Heterometrus spinifer: An Untapped Source of Anti-Tumor Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Consent and Use of Scorpions
2.2. Fecal Sample Collection
2.3. Dissection
2.4. Bacterial Identification
2.5. Conditioned Medium Preparation
2.6. Culture of Cancer and Normal Cells
2.7. Growth Inhibition Assays
2.8. Cytotoxicity Assays, Cell Staining and Survival Assays
2.9. Growth Inhibition Assays of Concentrated Active CM
2.10. Identification of Molecules(s) Exhibiting Anticancer Activity through Liquid Chromatography–Mass Spectrometry (LC–MS)
2.11. Statistical Analysis
3. Results
3.1. A Spectrum of Bacteria Identified from the Faeces and Gastrointestinal Tract of H. Spinifer
3.2. Conditioned Media from Feces of H. Spinifer Inhibited the Growth of HeLa Cells
3.3. Conditioned Media from Gastrointestinal Tract of H. Spinifer Inhibited the Growth of HeLa Cells
3.4. Conditioned Media from Feces of H. Spinifer Exhibited Limited Cytotoxic Effect Towards HeLa Cells
3.5. Conditioned Media HSG12 and HSG16 from the Gastrointestinal Tract of H. Spinifer Affected HeLa Cell Morphology
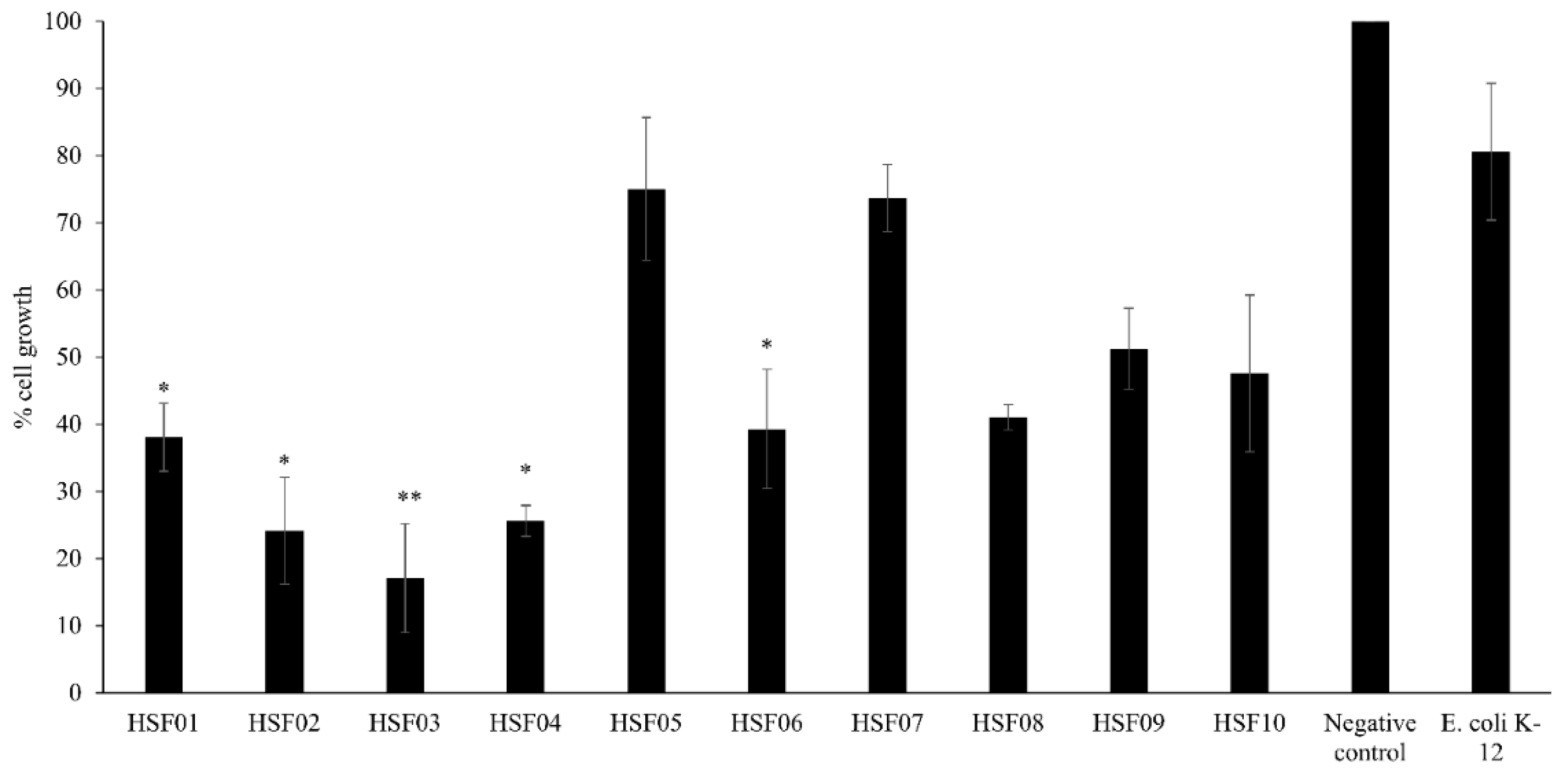
3.6. HSF02, HSF03, HSF05 and HSF08, from Feces of H. Spinifer, Affected the Survival of HeLa Cells
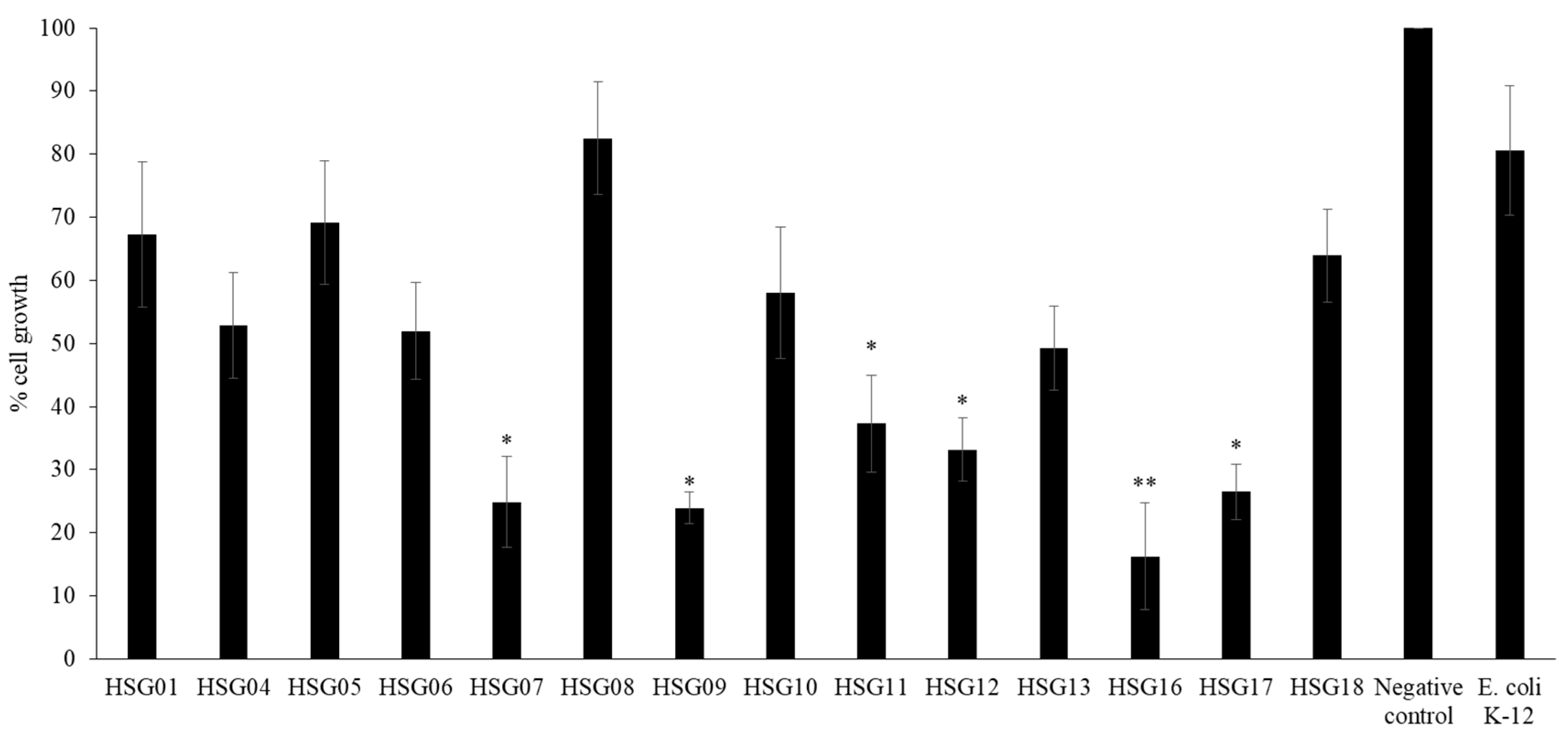
3.7. HSG12, HSG16 and HSG17 from Gastrointestinal Tract of H. Spinifer, Affected the Survival of HeLa Cells
