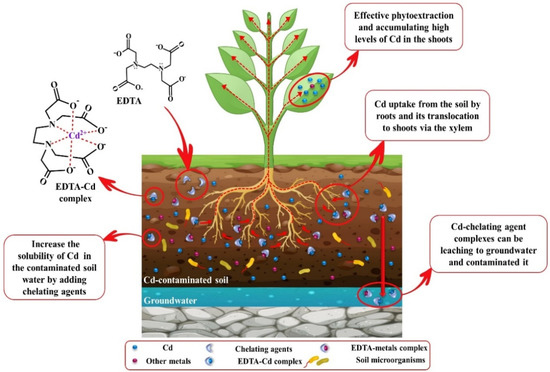
Figure 3.
A hypothetical model of phytoremediation by adding chelating agents to remediate Cd-contaminated soil.
Generally, the disadvantages of the application of chelating agents in soils can reduce the growth and biomass of the plant, as well as the adverse effects on the soil quality [213]. Moreover, some studies have reported that EDTA elevates Cd mobility in soils and its absorption by the roots, though EDTA is not able to overcome the restrictions of the translocation of Cd from the root to shoot [214,215]. Since the absorbed EDTA-Cd complex moves through the apoplastic pathway, the existence of the Casparian strip and suberin deposits disrupt the transfer of this complex from roots to aerial parts, so greater amounts of Cd are accumulated in roots than aerial parts [216,217]. Despite the enhanced levels of soluble Cd in the soil by chelators, regarding the existence of high amounts of Ca and Fe in the soil and their competition for binding to these chelators, higher amounts of these chelating agents must enter the soil to bind to Cd. On the other hand, plants able to absorb only a small portion of the soluble Cd, and a high amount of the soluble Cd-chelate complexes persist in the soil [217]. Notably, the Cd-chelate complex is stable across an extensive pH range, its leaching is unavoidable, and it could enter groundwater and contaminate it [218].
8. Phytochelatins and Metallothionein for Cadmium Phytoremediation
The binding of Cd to high-affinity chelators, including phytochelatins (PCs) and metallothioneins (MTs), and organic acids and amino acids within plant cells is one of the vital adaptative mechanisms [46]. Moreover, the character of these ligands varies, depending on their position inside the plants and plant age [219]. Notably, among metals, Cd is known as a potent stimulator of PCs in different types of plants [220]. Figure 4 represents a schematic summary of Cd tolerance and its accumulation by PCs in leaf cells of Cd-hyperaccumulating plants.
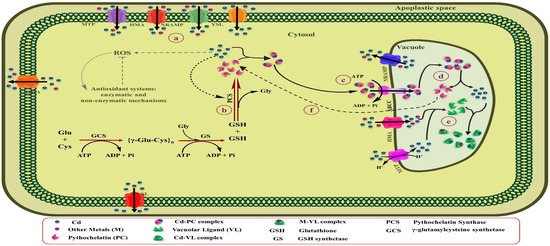
Figure 4.
Schematic overview of Cd tolerance and its accumulation by PCs in leaves cells of Cd-hyperaccumulating plants. (a) Elevated Cd concentration inside the cytosol. (b) The Cd can be directly bound to the PCS and stimulate the biosynthesis of PCs from GSH by activating this enzyme. (c) Once the Cd-PCs complexes are formed, they can finally be sequestered within vacuoles by transferring ABC transporters through the tonoplast. (d) Due to the low pH of vacuoles, Cd-PCs complexes disassociate, and Cd can be stabilized in vacuoles by binding to ligands, including organic acids and probably amino acids. (e) PCs may be by destroyed hydrolase enzymes inside the vacuole or returned to the cytosol, where they able to keep their role as Cd shuttles.
PCs are Cys-rich polypeptides that include duplicate units of γ-glutamyl-cysteine, followed by one glycine (Gly) in the C-terminal [(γ-Glu-Cys)n-Gly] with 2 to 11 repeating units. Albeit, depending on the plant species; Gly in the C-terminal can be substituted by Ala, Ser, Gln, or Glu. These metal ligands are enzymatically synthesized by γ-Glu-Cys dipeptide transpeptidase (PC synthase) from GSH in the cytosol to transfer Cd-PCs complexes to vacuoles or the apoplastic space by ATP-dependent pumps [221,222]. PC synthase is activated by direct Cd binding to the enzyme [223]. While the Cd concentration in the cytosol is elevated, PC synthase enhances tolerance and detoxification of Cd by biosynthesis of PCs [222]. Lee and Hwang [224] found that the overexpression of the NtPCS1 gene involved in PC synthase biosynthesis in transgenic tobacco plants results in high tolerance to Cd and As, and improved growth and development in these plants. By binding Cd to PCs, it forms a constant complex that is less toxic than the free Cd ions existing in the cells. Once the Cd-PCs complexes are formed inside the cytosol, they can finally be sequestered within vacuoles by transferring the ATP-binding cassette (ABC) transporters through the tonoplast (Figure 4) [225]. So far, three types of vacuolar ABC transporter, AtABCC1, AtABCC2 [143], and AtABCC3 [145], have been recognized in the Arabidopsis thaliana that are involved in the transfer of Cd-PCs complexes across the tonoplast into the vacuole and Cd tolerance [226]. Current research has determined that metals compartmentalize as a critical resistance mechanism to reduce oxidative stress and damage to the photosynthetic apparatus in the shoot cells of hyperaccumulator plants [227]. Similarly, it has been identified that mature leaves contain higher amounts of S-rich chelators (PCs and MTs) than in young leaves and can store high concentrations of Cd [228]. Sun et al. [229] found that the levels of PCs in leaf mesophilic tissues of the Cd hyperaccumulator Solanum nigrum were dramatically higher than the non-accumulator Solanum melongena, which caused the accumulation of significant amounts of Cd and their detoxification in shoots of the Cd-hyperaccumulator population. Similarly, analysis of the EDX spectrum in potato exposed to different Cd concentrations indicated that the highest amount of Cd was stored in the mesophilic tissue of leaves by binding to S-rich compounds [230]. A study on alfalfa under Cd stress conditions indicated that Cd tolerance in seedling roots was enhanced by increasing levels of the expression of genes associated with sulfur metabolism, especially genes implicated in GSH and PCs biosynthesis, also ABC transporters [231]. Due to the low pH of vacuoles, Cd-PCs complexes disassociate, and Cd can be stabilized in vacuoles by binding to ligands, including organic acids and probably amino acids [232]. PCs may be by destroyed hydrolase enzymes inside the vacuole or returned to the cytosol, where they able to keep their role as Cd shuttles [233]. PCs are also implicated in the long-distance transfer of excess Cd from the root to aerial parts to reduce Cd accumulation in roots [234]. For instance, PCs and GSH could play a role as long-distance Cd carriers by the xylem and phloem in rapeseed. Likewise, the rate of Cd transfer by the xylem depends on the Cd concentration in the root [235].
Like PCs, MTs belong to a small Cys-rich protein family with binding sites to metal that exists in an extensive range of organisms [236,237]. In contrast to PCs, which are biosynthesized enzymatically by PC synthase, MTs are directly produced by translating mRNA [238]. MT gene regulation is different in various plants and abiotic stress conditions, including heavy metal-induced expression (Figure 5) [239]. So far, four types of MTs have been recognized in plants, containing MT1, MT2, MT3, and MT4 types, which are categorized according to the arrangement of Cys residues in their C- and N-terminal domains [240]. Regarding the diversity of plants as well as the organization or distribution of Cys residues in the MT structure, there may be different isoforms for each type of MT, e.g., in A. thaliana, two isoforms (MT4a and MT4b) have been identified for MT4 [241]. The expression genes of MTs are varied in diverse plant tissues and different growth and development steps of plants [237]. Thus, the genes involved in MT-type1 produce are mostly expressed in roots. In contrast, the genes of MT-type2 are often expressed in the plant shoots and help to store high concentrations of heavy metals in shoots by biding to them, and besides, play an essential role in ROS tolerance. Besides, genes related to MT-type3 are usually involved in fruit ripening and are also expressed in leaves.
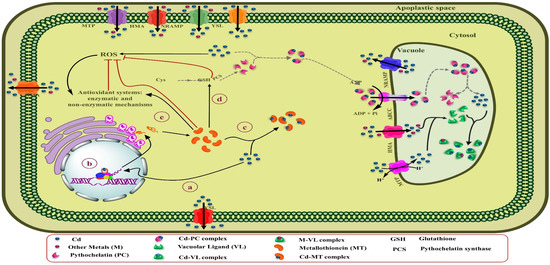
Figure 5.
Schematic overview of Cd detoxification and its accumulation by MTs in leaves cells of Cd hyperaccumulator plants. (a) By increasing the Cd concentration in the cytosol, (b) Cd can stimulate the expression of the MTs gene. (c) By binding MTs to Cd, they can play a substantial role in detoxifying and homeostasis Cd within the cytosol. (d) Additionally, MTs stimulate GSH biosynthesis as the main constituent of PCs. (e) MTs play an essential role in ROS scavenging and its tolerance.
In contrast, the expression of genes associated with MT-type4 has been observed in the seed development stage [237]. However, the role of different types of MTs as metal chelators has been identified, which can lead to the tolerance and accumulation of heavy metals in plants [238]. MTs have a great tendency to bind to heavy metals containing Cd, Zn, Cu, and As, and can eliminate them even at a low amount; nevertheless, in terms of the importance as a Cd chelator, MT is considered after PCs [242]. Generally, MTs play multiple roles in plants, such as tolerance and maintenance of the cellular ion balance by detoxification of heavy metals, ROS scavenging, damaged DNA repair, and as well as various physiological processes, including seed germination and fruit ripening in plants [240,243]. However, the mechanism of action of MT plants in stressful conditions relative to mammalian MT remains unknown. It has been established that MT2, in Coptis japonica, has 14 Cys-residues in its C- and N-terminal that can bind to four Cd(II) ions [239]. Li et al. [242], proved that in Ziziphus jujuba 24 h after Cd exposure, ZjMTs levels elevated and caused increased Cd tolerance and detoxification. This MTs also has six Cys residues in the C- and N-terminal structure and belongs to the MT-Type1 group [242]. It was found that the expression of a gene of CsMTL2 screened from cucumber fruit was regulated by induction of metal stress, especially Cd in the transformed cells of Escherichia. coli. Besides, the highest levels of Cd and Zn were observed consequent of heterologous overexpression of CsMTL2 in E. coli cells compared to the control [242]. In a similar experiment, the same results were obtained on the expression of CsMT4 screened from cucumber fruit in E. coli cells under Cd and Zn stress [244]. Overexpression of PjMT1 and PjMT2 genes transferred to tobacco plants from Prosopis juliflora caused significantly increased accumulation of Cd in transgenic tobacco plants compared to wild-type plants [245]. Overall, these outcomes suggest that different types of MTs are implicated in the tolerance and accumulation of high amounts of Cd in plant cells. Gonzalez-Mendoza et al. [246] examined Cd and Cu stress in Avicennia germinate plants, and they observed a direct relationship between the overexpression of AvPCS and AvMT2 genes and homeostasis and detoxification of Cd and Cu in these plant cells. Additionally, it was shown that the expression of MT genes in yeast strains screened form A. thaliana, which were exposed to several metal stress conditions, MT3 was the best candidate for metal phytoremediation [247]. Today, genetic engineering techniques can improve the effectiveness of phytoremediation in plants [248]. For instance, given the importance of vacuoles as a subcellular organelle for storing Cd, the engineering of vacuolar carriers in particular cells, as well as overexpression of proteins in the cell wall with a high affinity to bind to Cd as another site of Cd storage, can help accumulate more Cd in shoots [249]. Further research for understanding the mechanisms of tolerance and accumulation of Cd by genetic engineering techniques might bring a new milestone for the evolution of phytoremediation of Cd by plants.
9. Omics Approaches for Cadmium Phytoremediation
Omics technologies, such as genomics, transcriptomics, proteomics, and metabolomics, have been widely applied to study the genetic insights, metabolic pathways, and molecular changes in response to external heavy metal stress response, its transport, and accumulation (Table 4, Table 5 and Table 6).

Table 4.
Summary of experiments directed using transcriptomic approaches under Cd stress in plants.

Table 5.
