Potassium and Magnesium Mediate the Light and CO2 Photosynthetic Responses of Grapevines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Nutrient Treatments
2.3. Gas Exchange Measurements
2.4. Gas Exchange Along the Shoot
2.5. Photosynthetic Responses to Light
2.6. Photosynthetic Responses to Internal CO2
2.7. Chlorophyll Index
2.8. Biomass
2.9. Data Analysis
3. Results
3.1. Photosynthesis Along the Shoot
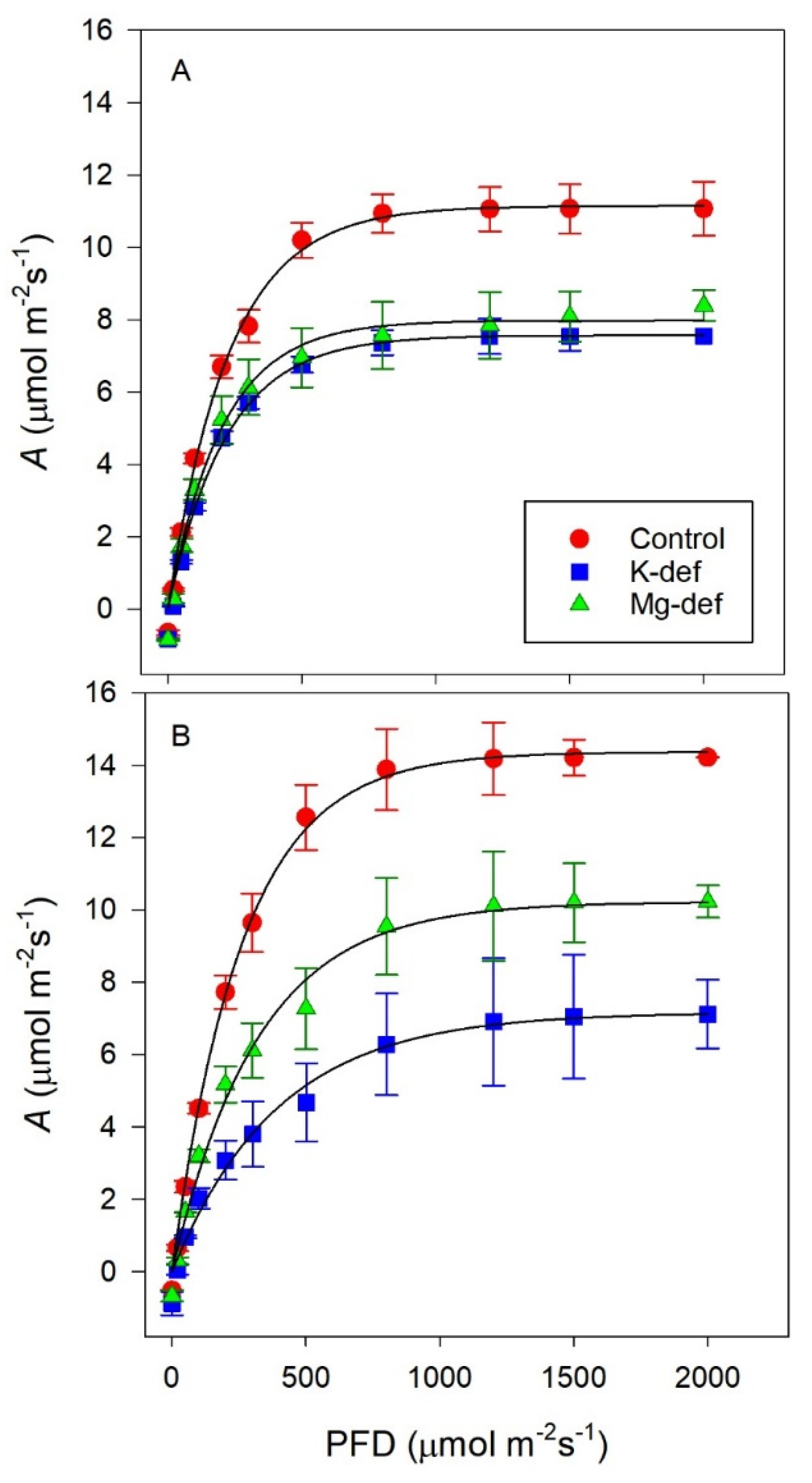
3.2. Photosynthetic Light Responses
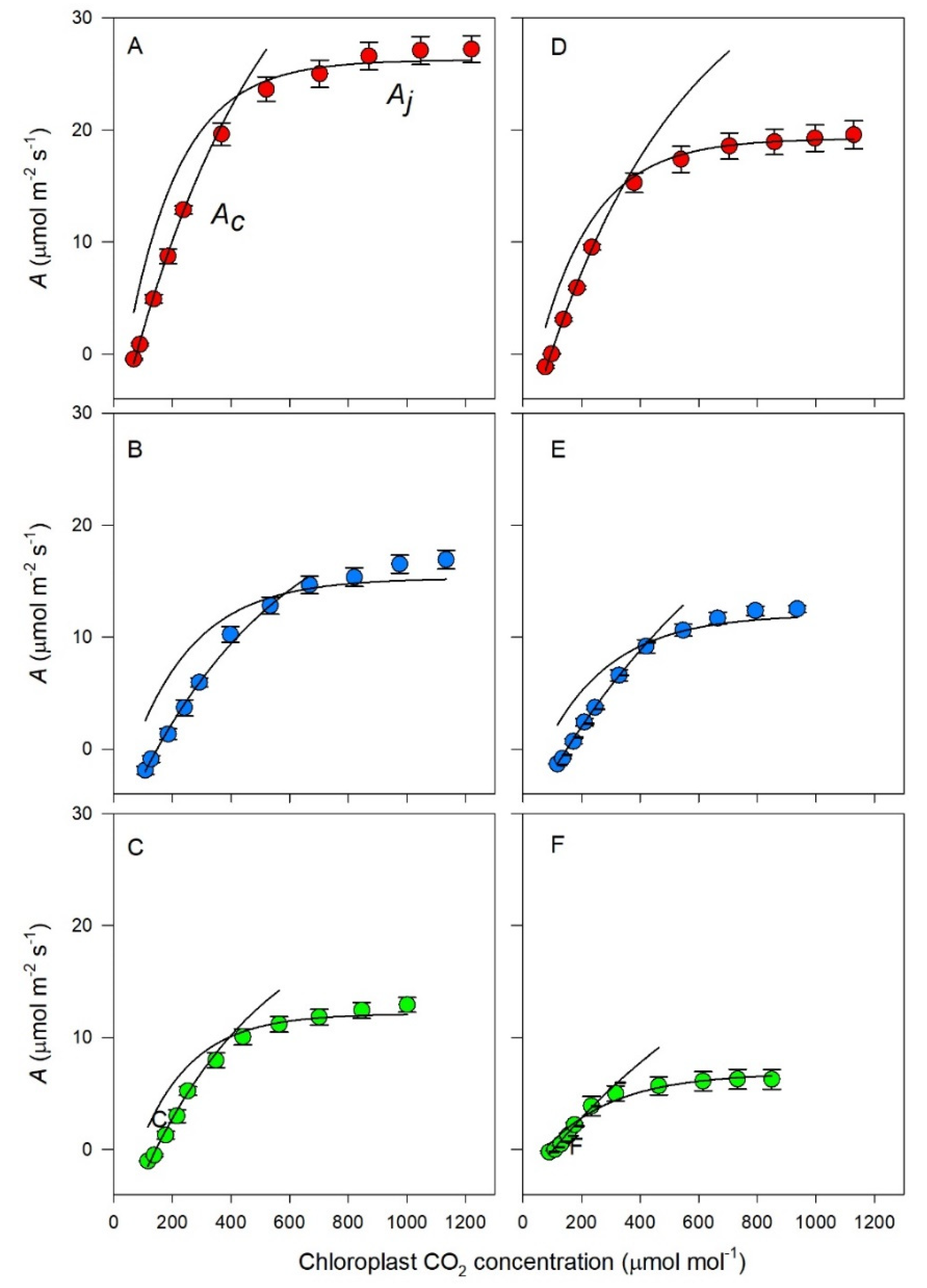
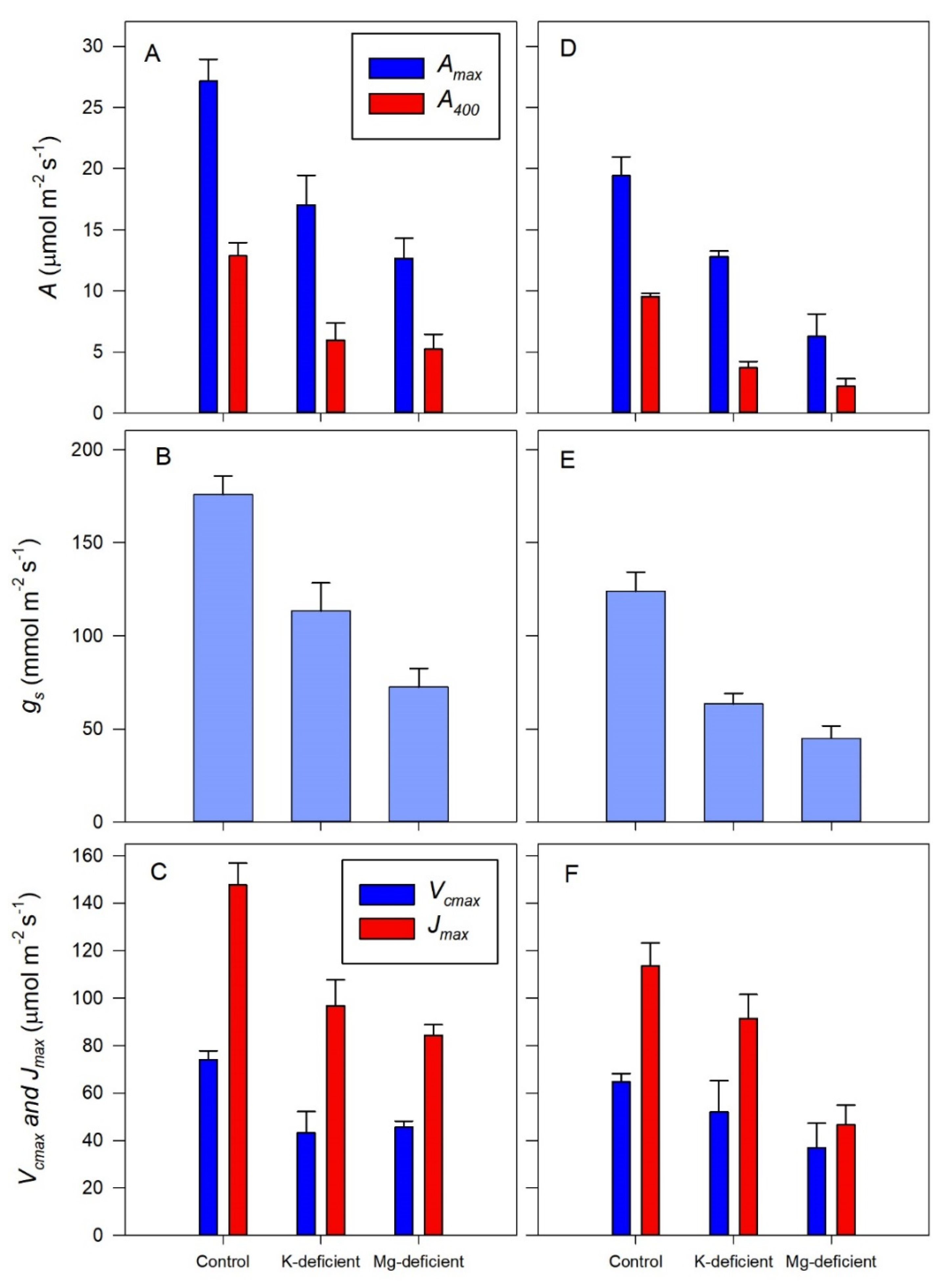
3.3. Photosynthetic Response to Chloroplast CO2
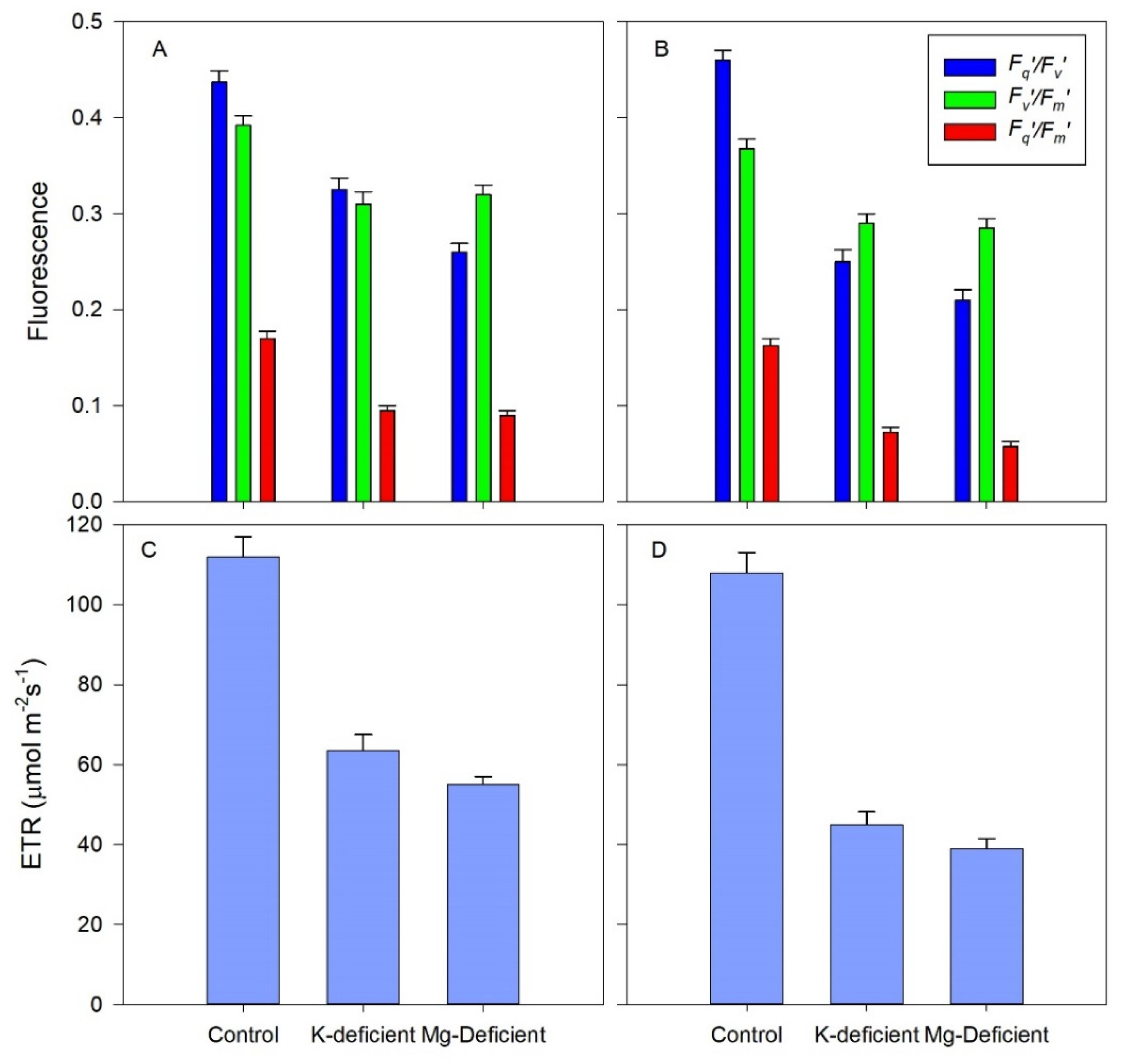
3.4. Chlorophyll Fluorescence Responses
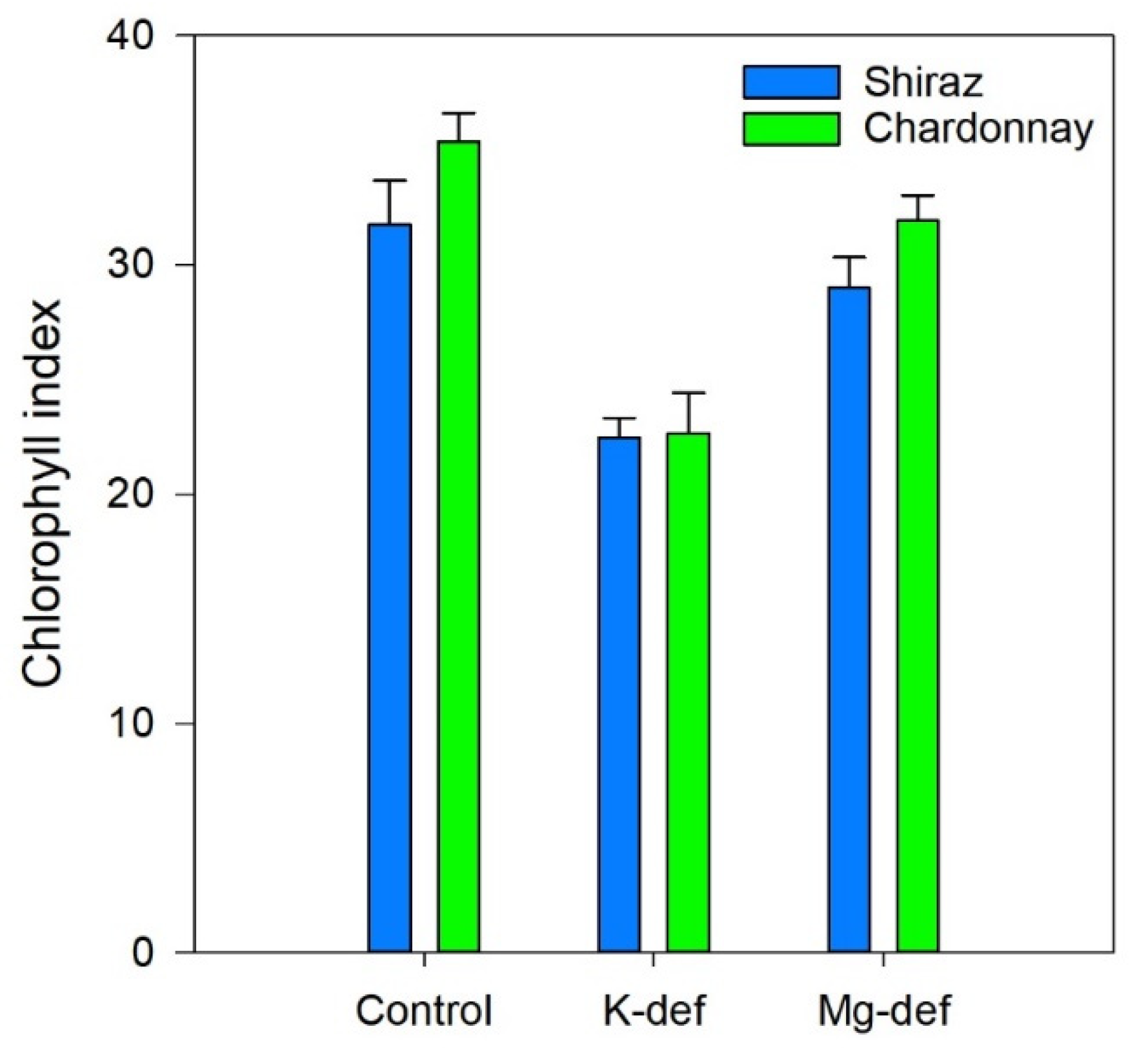
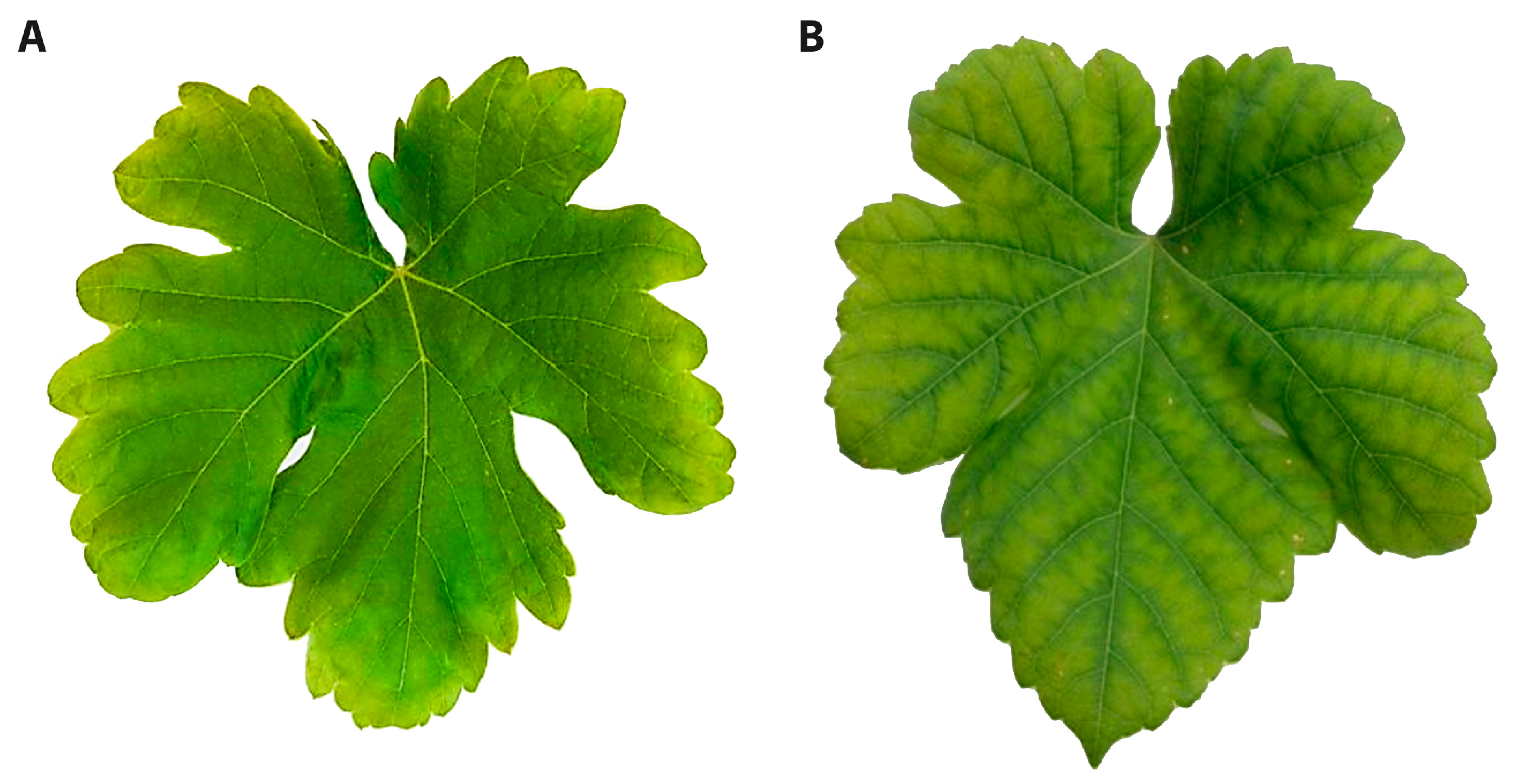
3.5. Chlorophyll Index
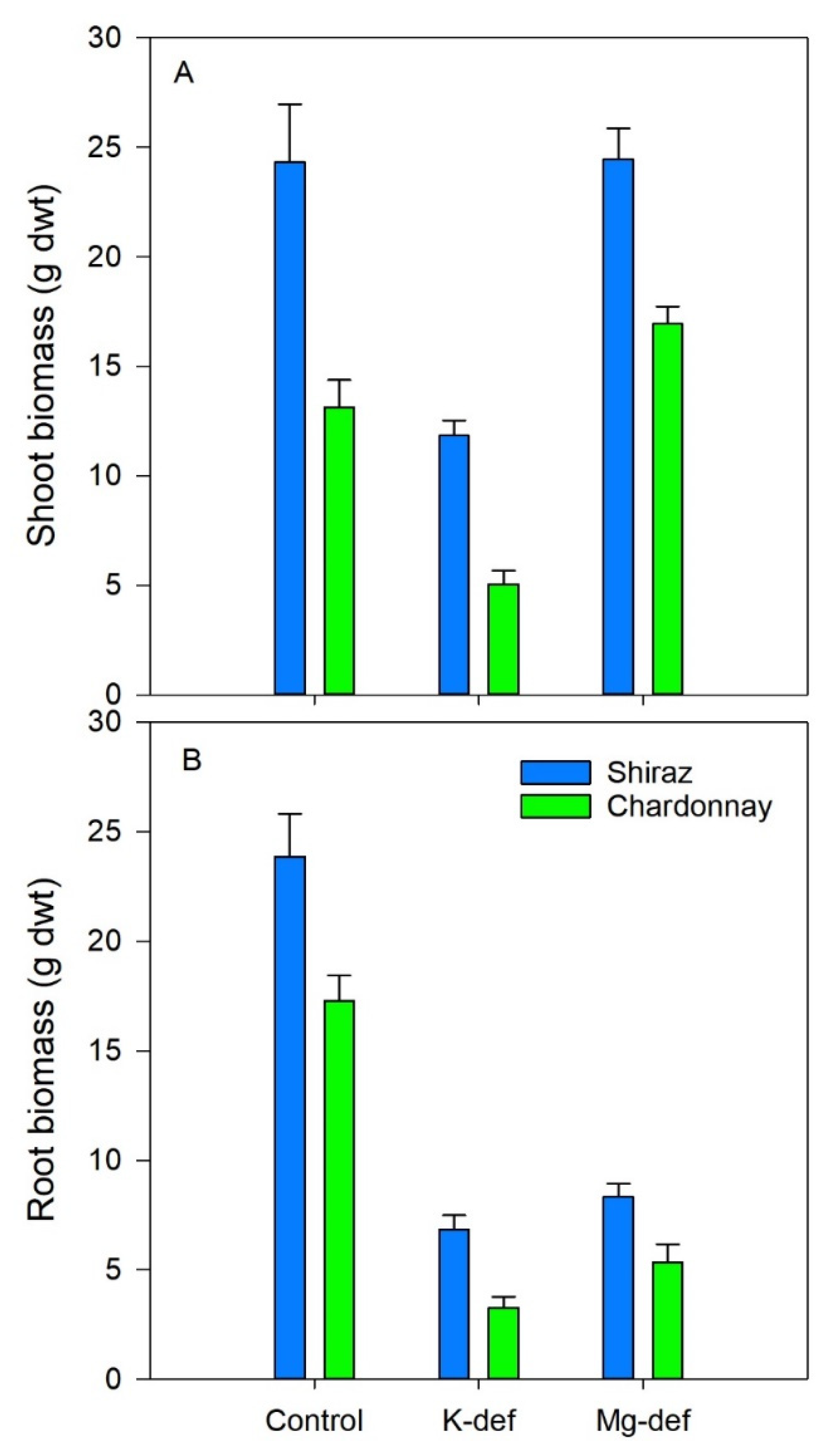
3.6. Biomass Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Coetzee, Z.A.; Walker, R.R.; Deloire, A.J.; Barril, C.; Clarke, S.J.; Rogiers, S.Y. Impact of reduced atmospheric CO2 and varied potassium supply on carbohydrate and potassium distribution in grapevine and grape berries (Vitis vinifera L.). Plant Physiol. Biochem. 2017, 120, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Martín, P.; del Álamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudillère, J.-P. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 2007, 59, 139–149. [Google Scholar] [CrossRef]
- Williams, C.M.J.; Maier, N.A.; Bartlett, L. Effect of molybdenum foliar sprays on yield, berry size, seed formation, and petiolar nutrient composition of “Merlot” grapevines. J. Plant Nutr. 2005, 27, 1891–1916. [Google Scholar] [CrossRef]
- Leibar, U.; Pascual, I.; Aizpurua, A.; Morales, F.; Unamunzaga, O. Grapevine nutritional status and K concentration of must under future expected climatic conditions texturally different soils. J. Soil Sci. Plant Nutr. 2017, 17, 385–397. [Google Scholar] [CrossRef]
- Hsiao, T.; Lauchli, A. Role of potassium in plant-water relations. In Advances in Plant Nutrition; Praeger: New York, NY, USA, 1986. [Google Scholar]