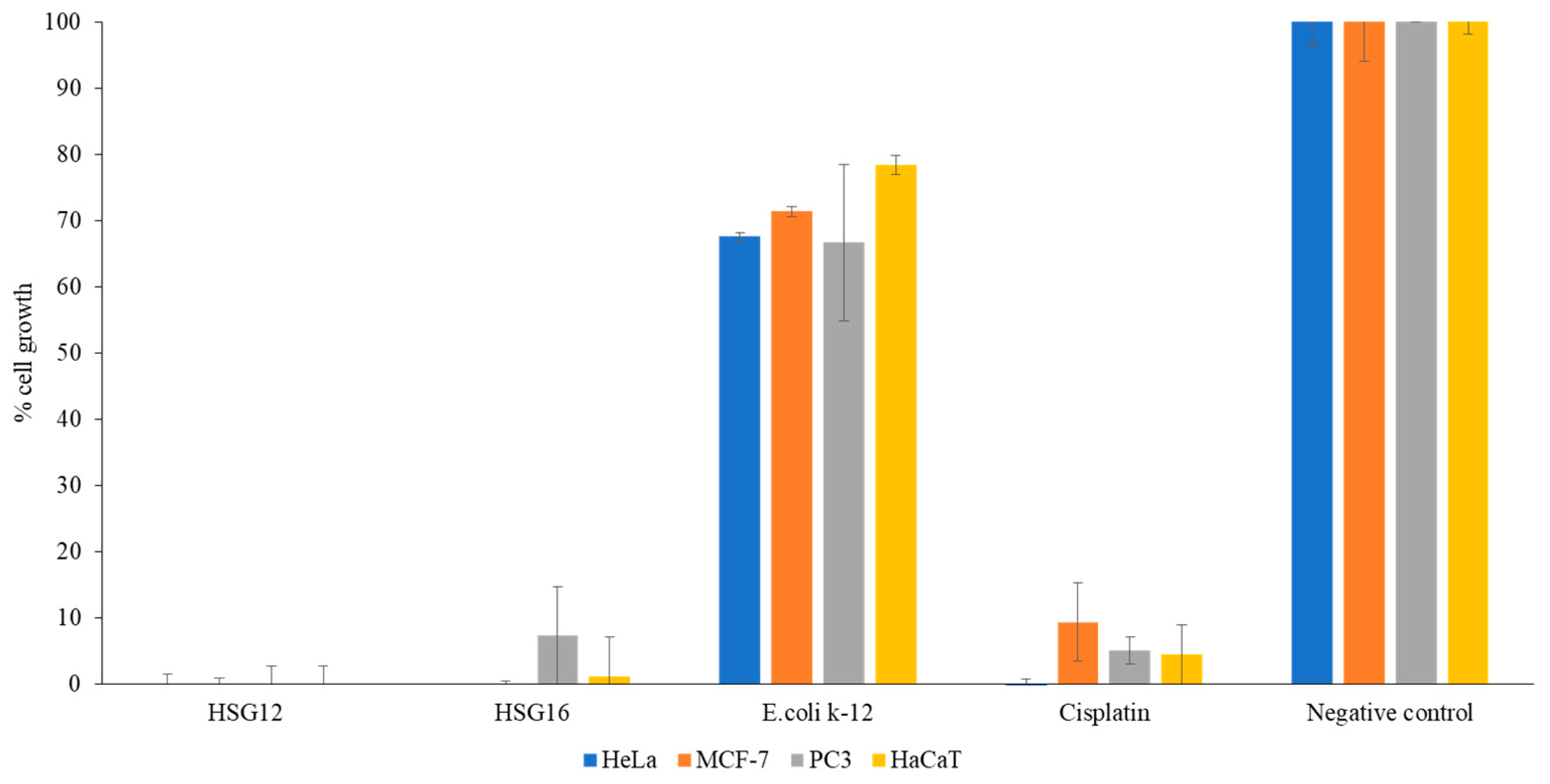
3.8. Concentrated CM from the Gastrointestinal Tract of H. Spinifer Inhibited the Growth of Cancer and Normal Cells
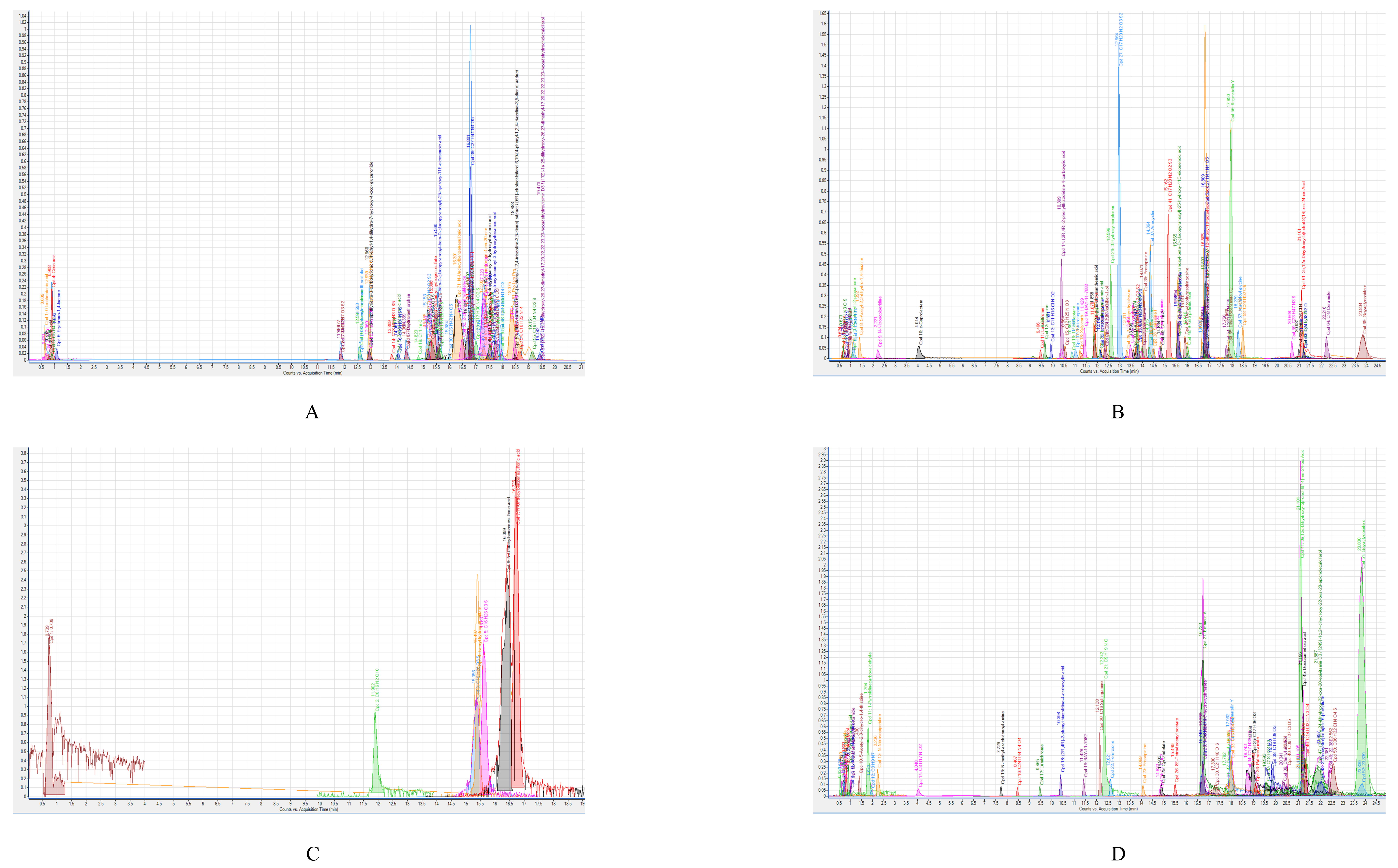
3.9. Concentrated CM from the Gastrointestinal Tract of H. Spinifer Possesses 72 and 38 Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
References
- World Health Organization. Global Cancer Observatory. 2018. Available online: https://gco.iarc.fr/ (accessed on 6 April 2019).