Summary of experiments directed using proteomics platforms under Cd stress in plants.

Table 6.
Summary of experiments directing metabolomics profile under Cd stress in plants.
9.1. Genomics
Genomics approaches’ advancement enhances the identification of multiple genes involved in phytoremediation, plant stress tolerance, and transport of heavy metals, such as DNA mismatch repair (MMR) in a soybean cultivar [250], targeted induced local lesions in genomes (TILLING) in rapeseed [8], clustered regularly interspaced short palindromic repeats (CRISPER/Cas9) in rice [251], and genome-wide association studies (GWAS) in rapeseed [252,253].
Navarro-Leon et al. [8] studied the role of the HMA4 gene of Brassica rapa through TILLING under 100 µM CdCl2 stress. The mutated plant showed increased Cd accumulation in the leaf with an increased level of GSH/GSSG and photosynthetic pigments and reduced biomass and oxidative stress. GWAS using the 60K Brassica Infinium® SNP array was performed with phenotypic and genotypic data to understand the mechanism of Cd tolerance in rapeseed. In total, 12 Cd-tolerant genotypes, 9 single nucleotide polymorphisms (SNPs) loci, and 7 genes linked to Cd tolerance were identified [252]. Chen et al. [253] performed GWAS of 419 rapeseed and identified 25 QTLs integrated with 98 SNPs that reside on 15 chromosomes linked to Cd-accumulated traits.
Ma et al. [254] studied Oryza nivara to detect quantitative trait loci (QTL) related to Cd tolerance. Seven QTLs residing on chromosome 2, 4, 6, and 8 were identified along with five genes related to oxidoreductase, terpene synthase, carboxypeptidase, and cysteine-rich receptor protein through GeneChip data. Ra44 was obtained as a Cd-tolerant line and further used to study the Cd tolerance in rice. GWAS analysis of 349 wild A. thaliana was performed to check the variability in Cd accumulation and the HMA3 locus was found as being potentially responsible for Cd accumulation in the leaf [255].
CRISPER/Cas9 technology was used to obtain three mutants (LCH1, LCH2, and LCH3) of the OSNramp5 gene from rice that were involved in the uptake of Cd and other metal ions from root cells [251]. Tang et al. [256] used the CRISPER/Cas9 system in the rice indica gene OsNramp5 to reduce Cd accumulation in rice for food safety, and demonstrated a 0.05 mg kg−1 decrease in the Cd concentration whereas the plant yield was not affected.
9.2. Transcriptomics
Different technologies are used for transcriptomic studies, i.e., microarray, RNA sequencing (RNA-Seq), cap analysis of gene expression (CAGE), next-generation sequencing (NGS), massive parallel signature sequencing (MPSS), and serial analysis of gene expression (SAGE), as shown in Table 4.
Wang et al. [257] studied the adaptability of the ornamental plant Verbena. bonariensis under Cd stress. Plants transcriptome analysis under Cd stress revealed 237,866 transcripts and 191,370 unigenes whose enrichment analysis revealed differentially expressed genes (DEGs) from all major process, especially lignin synthesis, anthocyanins synthase (ANS), and chalcone synthase (CHS), under Cd stress, confirming the plant has a great Cd phytoremediation property through Cd tolerance and distillation of it. B. juncea is a well-known plant used for phytoremediation studies utilized for microarray analysis to understand the functional genes associated with Pb and Cd stress. The microarray chip of A. thaliana probes was used to study the DEGs in roots of B. juncea (variety P78) [258].
The genome-wide transcriptomic profile of Brassica rapa var. parachinesis (Chinese flowering cabbage) was performed using Solexa sequencing. They identified 1404 upregulated genes and 1669 downregulated genes, and precisely three Cd tolerance genes identified as HMA3, HMA4, and Nramp1 [259]. In a recent study, Cd accumulation and distribution was investigated in the peanut plant through RNA-seq analysis. Here, 8cDNA libraries identified 4484 novel genes from which 6798 were grouped to Cd-related DEGs among two cultivars, first Fenghua 1 (low-Cd) and second Silihong (high-Cd). A total of 183 DEGs were to be found linked to ion transport-related proteins (ZIPs, MTPs, Nramps, and YSLs), among them 9 genes related to the cell wall and 9 genes specifically related to metal transport from which 4 were linked to endomembrane-tonoplast transported genes (MTP4, ABCC4, ABCC15, and ZIP11), four Cd efflux genes (YSL7, FRD3, PDR12, and ZIP1), and one Cd influx gene (IRT1); higher expression in Fenghua 1 might be related to the difference in Cd transport and accumulation among the two cultivars [260]. Creeping bentgrass transcriptional analysis showed four transcription factors (bZIP, WRKY, MYB, and ERF) linked with cd stress [261].
RNA-seq integrated with PacBio ISO-seq was developed to study the full transcriptomic data of Italian rye grass, a potential phytoremediation plant. Out of 2367 DEGs, the overexpression of the LmAUX1 gene significantly enhanced the Cd concentration in A. thaliana. The transcriptome analysis helped in the construction of full-length UniTransModels; out of this, 26.76% had isoforms [262]. RNA-seq analysis of Pokeweed revealed 10.63 Gb transcriptomic data consist of up to 97,000 unigenes covering 72 metabolic pathways. It had excellent phytoremediation ability, and different heavy metal-tolerance genes were identified, including nicotianamine synthase (8), ABC transporter (3), expansins (11), metallothioneine (3), ZRT/IRT protein (4), and aquaporins (4) [263].
9.3. Proteomics
Novel proteomic techniques providing a better understanding of the Cd-responsive proteins for plant stress improvement and phytoremediation are two-dimensional electrophoresis (2DE), sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE), matrix-assisted desorption ionization-time of flight (MALDI-TOF), inductively coupled plasma mass spectroscopy (ICP-MS), liquid chromatography-tandem MS (LC-MS/MS), high-performance liquid chromatography (HPLC), etc., to quantify large-scale proteomic profiles (Table 5).
Proteomic analysis of xylem tissue of Cd-treated rapeseed plant revealed the proteins related to energy production, carbohydrate metabolism, and redox reduction [267]. Cd stress caused a reduction in growth and photosynthesis when Populus. tremula x Populus. alba was exposed to 360 mg kg−1 Cd for 61 days [268]. Proteomic analysis (MALDI-TOF/TOF, 2DE) of a Cd-treated Sorghum bicolor plant revealed increased expression of 15 proteins while it downregulated 8 proteins. Mostly, Cd reduces the activity of ATP production, carbon fixation, and protein synthesis regulation [269]. Suspension cells of rice were subjected to iTRAQ and ICP-MS analysis to study improved Cd stress with silicon [270].
Under 500 µM Cd stress, Microsorum pteropus fern had a higher ability to hyperaccumulate Cd (up to 4000 mg kg−1) in its root and leaf dry mass. Proteomic analysis of fern leaves and roots through MALDI-TOF revealed eight proteins majorly involved in energy metabolism. It enhanced the antioxidant activity to reduce damage from Cd, whereas 20 proteins differentially expressed in leaves were mainly involved in the regulation of photosynthesis and cellular metabolism [271].
9.4. Metabolomics
Different analytical approaches have been developed to understand the plant metabolic response, including nuclear magnetic resonance spectroscopy (NMR), gas and liquid chromatography (GC and LC), and inductively joined mass spectrometry (Table 6). Amino acids, phenols, carotenoids, α-tocopherol, and glutathione are major metabolites whose synthesis varies under metal stress. Cd stress caused a vital change on the metabolism of amino acids, sugar, and organic acids [278,279]. Siriporansdulsil et al. [280] reported an increased level of proline under Cd stress in microalgae whereas an increased level of α-tocopherol was observed in A. thaliana under Cd stress [281]. Metabolic pathways and metabolite enhance Cd tolerance and absorption by plants by promoting PCs [282].
LC-MS/MS and HPLC analysis for metabolites and thiol compounds in Amaranthus hypochondriacus revealed that the plant accumulates 40 times more Cd inside leaves as compared to the control under Cd stress. Among 41 SDMs, 12 were significantly related to PCs in 3 metabolic pathways as Lue, Val, and Ile biosynthesis; Asp, Ala, and Glu metabolism; and Pro and Arg metabolism [282]. Navarro-Reig et al. [283] identified 112 metabolites in rice through LC-MS (HPLC joined with Q-Exactive mass spectrometer). Mengdi et al. [284] identified nine metabolic pathways in A. hypochondriacus (K472) through LC-MS analysis. The intermediate vegetative stage had the highest Cd tolerance ability, which expressed 29 significantly different metabolites involved in 4 metabolic processes, including purine metabolism; Gly, Thr, and Ser metabolism; Asp, Ala, and Glu metabolism; and Arg and Pro metabolism. Further, 100 μM Cd caused an increase in asparagine, tyrosine, and α-tocopherol levels in the leaves of a tomato plant but overall causes severe metabolic and physiological deformities in the plant whereas tomato could bear the exposure to 20 μM Cd adequately [285]. In radish, GC-MS analysis revealed that Cd stress caused an alteration in amino acid metabolism, energy production, and oxidative phosphorylation pathways [278].
10. Genetic Engineering for Cadmium Phytoremediation
A series of studies addressed the issue of remediating Cd through genetically engineered plants (Table 7) as it is known that metals with similar chemical and physical properties biologically antagonize each other [288]. Interestingly, there is a high resemblance between the ionic hydrated radius of Fe2+ (4.28 a.m.) and Cd2+ (4.26 a.m.) [248]. In line with this, a bts-1 lack-of-function mutant in Arabidopsis displayed a higher Cd accumulation capacity in its roots and shoots in comparison to wild-type plants. The enhanced Cd accumulation in the roots and shoots of the bts-1 mutant was because of the positive regulation of Fe nutrition via the upregulation of Fe-related genes [248]. In another study, the detoxification and accumulation of Cd in rice shoots was investigated. The CAL2 gene, which is located in the cell wall, has shown a good Cd chelation ability. To confirm this, ectopic overexpression of CAL2 in Arabidopsis and rice strongly induced their Cd accumulation capacity without affecting the uptake of other essential nutrients [289]. However, the CAL2 gene-overexpressing transgenic lines showed sensitivity to Cd stress. The reason could be the high abundance of accumulated Cd in the shoots of transgenic plants [289]. The Arabidopsis AtPDF2.5 was triggered when the plant was subjected to Cd stress. The results showed the responsive nature of AtPDF2.5 to Cd [35]. The AtPDF2.5 possessed eight cysteine residues, which are involved in the tolerance and chelation of Cd. To validate its role, the AtPDF2.5 gene-overexpressed plants were generated, which displayed tolerance to Cd and also enhanced the Cd accumulation in shoots. Further, AtPDF2.5 facilitates Cd efflux in the cytoplasm via its chelation activity [35]. Similarly, the Arabidopsis AtPDF2.6 possessed Cd chelation properties and were also induced significantly under Cd treatment [290]. Taken together, these lines of evidence affirmed the importance of genetic engineering in the production of Cd-tolerant crops, which could also be helpful in the phytoremediation of soil.

Table 7.
Role of genetically modified plants in the detoxification and phytoremediation of Cd in soil.
11. Employing Microbes for Cadmium Phytoremediation
Transformation of heavy metals from an unavailable to available form is an important factor that decides the fate of phytoremediation. Numerous microbes have been reported to initiate the phytoremediation of Cd by fractionating it in the soil and allow the plant to uptake it. Different classes of microbes (bacteria and fungi) play a crucial role in Cd phytoremediation by activating various mechanisms and producing different compounds, such as siderophores, organic acid, polymeric substances, bioaccumulation, and biosorption (Figure 6). The siderophores are the iron chelators with la ow molecular weight and generally help in the phytoremediation of Cd [297]. Organic acids, which enhance the bioavailability of Cd, are produced by soil microbes. Additionally, organic acid influences the soil pH level by keeping it low, which facilitate the phytoextraction process and is thus crucial for the phytoremediation of Cd [298]. The secretion of extracellular polymeric compounds (exopolysaccharides, mucopolysaccharides, and glomalin) reduces the mobility and bioavailability of Cd, which makes it an essential element in the Cd phytoremediation process [299]. Biosorption and bioaccumulation refer to the processes of metal absorption and accumulation by plants with the help of soil microbes [300]. The biosorption and bioaccumulation contribute largely to Cd phytoremediation by phytostabilizing Cd at the root zone. Below, we discuss the role of bacteria and fungi in promoting phytoremediation of Cd in soil.