- Liesche, J. How regulation of phloem transport could link potassium fertilization to increased growth. Tree Physiol. 2016, 36, 1–5. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Ann. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Ahmad, I.; Maathuis, F.J. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Boulton, R. The general relationship between potassium, sodium and pH in grape juice and wine. Am. J. Enol. Vitic. 1980, 31, 182–186. [Google Scholar]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H. Potassium concentration and pH inter-relationships in grape juice and wine of Chardonnay and Shiraz from a range of rootstocks in different environments. Aust. J. Grape Wine Res. 2012, 18, 183–193. [Google Scholar] [CrossRef]
- Mattick, L.R.; Shaulis, N.J.; Moyer, J.C. The effect of potassium fertilization on the acid content of ‘Concord’ grape juice. Am. J. Enol. Vitic. 1972, 23, 26–30. [Google Scholar]
- Onanuga, A.O.; Jiang, P.a.; Adl, S. Phosphorus, potassium and phytohormones promote chlorophyll production differently in two cotton (Gossypium hirsutum) varieties grown in hydroponic nutrient solution. J. Agric. Sci. 2012, 4, 157–166. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Humble, G.; Raschke, K. Stomatal opening quantitatively related to potassium transport: Evidence from electron probe analysis. Plant Physiol. 1971, 48, 447–453. [Google Scholar] [CrossRef]
- Thiel, G.; Wolf, A.H. Operation of K+-channels in stomatal movement. Trends Plant Sci. 1997, 2, 339–345. [Google Scholar] [CrossRef]
- Bednarz, C.; Oosterhuis, D.; Evans, R. Leaf photosynthesis and carbon isotope discrimination of cotton in response to potassium deficiency. Environ. Exp. Bot. 1998, 39, 131–139. [Google Scholar] [CrossRef]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiol. 2011, 31, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ren, T.; Pan, Y.; Li, X.; Cong, R.; Lu, J. Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci. Rep. 2016, 6, 21725. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weedon, M.M. