Development of a Pulsatile Flow-Generating Circulatory Assist Device (K-Beat) for Use with Veno-Arterial Extracorporeal Membrane Oxygenation in a Pig Model Study
Abstract
1. Introduction
2. Materials and Methods
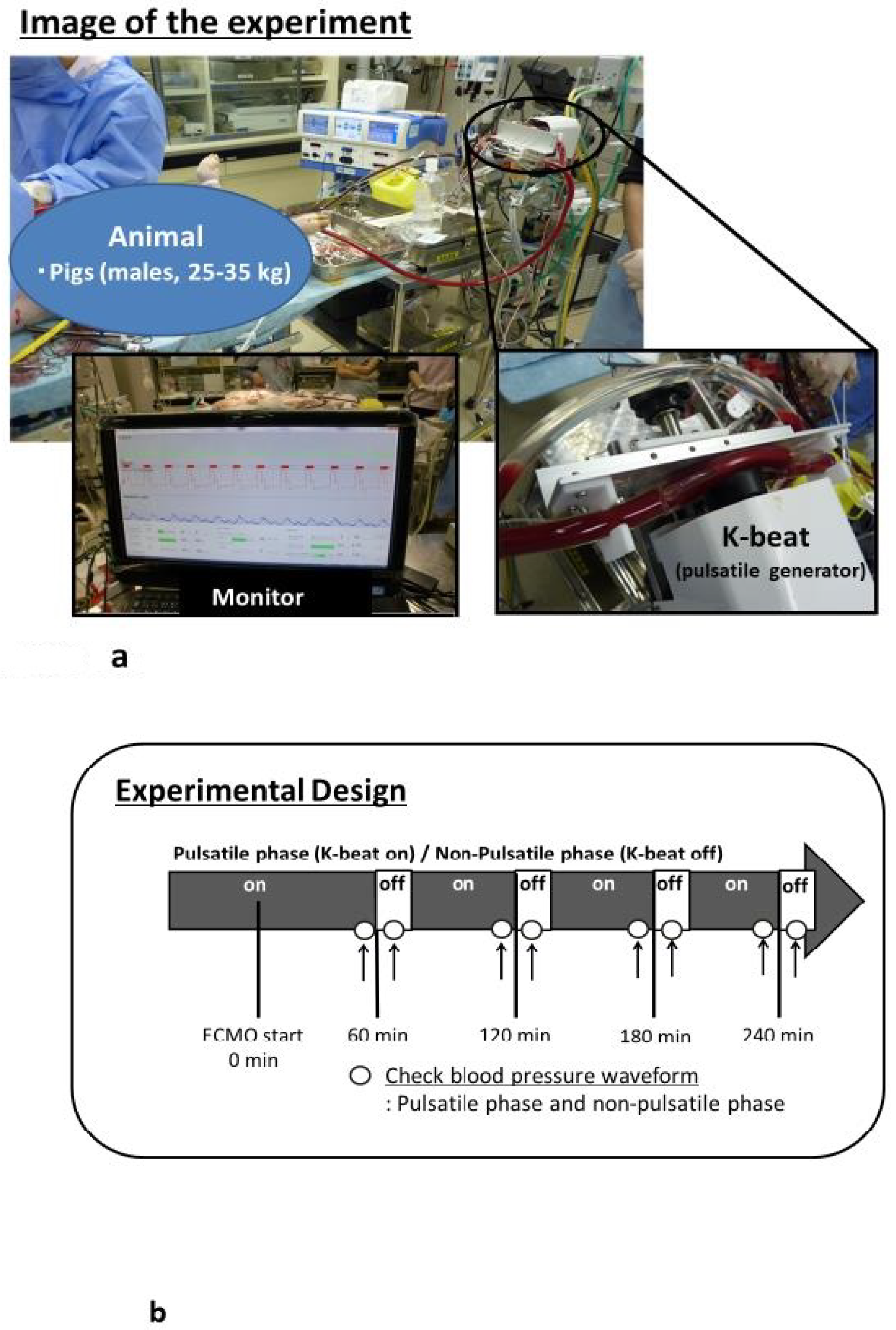
2.1. Ethical Approval
2.2. Anesthesia, Surgical Preparation, and V-A ECMO
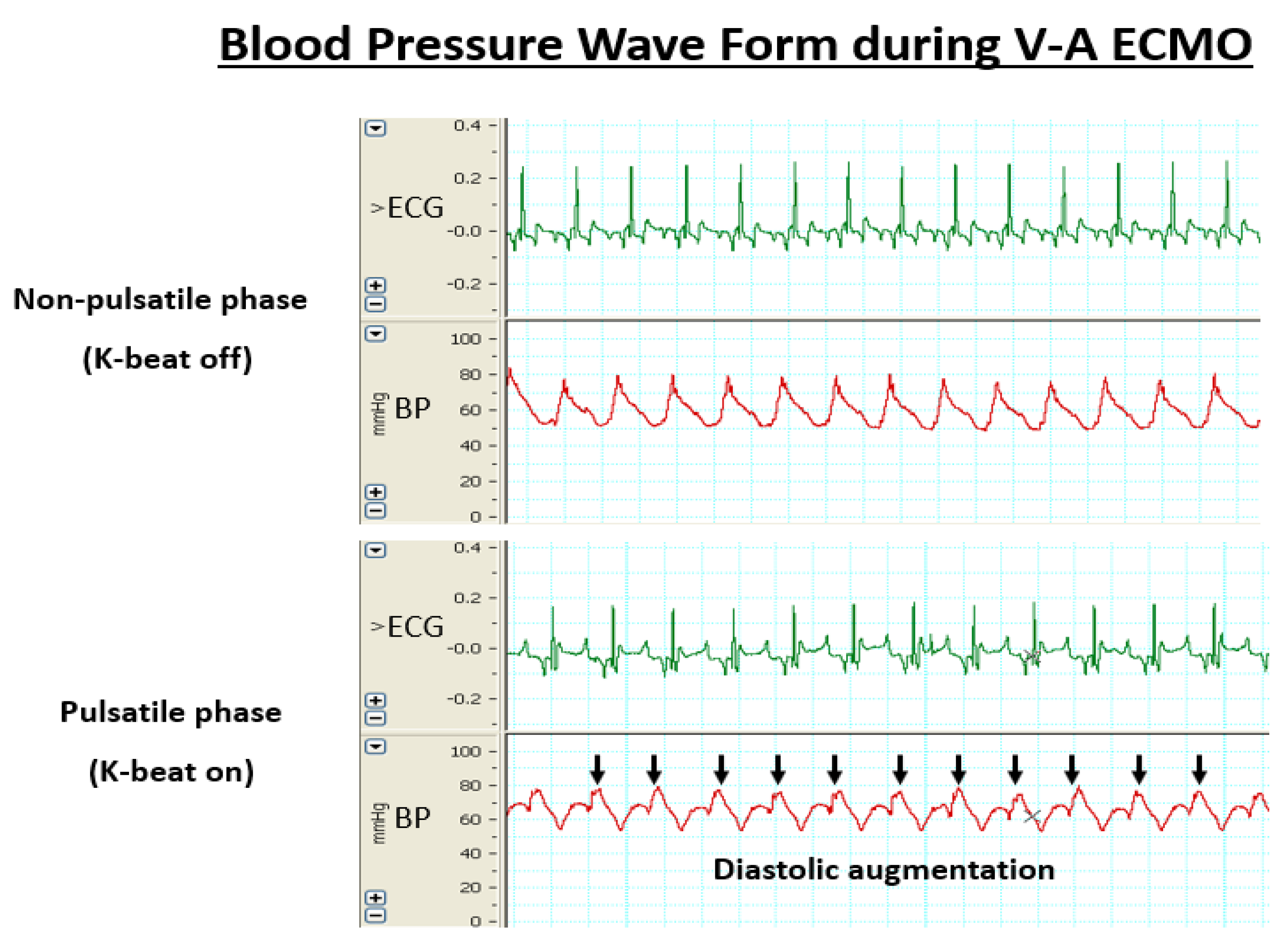
2.3. Experimental Design
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| V-A ECMO | Veno-arterial extracorporeal membrane oxygenation |
| ECG | Electrocardiogram |
| IABP | Intra-aortic balloon pumping |
| PaO2 | Arterial pressure of oxygen |
| PaCO2 | Arterial pressure of carbon dioxide |
References
- Reichman, R.T.; Joyo, C.I.; Dembitsky, W.P.; Griffith, L.D.; Adamson, R.M.; Daily, P.O.; Overlie, P.A.; Smith, S.C., Jr.; Jaski, B.E. Improved patient survival after cardiac arrest using cardiopulmonary support system. Ann. Thorac. Surg. 1990, 49, 101–105. [Google Scholar] [CrossRef]
- Hartz, R.; LoCicero, J., 3rd; Sanders, J.H., Jr.; Frederiksen, J.W.; Joob, A.W.; Michaelis, L.L. Clinical experiences with portable cardiopulmonary bypass in cardiac arrest patients. Ann. Thorac. Surg. 1990, 50, 437–441. [Google Scholar] [CrossRef]
- Mooney, M.R.; Arom, K.V.; Joyce, L.D.; Mooney, J.F.; Goldenberg, I.F.; Von Rueden, T.J.; Emery, R.W. Emergency cardiopulmonary bypass support in patients with cardiac arrest. J. Thorac. Cardiovasc. Surg. 1991, 101, 450–454. [Google Scholar] [CrossRef]
- Younger, J.G.; Schreiner, R.J.; Swaniker, F.; Hirschl, R.B.; Chapman, R.A.; Bartlett, R.H. Extracorporeal resuscitation of cardiac arrest. Ann. Emerg. Med. 1999, 6, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.; Liddel, M.; Davis, C. Extracorporeal life support-state of the art. Paediatr. Respir. Rev. 2003, 4, 147–152. [Google Scholar] [CrossRef]