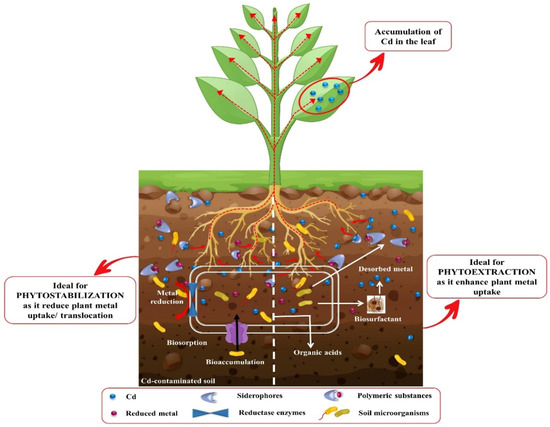
Figure 6.
Schematic illustration of Cd phytoremediation by microbes. In the phytoextraction process, the production of siderophores and organic acids by the soil microbes influences the phytoextraction capacity of the accumulator in a positive manner. The generation of polymeric substances by soil microbes keeps the Cd metal in a static form, which facilitates the biosorption and bioaccumulation process.
11.1. Role of Arbuscular Mycorrhizal Fungi
The arbuscular mycorrhizal fungi (AMF) is a portent regulator of plant growth under various stress conditions, including heavy metals. In general, the AMF makes a symbiotic association with the host plant by increasing the availability of solubilized P [301]. According to previous research, the uptake of Cd largely depends on the amount of accumulation of P [301]. This suggests the critical role of phytoremediation of Cd by AMF. For instance, the application of AMF in Cd-polluted soil significantly enhanced Cd accumulation and phytoavailability in the root and shoot tissues by lowering the soil pH and chemical fractions [302]. Additionally, it increased the tolerance of Solanum nigrum to Cd stress [302]. Eichhornia crassipes is considered as a metal hyperaccumulator and plays an important role in soil phytoremediation. The combination of E. crassipes with AMF reduced the Cd concentration in soil to a large extent. Additionally, the inoculation of AMF with E. crassipes substantially induced Cd accumulation and translocation in the roots and shoots [303]. In Table 8, we list studies featuring the role of AMF and other strains of fungi in Cd phytoremediation.

Table 8.
Enlisted studies of arbuscular mycorrhizal fungi (AMF)/fungi and its role in Cd phytoremediation.
Therefore, this phytoremediation strategy can be applied to minimize the Cd toxicity in soil and water [303]. The growth of Cassia italica Mill has been restricted by the increased amount of Cd in soil [304]. The inoculation of the AMF to Cassia italica Mill medicinal plant significantly hastened the tolerance to Cd by limiting its translocation to shoots. Meanwhile, a higher amount of Cd uptake was observed in the roots of the C. italica Mill plant inoculated with AMF [304]. Tomato fruits are mainly consumed throughout the world; however, the Cd toxicity severely affected the growth and yield of it. On the other hand, the accumulation of high amounts of Cd in tomato fruit could be extremely harmful to human health [305]. The inoculation of AMF in tomato plants reduced the Cd toxicity and prevented the translocation of Cd from the roots to the aerial parts of the plants [305]. The application of Cd could be a useful strategy to protect hazardous environmental changes, such as the removal of Cd from the soil, and reduced the usage of inorganic fertilizer.
11.2. Role of Plant Growth-Promoting Bacteria (PGRB) in the Phytoremediation of Cd
A plethora of studies highlighted the role of PGRB in the phytoremediation of toxic metals, including Cd (Table 9). Among the group of PGRB, some of them detoxify and break down heavy metals by releasing various essential binding compounds, such as organic acid, siderophores, exopolymers, and biosurfactants, which makes the metals available to the plants (Figure 6) [316]. For example, Arthrobacter inoculated to Ocimum gratissimum helps in the removal of Cd from the soil by inducing the uptake of Cd through roots [317]. Similarly, the Arthrobacter sp. was inoculated to the Glycine max under Cd-contaminated soil. The application of Arthrobacter sp. remarkably enhanced the bioavailability of Cd to the plant. Additionally, increased accumulation of Cd in the roots was also observed in the Arthrobacter sp.-inoculated plants [318]. The Bradyrhizobium sp. is a Cd-tolerant PGRB that reduces Cd toxicity and improves agronomic traits of plants grown in Cd-contaminated soil [319]. The inoculation of Bradyrhizobium sp. over Lolium multiflorum triggered the uptake of Cd from the soil and increased the biomass. The induction in Cd accumulation was also observed in the shoots of Lolium multiflorum [319]. The Mesorhizobium huakuii subsp. rengei B3 was inoculated to an Astragalus sinicus plant. The results showed a 19-fold increase in the cell-mediated Cd+2 binding capacity [320]. In rapeseed, the Arthrobacter sp. SrN1 and Bacillus altitudinis SrN9 was applied to alleviate the deleterious effects of Cd. The Arthrobacter sp. SrN1 and Bacillus altitudinis SrN9 inoculation not only boosted the resistance of rapeseed to Cd stress but also increased the uptake and translocation of Cd [321]. This indicates the potential role of Arthrobacter sp. SrN1 and Bacillus altitudinis SrN9 in Cd phytoremediation without altering the plant productivity. Likewise, enhanced Cd uptake was observed in the roots of the Sedum plumbizincicolaa plant after being inoculated with the bacterial strain Rhodococcus erythropolis NSX2 [322]. The application of PGRB to soil could be a useful, cost-effective, and environmentally friendly strategy of Cd phytoremediation. However, more work is required to understand and explore the different strains of microbes involved in the phytoremediation of Cd without hindering plant growth.

Table 9.
List of studies featuring plant growth-promoting bacteria (PGRB) role in Cd phytoremediation.
12. Conclusions and Future Perspectives
Phytoremediation of Cd provides a way forward for the restoration of the polluted environment and has provided many positive and desirable results. Through an extensive literature review, it is evident that Cd interferes with plant functions and as an external stimulus; it activates the defense mechanism through various physiological and metabolic pathways. The multiple phytoremediation strategies offer cost-efficient optimal prospects for the in-situ remediation of Cd in a most environment-friendly way. For a successful rehabilitation, it is essential to utilize the prominent physiological features of Cd hyperaccumulators for the extraction, transformation, and/or stabilization of Cd. Meanwhile, it is important to evaluate the effectiveness of phytoremediation technologies and integrate the available resources. This will not only facilitate the phytoextraction process but also boost the plant productivity in areas with suboptimal soil metal levels by utilizing multi-omics approaches, microbes’ potential, amendments (like AMF and PGPBs), and techniques, such as genetic engineering. Furthermore, the phytoremediation potential and improved tolerance to Cd could be counted as a first step towards leveraging the accumulation potential of plant species. On the other hand, it is equally important to consider the antagonistic and synergistic behavior of contaminants for remediation potential and ensure an ecologically responsible alternative for further use and/or processing of plants, which must be done under a strictly controlled environment.
Notably, the phytoremediation tool is still under the examination and progress phase, and numerous technical barriers need to be resolved. The multifaceted connections that occur under site-specific environments demand a multi-disciplinary approach for metal phytoextraction. This accomplishment will eventually depend upon utilization of a complete analytical tool to integrate the works of plant scientists, soil microbiologists, agronomists, and environmental engineers. Nevertheless, phytoremediation promises to be a vital waste managing choice for the present century.
Moreover, research is required to understand the molecular mechanism of hyperaccumulators in field trials at different locations, as a majority of the current research is based on lab studies. The molecular mechanism of soil amendment-mediated phytoremediation is also unclear. Therefore, omics approaches could be employed to elaborate on how AMF and PGPBs induce the phytoremediation of Cd by regulating numerous genetic, metabolic, and hormonal pathways. An exploration of different genetic pathways will not only enhance the phytoremediation capacity of food crops but also improve their productivity in Cd-contaminated soil.
Author Contributions
Constructed the main conceptual ideas and proof outline, M.H. (Mirza Hasanuzzaman); prepared the figures, A.R., S.N.K., R.S. and N.Z.; prepared the tables, A.R., M.H. (Madiha Habib), R.S. and N.Z.; prepared the final draft, A.R.; writing—original draft preparation, A.R., M.H. (Madiha Habib), S.N.K., Z.Z., N.Z., R.S., M.H. (Mirza Hasanuzzaman); proofread and improved the manuscript, M.H. (Mirza Hasanuzzaman). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are thankful to the researchers whose contributions have been cited in this review paper, which have helped us to prepare a constructive review. Moreover, we apologize to those authors whose admirable work could not be cited due to space limitations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of phytoremediation potential of five Cd (hyper) accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef] [PubMed]
- Baliardini, C.; Meyer, C.-L.; Salis, P.; Saumitou-Laprade, P.; Verbruggen, N. Cation EXCHANGER1 cosegregates with cadmium tolerance in the metal hyperaccumulator Arabidopsis halleri and plays a role in limiting oxidative stress in Arabidopsis spp. Plant Physiol. 2015, 169, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.L.R.; Salvato, F.; Alcântara, B.K.; Nalin, R.S.; Piotto, F.Â.; Azevedo, R.A. Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 2018, 27, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L.). Ind. Crops Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Shanying, H.; Xiaoe, Y.; Zhenli, H.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Navarro-León, E.; Oviedo-Silva, J.; Ruiz, J.M.; Blasco, B. Possible role of HMA4a TILLING mutants of Brassica rapa in cadmium phytoremediation programs. Ecotoxicol. Environ. Saf. 2019, 180, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; di Toppi, L.S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012, 57, 15–22. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Pan, L.-B.; Ma, J.; Wang, X.-L.; Hou, H. Heavy metals in soils from a typical county in Shanxi Province, China: Levels, sources and spatial distribution. Chemosphere 2016, 148, 248–254. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, S.; Khan, A.Z.; Khan, M.A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, T.; Ning, Z.; Li, H.; Tang, J.; Zhou, G. High cadmium concentration in soil in the Three Gorges region: Geogenic source and potential bioavailability. Appl. Geochem. 2013, 37, 149–156. [Google Scholar] [CrossRef]
- Khan, S.; Munir, S.; Sajjad, M.; Li, G. Urban park soil contamination by potentially harmful elements and human health risk in Peshawar City, Khyber Pakhtunkhwa, Pakistan. J. Geochem. Explor. 2016, 165, 102–110. [Google Scholar] [CrossRef]
- Nawab, J.; Khan, S.; Aamir, M.; Shamshad, I.; Qamar, Z.; Din, I.; Huang, Q. Organic amendments impact the availability of heavy metal (loid) s in mine-impacted soil and their phytoremediation by Penisitum americanum and Sorghum bicolor. Environ. Sci. Pollut. Res. 2016, 23, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef]
- Djordjevic, V.R.; Wallace, D.R.; Schweitzer, A.; Boricic, N.; Knezevic, D.; Matic, S.; Grubor, N.; Kerkez, M.; Radenkovic, D.; Bulat, Z.; et al. Environmental cadmium exposure and pancreatic cancer: Evidence from case control, animal and in vitro studies. Environ. Int. 2019, 128, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Méndez, A.; Reichman, S.M. Soil Pollution and Remediation. Int. J. Environ. Res. Public Health 2018, 15, 1657. [Google Scholar] [CrossRef]