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Haldimann, P.; Feller, U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose−1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Ann. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1986; p. 674. [Google Scholar]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Implications of adenylate kinase-governed equilibrium of adenylates on contents of free magnesium in plant cells and compartments. Biochem. J. 2001, 360, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Koblet, W. Stress-induced development of inflorescence necrosis and bunch-stem necrosis in Vitis vinifera L. in response to environmental and nutritional effects. Vitis 1995, 34, 145–150. [Google Scholar]
- Rupp, D.; Fox, R.; Tränkle, L. Foliar application of magnesium fertilizer in grapevines: Effects on wine quality. Acta Hortic. 2002, 594, 149–155. [Google Scholar] [CrossRef]
- Sinilal, B.; Ovadia, R.; Nissim-Levi, A.; Perl, A.; Carmeli-Weissberg, M.; Oren-Shamir, M. Increased accumulation and decreased catabolism of anthocyanins in red grape cell suspension culture following magnesium treatment. Planta 2011, 234, 61–71. [Google Scholar] [CrossRef]
- Conradie, W. Seasonal uptake of nutrients by Chenin blanc in sand culture: II. phosphorus, potassium, calcium and magnesium. S. Afr. J. Enol. Vitic. 1981, 2, 7–13. [Google Scholar] [CrossRef]
- Delas, J.; Pouget, R. Action de la concentration de la solution nutritive sur quelques caractéristiques physiologiques et technologiques chez Vitis vinifera L. cv.” Cabernet-Sauvignon”. II.-Composition minérale des organes végétatifs, du moût et du vin. Agronomie 1984, 4, 443–450. [Google Scholar] [CrossRef]
- Skinner, P.W.; Matthews, M.A. A novel interaction of magnesium translocation with the supply of phosphorus to roots of grapevine (Vitis vinifera L.). Plant Cell Environ. 1990, 13, 821–826. [Google Scholar] [CrossRef]