- Cremers, B.; Link, A.; Werner, C.; Gorhan, H.; Simundic, I.; Matheis, G.; Scheller, B.; Böhm, M.; Laufs, U. Pulsatile venoarterial perfusion using a novel synchronized cardiac assist device augments coronary artery blood flow during ventricular fibrillation. Artif. Organs 2015, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.M.; Rinösl, H.; Kühn, K.; Skhirtladze-Dworschak, K.; Felli, A.; Mouhieddine, M.; Menger, J.; Pataraia, E.; Ankersmit, H.J.; Dworschak, M. Non-pulsatile blood flow is associated with enhanced cerebrovascular carbon dioxide reactivity and an attenuated relationship between cerebral blood flow and regional brain oxygenation. Crit. Care 2019, 23, 426. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, J.; Paul, E.; Rosenfeldt, F.L. Early Gastrointestinal Complications From Ventricular Assist Devices is Increased by Non-Pulsatile Flow. Heart Lung Circ. 2020, 29, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Inamori, S.; Fujii, Y.; Oshita, T.; Kobayashi, Y.; Minamiyama, M.; Sasaki, S.; Murakami, T.; Sakuma, I.; Gunshin, M.; Suematsu, Y.; et al. Development of a pulsatile flow-generating circulatory-assist device. J. Artif. Organs 2010, 13, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.G.; Bruhn, P.S.; Cohen, S.E.; Gallagher, M.W.; Manart, F.; Moore, C.A.; Seifert, P.E.; Askari, P.; Banchieri, C. Emergent applications of cardiopulmonary support: A multiinstitutional experience. Ann. Thorac. Surg. 1992, 54, 699–704. [Google Scholar] [CrossRef]
- Wolfe, R.; Strother, A.; Wang, S.; Kunselman, A.R.; Ündar, A. Impact of pulsatility and flow rates on hemodynamic energy transmission in an adult extracorporeal life support system. Artif. Organs 2015, 39, E127–E137. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Izer, J.M.; Clark, J.B.; Patel, S.; Pauliks, L.; Kunselman, A.R.; Leach, D.; Cooper, T.K.; Wilson, R.P.; Ündar, A. In vivo hemodynamic performance evaluation of novel electrocardiogram-synchronized pulsatile and nonpulsatile extracorporeal life support systems in an adult swine model. Artif. Organs 2015, 39, E90–E101. [Google Scholar] [CrossRef] [PubMed]