- Maity, J.P.; Huang, Y.M.; Fan, C.-W.; Chen, C.-C.; Li, C.-Y.; Hsu, C.-M.; Chang, Y.-F.; Wu, C.-I.; Chen, C.-Y.; Jean, J.-S. Evaluation of remediation process with soapberry derived saponin for removal of heavy metals from contaminated soils in Hai-Pu, Taiwan. J. Environ. Sci. 2013, 25, 1180–1185. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Nair, A.; Dubey, K.V.; Singh, S.; Devotta, S. Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere 2007, 68, 1996–2002. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y. Transformation of heavy metals and dewaterability of waste activated sludge during the conditioning by Fe2+-activated peroxymonosulfate oxidation combined with rice straw biochar as skeleton builder. Chemosphere 2020, 238, 124628. [Google Scholar] [CrossRef]
- Ren, W.-X.; Li, P.-J.; Geng, Y.; Li, X.-J. Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J. Hazard. Mater. 2009, 167, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, M.; Sachdeva, S. Mycoremediation potential of Pleurotus species for heavy metals: A review. Bioresour. Bioprocessing 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.M.; Soleimani, N.; Mehrasbi, M.; Darabian, S.; Mohammadi, J.; Ramazani, A. Highly cadmium tolerant fungi: Their tolerance and removal potential. J. Environ. Health Sci. Eng. 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Bagot, D.; Lebeau, T.; Jezequel, K. Microorganisms for remediation of cadmium-contaminated soils. Environ. Chem. Lett. 2006, 4, 207–211. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Liu, T.; Xin, X.; Hu, H. Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 2017, 189, 599–608. [Google Scholar] [CrossRef]
- Virkutyte, J.; Sillanpää, M.; Latostenmaa, P. Electrokinetic soil remediation—Critical overview. Sci. Total Environ. 2002, 289, 97–121. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, X.; Jia, J.; Qu, L.; Wang, W. Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environ. Pollut. 2007, 150, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; He, X.; Liu, Y. Optimization analysis and mechanism exploration on the removal of cadmium from contaminated soil by electrokinetic remediation. Sep. Purif. Technol. 2020. [Google Scholar] [CrossRef]
- Azizollahi, Z.; Ghaderian, S.M.; Ghotbi-Ravandi, A.A. Cadmium accumulation and its effects on physiological and biochemical characters of summer savory (Satureja hortensis L.). Int. J. Phytoremediat. 2019, 21, 1241–1253. [Google Scholar] [CrossRef]
- Yu, S.; Sheng, L.; Zhang, C.; Deng, H. Physiological response of Arundo donax to cadmium stress by Fourier transform infrared spectroscopy. Spectrochim. Acta A 2018, 198, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.S.; Yang, Y.; Gu, T.; Wu, Z.; Zhang, Z. The Arabidopsis defensin gene AtPDF2.5 mediates cadmium tolerance and accumulation. Plant Cell Environ. 2019, 42, 2681–2695. [Google Scholar] [CrossRef]
- Gajewska, E.; SkŁodowska, M. Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol. Environ. Saf. 2010, 73, 996–1003. [Google Scholar] [CrossRef]
- Popova, L.P.; Maslenkova, L.T.; Yordanova, R.Y.; Ivanova, A.P.; Krantev, A.P.; Szalai, G.; Janda, T. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol. Biochem. 2009, 47, 224–231. [Google Scholar] [CrossRef]
- Yamada, M.; Malambane, G.; Yamada, S.; Suharsono, S.; Tsujimoto, H.; Moseki, B.; Akashi, K. Differential physiological responses and tolerance to potentially toxic elements in biodiesel tree Jatropha curcas. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Meena, M.; Aamir, M.; Kumar, V.; Swapnil, P.; Upadhyay, R. Evaluation of morpho-physiological growth parameters of tomato in response to Cd induced toxicity and characterization of metal sensitive NRAMP3 transporter protein. Environ. Exp. Bot. 2018, 148, 144–167. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Chun, C.-P.; Zhou, W.; Ling, L.-L.; Cao, L.; Fu, X.-Z.; Peng, L.-Z.; Li, Z.-G. Uptake of cadmium (Cd) by selected citrus rootstock cultivars. Sci. Hortic. 2020, 263, 109061. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Zheng, R.-L.; Li, H.-F.; Jiang, R.-F.; Zhang, F.-S. Cadmium accumulation in the edible parts of different cultivars of radish, Raphanus sativus L.; and carrot, Daucus carota var. sativa, grown in a Cd-contaminated soil. Bull. Environ. Cont. Toxicol. 2008, 81, 75–79. [Google Scholar] [CrossRef]
- Ulusu, Y.; Öztürk, L.; Elmastaş, M. Antioxidant capacity and cadmium accumulation in parsley seedlings exposed to cadmium stress. Russ. J. Plant Physiol. 2017, 64, 883–888. [Google Scholar] [CrossRef]
- Li, L.-Z.; Tu, C.; Peijnenburg, W.J.; Luo, Y.-M. Characteristics of cadmium uptake and membrane transport in roots of intact wheat (Triticum aestivum L.) seedlings. Environ. Pollut. 2017, 221, 351–358. [Google Scholar] [CrossRef]
- Rahman, M.F.; Ghosal, A.; Alam, M.F.; Kabir, A.H. Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf. 2017, 135, 165–172. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Khan, M.H.U.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yang, H.; Li, X.; Cui, Z. Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794. [Google Scholar] [CrossRef]
- Carvalho, M.E.; Piotto, F.A.; Gaziola, S.A.; Jacomino, A.P.; Jozefczak, M.; Cuypers, A.; Azevedo, R.A. New insights about cadmium impacts on tomato: Plant acclimation, nutritional changes, fruit quality and yield. Food Energy Secur. 2018, 7, e00131. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Zhao, Y.; Long, Y.; Liu, S.; Pan, Y. Physiological and biochemical mechanisms preventing Cd toxicity in the new hyperaccumulator Abelmoschus manihot. J. Plant Growth Regul. 2018, 37, 709–718. [Google Scholar] [CrossRef]
- Ahmed, H.; Häder, D.-P. Rapid ecotoxicological bioassay of nickel and cadmium using motility and photosynthetic parameters of Euglena gracilis. Environ. Exp. Bot. 2010, 69, 68–75. [Google Scholar] [CrossRef]
- Liu, M.; Bi, J.; Liu, X.; Kang, J.; Korpelainen, H.; Niinemets, Ü.; Li, C. Microstructural and physiological responses to cadmium stress under different nitrogen levels in Populus cathayana females and males. Tree Physiol. 2020, 40, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Shang, L.; Zhou, Z.; Wang, R. Cadmium accumulation and its effects on nutrient uptake and photosynthetic performance in cucumber (Cucumis sativus L.). Philipp. Agric. Sci. 2017, 100, 263–270. [Google Scholar]
- Rossi, L.; Bagheri, M.; Zhang, W.; Chen, Z.; Burken, J.G.; Ma, X. Using artificial neural network to investigate physiological changes and cerium oxide nanoparticles and cadmium uptake by Brassica napus plants. Environ. Pollut. 2019, 246, 381–389. [Google Scholar] [CrossRef]
- Iannone, M.F.; Rosales, E.P.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma 2010, 245, 15–27. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Gjorgieva, D.; Kadifkova-Panovska, T.; Mitrev, S.; Kovacevik, B.; Kostadinovska, E.; Bačeva, K.; Stafilov, T. Assessment of the genotoxicity of heavy metals in Phaseolus vulgaris L. as a model plant system by Random Amplified Polymorphic DNA (RAPD) analysis. J. Environ. Sci. Health 2012, 47, 366–373. [Google Scholar] [CrossRef]
- Surgun-Acar, Y. Determination of heavy metal-induced DNA damage in Pisum Sativum, L. at the molecular and population level. J. Anim. Plant Sci. 2018, 28, 1825–1834. [Google Scholar]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, W.; Liu, F.; Wan, Y. Physiological responses of peanut seedlings to exposure to low or high cadmium concentration and the alleviating effect of exogenous nitric oxide to high cadmium concentration stress. Plant Biosyst. 2020, 154, 405–412. [Google Scholar] [CrossRef]
- Çatav, Ş.S.; Genç, T.O.; Oktay, M.K.; Küçükakyüz, K. Cadmium toxicity in wheat: Impacts on element contents, antioxidant enzyme activities, oxidative stress, and genotoxicity. Bull. Environ. Cont. Toxicol. 2020, 104, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Barman, F.; Majumdar, S.; Arzoo, S.H.; Kundu, R. Genotypic variation among 20 rice cultivars/landraces in response to cadmium stress grown locally in West Bengal, India. Plant Physiol. Biochem. 2020, 148, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Ahmad, A.; Hayat, S. Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J. Biol. Sci. 2014, 21, 125–131. [Google Scholar] [CrossRef]
- Lee, H.J.; Abdula, S.E.; Jang, D.W.; Park, S.-H.; Yoon, U.-H.; Jung, Y.J.; Kang, K.K.; Nou, I.S.; Cho, Y.-G. Overexpression of the glutamine synthetase gene modulates oxidative stress response in rice after exposure to cadmium stress. Plant Cell Rep. 2013, 32, 1521–1529. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef]
- Ahmadpour, P.; Soleimani, M. Cadmium accumulation and translocation in Jatropha curcas grown in contaminated soils. JWSS-Isfahan Uni. Technol. 2015, 19, 179–190. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Yu, G.; Liu, J.; Long, Y.; Chen, Z.; Sunahara, G.I.; Jiang, P.; You, S.; Lin, H.; Xiao, H. Phytoextraction of cadmium-contaminated soils: Comparison of plant species and low molecular weight organic acids. Int. J. Phytoremediat. 2020, 22, 383–391. [Google Scholar] [CrossRef]
- El-Mahrouk, E.-S.M.; Eisa, E.A.-H.; Hegazi, M.A.; Abdel-Gayed, M.E.-S.; Dewir, Y.H.; El-Mahrouk, M.E.; Naidoo, Y. Phytoremediation of cadmium-, copper-, and lead-contaminated soil by Salix mucronata (Synonym Salix safsaf). HortScience 2019, 54, 1249–1257. [Google Scholar] [CrossRef]
- Silva, J.; Fernandes, A.; Junior, M.S.; Santos, C.; Lobato, A. Tolerance mechanisms in Cassia alata exposed to cadmium toxicity–potential use for phytoremediation. Photosynthetica 2018, 56, 495–504. [Google Scholar] [CrossRef]
- Ali, S.Y.; Banerjee, S.N.; Chaudhury, S. Phytoextraction of cadmium and lead by three vegetable-crop plants. Plant Sci. Today 2016, 3, 298–303. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Tang, J.; Hu, L.; Chen, X. Plant coexistence can enhance phytoextraction of cadmium by tobacco (Nicotiana tabacum L.) in contaminated soil. J. Environ. Sci. 2011, 23, 453–460. [Google Scholar] [CrossRef]
- Fan, K.-C.; Hsi, H.-C.; Chen, C.-W.; Lee, H.-L.; Hseu, Z.-Y. Cadmium accumulation and tolerance of mahogany (Swietenia macrophylla) seedlings for phytoextraction applications. J. Environ. Manag. 2011, 92, 2818–2822. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Shakoor, M.B.; Bharwana, S.A.; Rizvi, H.; Ehsan, S.; Tauqeer, H.M.; Iftikhar, U.; Hannan, F. EDTA assisted phytoremediation of cadmium, lead and zinc. Int. J. Agron. Plant Prod. 2013, 4, 2833–2846. [Google Scholar]
- Hassan, M.; Dagari, M.; Babayo, A. Effect of citric acid on cadmium ion uptake and stress response of hydroponically grown jute mallow (Corchorus olitorius). J. Environ. Anal. Toxicol. 2016, 6. [Google Scholar] [CrossRef]
- Anderson, C.; Brooks, R.; Stewart, R.; Simcock, R.; Robinson, B. The phytoremediation and phytomining of heavy metals. In Proceedings of the Pacrim International Congress on Earth Science, Exploration and Mining Around Pacific Rim, Bali, Indonesia, 10–13 October 1999; pp. 127–135. [Google Scholar]
- Ranieri, E.; Moustakas, K.; Barbafieri, M.; Ranieri, A.C.; Herrera-Melián, J.A.; Petrella, A.; Tommasi, F. Phytoextraction technologies for mercury-and chromium-contaminated soil: A review. J. Chem. Technol. Biotechnol. 2020, 95, 317–327. [Google Scholar] [CrossRef]