- Holzapfel, B.P.; Smith, J.P.; Field, S.K. Seasonal vine nutrient dynamics and distribution of Shiraz grapevines. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Baby, T.; Hocking, B.; Tyerman, S.D.; Gilliham, M.; Collins, C. Modified method for producing grapevine plants in controlled environments. Am. J. Enol. Vitic. 2014, 65, 261–267. [Google Scholar] [CrossRef]
- Garcia, M.; Daverede, C.; Gallego, P.; Toumi, M. Effect of various potassium-calcium ratios on cation nutrition of grape grown hydroponically. J. Plant Nutr. 1999, 22, 417–425. [Google Scholar] [CrossRef]
- Robinson, J.B. Grapevine Nutrition. In Viticulture -2 Practises; Coombe, B.G., Dry, P., Eds.; Australian Industrial Publishers Pty Ltd.: Adelaide, Australia, 1992. [Google Scholar]
- Greer, D.H.; Halligan, E.A. Photosynthetic and fluorescence light responses for kiwifruit (Actinidia deliciosa) leaves at different stages of development on vines grown at two different photon flux densities. Funct. Plant Biol. 2001, 28, 373–382. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.v.; Berry, J. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L.; Flexas, J.; von Caemmerer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating mesophyll conductance to CO2: Methodology, potential errors, and recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Diaz-Espejo, A.; GalmÉS, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef]
- Terry, N.; Ulrich, A. Effects of potassium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1973, 51, 783–786. [Google Scholar] [CrossRef]
- Hartt, C.E. Effect of potassium deficiency upon translocation of 14C in attached blades and entire plants of sugarcane. Plant Physiol. 1969, 44, 1461–1469. [Google Scholar] [CrossRef][Green Version]
- Kanai, S.; Ohkura, K.; Adu-Gyamfi, J.; Mohapatra, P.; Nguyen, N.; Saneoka, H.; Fujita, K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- Ridolfi, M.; Garrec, J.-P. Consequences of an excess Al and a deficiency in Ca and Mg for stomatal functioning and net carbon assimilation of beech leaves. Ann. For. Sci. 2000, 57, 209–218. [Google Scholar] [CrossRef]
