Abstract
Potassium (K) and magnesium (Mg) deficiency are common stresses that can impact on grape yield and quality, but their effects on photosynthesis have received little attention. Understanding the diffusional and biochemical limitations to photosynthetic constraints will help to guide improvements in cultural practices. Accordingly, the photosynthetic response of Vitis vinifera cvs. Shiraz and Chardonnay to K or Mg deficiency was assessed under hydroponic conditions using miniature low-nutrient-reserve vines. Photosynthesis was at least partly reduced by a decline in stomatal conductance. Light and CO2-saturated photosynthesis, maximum rate of ribulose 1.5 bisphospate (RuBP) carboxylation (Vcmax) and maximum rate of electron transport (Jmax) all decreased under K and Mg deficiency. Likewise, chlorophyll fluorescence and electron transport were lower under both nutrient deficiencies while dark respiration increased. K deficiency drastically reduced shoot biomass in both cultivars, while root biomass was greatly reduced under both Mg and K deficiency. Taken together, these results indicate that the decrease in biomass was likely due to both stomatal and biochemical limitations in photosynthesis. Optimising photosynthesis through adequate nutrition will thus support increases in biomass with carry-on positive effects on crop yields.
1. Introduction
Nutrients are critical for optimum plant growth and development. In grapevines, deficiency of a particular nutrient can alter canopy development, root functioning, flowering, fruit set, ripening and final fruit composition, and thus yield and wine style [1,2,3,4,5]. The beautiful red soils of many grapevine-growing regions in Australia are aged and naturally nutrient-deficient. Even though grapevines are moderate in their nutrient requirements, nutrient starvation can also occur through leaching, run-off, immobilization by clay particles or long-term crop removal. Organic or manufactured fertiliser application for optimal vine performance is a common practise, however, nutrient availability and uptake is influenced by other physical and structural characteristics of the soil, including pH and water content. Consumers and legislators are putting increased pressure for sustainable production practises including the better management of fertilisers [6]. Vine nutrient requirements for optimal crop quality without excess vegetative vigour have received considerable attention, however, there is surprisingly little information on nutrient requirements for the ideal metabolic and physiological performance to help achieve this goal.
Potassium (K) is an essential element that drives growth, maintains turgor and is involved in phloem transport [7,8]. It regulates the cation–anion balance within cells, cytoplasmic pH, membrane potential and activates enzymes [9,10,11]. Potassium is the predominant cation of grape berries and is important to its sugar:acid balance and colour [12,13,14,15]. Moreover, this element is vital to photosynthesis. Potassium promotes chlorophyll synthesis [16] and assimilates export from leaves [17,18]. It is well established that K regulates stomatal behaviour [19,20], and because stomatal closure leads to a barrier in CO2 diffusion, it has been demonstrated in several crop plants that leaf net assimilation is curtailed through a decrease in stomatal conductance under K deficiency [21]. Nonstomatal limitations in photosynthesis have also been demonstrated in species such as Carya cathayensis Sarg. [22] and Brassica napus [23] grown under limited K. The maximum rates of carboxylation (Vcmax) and the regeneration of the carboxylating substrate ribulose 1,5-bisphosphate (RuBP) directed by the electron transport capacity (Jmax) are reduced in grapevines exposed to heat and drought [24] and in other species [25], however, how these parameters are affected by nutrient deficiencies is much less understood.
Magnesium (Mg) has many fundamental roles in plant metabolism [26,27]. This macro-element regulates cellular pH and the cation–ion balance and acts as a co-factor for enzymes involved in the formation of DNA and RNA, respiration, N assimilation, transport proteins and photosynthesis, including the protein rubisco [28,29,30]. A substantial proportion of a plant’s Mg is bound up in leaves as it is an essential component of chlorophyll a/b in the light harvesting complexes [31]. Magnesium has other roles associated with photosynthesis that are related to charge and membrane mobility [32] and is involved in energy transfer via adenosine triphosphate [33]. Similar to K, Mg deficiency can result in the accumulation of sucrose and starch in leaves through an inhibition of phloem loading [28,30,34]. From a viticultural perspective, Mg may help avoid bunch stem necrosis [35,36] and protect anthocyanins from catabolism in cell vacuoles [37]. Relative to K, berries are not a strong sink for Mg and most of it is partitioned to the roots and vegetative components [38]. Magnesium deficiency can occur in vines growing in sandy, acidic soils as well as in calcareous soils with high pH [39] due to competition with other cations. Even though grapevines are a model for perennial horticultural crops, the impact of Mg deficiency on photosynthesis in grapevines has received limited attention (with the exception of [40]).
The relative importance of diffusional and biochemical limitations to photosynthetic constraints during K or Mg deficiency requires clarification in grapevines to ensure more sustainable uses of these nutrients. In order to avoid the complexity of the soil–vine system, Shiraz and Chardonnay vines were grown hydroponically in Mg or K deficiency and leaf stomatal conductance, and photosynthetic capacity and biomass accumulation were monitored. Mature grapevines store ample nutrient reserves within the perennial woody components [41], and thus a nutrient deficiency response may not be apparent for some time. We, therefore, used miniature vines grown from the small rooted canes in this study to overcome this limitation.
2. Materials and Methods
2.1. Plant Growth Conditions
This study was undertaken at the National Wine and Grape Industry Centre plant growth facilities at Charles Sturt University in the Riverina, NSW, Australia. After five weeks of rooting, one-year-old dormant cuttings of Vitis vinifera cv. Chardonnay and cv. Shiraz vines were transferred into 2.5 L pots containing perlite and established in greenhouse conditions under natural light with an average air temperature of 25 °C during the day and 15 °C during the night. The vines were placed in a randomised block design across three tables, with each table supporting three vines of each treatment. Bud break occurred in these vines in September. Two shoots were retained on each plant and staked vertically.
2.2. Nutrient Treatments
Nutrient treatments were made up in separate tanks and pumped to the pots along separate fertigation lines. Fertigation was initiated 1 week prior to budbreak and occurred daily to the point of run-off, where one was a full nutrient treatment (control) based on modified half-strength Hoagland’s solution [42] and the others were the same nutrients, except, in one treatment, potassium was eliminated (K-deficient) and in the remaining treatment, magnesium was eliminated (Mg-deficient). The nitrate and phosphate concentrations were equal across the treatments. The Mg and K content of dried petiole samples collected at the 10-leaf stage from eight replicate plants were assessed by ICP-OES at a commercial diagnostic lab (Charles Sturt University, Wagga Wagga, NSW, Australia) and presented in Table 1. Mg uptake was greater in the K-deficient vines, as previously reported in similar studies [21,43], but still in the adequate range [44]. N was determined on a 50-mg sample with a VarioMAX combustion analyser (Elementar, Hanau, Germany). All other nutrients were in the adequate range and averaged at 33.4 N, 36.1 Ca, 3.25 P, 1.65 S, 0.018 B, 0.011 Cu, 0.047 Fe, 0.085 Mn, 0.08 Mo and 0.042 g kg−1 Zn.