- Cancer Research UK. Cancer Incidence Statistics. 2019. Available online: https://www.cancerresearchuk.org/health-professional/data-and-statistics (accessed on 9 April 2019).
- Bertram, J.S. The molecular biology of cancer. Mol. Asp. Med. 2000, 21, 167–223. [Google Scholar] [CrossRef]
- Weinberg, R.A. Garland Science; The Biology of Cancer Taylor & Francis Group, LLC: London, UK, 2007; p. 864. ISBN 10 0-8153-4076-1/ISBN-13. [Google Scholar]
- Langie, S.A.; Koppen, G.; Desaulniers, D.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Azqueta, A.; Bisson, W.H.; Brown, D.; Brunborg, G.; et al. Causes of genome instability: The effect of low dose chemical exposures in modern society. Carcinogenesis 2015, 36, S61–S88. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Animals living in polluted environments are a potential source of anti-tumor molecule (s). Cancer Chemoth. Pharm. 2017, 80, 919–924. [Google Scholar] [CrossRef]
- Lee, S.; Siddiqui, R.; Khan, N.A. Animals living in polluted environments are potential source of antimicrobials against infectious agents. Pathog. Glob. Health 2012, 106, 218–223. [Google Scholar] [CrossRef]
- Lehner, A.F.; Rumbeiha, W.; Shlosberg, A.; Stuart, K.; Johnson, M.; Domenech, R.; Langner, H. Diagnostic analysis of veterinary dried blood spots for toxic heavy metals exposure. J. Anal. Toxicol. 2013, 37, 406–422. [Google Scholar] [CrossRef]
- Siddiqui, R.; Jeyamogan, S.; Ali, S.M.; Abbas, F.; Sagathevan, K.A.; Khan, N.A. Crocodiles and alligators: Antiamoebic and antitumor compounds of crocodiles. Exp. Parasitol. 2017, 183, 194–200. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Siddiqui, R.; Sagathevan, K.A.; Khan, N.A. Gut bacteria of animals/pests living in polluted environments are a potential source of antibacterials. Appl. Microbiol. Biotechnol. 2019, 103, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Sagathevan, K.; Khan, N.A. Gut bacteria of cockroaches are a potential source of antibacterial compound (s). Lett. Appl. Microbiol. 2018, 66, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Gwee, M.C.E.; Cheah, L.S.; Gopalakrishnakone, P.; Wong, P.T.H.; Gong, J.P.; Kini, R.M. Studies on Venoms from the Black Scorpion Hetero-Metrus Longimanus and Some Other Scorpion Species. J. Toxicol. Toxin Rev. 1996, 15, 37–57. [Google Scholar] [CrossRef]
- Prendini, L. A new genus and species of bothriurid scorpion from the Brandberg Massif, Namibia, with a reanalysis of bothriurid phylogeny and a discussion of the phylogenetic position of Lisposoma Lawrence. Syst. Entomol. 2003, 28, 149–172. [Google Scholar] [CrossRef]
- Cappello, T.; Giannetto, A.; Parrino, V.; Maisano, M.; Oliva, S.; De Marco, G.; Guerriero, G.; Mauceri, A.; Fasulo, S. Baseline levels of metabolites in different tissues of mussel Mytilus galloprovincialis (Bivalvia: Mytilidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 26, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish Immunol. 2019, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Saoca, C.; Costa, G.; Zumbo, A.; Piccione, G.; Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 2019, 513, 734398. [Google Scholar] [CrossRef]
- Soopramanien, M.; Mungroo, M.R.; Sagathevan, K.A.; Khan, N.A.; Siddiqui, R. Invertebrates living in polluted environments are potential source of novel anticancer agents. Marmara Pharm. J. 2019, 23. [Google Scholar] [CrossRef]