| HR | MAP | Hb | pH | Na | K | Cl | ||
|---|---|---|---|---|---|---|---|---|
| (beats/min) | (mm/Hg) | (g/dL) | (mEq/L) | (mEq/L) | (mEq/L) | |||
| Case 1 | pre | 87 | 68 | 11.2 | 7.356 | 141 | 4.3 | 106 |
| 240 min | 85 | 70 | 9.2 | 7.352 | 142 | 5.3 | 106 | |
| Case 2 | pre | 80 | 73 | 12.9 | 7.401 | 138 | 4.6 | 110 |
| 240 min | 82 | 71 | 8.2 | 7.361 | 140 | 5.4 | 108 | |
| Case 3 | pre | 75 | 64 | 10.7 | 7.387 | 138 | 3.9 | 110 |
| 240 min | 79 | 67 | 7.4 | 7.346 | 139 | 5.2 | 109 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Akamatsu, N.; Yamasaki, Y.; Miki, K.; Banno, M.; Minami, K.; Inamori, S. Development of a Pulsatile Flow-Generating Circulatory Assist Device (K-Beat) for Use with Veno-Arterial Extracorporeal Membrane Oxygenation in a Pig Model Study. Biology 2020, 9, 121. https://doi.org/10.3390/biology9060121
Fujii Y, Akamatsu N, Yamasaki Y, Miki K, Banno M, Minami K, Inamori S. Development of a Pulsatile Flow-Generating Circulatory Assist Device (K-Beat) for Use with Veno-Arterial Extracorporeal Membrane Oxygenation in a Pig Model Study. Biology. 2020; 9(6):121. https://doi.org/10.3390/biology9060121
Chicago/Turabian StyleFujii, Yutaka, Nobuo Akamatsu, Yasunori Yamasaki, Kota Miki, Masayuki Banno, Kenta Minami, and Shuji Inamori. 2020. "Development of a Pulsatile Flow-Generating Circulatory Assist Device (K-Beat) for Use with Veno-Arterial Extracorporeal Membrane Oxygenation in a Pig Model Study" Biology 9, no. 6: 121. https://doi.org/10.3390/biology9060121
APA StyleFujii, Y., Akamatsu, N., Yamasaki, Y., Miki, K., Banno, M., Minami, K., & Inamori, S. (2020). Development of a Pulsatile Flow-Generating Circulatory Assist Device (K-Beat) for Use with Veno-Arterial Extracorporeal Membrane Oxygenation in a Pig Model Study. Biology, 9(6), 121. https://doi.org/10.3390/biology9060121