Table 1.
Magnesium and K content of Shiraz and Chardonnay petiole samples collected at the 10-leaf stage (g kg−1 dry weight) from grapevines grown hydroponically in greenhouse conditions.
2.3. Gas Exchange Measurements
All gas exchange measurements were undertaken with the LiCor LI-6400 XT system (Li-Cor Biosciences, Lincoln, Nebraska) in one configuration with the standard LED lighting system (LI-6400-02B) and the other configuration with the LI-6400-40 fluorometer attached to the cuvette. In all cases, the leaf temperature was maintained at 25 °C, the photon flux density (PFD) was set at 1500 µmol m−2 s−1 except when varied in the light responses and the CO2 concertation was maintained at 400 µmol mol−1. The leaf-air vapour pressure deficit was not controlled but remained at about 1.5 kPa. The red to blue ratio was maintained at 9:1 in both the standard LED lighting system and the fluorometer. The area of illumination was 2 cm2.
2.4. Gas Exchange Along the Shoot
At about mid-season, when the shoots on each vine had up to 15 leaves, for each treatment, the gas exchange was measured on every leaf of the randomly selected vines of both cultivars, at least until the leaves were too small to fully cover the chamber. All measurements were undertaken as above on three vines per treatment for each cultivar. The most basal bud was not counted as a node.
2.5. Photosynthetic Responses to Light
Gas exchange was measured at the constant conditions above on the youngest fully expanded leaves on shoots of randomly chosen vines in each treatment. For each light response, the PFD was initially set at 1500 µmol m−2 s−1 and, when the rates were steady, the PFD was decreased progressively in selected steps until approximately 1 µmol m−2 s−1 (dark). The procedure was repeated 2–3 times for each treatment.
2.6. Photosynthetic Responses to Internal CO2
On each occasion, fully expanded leaves were used and the gas exchange conditions were as above. For each A/ci response, the reference CO2 concentration was set at 400 µmol mol−1 until the rates were steady, and then the CO2 concentration was reduced in 50–100 µmol mol−1 steps to 50 µmol mol−1. Thereafter the CO2 concentration was increased back to 400 µmol mol−1 to ensure that rates had returned close to the initial rates, and then the CO2 concentration was increased in 100–200 µmol mol−1 to 1600 µmol mol−1. Each response was determined for leaves on each of the nutrient treatments and replicated 3–4 times.
Simultaneous chlorophyll fluorescence was measured during each of the A/ci responses. Throughout each response and at each change in CO2 concentration, the steady-state fluorescence in the light (Fs) and the maximal fluorescence in the light (Fm’) were measured. Then, the actinic light was turned off briefly and low-intensity far-red light was turned on for 3 s to enable measurement of the light-adapted minimal fluorescence (Fo’) to be measured.
2.7. Chlorophyll Index
The chlorophyll index (CI) was measured of leaves on node position 8 from the shoot base with a chlorophyll meter (SPAD-502, Minolta, Osaka, Japan). The SPAD measurement per leaf consisted of an average of three readings (one reading per apical leaf lobe and both lateral leaf lobes).
2.8. Biomass
Twelve weeks after budburst, the vines were dismembered into roots and shoots (stems and leaves). The original cane from which the roots and shoots originated was omitted from the biomass assessment. The vine components were dried in a forced-air oven at 60 °C and dry weight was assessed once stable.
2.9. Data Analysis
All data were analysed using a general linear model (GLM) approach using SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA) and least squares means and standard errors were determined assuming a fully randomised experimental design. The photosynthetic light responses were analysed using non-linear regression with SAS to fit a hyperbolic tangent function [45].
For the fitting of the A/ci data to the C3 photosynthetic model of Farquhar et al. [46], the apparent maximum rates of RuBP carboxylation (Vcmax) and the apparent maximum rates of RuBP regeneration (Jmax) were determined following the procedure of Greer and Weedon [24]. The temperature dependencies of Vcmax and Jmax were adopted from Sharkey et al. [47] and all data were analysed using non-linear regressions with SAS. For the determination of the mesophyll conductance (gm) to calculate chloroplast CO2 concentration, the procedures of Pons et al. [48] and Flexas et al. [49], using the electron transport rate estimated from the simultaneous chlorophyll fluorescence measurements, were adopted.
3. Results
3.1. Photosynthesis Along the Shoot
Node position along the stem had a significant impact on leaf Anet in both cultivars (p < 0.01) with nutrient deficiency resulting in the lowest values for those leaves located along the middle of the shoot (p < 0.01) (Figure 1). Potassium deficiency decreased Anet by up to 50% from nodes 1 to 5 in Chardonnay, however, this was not apparent in Shiraz. Middle-aged Shiraz leaves, between nodes 6−10, were negatively affected by K deficiency, with 30–75% lower Anet relative to the control. In Chardonnay, these middle-aged leaves had about 40–80% reduction in Anet. The assimilation of youngest leaves of the K-deficient vines (node 12 onwards for Shiraz) was not different from the control. In the Mg-deficient vines, Anet was 80% lower in both old and middle-aged leaves, but the younger leaves from node 11 (Shiraz) or 14 (Chardonnay) onwards were not different from the control.
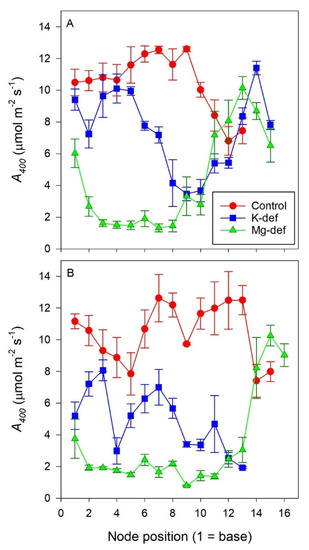
Figure 1.
Anet of Chardonnay (A) and Shiraz (B) leaves with node position along the shoot for vines grown hydroponically in greenhouse conditions in control, K-deficient and Mg-deficient nutrient solutions.