- Hariadi, Y.; Shabala, S. Screening broad beans (Vicia faba) for magnesium deficiency. II. Photosynthetic performance and leaf bioelectrical responses. Funct. Plant Biol. 2004, 31, 539–549. [Google Scholar] [CrossRef]
- McSwain, B.D.; Tsujimoto, H.Y.; Arnon, D.I. Effects of magnesium and chloride ions on light-induced electron transport in membrane fragments from a blue-green alga. Biochim. Biophys. Acta 1976, 423, 313–322. [Google Scholar] [CrossRef]
- Welch, R.M. Effects of nutrient deficiencies on seed production and quality. Adv. Plant Nutr. 1986, 2, 205–247. [Google Scholar]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.; Beverley, O.; Keller, M. Mineral sinks within ripening grape berries (Vitis vinifera L). Vitis 2006, 45, 115–123. [Google Scholar]
- Pastore, C.; Zenoni, S.; Tornielli, G.B.; Allegro, G.; Dal Santo, S.; Valentini, G.; Intrieri, C.; Pezzotti, M.; Filippetti, I. Increasing the source/sink ratio in Vitis vinifera (cv Sangiovese) induces extensive transcriptome reprogramming and modifies berry ripening. BMC Genom. 2011, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Bertamini, M.; Nedunchezhian, N. Leaf age effects on chlorophyll, Rubisco, photosynthetic electron transport activities and thylakoid membrane protein in field grown grapevine leaves. J. Plant Physiol. 2002, 159, 799–803. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M.; Weston, C. Reductions in biomass accumulation, photosynthesis in situ and net carbon balance are the costs of protecting Vitis vinifera ‘Semillon’grapevines from heat stress with shade covering. AoB Plants 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Peaslee, D.E.; Moss, D.N. Stomatal conductivities in K-deficient leaves of maize (Zea mays, L.). Crop Sci. 1968, 8, 427–430. [Google Scholar] [CrossRef]
- Laing, W.; Greer, D.; Sun, O.; Beets, P.; Lowe, A.; Payn, T. Physiological impacts of Mg deficiency in Pinus radiata: Growth and photosynthesis. New Phytol. 2000, 146, 47–57. [Google Scholar] [CrossRef]