- Wang, B.J.; Liu, Y.; Jiang, J.T.; Liu, B.; Liu, S.J. Microbial diversity in scorpion intestine (Buthus martensii) Karsch. Acta Microbiol. Sin. 2007, 47, 888–893. [Google Scholar]
- Cheng, X.; Wang, B.; Jin, Z.; Ma, D.; Yang, W.; Zhao, R.; Jing, X.; Shen, B.; Peng, C.; Qiu, W. Pseudomonas aeruginosa-mannose-sensitive hemagglutinin inhibits pancreatic cancer cell proliferation and induces apoptosis via the EGFR pathway and caspase signaling. Oncotarget 2016, 7, 77916. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Lei, S.; Shao, D.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Sun, H.; et al. Iturin A-like lipopeptides from Bacillus subtilis trigger apoptosis, paraptosis, and autophagy in Caco-2 cells. J. Cell. Physiol. 2019, 234, 6414–6427. [Google Scholar] [CrossRef]
- Tichomirowa, M.; Theodoropoulou, M.; Lohrer, P.; Schaaf, L.; Losa, M.; Uhl, E.; Lange, M.; Arzt, E.; Stalla, G.K.; Renner, U. Bacterial endotoxin (lipopolysaccharide) stimulates interleukin-6 production and inhibits growth of pituitary tumor cells expressing the toll-like receptor 4. J. Neuroendocrinol. 2005, 17, 152–160. [Google Scholar] [CrossRef]
- Fani, S.; Kamalidehghan, B.; Lo, K.M.; Nigjeh, S.E.; Keong, Y.S.; Dehghan, F.; Soori, R.; Abdulla, M.A.; Chow, K.M.; Ali, H.M.; et al. Anticancer activity of a monobenzyltin complex C1 against MDA-MB-231 cells through induction of Apoptosis and inhibition of breast cancer stem cells. Sci. Rep. 2016, 6, 38992. [Google Scholar] [CrossRef] [PubMed]
- Arimochi, H.; Morita, K. Characterization of cytotoxic actions of tricyclic antidepressants on human HT29 colon carcinoma cells. Eur. J. Pharmacol. 2006, 541, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Van Mellaert, L.; Barbé, S.; Anné, J. Clostridium spores as anti-tumor agents. Trends Microbiol. 2006, 14, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Klier, U.; Maletzki, C.; Göttmann, N.; Kreikemeyer, B.; Linnebacher, M. Avitalized bacteria mediate tumor growth control via activation of innate immunity. Cell. Immunol. 2011, 269, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Ho, W.S. Crocodile blood extract induces the apoptosis of lung cancer cells through PTEN activity. Oncol. Rep. 2016, 36, 1457–1466. [Google Scholar] [CrossRef][Green Version]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Ong, Q.; Guo, S.; Zhang, K.; Cui, B. U0126 protects cells against oxidative stress independent of its function as a MEK inhibitor. ACS Chem. Neurosci. 2015, 6, 130–137. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Eychène, A. The Raf/MEK/ERK pathway: New concepts of activation. Biol. Cell 2001, 93, 53–62. [Google Scholar] [CrossRef]
- Marampon, F.; Bossi, G.; Ciccarelli, C.; Di Rocco, A.; Sacchi, A.; Pestell, R.G.; Zani, B.M. MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol. Cancer Ther. 2009, 8, 543–551. [Google Scholar] [CrossRef]
- Gao, J.; Niwa, K.; Takemura, M.; Sun, W.; Onogi, K.; Wu, Y.; Seishima, M.; Mori, H.; Tamaya, T. Significant anti-proliferation of human endometrial cancer cells by combined treatment with a selective COX-2 inhibitor NS398 and specific MEK inhibitor U0126. Int. J. Oncol. 2005, 26, 737–744. [Google Scholar] [CrossRef]
- Sun, W.H.; Su, H.; Zhang, L.J.; Shao, Y.; Xu, H.C.; Zhang, T.; Xue, Y.P.; Ding, G.X.; Cheng, Y.L. Effects of gastrin receptor antagonist and cyclooxygenase-2 inhibitor on proliferation and apoptosis of gastric cancer cell. Natl. Med. J. China 2006, 86, 250. [Google Scholar]