3.2. Photosynthetic Light Responses
The photosynthetic light responses (Figure 2A) indicated that the K and Mg nutrient deficiencies in the Shiraz vines reduced light saturated photosynthesis by similar amounts compared to the control vines. This is shown more generally in Table 1, where the analyses of the fitting of the hyperbolic tangent function to the light responses are shown. However, respiration rates were higher in the nutrient deficient vines, while the PFD at saturation was statistically lower compared to the control vines. Although statistically different, the apparent photon yields were largely unaffected by the nutrient deficiency.
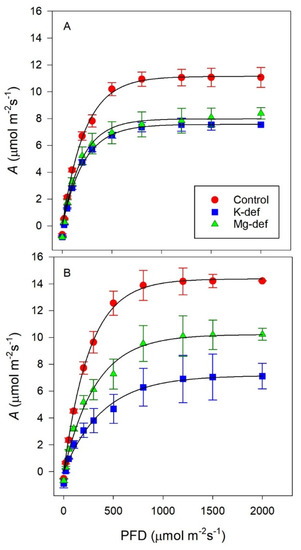
Figure 2.
Light response curves for Shiraz (A) and Chardonnay (B) vines grown hydroponically in greenhouse conditions in control, K-deficient and Mg-deficient nutrient solutions.
By contrast for the Chardonnay vines (Table 2), the potassium deficiency reduced photosynthesis, especially Amax, much more than occurred in the magnesium-deficient treatment. Compared to the control vines, however, those vines with reduced nutrients had significantly lower light-saturated photosynthetic rates (Asat), were light-saturated at higher PFDs but had no significant differences in respiration rates (Rd). The efficiency of photosynthesis was determined by the reduced apparent photon yield (ϕI) in the nutrient-deficient vines.

Table 2.
Attributes of the curve fitting to photosynthetic light responses.
3.3. Photosynthetic Response to Chloroplast CO2
The fitting of the C3 photosynthesis model to the photosynthetic response to chloroplast CO2 concentration of the control and treated Shiraz and Chardonnay vines (Figure 3) was highly significant (p < 0.001; r2 = 0.95 − 0.98). For the Shiraz vines, the carboxylation of RuBP was clearly limiting assimilation below about 400 µmol mol−1 for each treatment, and regeneration of RuBP was limiting at the higher CO2 concentrations. Notably, the response to CO2 became progressively diminished from the control to the K-deficient vines to the Mg-deficient vines, with a clear reduction in the CO2-saturated assimilation rates. A similar pattern occurred with the Chardonnay vines except that, for all treatments, the CO2-saturated assimilation rates were markedly lower compared with those for Shiraz. It was also apparent for the Mg-deficient Chardonnay vines that at low CO2 there was co-limitation by RuBP carboxylation and regeneration, but the assimilation rates were markedly reduced.
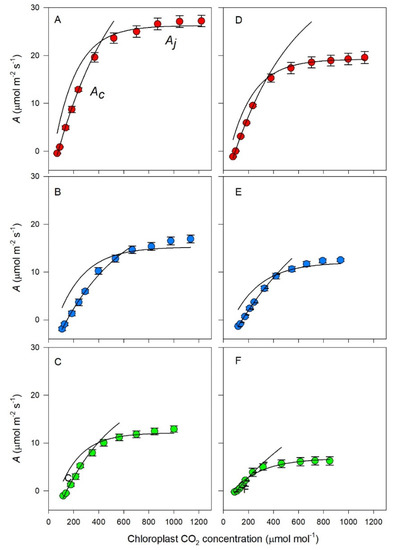
Figure 3.
Rates of light-saturated photosynthesis (mean ± s.e.) of leaves as a function of the chloroplast CO2 concentration. Shiraz (A–C) and Chardonnay (D–F) vines grown hydroponically in greenhouse conditions under control (A,D), K-deficient (B,C) or Mg-deficient (C,D) conditions. The lines fitted to these data are from the C3 model of photosynthesis according to Farquhar et al. (1980). Aj = RuBP regeneration-limited photosynthesis, Ac = Rubisco-limited photosynthesis
The effect of the nutrient deficiency on the A/cc (assimilation as a function of chloroplast CO2 concentration) responses is shown more generally in Figure 4 where the light and CO2-saturated assimilation rates (Amax) and the assimilation rates at ambient (400 µmol mol−1) CO2 concentrations (A400) for both grapevine cultivars declined markedly. For example, Amax declined by 37% with the K-deficient Shiraz vines and by another 30% for the Mg-deficient vines, compared to the control vines. Comparable reductions in Amax were 37 and 50% for the Chardonnay vines. Thus, a comparable reduction in Amax occurred with K deficiency in both cultivars, but Chardonnay assimilation was much more sensitive to Mg deficiency than Shiraz assimilation.
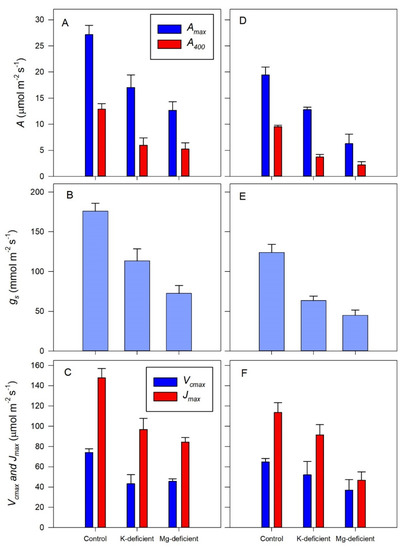
Figure 4.
Leaf net assimilation (Anet) (A,D), stomatal conductance (gs) (B,E) and maximum rates of ribulose 1,5-bisphosphate carboxylation (Vcmax) and electron transport (Jmax) (C,F) of grapevines grown hydroponically in greenhouse conditions in control, K-deficient or Mg-deficient nutrient conditions (mean ± s.e.).
When CO2 was limiting assimilation (A400), the effects of the nutrient deficiencies were much greater than for Amax, where a 53% reduction occurred for the Shiraz vines and a 61% reduction occurred with the Chardonnay vines in the K-deficient vines compared to the control vines. However, the Mg deficiency caused only a slightly greater reduction in A400, more in Chardonnay than in Shiraz, compared to the controls. It was apparent that Chardonnay assimilation was again more sensitive to the Mg deficiency, as the rates were negligible.