- Pierce, J. Determinants of substrate specificity and the role of metal in the reactions of ribulosebisphosphate carboxylase/oxygenase. Plant Physiol. 1986, 81, 943–945. [Google Scholar] [CrossRef][Green Version]
- Sugiyama, T.; Nakayama, N.; Akazawa, T. Structure and function of chloroplast proteins: V. Homotropic effect of bicarbonate in RuDP carboxylase reaction and the mechanism of activation by magnesium ions. Arch. Biochem. Biophys. 1968, 126, 737–745. [Google Scholar] [CrossRef]
- Ishijima, S.; Uchibori, A.; Takagi, H.; Maki, R.; Ohnishi, M. Light-induced increase in free Mg2+ concentration in spinach chloroplasts: Measurement of free Mg2+ by using a fluorescent probe and necessity of stromal alkalinization. Arch. Biochem. Biophys. 2003, 412, 126–132. [Google Scholar] [CrossRef]
- Oja, V.; Laisk, A.; Heber, U. Light-induced alkalization of the chloroplast stroma in vivo as estimated from the CO2 capacity of intact sunflower leaves. Biochim. Biophys. Acta Bioenerg. 1986, 849, 355–365. [Google Scholar] [CrossRef]
- Weng, X.-Y.; Zheng, C.-J.; Xu, H.-X.; Sun, J.-Y. Characteristics of photosynthesis and functions of the water–water cycle in rice (Oryza sativa) leaves in response to potassium deficiency. Physiol. Plant. 2007, 131, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Cakmak, I. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Katz, J.J.; Norris, J.R.; Shipman, L.L.; Thurnauer, M.C.; Wasielewski, M.R. Chlorophyll function in the photosynthetic reaction center. Ann. Rev. Biophys. Bioeng. 1978, 7, 393–434. [Google Scholar] [CrossRef]
- Tester, M.; Blatt, M.R. Direct measurement of K+ channels in thylakoid membranes by incorporation of vesicles into planar lipid bilayers. Plant Physiol. 1989, 91, 249–252. [Google Scholar] [CrossRef]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Cui, J.; Abadie, C.; Carroll, A.; Lamade, E.; Tcherkez, G. Responses to K deficiency and waterlogging interact via respiratory and nitrogen metabolism. Plant Cell Environ. 2019, 42, 647–658. [Google Scholar] [CrossRef]
- Bottrill, D.E.; Possingham, J.V.; Kriedemann, P.E. The effect of nutrient deficiencies on phosynthesis and respiration in spinach. Plant Soil 1970, 32, 424–438. [Google Scholar] [CrossRef]