- Ernst, W.H. Phytoextraction of mine wastes—Options and impossibilities. Geochemistry 2005, 65, 29–42. [Google Scholar] [CrossRef]
- Robinson, B.; Anderson, C.; Dickinson, N. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Khorasani, N.; Yousefi, N.; Karami, M. Findings on the phytoextraction and phytostabilization of soils contaminated with heavy metals. Biol. Trace Elem. Res. 2011, 144, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Andrade Júnior, W.V.; de Oliveira Neto, C.F.; Santos Filho, B.G.d.; do Amarante, C.B.; Cruz, E.D.; Okumura, R.S.; Barbosa, A.V.C.; de Sousa, D.J.P.; Teixeira, J.S.S.; Botelho, A.D.S. Effect of cadmium on young plants of Virola surinamensis. AoB Plants 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Zgorelec, Z.; Bilandzija, N.; Knez, K.; Galic, M.; Zuzul, S. cadmium and Mercury phytostabilization from soil using Miscanthus× giganteus. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Application of white mustard and oats in the phytostabilisation of soil contaminated with cadmium with the addition of cellulose and urea. J. Soil Sediment. 2020, 20, 931–942. [Google Scholar] [CrossRef]
- Lan, M.-M.; Liu, C.; Liu, S.-J.; Qiu, R.-L.; Tang, Y.-T. Phytostabilization of Cd and Pb in Highly polluted Farmland soils using ramie and amendments. Int. J. Environ. Res. Public Health 2020, 17, 1661. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.; Poonia, P. Phytostabilization of metalliferous mine waste. J. Indus. Pollut. Control 2013, 29, 183–192. [Google Scholar]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian. J. Energy Environ. 2005, 6, 18. [Google Scholar]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- Galal, T.M.; Eid, E.M.; Dakhil, M.A.; Hassan, L.M. Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. Int. J. Phytoremediat. 2018, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.; Pandey, V.C.; Srivastava, P.; Rakesh, P.; Chandran, S.; Singh, N.; Thomas, A. Phytofiltration of cadmium from water by Limnocharis flava (L.) Buchenau grown in free-floating culture system. J. Hazard. Mater. 2009, 170, 791–797. [Google Scholar] [CrossRef]
- Islam, M.S.; Saito, T.; Kurasaki, M. Phytofiltration of arsenic and cadmium by using an aquatic plant, Micranthemum umbrosum: Phytotoxicity, uptake kinetics, and mechanism. Ecotoxicol. Environ. Saf. 2015, 112, 193–200. [Google Scholar] [CrossRef]
- Dürešová, Z.; Šuňovská, A.; Horník, M.; Pipíška, M.; Gubišová, M.; Gubiš, J.; Hostin, S. Rhizofiltration potential of Arundo donax for cadmium and zinc removal from contaminated wastewater. Chem. Pap. 2014, 68, 1452–1462. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G. Heavy metal removal in phytofiltration and phycoremediation: The need to differentiate between bioadsorption and bioaccumulation. New Biotech. 2012, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, H.; Lu, H.; Jiang, S.; Dai, M.; Liu, J.; Yan, C. Rhizodegradation potential and tolerance of Avicennia marina (Forsk.) Vierh in phenanthrene and pyrene contaminated sediments. Mar. Pollut. Bull. 2016, 110, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Yanai, J.; Zhao, F.-J.; McGrath, S.P.; Kosaki, T. Effect of soil characteristics on Cd uptake by the hyperaccumulator Thlaspi caerulescens. Environ. Pollut. 2006, 139, 167–175. [Google Scholar] [CrossRef]
- Sharma, H.D.; Reddy, K.R. Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Technologies; John Wiley and Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, X.; Yang, X.; Cui, Z. Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere 2019, 224, 716–725. [Google Scholar] [CrossRef]
- Braud, A.M.; Gaudin, P.; Hazotte, A.; Le Guern, C.; Lebeau, T. Chelate-assisted phytoextraction of lead using Fagopyrum esculentum: Laboratory vs. field experiments. Int. J. Phytoremediat. 2019, 21, 1072–1079. [Google Scholar] [CrossRef]
- Bani, A.; Echevarria, G. Can organic amendments replace chemical fertilizers in nickel agromining cropping systems in Albania? Int. J. Phytoremediat. 2019, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bingöl, N.A.; Özmal, F.; Akın, B. Phytoremediation and Biosorption Potential of Lythrum salicaria L. for Nickel Removal from Aqueous Solutions. Pol. J. Environ. Stud. 2017, 26. [Google Scholar] [CrossRef]
- da Conceição Gomes, M.A.; Hauser-Davis, R.A.; de Souza, A.N.; Vitória, A.P. Metal phytoremediation: General strategies, genetically modified plants and applications in metal nanoparticle contamination. Ecotoxicol. Environ. Saf. 2016, 134, 133–147. [Google Scholar] [CrossRef]
- Nasr, M. Phytomanagement in Egypt: A Sustainable Approach for Clean Environment Coupled with Meeting Future Energy Demand. In Waste Management in MENA Regions; Springer: Berlin/Heidelberg, Germany, 2020; pp. 93–109. [Google Scholar]
- Mesjasz-Przybyłowicz, J.; Nakonieczny, M.; Migula, P.; Augustyniak, M.; Tarnawska, M.; Reimold, U.; Koeberl, C.; Przybyłowicz, W.; Głowacka, E. Uptake of cadmium, lead nickel and zinc from soil and water solutions by the nickel hyperaccumulator Berkheya coddii. Acta Biol. Cracoviensia Ser. Bot. 2004, 46, 75–85. [Google Scholar]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Ye, Z.; Lan, C.; Xie, Z.; Shu, W. Chemically assisted phytoextraction of heavy metal contaminated soils using three plant species. Plant Soil 2005, 276, 153–162. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef] [PubMed]
- Di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Yang, M.; Lin, X.; Yang, X. Impact of Cd on growth and nutrient accumulation of different plant species. Chin. J. Appl. Ecol. 1998, 9, 89–94. [Google Scholar]
- Khaokaew, S.; Landrot, G. A field-scale study of cadmium phytoremediation in a contaminated agricultural soil at Mae Sot District, Tak Province, Thailand: (1) Determination of Cd-hyperaccumulating plants. Chemosphere 2015, 138, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.M.; Lee, D.A.; Schroeder, J.I. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 10118–10123. [Google Scholar] [CrossRef]
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Lux, A.; Vaculík, M.; Martinka, M.; Lišková, D.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Cadmium induces hypodermal periderm formation in the roots of the monocotyledonous medicinal plant Merwilla plumbea. Ann. Bot. 2011, 107, 285–292. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Ma, J.F.; Yamaji, N.; Ueno, D.; Nomoto, K.; Iwashita, T. A specific transporter for iron (III)–phytosiderophore in barley roots. Plant J. 2006, 46, 563–572. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef] [PubMed]
- Nevo, Y.; Nelson, N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Hodoshima, H.; Miyano, Y.; Shoji, K.; Shimada, H.; Goto, F. Cadmium inducible Fe deficiency responses observed from macro and molecular views in tobacco plants. Plant Cell Rep. 2006, 25, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J.F. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Peijnenburg, W.J.; Zhao, J.; Chen, X.; Yu, J.; Wu, H. Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicol. Environ. Saf. 2012, 75, 1–7. [Google Scholar] [CrossRef]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trend. Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Migocka, M.; Kosieradzka, A.; Papierniak, A.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Garbiec, A.; Filleur, S. Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. J. Exp. Bot. 2015, 66, 1001–1015. [Google Scholar] [CrossRef]
- Migocka, M.; Papierniak, A.; Kosieradzka, A.; Posyniak, E.; Maciaszczyk-Dziubinska, E.; Biskup, R.; Garbiec, A.; Marchewka, T. Cucumber metal tolerance protein Cs MTP 9 is a plasma membrane H+-coupled antiporter involved in the Mn2+ and Cd2+ efflux from root cells. Plant J. 2015, 84, 1045–1058. [Google Scholar] [CrossRef]
- Lekeux, G.; Crowet, J.-M.; Nouet, C.; Joris, M.; Jadoul, A.; Bosman, B.; Carnol, M.; Motte, P.; Lins, L.; Galleni, M. Homology modeling and in vivo functional characterization of the zinc permeation pathway in a heavy metal P-type ATPase. J. Exp. Bot. 2019, 70, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sasaki, A.; Xia, J.X.; Yokosho, K.; Ma, J.F. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ishikawa, S.; Inagaki, K.; Chiba, K.; Hayashi, H.; Yanagisawa, S.; Yoneyama, T. Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2010, 56, 839–847. [Google Scholar] [CrossRef]
- Harris, W.R.; Sammons, R.D.; Grabiak, R.C. A speciation model of essential trace metal ions in phloem. J. Inorganic Biochem. 2012, 116, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Kamiya, T.; Clemens, S.; Fujiwara, T. Characterization of OsLCT1, a cadmium transporter from indica rice (Oryza sativa). Physiol. Plant. 2014, 151, 339–347. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Lu, L.; Zhu, Z.; Yang, X. Functional analysis of CAX2-like transporters isolated from two ecotypes of Sedum alfredii. Biol. Plant. 2016, 60, 37–47. [Google Scholar] [CrossRef]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef]
- Korenkov, V.; King, B.; Hirschi, K.; Wagner, G.J. Root-selective expression of AtCAX4 and AtCAX2 results in reduced lamina cadmium in field-grown Nicotiana tabacum L. Plant Biotechnol. J. 2009, 7, 219–226. [Google Scholar] [CrossRef]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Milner, M.J.; Yamaji, N.; Yokosho, K.; Koyama, E.; Clemencia Zambrano, M.; Kaskie, M.; Ebbs, S.; Kochian, L.V.; Ma, J.F. Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J. 2011, 66, 852–862. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Shohag, M.J.I.; Tian, S.; Song, H.; Feng, Y.; Yang, X. Enhanced expression of SaHMA3 plays critical roles in Cd hyperaccumulation and hypertolerance in Cd hyperaccumulator Sedum alfredii Hance. Planta 2016, 243, 577–589. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Let. 2007, 581, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Mori, S.; Baba, K.; Kaburagi-Yada, S.; Arao, T.; Kitajima, N.; Hokura, A.; Terada, Y. Cadmium distribution in the root tissues of solanaceous plants with contrasting root-to-shoot Cd translocation efficiencies. Environ. Exp. Bot. 2011, 71, 198–206. [Google Scholar] [CrossRef]
- Seregin, I.; Ivanov, V. Histochemical investigation of cadmium and lead distribution in plants. Russ. J. Plant Physiol. 1997, 44, 791–796. [Google Scholar]
- Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R. Use of fungi in mitigating cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, UK, 2019; pp. 397–426. [Google Scholar]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. Zinc TRANSPORTER5 and zinc TRANSPORTER9 function synergistically in zinc/cadmium uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, S.; Liu, B.; Zhang, M.; Wu, K. Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep. 2012, 31, 67–79. [Google Scholar] [CrossRef]
- Song, Y.; Hudek, L.; Freestone, D.; Puhui, J.; Michalczyk, A.; Senlin, Z.; Ackland, M. Comparative analyses of cadmium and zinc uptake correlated with changes in natural resistance-associated macrophage protein (NRAMP) expression in Solanum nigrum L. and Brassica rapa. Environ. Chem. 2014, 11, 653–660. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.-Y.; Lee, Y.; An, G. Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol. 2007, 145, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Bækgaard, L.; Mikkelsen, M.D.; Sørensen, D.M.; Hegelund, J.N.; Persson, D.P.; Mills, R.F.; Yang, Z.; Husted, S.; Andersen, J.P.; Buch-Pedersen, M. A combined zinc/cadmium sensor and zinc/cadmium export regulator in a heavy metal pump. J. Biol. Chem. 2010, 285, 31243–31252. [Google Scholar] [CrossRef]