In part, the changes in assimilation induced by nutrient deficiency were correlated with a similar impact on stomatal conductance (Figure 4B,E) in both cultivars. However, the Shiraz vines had intrinsically more open stomata than the Chardonnay vines, for example, the control Shiraz vines had average stomatal conductance of 176 ± 10 mmol m−2 s−1 compared to the control Chardonnay vines with an average conductance of 99 ± 8 mmol m−2 s−1, which is statistically (p < 0.01) lower. For the K deficiency, stomatal conductance declined by 35 and 51% compared to the control vines but by a further 36 and 70% reduction for the Mg-deficient vines. Thus, stomata of these Chardonnay vines with Mg–deficiency were for all intents closed, matching the almost negligible assimilation.
Consistent with the cultivar differences in assimilation, the apparent maximum rates of RuBP carboxylation (Vcmax) also differed significantly (p < 0.05), from 74.1 ± 3.6 µmol m−2 s−1 in Shiraz leaves to 64.8 ± 3.4 µmol m−2 s−1 in the Chardonnay leaves. However, the Chardonnay vines with K deficiency had only a 19% reduction in carboxylation rates compared to 41% reduction for the Shiraz vines. By contrast, Mg deficiency had no more effect on the maximum rates of RuBP carboxylation than the K deficiency in the Shiraz leaves, whereas the rates of carboxylation declined by a further 28% with Mg deficiency in the Chardonnay leaves.
A generally similar response occurred with the apparent maximum rates of RuBP regeneration (Jmax), again with statistically (p < 0.05) higher rates in the control Shiraz leaves (148 ± 9 µmol m−2 s−1) compared to the control Chardonnay leaves (113 ± 9 µmol m−2 s−1). As with Vcmax, the K deficiency had a greater impact on rates of RuBP regeneration (34% reduction) in the Shiraz leaves compared to the Chardonnay leaves (19%) and the Mg deficiency was also comparable, with 13% lower RuBP regeneration rates in the Shiraz leaves and 49% lower rates in the Chardonnay leaves.
3.4. Chlorophyll Fluorescence Responses
The maximum efficiency of PSII in the light-adapted state (Fv′/Fm′) did not differ markedly between the two nutrient deficiencies (Figure 5) but was 20–24% lower than the control vines of both cultivars. The actual quantum efficiency of PSII electron transport (Fq′/Fm′) was highest in the control vines but was reduced in both K-deficient and Mg-deficient vines by 27 and 41% in the Shiraz vines and by 56 and 65% in the Chardonnay vines, thus nutrient deficiencies had a greater effect on Chardonnay photochemistry than on the Shiraz vines. Photochemical quenching (Fq′/Fv′) increased by about 45% in the K-deficient vines for both cultivars, and a further 10% increase occurred for the Mg-deficient vines, but there were few differences in photochemical quenching between the cultivars. ETR declined in the leaves with the absence of K and Mg nutrients, by 45 and 52%, respectively, in the Shiraz vines and by 56 and 65% in the Chardonnay vines, consistent with the lower photochemical efficiency in the Chardonnay vines.
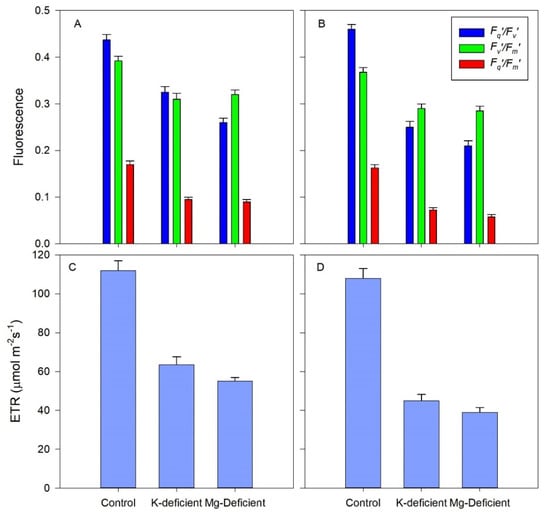
Figure 5.
Fluorescence attributes and electron transport rate (ETR) of grapevine leaves grown hydroponically in greenhouse conditions and grown in control, K-deficient or Mg-deficient nutrient solutions. Photochemical quenching (Fq′/Fv′), maximum efficiency of PSII photochemistry in the light if all PSII centres were open (Fv′/Fm′), and PSII operating efficiency in the light (Fq′/Fm′) in Shiraz (A) and Chardonnay (B). ETR in Shiraz (C) or Chardonnay (D).
3.5. Chlorophyll Index
In both cultivars, the leaf chlorophyll index was more strongly depressed in the K-deficient vines (30–35%) than the Mg-deficient vines (<10%) (Figure 6). Potassium deficiency resulted in chlorosis on the leaf margins, while Mg deficiency triggered interveinal chlorosis (Figure 7). Over time, necrotic areas formed on the margins (K) or in the leaf interior (Mg). No wilting was apparent.
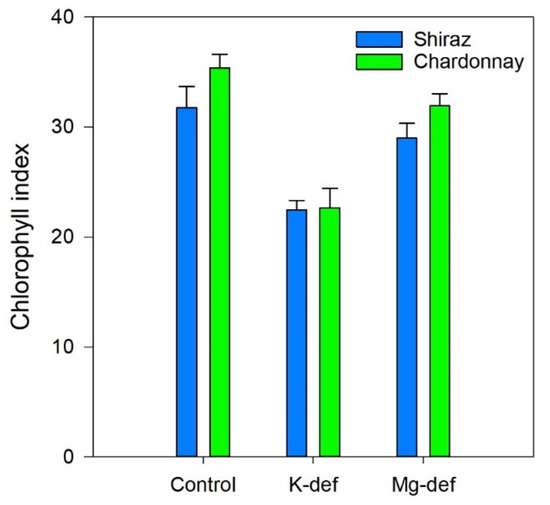
Figure 6.
Chlorophyll index of Chardonnay and Shiraz vines after 3 months of hydroponic greenhouse conditions and grown in control, K-deficient and Mg-deficient nutrient solutions.
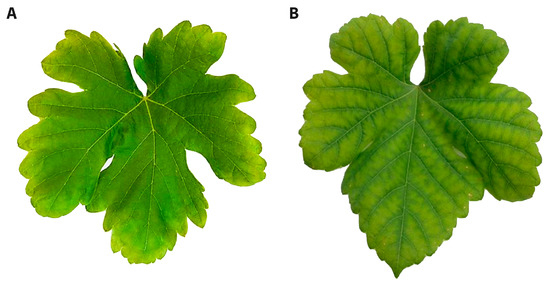
Figure 7.