- Cui, J.; Davanture, M.; Zivy, M.; Lamade, E.; Tcherkez, G. Metabolic responses to potassium availability and waterlogging reshape respiration and carbon use efficiency in oil palm. New Phytol. 2019, 223, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Ulrich, A. Effects of magnesium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1974, 54, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Andrianteranagna, M.; Cuellar, T.; Cherel, I.; Gibrat, R.; Boeglin, M.; Moreau, B.; Paris, N.; Verdeil, J.L.; Zimmermann, S.; et al. Characterization of the grapevine Shaker K+ channel VvK3.1 supports its function in massive potassium fluxes necessary for berry potassium loading and pulvinus-actuated leaf movements. New Phytol. 2019, 222, 286–300. [Google Scholar] [CrossRef]
- Roberts, T.M.; Skeffington, R.A.; Blank, L.W. Causes of Type 1 spruce decline in Europe. For. Int. J. For. Res. 1989, 62, 179–222. [Google Scholar] [CrossRef]
- Pervez, H.; Ashraf, M.; Makhdum, M.I. Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirsutum L.). Photosynthetica 2004, 42, 251–255. [Google Scholar] [CrossRef]
- Claussen, M.; Lüthe, H.; Blatt, M.; Böttger, M. Auxin-induced growth and its linkage to potassium channels. Planta 1997, 201, 227–234. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Klouvatou, A.; Vekkos, K.A.; Manetas, Y.; Karabourniotis, G. The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann. Bot. 2006, 98, 257–265. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Clarke, S.J.; Rogiers, S.Y. The role of fruit exposure in the late season decline of grape berry mesocarp cell vitality. Plant Physiol. Biochem. 2019, 135, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liao, S.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Effect of water stress and elevated temperature on hypoxia and cell death in the mesocarp of Shiraz berries. Aust. J. Grape Wine Res. 2018, 24, 487–497. [Google Scholar] [CrossRef]