- Moons, A. Ospdr9, which encodes a PDR-type ABC transporter, is induced by heavy metals, hypoxic stress and redox perturbations in rice roots. FEBS Lett. 2003, 553, 370–376. [Google Scholar] [CrossRef]
- Hirschi, K.D.; Korenkov, V.D.; Wilganowski, N.L.; Wagner, G. Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 2000, 124, 125–134. [Google Scholar] [CrossRef]
- Shigaki, T.; Pittman, J.K.; Hirschi, K.D. Manganese Specificity Determinants in the Arabidopsis Metal/H+ Antiporter CAX2. J. Biol. Chem. 2003, 278, 6610–6617. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Tan, J.; Zhang, Y.; Liang, S.; Xiang, S.; Wang, H.; Chai, T. Isolation and characterization of a novel cadmium-regulated yellow stripe-like transporter (SnYSL3) in Solanum nigrum. Plant Cell Rep. 2017, 36, 281–296. [Google Scholar] [CrossRef]
- Koike, S.; Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004, 39, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Zheng, L.; Li, Y.; Zhou, X.; Li, J.; Gu, D.; Xu, E.; Lu, Y.; Chen, X. The intracellular transporter AtNRAMP6 is involved in Fe homeostasis in Arabidopsis. Front. Plant Sci. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol. Cell. 2010, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef]
- Song, W.-Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shim, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef] [PubMed]
- Mary, V.; Ramos, M.S.; Gillet, C.; Socha, A.L.; Giraudat, J.; Agorio, A.; Merlot, S.; Clairet, C.; Kim, S.A.; Punshon, T. Bypassing iron storage in endodermal vacuoles rescues the iron mobilization defect in the natural resistance associated-macrophage protein3natural resistance associated-macrophage protein4 double mutant. Plant Physiol. 2015, 169, 748–759. [Google Scholar] [CrossRef]
- Hassan, Z.; Aarts, M.G. Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ. Exp. Bot. 2011, 72, 53–63. [Google Scholar] [CrossRef]
- Kawachi, M.; Kobae, Y.; Kogawa, S.; Mimura, T.; Krämer, U.; Maeshima, M. Amino acid screening based on structural modeling identifies critical residues for the function, ion selectivity and structure of Arabidopsis MTP1. FEBS J. 2012, 279, 2339–2356. [Google Scholar] [CrossRef]
- Deng, F.; Yamaji, N.; Xia, J.; Ma, J.F. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar] [CrossRef]
- Chen, Z.; Fujii, Y.; Yamaji, N.; Masuda, S.; Takemoto, Y.; Kamiya, T.; Yusuyin, Y.; Iwasaki, K.; Kato, S.-I.; Maeshima, M. Mn tolerance in rice is mediated by MTP8. 1, a member of the cation diffusion facilitator family. J. Exp. Bot. 2013, 64, 4375–4387. [Google Scholar] [CrossRef]
- Bahmani, R.; Kim, D.; Na, J.; Hwang, S. Expression of the tobacco non-symbiotic class 1 hemoglobin gene Hb1 reduces cadmium levels by modulating Cd transporter expression through decreasing nitric oxide and ROS level in Arabidopsis. Front. Plant Sci. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yang, M.; Li, Y.; Tian, J.; Zhang, Y.; Liang, L.; Liu, Z.; Chen, K.; Li, Y.; Lv, K. Comprehensive analysis of variation of cadmium accumulation in rice and detection of a new weak allele of OsHMA3. J. Exp. Bot. 2019, 70, 6389–6400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, K.-F.; Poysa, V.; Shi, C.; Zhou, Y.-H. A single point mutation in GmHMA3 affects cadimum (Cd) translocation and accumulation in soybean seeds. Mol. Plant 2012, 5, 1154–1156. [Google Scholar] [CrossRef][Green Version]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.N.; Carvalho, M.E.A.; Piotto, F.A.; Batagin-Piotto, K.D.; Nogueira, M.L.; Gaziola, S.A.; Azevedo, R.A. Antioxidant defense response in plants to cadmium stress. In Cadmium Tolerance in Plants: Agronomic, Molecular, Signaling, and Omic Approaches; Hasanuzzaman, M., Prasad, M.N.V., Nahar, L., Eds.; Academic Press: New York, NY, USA, 2019; pp. 423–461. [Google Scholar]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G. Glutamate signalling in roots. J. Exp. Bot. 2014, 65, 779–787. [Google Scholar] [CrossRef]
- Nie, J.; Liu, Y.; Zeng, G.; Zheng, B.; Tan, X.; Liu, H.; Xie, J.; Gan, C.; Liu, W. Cadmium accumulation and tolerance of Macleaya cordata: A newly potential plant for sustainable phytoremediation in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 10189–10199. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Pan, C.; Lu, H.; Yu, J.; Liu, J.; Liu, Y.; Yan, C. Identification of Cadmium-responsive Kandelia obovata SOD family genes and response to Cd toxicity. Environ. Exp. Bot. 2019, 162, 230–238. [Google Scholar] [CrossRef]
- Jung, H.-i.; Lee, B.-R.; Chae, M.-J.; Kong, M.-S.; Lee, C.-H.; Kang, S.-S.; Kim, Y.-H. Sulfur alleviates cadmium toxicity in rice (Oryza sativa L.) seedlings by altering antioxidant levels. J. Crop Sci. Biotechnol. 2017, 20, 213–220. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Ahmad, P. Contrasting tolerance among soybean genotypes subjected to different levels of cadmium stress. Pak. J. Bot. 2017, 49, 903–911. [Google Scholar]
- Gratao, P.L.; Monteiro, C.C.; Tezotto, T.; Carvalho, R.F.; Alves, L.R.; Peters, L.P.; Azevedo, R.A. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 2015, 28, 803–816. [Google Scholar] [CrossRef]
- Nouairi, I.; Ammar, W.B.; Youssef, N.B.; Miled, D.D.B.; Ghorbal, M.H.; Zarrouk, M. Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol. Plant. 2009, 31, 237–247. [Google Scholar] [CrossRef]
- Iqbal, N.; Masood, A.; Nazar, R.; Syeed, S.; Khan, N.A. Photosynthesis, growth and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in cadmium tolerance. Agric. Sci. China 2010, 9, 519–527. [Google Scholar] [CrossRef]
- Luo, H.; Li, H.; Zhang, X.; Fu, J. Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 2011, 20, 770–778. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: An evaluation of the role of antioxidant machinery. Plant Signal. Behave. 2011, 6, 293–300. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef]
- Mesnoua, M.; Mateos-Naranjo, E.; Barcia-Piedras, J.M.; Pérez-Romero, J.A.; Lotmani, B.; Redondo-Gómez, S. Physiological and biochemical mechanisms preventing Cd-toxicity in the hyperaccumulator Atriplex halimus L. Plant Physiol. Biochem. 2016, 106, 30–38. [Google Scholar] [CrossRef]
- Asopa, P.P.; Bhatt, R.; Sihag, S.; Kothari, S.; Kachhwaha, S. Effect of cadmium on physiological parameters of cereal and millet plants—A comparative study. Int. J. Phytoremediat. 2017, 19, 225–230. [Google Scholar] [CrossRef]
- Ashrafzadeh, S.; Leung, D.W. Novel potato plants with enhanced cadmium resistance and antioxidative defence generated after in vitro cell line selection. PLoS ONE 2017, 12, e0185621. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, Y.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol. Environ. Saf. 2019, 177, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sozoniuk, M.; Nowak, M.; Dudziak, K.; Bulak, P.; Leśniowska-Nowak, J.; Kowalczyk, K. Antioxidative system response of pedunculate oak (Quercus robur L.) seedlings to Cd exposure. Physiol. Mol. Biol. Plants 2019, 25, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, L.; Tian, S.; Li, S.; Liu, X.; Gao, X.; Zhou, W.; Lin, X. Cadmium-induced nitric oxide burst enhances Cd tolerance at early stage in roots of a hyperaccumulator Sedum alfredii partially by altering glutathione metabolism. Sci. Total Environ. 2019, 650, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Exp. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, D.; Yao, G.; Huang, X.; Wei, L.; Liu, Y.; Wu, Q.; Mai, X.; Liu, G.; Xiao, T. Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils. Int. J. Environ. Res. Public Health 2020, 17, 497. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Uddin, M.; Ara-Sharmeen, I.; Alharby, F.H.; Alzahrani, Y.; Hakeem, K.R.; Zhang, L. Assisting phytoremediation of heavy metals using chemical amendments. Plants 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Shen, Z.; Li, X. Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 2005, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Engelen, D.L.; Sharpe-Pedler, R.C.; Moorhead, K.K. Effect of chelating agents and solubility of cadmium complexes on uptake from soil by Brassica juncea. Chemosphere 2007, 68, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.; Bauer, U.; Ebel, M.; Schaeffer, A. The influence of EDDS and EDTA on the uptake of heavy metals of Cd and Cu from soil with tobacco Nicotiana tabacum. Chemosphere 2007, 68, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A potential candidate for phytoremediation of metals—A review. Plants 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, X.; Guo, Z.; Xiao, X.; Peng, C.; Yang, J.; Zhou, C.; Zeng, P. Chelator-assisted phytoextraction of arsenic, cadmium and lead by Pteris vittata L. and soil microbial community structure response. Int. J. Phytoremed. 2019, 21, 1032–1040. [Google Scholar] [CrossRef]
- Turgut, C.; Pepe, M.K.; Cutright, T.J. The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus. Environ. Pollut. 2004, 131, 147–154. [Google Scholar] [CrossRef]
- Bian, X.; Cui, J.; Tang, B.; Yang, L. Chelant-induced phytoextraction of heavy metals from contaminated soils: A review. Pol. J. Environ. Stud. 2018, 27, 2417–2424. [Google Scholar] [CrossRef]
- Wong, J.W.; Wong, W.W.; Wei, Z.; Jagadeesan, H. Alkaline biosolids and EDTA for phytoremediation of an acidic loamy soil spiked with cadmium. Sci. Total Environ. 2004, 324, 235–246. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Sinegani, A.S. Chelate-assisted phytoextraction of cadmium from a mine soil by negatively charged sunflower. Int. J. Environ. Sci. Technol. 2014, 11, 695–702. [Google Scholar] [CrossRef]
- Redjala, T.; Sterckeman, T.; Morel, J.L. Cadmium uptake by roots: Contribution of apoplast and of high-and low-affinity membrane transport systems. Environ. Exp. Bot. 2009, 67, 235–242. [Google Scholar] [CrossRef]
- Tack, F.M.; Meers, E. Assisted phytoextraction: Helping plants to help us. Elements 2010, 6, 383–388. [Google Scholar] [CrossRef]
- Nowack, B.; Schulin, R.; Robinson, B.H. Critical assessment of chelant-enhanced metal phytoextraction. Environ. Sci. Technol. 2006, 40, 5225–5232. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Filiz, E.; Saracoglu, I.A.; Ozyigit, I.I.; Yalcin, B. Comparative analyses of phytochelatin synthase (PCS) genes in higher plants. Biotechnol. Biotechnol. Equip. 2019, 33, 178–194. [Google Scholar] [CrossRef]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.-P.; Rea, P.A. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J. Biol. Chem. 2000, 275, 31451–31459. [Google Scholar] [CrossRef]
- Lee, B.D.; Hwang, S. Tobacco phytochelatin synthase (NtPCS1) plays important roles in cadmium and arsenic tolerance and in early plant development in tobacco. Plant Biotechnol. Rep. 2015, 9, 107–114. [Google Scholar] [CrossRef]
- Franchi, N.; Piccinni, E.; Ferro, D.; Basso, G.; Spolaore, B.; Santovito, G.; Ballarin, L. Characterization and transcription studies of a phytochelatin synthase gene from the solitary tunicate Ciona intestinalis exposed to cadmium. Aquatic Toxicol. 2014, 152, 47–56. [Google Scholar] [CrossRef]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.-Y.; Li, R. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef]