K (A) and Mg (B) deficiency symptoms on mature leaves of Shiraz vines grown hydroponically in greenhouse conditions.
3.6. Biomass Accumulation
Biomass accumulation was curtailed in the deficient vines (Figure 8). Potassium deficiency reduced root growth by 70–80% and shoot growth by 50–60% in both cultivars (p < 0.001). Magnesium deficiency reduced root growth by 65–70% in both cultivars (p < 0.001) but shoot growth was not adversely affected in either cultivar. However, there were intrinsic cultivar differences, with Shiraz vines outperforming both root and shoot growth compared to the Chardonnay vines in all growth conditions.
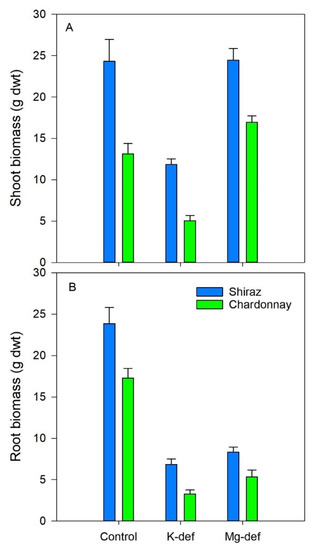
Figure 8.
Shoot (A) and root (B) biomass (dry weight) of Chardonnay and Shiraz vines after 3 months of growth hydroponically in greenhouse conditions and grown in control, K-deficient and Mg-deficient solutions.
4. Discussion
Magnesium and potassium deficiency markedly impaired photosynthesis in both Shiraz and Chardonnay vines when grown in these nutrient deficient conditions. This was evidenced by declines in A400 (photosynthesis at ambient CO2), Amax (CO2-saturated photosynthesis) and Asat (PFD saturating photosynthesis). Reduced photosynthesis in response to K deficiency has been well reported in cotton [18], sugar beet [50], sugarcane [51] and tomato [52] while photosynthesis depreciation in Mg-deficient plants has been also observed in arabidopsis [34], sugar beet [53], beech [54], broad bean [55] and blue-green algae [56] among others. Thus, both K and Mg deficiencies induce widespread problems affecting productivity through inefficient harvest of solar energy thereby reducing photoassimilate supply.
In our vines, the severity of the decline in photosynthesis was dependent on leaf location along the shoot. The older and middle-aged fully expanded leaves of both cultivars were severely affected by the Mg deficiency. Potassium deficiency also reduced Anet in older and middle-aged leaves of Chardonnay but in Shiraz vines, the older leaves were not as severely compromised. Those leaves that were still expanding and not fully mature were not affected by the nutrient deficiencies, suggesting that at least in Chardonnay both K and Mg were mobile and transported to the growing sinks from the older leaves or other storage sites in the vine. Other studies have shown similar mobilities for these nutrients [57]. This has important implications for reproductive growth, as flowers and berries can be a strong sink for nutrients through to maturity [58] at the expense of other growth points [59]. Our vines did not bear any inflorescences, and this is likely why the new leaves were favoured as sinks. The lower mobility of K in Shiraz may hinder partitioning to the crop, and thus inadequate ripening may be more prominent in this variety relative to Chardonnay. These vines were still young and, if the measurements had been carried out later in the season, a decline in A in the older leaves may have eventuated, as is common during normal aging and senescence [60]. Heavy shading of the internal canopy will also result in lower assimilation [61], but the basal leaves of our miniature vines with vertically trained shoots were well exposed to light.
The decline in net assimilation in response to Mg and K deficiency can, at least, be partly attributed to a reduced stomatal conductance (gs) in both Shiraz and Chardonnay vines. The decline in gs with K deficiency is well-documented [20,21,62], however, some studies have shown either no or the opposite stomatal response, and this may be related to the extent of K starvation or interspecific differences [22]. Lower gs in Mg-deficient pine seedlings was accompanied by reductions in the photochemical yield [63], indicating reductions in PSII photochemical performance, in keeping with the present results. In our vines, nutrient starvation not only decreased the stomatal elements of the photosynthetic response, but the non-stomatal attributes were also downgraded. The role of these attributes in CO2 fixation was clear. At low CO2 (less than 400 ppm), the carboxylation of RuBP limited assimilation, but the regeneration of RuBP was limiting at the higher CO2 concentrations.
In Chardonnay under Mg deficiency, there was co-limitation by RuBP carboxylation and regeneration at low CO2. The apparent maximum rates of RuBP carboxylation (Vcmax) were also severely reduced in response to both nutrient deficiencies. Again, Chardonnay carboxylation was more sensitive to Mg deficiency than K deficiency, but Shiraz carboxylation was apparently equally sensitive to both nutrients. The apparent maximum rates of assimilate electron transport (Jmax) followed similar trends to Vcmax, indicative of impeded RuBP regeneration by these nutrient deficiencies. It is well established that Mg modulates RuBP carboxylase [64,65]. Proton pumping from the stroma into the intrathylakoid space is counterbalanced by Mg2+ transport in the opposite direction [66,67]. Vcmax and Jmax limitations in photosynthesis were also apparent in K-deficient rice [68] and hickory seedlings, more so than gm or gs [22].
Leaf chlorophyll content was apparently lower under Mg- and especially K-deficient vines, as evidenced by the chlorophyll index data as well as visual symptoms. Typical symptoms of K deficiency include chlorosis that initiates at the leaf margins, which then spreads inwards, while, in Mg deficiency, interveinal chlorosis is characteristic, and this may be exacerbated by high light through ROS destruction of chlorophyll and membranes [69]. Because sucrose export from leaves was inhibited by a deficiency in these elements [17], chlorophyll degradation may have occurred through feedback inhibition. Chlorophyll fluorescence is a well-accepted indicator of PSII function. Both cultivars had lower chlorophyll fluorescence attributes, including decreased efficiency of PSII photochemistry, increased photochemical quenching and decreased electron transport rates (ETR) under both nutrient deficiencies. Other studies have confirmed that Mg deficiency can impair PSII functioning [56] or both PSI and PSII photochemistry [53]. Magnesium is the central molecule of chlorophyll that absorbs photons and initiates electron flow [70]. Potassium- offers no structural purpose; however, it acts as a counter-ion to light-induced H+ flux across the thylakoid membrane [71]. It also establishes the trans-membrane pH gradient for the synthesis of ATP [30,72]. Consistent with this, these present data confirm the deleterious effects of these nutrient deficiencies on the photochemical and metabolic processes of photosynthesis in the leaves of the Shiraz and Chardonnay vines.