- Hoosein, N.M.; Kiener, P.A.; Curry, R.C.; Rovati, L.C.; McGilbra, D.K.; Brattain, M.G. Antiproliferative effects of gastrin receptor antagonists and antibodies to gastrin on human colon carcinoma cell lines. Cancer Res. 1988, 48, 7179–7183. [Google Scholar] [PubMed]
- Wisetsai, A.; Lekphrom, R.; Schevenels, F.T. A novel cyclohexenone from Trachyspermum roxburghianum. Nat. Prod. Res. 2018, 32, 2499–2504. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Zahli, R.; Abrini, J. Evaluation of antimicrobial activity of four organic acids used in chicks feed to control Salmonella typhimurium: Suggestion of amendment in the search standard. Int. J. Microbiol. 2018, 2018. [Google Scholar] [CrossRef]
- Li, M.H.; Luo, Y.H.; Lin, C.F.; Chang, Y.T.; Lu, S.L.; Kuo, C.F.; Hong, J.S.; Lin, Y.S. Dextromethorphan efficiently increases bactericidal activity, attenuates inflammatory responses, and prevents group A streptococcal sepsis. Antimicrob. Agents Chemother. 2011, 55, 967–973. [Google Scholar] [CrossRef]
- Ksouri, A.; Dob, T.; Belkebir, A.; Dahmane, D.; Nouasri, A. Volatile compounds and biological activities of aerial parts of Pituranthos scoparius (Coss and Dur) Schinz (Apiaceae) from Hoggar, southern Algeria. Trop. J. Pharm. Res. 2017, 16, 51–58. [Google Scholar] [CrossRef]
- Lutsar, I.; Friedland, I.R.; Jafri, H.S.; Wubbel, L.; Ahmed, A.; Trujillo, M.; McCoig, C.C.; McCracken, G.H., Jr. Factors influencing the anti-inflammatory effect of dexamethasone therapy in experimental pneumococcal meningitis. J. Antimicrob. Chemother. 2003, 52, 651–655. [Google Scholar] [CrossRef]
- Shammi, T.; Khalid, M.I. Study of antimicrobial activity of two common anti-cough formulations sold in Bangladesh. Stamford J. Microbiol. 2017, 7, 7–9. [Google Scholar] [CrossRef]
- Li, Z.; Bao, X.; Bai, X.; Zhang, G.; Wang, J.; Zhu, M.; Wang, Y.; Shang, J.; Sheng, C.; Zhang, D.; et al. Design, synthesis, and Biological evaluation of phenol Bioisosteric Analogues of 3-Hydroxymorphinan. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Magliaro, B.C.; Saldanha, C.J. Clozapine protects PC-12 cells from death due to oxidative stress induced by hydrogen peroxide via a cell-type specific mechanism involving inhibition of extracellular signal-regulated kinase phosphorylation. Brain Res. 2009, 1283, 14–24. [Google Scholar] [CrossRef]
- Satoh, T.; Nakatsuka, D.; Watanabe, Y.; Nagata, I.; Kikuchi, H.; Namura, S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci. Lett. 2000, 288, 163–166. [Google Scholar] [CrossRef]
- Zhang, W.; Shin, E.J.; Wang, T.; Lee, P.H.; Pang, H.; Wie, M.B.; Kim, W.K.; Kim, S.J.; Huang, W.H.; Wang, Y.; et al. 3-Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. FASEB J. 2006, 20, 2496–2511. [Google Scholar] [CrossRef]
- Yoneda, Y.; Kuramoto, N.; Azuma, Y.; Inoue, K.; Ogita, K.; Mitani, A.; Yanase, H.; Masuda, S.; Zhang, L.; Kataoka, K. Prolongation by bifemelane of potentiation of AP1 DNA binding in hippocampal CA1 subfield of gerbils with transient forebrain ischemia. J. Neurosci. Res. 1998, 51, 574–582. [Google Scholar] [CrossRef]