- Iqbal, M.; Ahmad, A.; Ansari, M.; Qureshi, M.; Aref, I.M.; Khan, P.R.; Hegazy, S.S.; El-Atta, H.; Husen, A.; Hakeem, K.R. Improving the phytoextraction capacity of plants to scavenge metal (loid)-contaminated sites. Environ. Rev. 2015, 23, 44–65. [Google Scholar] [CrossRef]
- Küpper, H.; Mijovilovich, A.; Meyer-Klaucke, W.; Kroneck, P.M. Tissue-and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004, 134, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-L.; Zhou, Q.-X.; Sun, F.-H.; Jin, C.-X. Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ. Exp. Bot. 2007, 60, 468–476. [Google Scholar] [CrossRef]
- Xu, D.; Chen, Z.; Sun, K.; Yan, D.; Kang, M.; Zhao, Y. Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotoxicol. Environ. Saf. 2013, 97, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Yao, P.; Pan, J.; Dai, C.; Cao, H.; Chen, Z.; Zhang, S.; Xu, S.; Shen, W. Transcriptome analysis reveals insight into molecular hydrogen-induced cadmium tolerance in alfalfa: The prominent role of sulfur and (homo) glutathione metabolism. BMC Plant Biol. 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Z.; Wang, T.; Mantri, N.; Huang, H.; Li, H.; Tao, Z.; Guo, Q. Physiological and transcriptomic analyses of cadmium stress response in Dendrobium officinale seedling. Plant Physiol. Biochem. 2020, 148, 152–165. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2010, 5, 663–667. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Butko, E.; Springer, F.; Torpey, J.W.; Komives, E.A.; Kehr, J.; Schroeder, J.I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008, 54, 249–259. [Google Scholar] [CrossRef]
- Freisinger, E. Plant MTs—Long neglected members of the metallothionein superfamily. Dalton Trans. 2008, 47, 6663–6675. [Google Scholar] [CrossRef]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Metallomics 2018, 10, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef]
- Singh, G.; Tripathi, S.; Shanker, K.; Sharma, A. Cadmium-induced conformational changes in type 2 metallothionein of medicinal plant Coptis japonica: Insights from molecular dynamics studies of apo, partially and fully metalated forms. J. Biomol. Struct. Dyn. 2019, 37, 1520–1533. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, M.; Wang, S.; Ma, G.; Huang, X.; Qiao, C.; Wang, R.; Xu, X.; Liang, Y.; Lu, K. Genome-wide characterization and analysis of metallothionein family genes that function in metal stress tolerance in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2181. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-J.; Meetam, M.; Goldsbrough, P.B. Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 2008, 146, 1697–1706. [Google Scholar] [CrossRef]
- Li, L.-S.; Meng, Y.-P.; Cao, Q.-F.; Yang, Y.-Z.; Wang, F.; Jia, H.-S.; Wu, S.-B.; Liu, X.-G. Type 1 metallothionein (ZjMT) is responsible for heavy metal tolerance in Ziziphus jujuba. Biochemistry 2016, 81, 565–573. [Google Scholar] [CrossRef][Green Version]
- Pan, Y.; Pan, Y.; Zhai, J.; Xiong, Y.; Li, J.; Du, X.; Su, C.; Zhang, X. Cucumber metallothionein-like 2 (CsMTL2) exhibits metal-binding properties. Genes 2016, 7, 106. [Google Scholar] [CrossRef]
- Duan, L.; Yu, J.; Xu, L.; Tian, P.; Hu, X.; Song, X.; Pan, Y. Functional characterization of a type 4 metallothionein gene (CsMT4) in cucumber. Hortic. Plant J. 2019, 5, 120–128. [Google Scholar] [CrossRef]
- Balasundaram, U.; Venkataraman, G.; George, S.; Parida, A. Metallothioneins from a hyperaccumulating plant Prosopis juliflora show difference in heavy metal accumulation in transgenic tobacco. Int. J. Agric. Environ. Biotechnol. 2014, 7, 241–246. [Google Scholar] [CrossRef]
- Gonzalez-Mendoza, D.; Moreno, A.Q.; Zapata-Perez, O. Coordinated responses of phytochelatin synthase and metallothionein genes in black mangrove, Avicennia germinans, exposed to cadmium and copper. Aquatic Toxicol. 2007, 83, 306–314. [Google Scholar] [CrossRef]
- Ruta, L.L.; Lin, Y.-F.; Kissen, R.; Nicolau, I.; Neagoe, A.D.; Ghenea, S.; Bones, A.M.; Farcasanu, I.C. Anchoring plant metallothioneins to the inner face of the plasma membrane of Saccharomyces cerevisiae cells leads to heavy metal accumulation. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Du, W.X.; Fang, X.Z.; Zhang, L.L.; Jin, C.W. Knockdown of BTS may provide a new strategy to improve cadmium-phytoremediation efficiency by improving iron status in plants. J. Hazard. Mater. 2020, 384, 121473. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Palmgren, M.G.; Krämer, U. A long way ahead: Understanding and engineering plant metal accumulation. Trend. Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.; Du, Y.; Rogers, H.J.; Wu, Z.; Jia, S.; Yao, X.; Xie, F.; Liu, W. MSH2 and MSH6 in Mismatch Repair System Account for Soybean (Glycine max (L.) Merr.) Tolerance to Cadmium Toxicity by Determining DNA Damage Response. J. Agric. Food Chem. 2020, 68, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Fu, Y.; Xie, H.; Song, S.; Qiu, M.; Wen, J.; Chen, M.; Chen, G.; Tian, Y. Mutation at different sites of metal transporter gene OsNramp5 affects Cd accumulation and related agronomic traits in rice (Oryza sativa L.). Front. Plant Sci. 2019, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiao, X.; Yan, G.; Hu, J.; Cheng, X.; Li, L.; Li, H.; Wu, X. Association mapping of cadmium-tolerant QTLs in Brassica napus L. and insight into their contributions to phytoremediation. Environ. Exp. Bot. 2018, 155, 420–428. [Google Scholar] [CrossRef]
- Chen, L.; Wan, H.; Qian, J.; Guo, J.; Sun, C.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Song, L. Genome-wide association study of cadmium accumulation at the seedling stage in rapeseed (Brassica napus L.). Front. Plant Sci. 2018, 9, 375. [Google Scholar] [CrossRef]
- Ma, X.; Chen, X.; Zhao, J.; Wang, S.; Tan, L.; Sun, C.; Liu, F. Identification of QTLs related to cadmium tolerance from wild rice (Oryza nivara) using a high-density genetic map for a set of introgression lines. Euphytica 2019, 215, 205. [Google Scholar] [CrossRef]
- Chao, D.-Y.; Silva, A.; Baxter, I.; Huang, Y.S.; Nordborg, M.; Danku, J.; Lahner, B.; Yakubova, E.; Salt, D.E. Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1002923. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Wang, M.-q.; Bai, Z.-Y.; Xiao, Y.-F.; Li, Y.; Liu, Q.-L.; Zhang, L.; Pan, Y.-Z.; Jiang, B.-B.; Zhang, F. Transcriptomic analysis of Verbena bonariensis roots in response to cadmium stress. BMC Genom. 2019, 20, 877. [Google Scholar] [CrossRef] [PubMed]
- Dalyan, E.; Yüzbaşıoğlu, E.; Keskin, B.C.; Yıldızhan, Y.; Memon, A.; Ünal, M.; Yüksel, B. The identification of genes associated with Pb and Cd response mechanism in Brassica juncea L. by using Arabidopsis expression array. Environ. Exp. Bot. 2017, 139, 105–115. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Zou, D.; Zhao, J.; Fan, L.; Wu, T. Transcriptome profile analysis of cadmium tolerance in Chinese flowering cabbage. Hortic. Environ. Biotechnol. 2017, 58, 56–65. [Google Scholar] [CrossRef]
- Yu, R.; Ma, Y.; Li, Y.; Li, X.; Liu, C.; Du, X.; Shi, G. Comparative transcriptome analysis revealed key factors for differential cadmium transport and retention in roots of two contrasting peanut cultivars. BMC Genom. 2018, 19, 938. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Bai, Y.; Chao, Y.; Sun, X.; He, C.; Liang, X.; Xie, L.; Han, L. Genome-wide analysis reveals four key transcription factors associated with cadmium stress in creeping bentgrass (Agrostis stolonifera L.). PeerJ 2018, 6, e5191. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, Y.; He, Y.; Cao, Q.; Zhang, T.; Lou, L.; Cai, Q. Full-length transcriptome assembly of italian ryegrass root integrated with RNA-Seq to identify genes in response to plant cadmium stress. Int. J. Mol. Sci. 2020, 21, 1067. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, J.; Zhang, H.; Li, J.; Zhao, Q.; Xu, J. Transcriptome analysis of Phytolacca americana L. in response to cadmium stress. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, C.; Xia, X.; Li, M.; Li, H.; Wang, Y.; Wang, S.; Wang, C.; Ma, Y.; Zhou, G. Comparative transcriptome analysis of duckweed (Landoltia punctata) in response to cadmium provides insights into molecular mechanisms underlying hyperaccumulation. Chemosphere 2018, 190, 154–165. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, S.; Cheng, Z.; Li, T.; Jia, Y.; Wang, G.; Yang, Z.; Xian, J.; Yang, Y.; Zhou, W. Transcriptome analysis revealed cadmium accumulation mechanisms in hyperaccumulator Siegesbeckia orientalis L. Environ. Sci. Pollut. Res. 2020, 27, 18853–18865. [Google Scholar] [CrossRef]
- Liu, A.; Zhou, Z.; Yi, Y.; Chen, G. Transcriptome analysis reveals the roles of stem nodes in cadmium transport to rice grain. BMC Genom. 2020, 21, 1–16. [Google Scholar] [CrossRef]
- Jien, S.H.; Lin, Y.H. Proteins in xylem exudates from rapeseed plants (Brassica napus L.) play a crucial role in cadmium phytoremediation. Clean 2018, 46, 1700164. [Google Scholar] [CrossRef]
- Durand, T.C.; Sergeant, K.; Planchon, S.; Carpin, S.; Label, P.; Morabito, D.; Hausman, J.F.; Renaut, J. Acute metal stress in Populus tremula× P. alba (717-1B4 genotype): Leaf and cambial proteome changes induced by cadmium2+. Proteomics 2010, 10, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Cho, S.-W.; Kwon, S.J.; Kamal, A.H.M.; Kim, S.-W.; Oh, M.-W.; Lee, M.-S.; Chung, K.-Y.; Xin, Z.; Woo, S.-H. Morpho-physiological and proteome level responses to cadmium stress in sorghum. PLoS ONE 2016, 11, e0150431. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sheng, H.; Li, X.; Wang, L. iTRAQ-based proteomic analysis reveals the mechanisms of silicon-mediated cadmium tolerance in rice (Oryza sativa) cells. Plant Physiol. Biochem. 2016, 104, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.-Y.; Yan, Y.-Y.; Yang, B.; Li, X.-Y.; Xu, F.-L. Differential expression of proteins in the leaves and roots of cadmium-stressed Microsorum pteropus, a novel potential aquatic cadmium hyperaccumulator. Sci. Total Environ. 2018, 642, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.M.; Chacón-Madrid, K.; Galazzi, R.M.; Campos, B.K.; Arruda, S.C.; Azevedo, R.A.; Arruda, M.A. Evaluation of silicon influence on the mitigation of cadmium-stress in the development of Arabidopsis thaliana through total metal content, proteomic and enzymatic approaches. J. Trace Elem. Med. Biol. 2017, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Li, S.; Huang, F.; Qiu, J.; Zhang, J.; Sheng, Z.; Tang, S.; Wei, X.; Hu, P. The phosphoproteomic response of rice seedlings to cadmium stress. Int. J. Mol. Sci. 2017, 18, 2055. [Google Scholar] [CrossRef]
- Gong, B.; Nie, W.; Yan, Y.; Gao, Z.; Shi, Q. Unravelling cadmium toxicity and nitric oxide induced tolerance in Cucumis sativus: Insight into regulatory mechanisms using proteomics. J. Hazard. Mater. 2017, 336, 202–213. [Google Scholar] [CrossRef]
- Borges, K.L.R.; Salvato, F.; Loziuk, P.L.; Muddiman, D.C.; Azevedo, R.A. Quantitative proteomic analysis of tomato genotypes with differential cadmium tolerance. Environ. Sci. Pollut. Res. 2019, 26, 26039–26051. [Google Scholar] [CrossRef]
- Pernía, B.; Calabokis, M.; Noris, K.; Bubis, J.; Guerra, M.; Castrillo, M. Effects of cadmium in plants of Sphagneticola trilobata (L.) Pruski. Bioagro 2019, 31, 133–142. [Google Scholar]