Dark respiration was higher in the nutrient-deficient Shiraz vines, although no differences were apparent in Chardonnay, perhaps indicative of increased metabolism to counteract the nutrient deficiencies, at least in Shiraz vines. Consistent with this, sunflower [73], spinach [74] and oil palm [75] have also showed higher respiration in K deficiency, while sugar beet had higher respiration in Mg deficiency [76]. Both growth and cellular maintenance contribute to respiration and, since growth was depressed under nutrient deficiency, it seems likely that maintenance respiration may have been upregulated. Considering that Mg is important to enzymes in glycolysis, the pentose phosphate pathway and the tricarboxylic acid cycle, we hypothesised its deficiency would have down-regulated respiration. Perhaps processes that compensate for the ion deficiency are upregulated in an attempt to maintain homeostasis, and these are not as energy-efficient.
Root growth of the Shiraz and Chardonnay vines was severely stunted in both K and Mg deficiencies. This was in contrast to N and P deficiencies, which favour root growth [77]. Curtailed phloem export from the leaves, in particular sucrose- and Mg-containing amino acids, may have contributed to the decline in root growth and root to shoot ratio [30,77]. The roots of K-deficient bean plants had lower sucrose and starch than those grown in adequate K [17], conforming to the observation that K is specifically required for phloem loading in grapevines [78]. Weakened root growth is typical for Mg deficiency [79] and, again, impairment of carbohydrate export from source to sink through slow ATPase-driven phloem loading of sucrose [72] may be one of the underlying factors contributing to reduced root growth. Shoot growth was compromised under K deficiency in both cultivars, and was probably a consequence of the restricted photosynthesis described here, but K also maintains the pressure potential required to drive cellular expansion [80]. Considering that K activates membrane bound proton-pumping ATPases [17] and is the main solute required in vacuoles for cell extension [81], reduced leaf and stem growth rates may explain the lower biomass of these vines. Potassium deficiency has also altered water relations in tomato, as evidenced by greater stem and fruit shrinkage, and the biomass of all organs was reduced [52].
Overall, the results suggest that both cultivars responded negatively to K and Mg deficiency, but Chardonnay may be somewhat more sensitive. In the field, Shiraz tends to have a more vigorous growth habitat than Chardonnay, and this agrees with our biomass results under all three treatments. The leaf morphology of grapevine cultivars is relatively plastic and highly sensitive to environment, and underlying biochemical changes such as anthocyanin production can occur in response to abiotic stresses [82]. Even though Chardonnay is a green-skinned cultivar, its leaf petioles are apt to be more reddish in appearance than that of Shiraz, which produces red berries. This was, however, not visibly amplified by the nutrient deficiencies and the laminas also did not have obvious differences in coloration. Further work is required to better understand the underlying biochemical differences driving the altered photochemistry in these two cultivars. The lower apparent sensitivity of Shiraz gs to nutrient deficiency may be related to this cultivar’s propensity to a more open stomata under optimal conditions. Previous studies have demonstrated that Shiraz leans towards anisohydry, with oscillating plant water status in response to fluctuations in soil moisture or evaporative demand [83], and an investigation of how water-stressed vines respond to nutrient deficiencies would be worthwhile. Shiraz also tends to ripen later than Chardonnay and berries undergo shriveling and senescence in warm climates once berries are mature [84]. Warm temperatures and drought [85] exacerbate the associated programmed cell death. Nutrient imbalances may also cause localized cell death and tissue necrosis [69] and the laminas of both cultivars ultimately suffered such symptoms despite any protective mechanisms that may have been in place.
5. Conclusions
The relationship between vine nutritional composition and vine functioning is often not evident for a specific nutrient. With our system, we have demonstrated that both K and Mg deficiency resulted in severe photosynthetic limitations, with consequent repercussions on above- and below- ground growth. Potassium and Mg starvation reduced stomatal conductance, but there were also biochemical limitations through both reduced Rubisco activity and RuBP regeneration, directly impairing assimilation. PSII functioning was also comprised, suggesting that some reduction in light interception occurred. This was in keeping with some apparent leaf chlorosis caused by the nutrient deficiencies. Because K is necessary for assimilate translocation, it is possible that feedback inhibition of sucrose accumulation in leaves also contributed to reduced photosynthesis. Given these results, and the known increased demand for assimilates by the reproductive system of grapevines [86], characterisation of the source-sink relationship in vines supporting bunch growth with these nutrients in deficit is warranted to achieve the goal of sustainable productivity in vineyards.
Author Contributions
Conceptualisation, S.Y.R.; Methodology, D.H.G., S.Y.R., T.B. and F.J.M.; Investigation, D.H.G., S.Y.R., T.B. and F.J.M.; Formal analysis, D.H.G. and S.Y.R., Writing—original draft preparation, S.R. and D.H.G.; Writing—review and editing, D.H.G., S.Y.R., T.B. and F.J.M.; Funding acquisition, S.Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Wine Australia, NSW Department of Primary Industries and Charles Sturt University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Coetzee, Z.A.; Walker, R.R.; Deloire, A.J.; Barril, C.; Clarke, S.J.; Rogiers, S.Y. Impact of reduced atmospheric CO2 and varied potassium supply on carbohydrate and potassium distribution in grapevine and grape berries (Vitis vinifera L.). Plant Physiol. Biochem. 2017, 120, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Martín, P.; del Álamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudillère, J.-P. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 2007, 59, 139–149. [Google Scholar] [CrossRef]
- Williams, C.M.J.; Maier, N.A.; Bartlett, L. Effect of molybdenum foliar sprays on yield, berry size, seed formation, and petiolar nutrient composition of “Merlot” grapevines. J. Plant Nutr. 2005, 27, 1891–1916. [Google Scholar] [CrossRef]