- Greer, D.H. Short-term temperature dependency of the photosynthetic and PSII photochemical responses to photon flux density of leaves of Vitis vinifera cv. Shiraz vines grown in field conditions with and without fruit. Funct. Plant Biol. 2019, 46, 634–648. [Google Scholar] [CrossRef] [PubMed]

| Shiraz | Chardonnay | |||
|---|---|---|---|---|
| Treatment | Mg | K | Mg | K |
| Control | 6.2 | 41.7 | 7.1 | 33.6 |
| Mg deficiency | 1.2 | 41.8 | 1.2 | 41.1 |
| K deficiency | 18.5 | 2.8 | 17.3 | 3.9 |
| Control | −K | −Mg | p | ||
|---|---|---|---|---|---|
| Shiraz | Amax (µmol m−2 s−1) | 11.02 ± 0.7 | 8.00 ± 0.08 | 8.17 ± 0.19 | <0.001 |
| ϕI (mol mol−1) | 0.035 ± 0.000 | 0.030 ± 0.000 | 0.034 ± 0.001 | <0.001 | |
| Rd (µmol m−2 s−1) | 0.19 ± 0.01 | 0.47 ± 0.00 | 0.37 ± 0.03 | <0.001 | |
| Asat(µmol m−2 s−1) | 840 ± 13 | 697 ± 5 | 637 ± 9 | <0.001 | |
| Chard | Amax (µmol m−2 s−1) | 14.1 ± 0.3 | 7.0 ± 0.6 | 9.9 ± 0.4 | <0.001 |
| ϕI (mol mol−1) | 0.039 ± 0.001 | 0.012 ± 0.002 | 0.020 ± 0.002 | <0.001 | |
| Rd (µmol m−2 s−1) | 0.081 ± 0.040 | 0.014 ± 0.001 | 0.35 ± 0.14 | ns | |
| Asat (µmol m−2 s−1) | 963 ± 26 | 1253 ± 53 | 1364 ± 103 | <001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogiers, S.Y.; Greer, D.H.; Moroni, F.J.; Baby, T. Potassium and Magnesium Mediate the Light and CO2 Photosynthetic Responses of Grapevines. Biology 2020, 9, 144. https://doi.org/10.3390/biology9070144
Rogiers SY, Greer DH, Moroni FJ, Baby T. Potassium and Magnesium Mediate the Light and CO2 Photosynthetic Responses of Grapevines. Biology. 2020; 9(7):144. https://doi.org/10.3390/biology9070144
Chicago/Turabian StyleRogiers, Suzy Y., Dennis H. Greer, Francesca J. Moroni, and Tintu Baby. 2020. "Potassium and Magnesium Mediate the Light and CO2 Photosynthetic Responses of Grapevines" Biology 9, no. 7: 144. https://doi.org/10.3390/biology9070144
APA StyleRogiers, S. Y., Greer, D. H., Moroni, F. J., & Baby, T. (2020). Potassium and Magnesium Mediate the Light and CO2 Photosynthetic Responses of Grapevines. Biology, 9(7), 144. https://doi.org/10.3390/biology9070144