- Liu, S.; Li, Y.; Liu, L.; Min, J.; Liu, W.; Li, X.; Pan, X.; Lu, X.; Deng, Q. Comparative proteomics in rice seedlings to characterize the resistance to cadmium stress by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) with isobaric tag for relative and absolute quantitation (iTRAQ). Anal. Lett. 2020, 53, 807–820. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Shen, H.; Wang, J.; Liu, W.; Zhu, X.; Wang, R.; Sun, X.; Liu, L. Metabolomic analysis with GC-MS to reveal potential metabolites and biological pathways involved in Pb and Cd stress response of radish roots. Sci. Rep. 2015, 5, 18296. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Sadia, B.; Raza, A.; Khalid Hameed, M.; Saleem, F. Metabolomics: A way forward for crop improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef]
- Collin, V.C.; Eymery, F.; Genty, B.; Rey, P.; Havaux, M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ. 2008, 31, 244–257. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Lai, X.; Dai, H.; Sun, H.; Zhou, X.; Chen, T. Metabolic responses and their correlations with phytochelatins in Amaranthus hypochondriacus under cadmium stress. Environ. Pollut. 2019, 252, 1791–1800. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; Piña, B.; Moyano, E.; Galceran, M.T.; Tauler, R. Metabolomic analysis of the effects of cadmium and copper treatment in Oryza sativa L. using untargeted liquid chromatography coupled to high resolution mass spectrometry and all-ion fragmentation. Metallomics 2017, 9, 660–675. [Google Scholar] [CrossRef]
- Mengdi, X.; Haibo, D.; Jiaxin, L.; Zhe, X.; Yi, C.; Xuan, L.; Haiyan, M.; Hui, S.; Tianqi, A.; Yunzhen, L. Metabolomics reveals the “Invisible” detoxification mechanisms of Amaranthus hypochondriacus at three ages upon exposure to different levels of cadmium. Ecotoxicol. Environ. Saf. 2020, 195, 110520. [Google Scholar] [CrossRef]
- Hediji, H.; Djebali, W.; Cabasson, C.; Maucourt, M.; Baldet, P.; Bertrand, A.; Zoghlami, L.B.; Deborde, C.; Moing, A.; Brouquisse, R. Effects of long-term cadmium exposure on growth and metabolomic profile of tomato plants. Ecotoxicol. Environ. Saf. 2010, 73, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Renault, D. Effects of cadmium, inorganic mercury and methylmercury on the physiology and metabolomic profiles of shoots of the macrophyte Elodea nuttallii. Environ. Pollut. 2020, 257, 113557. [Google Scholar] [CrossRef]
- Mwamba, T.; Islam, F.; Ali, B.; Lwalaba, J.; Gill, R.; Zhang, F.; Farooq, M.; Ali, S.; Ulhassan, Z.; Huang, Q. Comparative metabolomic responses of low-and high-cadmium accumulating genotypes reveal the cadmium adaptive mechanism in Brassica napus. Chemosphere 2020, 250, 126308. [Google Scholar] [CrossRef]
- He, X.L.; Fan, S.K.; Zhu, J.; Guan, M.Y.; Liu, X.X.; Zhang, Y.S.; Jin, C.W. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 2017, 416, 453–462. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, J.-S.; Xiao, Y.; Yao, J.; Wu, Z.; Yang, Y.; Ismail, A.M. Overexpression of a defensin-like gene CAL2 enhances cadmium accumulation in plants. Front. Plant Sci. 2020, 11, 217. [Google Scholar]
- Luo, J.-S.; Gu, T.; Yang, Y.; Zhang, Z. A non-secreted plant defensin AtPDF2. 6 conferred cadmium tolerance via its chelation in Arabidopsis. Plant Mol. Biol. 2019, 100, 561–569. [Google Scholar] [CrossRef]
- Reisinger, S.; Schiavon, M.; Terry, N.; Pilon-Smits, E.A. Heavy metal tolerance and accumulation in Indian mustard (Brassica juncea L.) expressing bacterial γ-glutamylcysteine synthetase or glutathione synthetase. Int. J. Phytoremediat. 2008, 10, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.F.; Du, S.T.; Lu, K.X.; Liu, W.J.; Lin, X.Y.; Jin, C.W. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2012, 63, 3127–3136. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Guan, M.Y.; Lu, K.X.; Du, S.T.; Fan, S.K.; Ye, Y.-Q.; Lin, X.Y.; Jin, C.W. Inhibition of nitrate transporter 1.1-controlled nitrate uptake reduces cadmium uptake in Arabidopsis. Plant Physiol. 2014, 166, 934–944. [Google Scholar] [CrossRef]
- Siemianowski, O.; Barabasz, A.; Kendziorek, M.; Ruszczyńska, A.; Bulska, E.; Williams, L.E.; Antosiewicz, D.M. HMA4 expression in tobacco reduces Cd accumulation due to the induction of the apoplastic barrier. J. Exp. Bot. 2014, 65, 1125–1139. [Google Scholar] [CrossRef]
- Yan, J.; Wang, P.; Wang, P.; Yang, M.; Lian, X.; Tang, Z.; Huang, C.F.; Salt, D.E.; Zhao, F.J. A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant Cell Environ. 2016, 39, 1941–1954. [Google Scholar] [CrossRef]
- Song, J.; Feng, S.J.; Chen, J.; Zhao, W.T.; Yang, Z.M. A cadmium stress-responsive gene AtFC1 confers plant tolerance to cadmium toxicity. BMC Plant Biol. 2017, 17, 187. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Chao, Y.; Wang, S.; Tang, Y.-T.; Qiu, R.-L. Metal-tolerant Enterobacter sp. strain EG16 enhanced phytoremediation using Hibiscus cannabinus via siderophore-mediated plant growth promotion under metal contamination. Plant Soil 2017, 413, 203–216. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, X.-F.; Wang, L.-L.; Li, Q.-S.; Zhou, T.; Chen, Y.-K.; Zhao, Z.-Y.; He, B.-Y. Effect of phosphate-solubilizing bacteria on the mobility of insoluble cadmium and metabolic analysis. Int. J. Environ. Res. Public Health 2018, 15, 1330. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Sandhya, S.; Prasad, M.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Mushtaq, H.; Ali, H.; Munis, M.F.H.; Javed, M.T.; Chaudhary, H.J. Diazotrophs-assisted phytoremediation of heavy metals: A novel approach. Environ. Sci. Pollut. Res. 2015, 22, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, B.; Ohtomo, R. Mycorrhizal effects on growth, P uptake and Cd tolerance of the host plant vary among different AM fungal species. Soil Sci. Plant Nutr. 2015, 61, 359–368. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F.; You, Y.; Wang, Y.; Yang, D. Integration of earthworms and arbuscular mycorrhizal fungi into phytoremediation of cadmium-contaminated soil by Solanum nigrum L. J. Hazard. Mater. 2020, 389. [Google Scholar] [CrossRef]
- Gunathilakae, N.; Yapa, N.; Hettiarachchi, R. Effect of arbuscular mycorrhizal fungi on the cadmium phytoremediation potential of Eichhornia crassipes (Mart.) Solms. Groundwater Sustain. Dev. 2018, 7, 477–482. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.; Alqarawi, A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.; Alqarawi, A.; Al Huqail, A.A.; Egamberdieva, D.; Wirth, S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016, 23, 272–281. [Google Scholar] [CrossRef]
- Bissonnette, L.; St-Arnaud, M.; Labrecque, M. Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant Soil 2010, 332, 55–67. [Google Scholar] [CrossRef]
- Gao, Y.; Miao, C.; Mao, L.; Zhou, P.; Jin, Z.; Shi, W. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. J. Hazard. Mater. 2010, 181, 771–777. [Google Scholar] [CrossRef]
- Ling-Zhi, L.; Zong-Qiang, G.; ZHANG, Y.-L.; Pei-Jun, L. Growth, cadmium accumulation and physiology of marigold (Tagetes erecta L.) as affected by arbuscular mycorrhizal fungi. Pedosphere 2011, 21, 319–327. [Google Scholar]
- Bhaduri, A.M.; Fulekar, M. Assessment of arbuscular mycorrhizal fungi on the phytoremediation potential of Ipomoea aquatica on cadmium uptake. 3 Biotech 2012, 2, 193–198. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Millsp. under NaCl and Cd stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Hassan, S.E.; Hijri, M.; St-Arnaud, M. Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. New Biotechnol. 2013, 30, 780–787. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Z.; Zhang, Y.; Li, P. Growth, cadmium uptake and accumulation of maize (Zea mays L.) under the effects of arbuscular mycorrhizal fungi. Ecotoxicology 2014, 23, 1979–1986. [Google Scholar] [CrossRef]
- Ali, N.; Masood, S.; Mukhtar, T.; Kamran, M.A.; Rafique, M.; Munis, M.F.H.; Chaudhary, H.J. Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linum usitatissimum in association with Glomus intraradices. Environ. Monit. Assess. 2015, 187, 311. [Google Scholar]
- Jiang, Q.-Y.; Zhuo, F.; Long, S.-H.; Zhao, H.-D.; Yang, D.-J.; Ye, Z.-H.; Li, S.-S.; Jing, Y.-X. Can arbuscular mycorrhizal fungi reduce Cd uptake and alleviate Cd toxicity of Lonicera japonica grown in Cd-added soils? Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Jin, Z.; Deng, S.; Wen, Y.; Jin, Y.; Pan, L.; Zhang, Y.; Black, T.; Jones, K.C.; Zhang, H.; Zhang, D. Application of Simplicillium chinense for Cd and Pb biosorption and enhancing heavy metal phytoremediation of soils. Sci. Total Environ. 2019, 697, 134148. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Prapagdee, B.; Khonsue, N. Bacterial-assisted cadmium phytoremediation by Ocimum gratissimum L. in polluted agricultural soil: A field trial experiment. Int. J. Environ. Sci. Technol. 2015, 12, 3843–3852. [Google Scholar] [CrossRef]
- Rojjanateeranaj, P.; Sangthong, C.; Prapagdee, B. Enhanced cadmium phytoremediation of Glycine max L. through bioaugmentation of cadmium-resistant bacteria assisted by biostimulation. Chemosphere 2017, 185, 764–771. [Google Scholar] [CrossRef]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214. [Google Scholar] [CrossRef]
- Sriprang, R.; Hayashi, M.; Ono, H.; Takagi, M.; Hirata, K.; Murooka, Y. Enhanced accumulation of Cd2+ by a Mesorhizobium sp. transformed with a gene from Arabidopsis thaliana coding for phytochelatin synthase. Appl. Environ. Microbiol. 2003, 69, 1791–1796. [Google Scholar] [CrossRef]
- Pan, F.; Meng, Q.; Luo, S.; Shen, J.; Chen, B.; Khan, K.Y.; Japenga, J.; Ma, X.; Yang, X.; Feng, Y. Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth-promoting bacteria isolated from Cd hyperaccumulator Sedum alfredii Hance. Int. J. Phytoremediat. 2017, 19, 281–289. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Wang, B.; Hou, J.; Luo, Y.; Tang, C.; Franks, A.E. Plant growth-promoting rhizobacteria enhance the growth and Cd uptake of Sedum plumbizincicola in a Cd-contaminated soil. J. Soil Sediments 2015, 15, 1191–1199. [Google Scholar] [CrossRef]
- Wan, Y.; Luo, S.; Chen, J.; Xiao, X.; Chen, L.; Zeng, G.; Liu, C.; He, Y. Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere 2012, 89, 743–750. [Google Scholar] [CrossRef]
- Chen, Z.-j.; Sheng, X.-f.; He, L.-y.; Huang, Z.; Zhang, W.-h. Effects of root inoculation with bacteria on the growth, Cd uptake and bacterial communities associated with rape grown in Cd-contaminated soil. J. Hazard. Mater. 2013, 244, 709–717. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.; Li, X.; Wan, Y.; Chen, J.; Liu, C. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Kamran, M.A.; Syed, J.H.; Eqani, S.A.M.A.S.; Munis, M.F.H.; Chaudhary, H.J. Effect of plant growth-promoting rhizobacteria inoculation on cadmium (Cd) uptake by Eruca sativa. Environ. Sci. Pollut. Res. 2015, 22, 9275–9283. [Google Scholar] [CrossRef]
- Złoch, M.; Kowalkowski, T.; Tyburski, J.; Hrynkiewicz, K. Modeling of phytoextraction efficiency of microbially stimulated Salix dasyclados L. in the soils with different speciation of heavy metals. Int. J. Phytoremediat. 2017, 19, 1150–1164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiao, J.; Fan, X.; Sun, H.; Zhang, Y.; Jiang, J.; Liu, C. Endophytic bacterium Pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars. Front. Plant Sci. 2017, 7, 2068. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Panda, S.; Basu, A.; Dhal, N. Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int. J. Phytoremediat. 2018, 20, 682–691. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).