- Leibar, U.; Pascual, I.; Aizpurua, A.; Morales, F.; Unamunzaga, O. Grapevine nutritional status and K concentration of must under future expected climatic conditions texturally different soils. J. Soil Sci. Plant Nutr. 2017, 17, 385–397. [Google Scholar] [CrossRef]
- Hsiao, T.; Lauchli, A. Role of potassium in plant-water relations. In Advances in Plant Nutrition; Praeger: New York, NY, USA, 1986. [Google Scholar]
- Liesche, J. How regulation of phloem transport could link potassium fertilization to increased growth. Tree Physiol. 2016, 36, 1–5. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Ann. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Ahmad, I.; Maathuis, F.J. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Boulton, R. The general relationship between potassium, sodium and pH in grape juice and wine. Am. J. Enol. Vitic. 1980, 31, 182–186. [Google Scholar]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H. Potassium concentration and pH inter-relationships in grape juice and wine of Chardonnay and Shiraz from a range of rootstocks in different environments. Aust. J. Grape Wine Res. 2012, 18, 183–193. [Google Scholar] [CrossRef]
- Mattick, L.R.; Shaulis, N.J.; Moyer, J.C. The effect of potassium fertilization on the acid content of ‘Concord’ grape juice. Am. J. Enol. Vitic. 1972, 23, 26–30. [Google Scholar]
- Onanuga, A.O.; Jiang, P.a.; Adl, S. Phosphorus, potassium and phytohormones promote chlorophyll production differently in two cotton (Gossypium hirsutum) varieties grown in hydroponic nutrient solution. J. Agric. Sci. 2012, 4, 157–166. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Humble, G.; Raschke, K. Stomatal opening quantitatively related to potassium transport: Evidence from electron probe analysis. Plant Physiol. 1971, 48, 447–453. [Google Scholar] [CrossRef]
- Thiel, G.; Wolf, A.H. Operation of K+-channels in stomatal movement. Trends Plant Sci. 1997, 2, 339–345. [Google Scholar] [CrossRef]
- Bednarz, C.; Oosterhuis, D.; Evans, R. Leaf photosynthesis and carbon isotope discrimination of cotton in response to potassium deficiency. Environ. Exp. Bot. 1998, 39, 131–139. [Google Scholar] [CrossRef]
- Jin, S.H.; Huang, J.Q.; Li, X.Q.; Zheng, B.S.; Wu, J.S.; Wang, Z.J.; Liu, G.H.; Chen, M. Effects of potassium supply on limitations of photosynthesis by mesophyll diffusion conductance in Carya cathayensis. Tree Physiol. 2011, 31, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ren, T.; Pan, Y.; Li, X.; Cong, R.; Lu, J. Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci. Rep. 2016, 6, 21725. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weedon, M.M. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Haldimann, P.; Feller, U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose−1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Ann. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1986; p. 674. [Google Scholar]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Implications of adenylate kinase-governed equilibrium of adenylates on contents of free magnesium in plant cells and compartments. Biochem. J. 2001, 360, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Koblet, W. Stress-induced development of inflorescence necrosis and bunch-stem necrosis in Vitis vinifera L. in response to environmental and nutritional effects. Vitis 1995, 34, 145–150. [Google Scholar]
- Rupp, D.; Fox, R.; Tränkle, L. Foliar application of magnesium fertilizer in grapevines: Effects on wine quality. Acta Hortic. 2002, 594, 149–155. [Google Scholar] [CrossRef]
- Sinilal, B.; Ovadia, R.; Nissim-Levi, A.; Perl, A.; Carmeli-Weissberg, M.; Oren-Shamir, M. Increased accumulation and decreased catabolism of anthocyanins in red grape cell suspension culture following magnesium treatment. Planta 2011, 234, 61–71. [Google Scholar] [CrossRef]
- Conradie, W. Seasonal uptake of nutrients by Chenin blanc in sand culture: II. phosphorus, potassium, calcium and magnesium. S. Afr. J. Enol. Vitic. 1981, 2, 7–13. [Google Scholar] [CrossRef]
- Delas, J.; Pouget, R. Action de la concentration de la solution nutritive sur quelques caractéristiques physiologiques et technologiques chez Vitis vinifera L. cv.” Cabernet-Sauvignon”. II.-Composition minérale des organes végétatifs, du moût et du vin. Agronomie 1984, 4, 443–450. [Google Scholar] [CrossRef]
- Skinner, P.W.; Matthews, M.A. A novel interaction of magnesium translocation with the supply of phosphorus to roots of grapevine (Vitis vinifera L.). Plant Cell Environ. 1990, 13, 821–826. [Google Scholar] [CrossRef]
- Holzapfel, B.P.; Smith, J.P.; Field, S.K. Seasonal vine nutrient dynamics and distribution of Shiraz grapevines. OENO One 2019, 53. [Google Scholar] [CrossRef]
- Baby, T.; Hocking, B.; Tyerman, S.D.; Gilliham, M.; Collins, C. Modified method for producing grapevine plants in controlled environments. Am. J. Enol. Vitic. 2014, 65, 261–267. [Google Scholar] [CrossRef]
- Garcia, M.; Daverede, C.; Gallego, P.; Toumi, M. Effect of various potassium-calcium ratios on cation nutrition of grape grown hydroponically. J. Plant Nutr. 1999, 22, 417–425. [Google Scholar] [CrossRef]
- Robinson, J.B. Grapevine Nutrition. In Viticulture -2 Practises; Coombe, B.G., Dry, P., Eds.; Australian Industrial Publishers Pty Ltd.: Adelaide, Australia, 1992. [Google Scholar]
- Greer, D.H.; Halligan, E.A. Photosynthetic and fluorescence light responses for kiwifruit (Actinidia deliciosa) leaves at different stages of development on vines grown at two different photon flux densities. Funct. Plant Biol. 2001, 28, 373–382. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.v.; Berry, J. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L.; Flexas, J.; von Caemmerer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating mesophyll conductance to CO2: Methodology, potential errors, and recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Diaz-Espejo, A.; GalmÉS, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef]
- Terry, N.; Ulrich, A. Effects of potassium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1973, 51, 783–786. [Google Scholar] [CrossRef]
- Hartt, C.E. Effect of potassium deficiency upon translocation of 14C in attached blades and entire plants of sugarcane. Plant Physiol. 1969, 44, 1461–1469. [Google Scholar] [CrossRef][Green Version]
- Kanai, S.; Ohkura, K.; Adu-Gyamfi, J.; Mohapatra, P.; Nguyen, N.; Saneoka, H.; Fujita, K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef]