- Droebner, K.; Pleschka, S.; Ludwig, S.; Planz, O. Antiviral activity of the MEK-inhibitor U0126 against pandemic H1N1v and highly pathogenic avian influenza virus in vitro and in vivo. Antivir. Res. 2011, 92, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Planz, O.; Pleschka, S.; Ludwig, S. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 2001, 75, 4871–4877. [Google Scholar] [CrossRef][Green Version]
- Matthys, H.; Bleicher, B.; Bleicher, U. Dextromethorphan and codeine: Objective assessment of antitussive activity in patients with chronic cough. J. Int. Med. Res. 1983, 11, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Furusawa, I.; Matsuura, K.; Shishiyama, J. Mode of action of ferimzone, a novel systemic fungicide for rice diseases: Biological properties against Pyricularia oryzae in vitro. Phytopathology 1989, 79, 827–832. [Google Scholar] [CrossRef]
| Gram Stain | Bacteria | |
|---|---|---|
| Scorpion faecal samples | ||
| HSF01 | Gram negative | Enterobacter gergoviae |
| HSF02 | Gram negative | Klebsiella pneumoniae |
| HSF03 | Gram-negative | Acinetobacter baumannii |
| HSF04 | Gram-positive | Lactobacillus fuchuensis |
| HSF05 | Gram-negative | Pseudomonas aeruginosa |
| HSF06 | Gram-positive | Lysinibacillus fusiformis |
| HSF07 | Gram-negative | Pseudomonas aeruginosa |
| HSF08 | Gram-negative | Pseudomonas stutzeri |
| HSF09 | Gram-negative | Pseudomonas aeruginosa |
| HSF10 | Gram-negative | Enterobacter cloacae |
| Scorpion gut | ||
| HSG01 | Gram-positive | Coagulase negative Staphylococcus spp. |
| HSG04 | Gram-positive | Coagulase negative Staphylococcus spp. |
| HSG05 | Gram-positive | Bacillus pumilus |
| HSG06 | Gram-negative | Pseudomonas aeruginosa |
| HSG07 | Gram-positive | Gram-positive bacilli |
| HSG08 | Gram-positive | Coagulase negative Staphylococcus spp. |
| HSG09 | Gram-positive | Staphylococcus sciuri |
| HSG10 | Gram-negative | Burkholderia cenocepacia |
| HSG11 | Gram-negative | Pseudomonas aeruginosa |
| HSG12 | Gram-negative | Pseudomonas aeruginosa |
| HSG13 | Gram-negative | Enterobacter gergoviae |
| HSG16 | Gram-positive | Bacillus subtilis |
| HSG17 | Gram-negative | Serratia marcescens |
| HSG18 | Gram-positive | Coagulase negative Staphylococcus spp. |
| Bacteria | Number of Molecules | ||||
|---|---|---|---|---|---|
| Detected | Identified | Reported Activity | Anticancer Activity | Unidentified | |
| H. spinifer | |||||
| P. aeruginosa (HSG12) | 72 | 32 | 11 (Table S1A) | 2 U-0126, Proglumide | 40 (Table S1B) |
| B. subtilis (HSG16) | 38 | 15 | 6 (Table S2A) | 1 3-Butylidene-7-hydroxyphthalide | 23 (Table S2B) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soopramanien, M.; Khan, N.A.; Ghimire, A.; Sagathevan, K.; Siddiqui, R. Heterometrus spinifer: An Untapped Source of Anti-Tumor Molecules. Biology 2020, 9, 150. https://doi.org/10.3390/biology9070150
Soopramanien M, Khan NA, Ghimire A, Sagathevan K, Siddiqui R. Heterometrus spinifer: An Untapped Source of Anti-Tumor Molecules. Biology. 2020; 9(7):150. https://doi.org/10.3390/biology9070150
Chicago/Turabian StyleSoopramanien, Morhanavallee, Naveed Ahmed Khan, Ajnish Ghimire, Kuppusamy Sagathevan, and Ruqaiyyah Siddiqui. 2020. "Heterometrus spinifer: An Untapped Source of Anti-Tumor Molecules" Biology 9, no. 7: 150. https://doi.org/10.3390/biology9070150
APA StyleSoopramanien, M., Khan, N. A., Ghimire, A., Sagathevan, K., & Siddiqui, R. (2020). Heterometrus spinifer: An Untapped Source of Anti-Tumor Molecules. Biology, 9(7), 150. https://doi.org/10.3390/biology9070150