- Ridolfi, M.; Garrec, J.-P. Consequences of an excess Al and a deficiency in Ca and Mg for stomatal functioning and net carbon assimilation of beech leaves. Ann. For. Sci. 2000, 57, 209–218. [Google Scholar] [CrossRef]
- Hariadi, Y.; Shabala, S. Screening broad beans (Vicia faba) for magnesium deficiency. II. Photosynthetic performance and leaf bioelectrical responses. Funct. Plant Biol. 2004, 31, 539–549. [Google Scholar] [CrossRef]
- McSwain, B.D.; Tsujimoto, H.Y.; Arnon, D.I. Effects of magnesium and chloride ions on light-induced electron transport in membrane fragments from a blue-green alga. Biochim. Biophys. Acta 1976, 423, 313–322. [Google Scholar] [CrossRef]
- Welch, R.M. Effects of nutrient deficiencies on seed production and quality. Adv. Plant Nutr. 1986, 2, 205–247. [Google Scholar]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.; Beverley, O.; Keller, M. Mineral sinks within ripening grape berries (Vitis vinifera L). Vitis 2006, 45, 115–123. [Google Scholar]
- Pastore, C.; Zenoni, S.; Tornielli, G.B.; Allegro, G.; Dal Santo, S.; Valentini, G.; Intrieri, C.; Pezzotti, M.; Filippetti, I. Increasing the source/sink ratio in Vitis vinifera (cv Sangiovese) induces extensive transcriptome reprogramming and modifies berry ripening. BMC Genom. 2011, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Bertamini, M.; Nedunchezhian, N. Leaf age effects on chlorophyll, Rubisco, photosynthetic electron transport activities and thylakoid membrane protein in field grown grapevine leaves. J. Plant Physiol. 2002, 159, 799–803. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M.; Weston, C. Reductions in biomass accumulation, photosynthesis in situ and net carbon balance are the costs of protecting Vitis vinifera ‘Semillon’grapevines from heat stress with shade covering. AoB Plants 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Peaslee, D.E.; Moss, D.N. Stomatal conductivities in K-deficient leaves of maize (Zea mays, L.). Crop Sci. 1968, 8, 427–430. [Google Scholar] [CrossRef]
- Laing, W.; Greer, D.; Sun, O.; Beets, P.; Lowe, A.; Payn, T. Physiological impacts of Mg deficiency in Pinus radiata: Growth and photosynthesis. New Phytol. 2000, 146, 47–57. [Google Scholar] [CrossRef]
- Pierce, J. Determinants of substrate specificity and the role of metal in the reactions of ribulosebisphosphate carboxylase/oxygenase. Plant Physiol. 1986, 81, 943–945. [Google Scholar] [CrossRef][Green Version]
- Sugiyama, T.; Nakayama, N.; Akazawa, T. Structure and function of chloroplast proteins: V. Homotropic effect of bicarbonate in RuDP carboxylase reaction and the mechanism of activation by magnesium ions. Arch. Biochem. Biophys. 1968, 126, 737–745. [Google Scholar] [CrossRef]
- Ishijima, S.; Uchibori, A.; Takagi, H.; Maki, R.; Ohnishi, M. Light-induced increase in free Mg2+ concentration in spinach chloroplasts: Measurement of free Mg2+ by using a fluorescent probe and necessity of stromal alkalinization. Arch. Biochem. Biophys. 2003, 412, 126–132. [Google Scholar] [CrossRef]
- Oja, V.; Laisk, A.; Heber, U. Light-induced alkalization of the chloroplast stroma in vivo as estimated from the CO2 capacity of intact sunflower leaves. Biochim. Biophys. Acta Bioenerg. 1986, 849, 355–365. [Google Scholar] [CrossRef]
- Weng, X.-Y.; Zheng, C.-J.; Xu, H.-X.; Sun, J.-Y. Characteristics of photosynthesis and functions of the water–water cycle in rice (Oryza sativa) leaves in response to potassium deficiency. Physiol. Plant. 2007, 131, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Cakmak, I. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Katz, J.J.; Norris, J.R.; Shipman, L.L.; Thurnauer, M.C.; Wasielewski, M.R. Chlorophyll function in the photosynthetic reaction center. Ann. Rev. Biophys. Bioeng. 1978, 7, 393–434. [Google Scholar] [CrossRef]
- Tester, M.; Blatt, M.R. Direct measurement of K+ channels in thylakoid membranes by incorporation of vesicles into planar lipid bilayers. Plant Physiol. 1989, 91, 249–252. [Google Scholar] [CrossRef]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Cui, J.; Abadie, C.; Carroll, A.; Lamade, E.; Tcherkez, G. Responses to K deficiency and waterlogging interact via respiratory and nitrogen metabolism. Plant Cell Environ. 2019, 42, 647–658. [Google Scholar] [CrossRef]
- Bottrill, D.E.; Possingham, J.V.; Kriedemann, P.E. The effect of nutrient deficiencies on phosynthesis and respiration in spinach. Plant Soil 1970, 32, 424–438. [Google Scholar] [CrossRef]
- Cui, J.; Davanture, M.; Zivy, M.; Lamade, E.; Tcherkez, G. Metabolic responses to potassium availability and waterlogging reshape respiration and carbon use efficiency in oil palm. New Phytol. 2019, 223, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Ulrich, A. Effects of magnesium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1974, 54, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Andrianteranagna, M.; Cuellar, T.; Cherel, I.; Gibrat, R.; Boeglin, M.; Moreau, B.; Paris, N.; Verdeil, J.L.; Zimmermann, S.; et al. Characterization of the grapevine Shaker K+ channel VvK3.1 supports its function in massive potassium fluxes necessary for berry potassium loading and pulvinus-actuated leaf movements. New Phytol. 2019, 222, 286–300. [Google Scholar] [CrossRef]
- Roberts, T.M.; Skeffington, R.A.; Blank, L.W. Causes of Type 1 spruce decline in Europe. For. Int. J. For. Res. 1989, 62, 179–222. [Google Scholar] [CrossRef]
- Pervez, H.; Ashraf, M.; Makhdum, M.I. Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirsutum L.). Photosynthetica 2004, 42, 251–255. [Google Scholar] [CrossRef]
- Claussen, M.; Lüthe, H.; Blatt, M.; Böttger, M. Auxin-induced growth and its linkage to potassium channels. Planta 1997, 201, 227–234. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Klouvatou, A.; Vekkos, K.A.; Manetas, Y.; Karabourniotis, G. The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann. Bot. 2006, 98, 257–265. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Clarke, S.J.; Rogiers, S.Y. The role of fruit exposure in the late season decline of grape berry mesocarp cell vitality. Plant Physiol. Biochem. 2019, 135, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liao, S.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Effect of water stress and elevated temperature on hypoxia and cell death in the mesocarp of Shiraz berries. Aust. J. Grape Wine Res. 2018, 24, 487–497. [Google Scholar] [CrossRef]
- Greer, D.H. Short-term temperature dependency of the photosynthetic and PSII photochemical responses to photon flux density of leaves of Vitis vinifera cv. Shiraz vines grown in field conditions with and without fruit. Funct. Plant Biol. 2019, 46, 634–648. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).