A Perspective on Body Size and Abundance Relationships across Ecological Communities
Abstract
1. Introduction
2. Case Studies
2.1. Across Climate Zones (Biogeographic Regions)
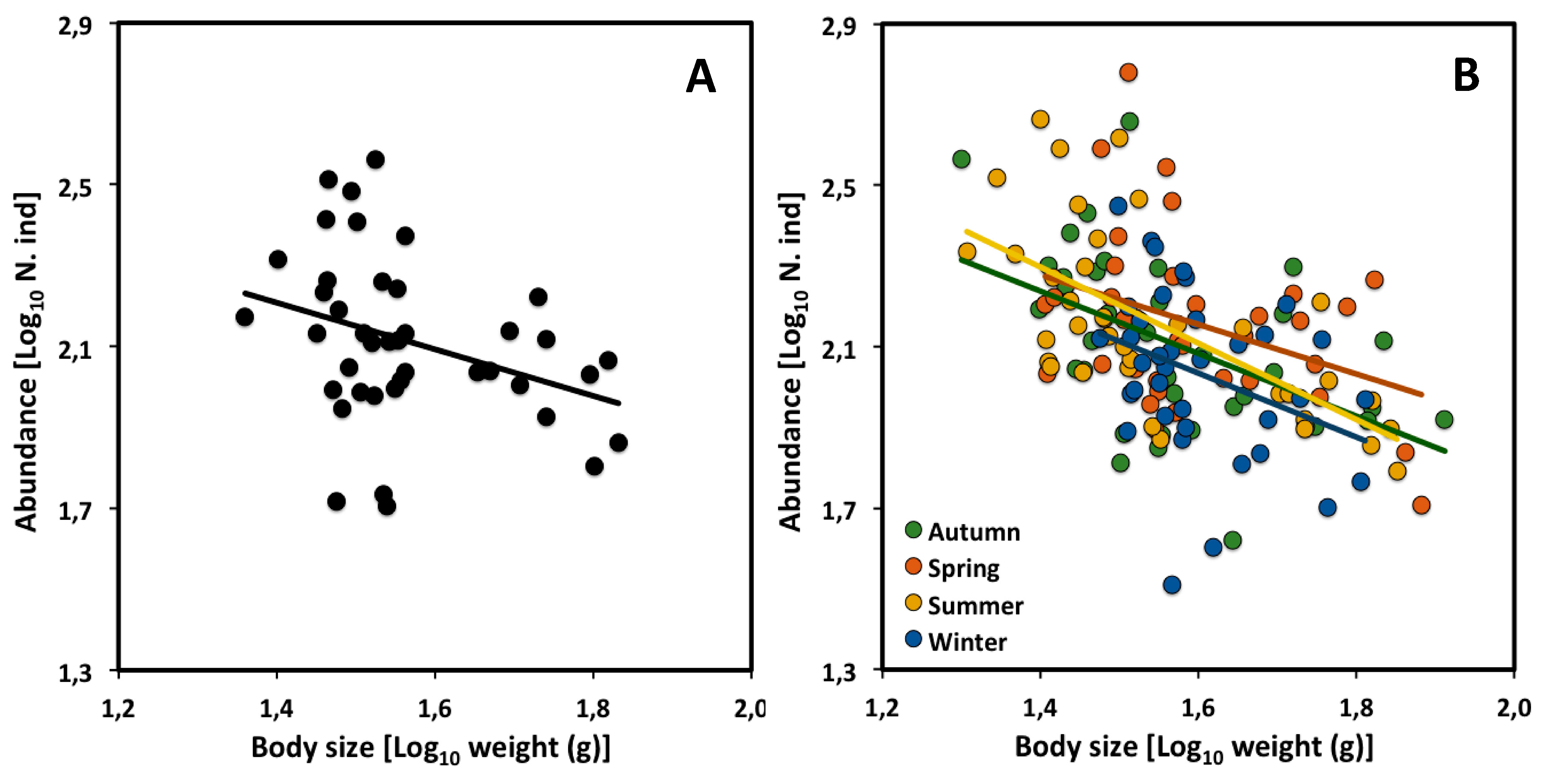
2.2. Across Seasons
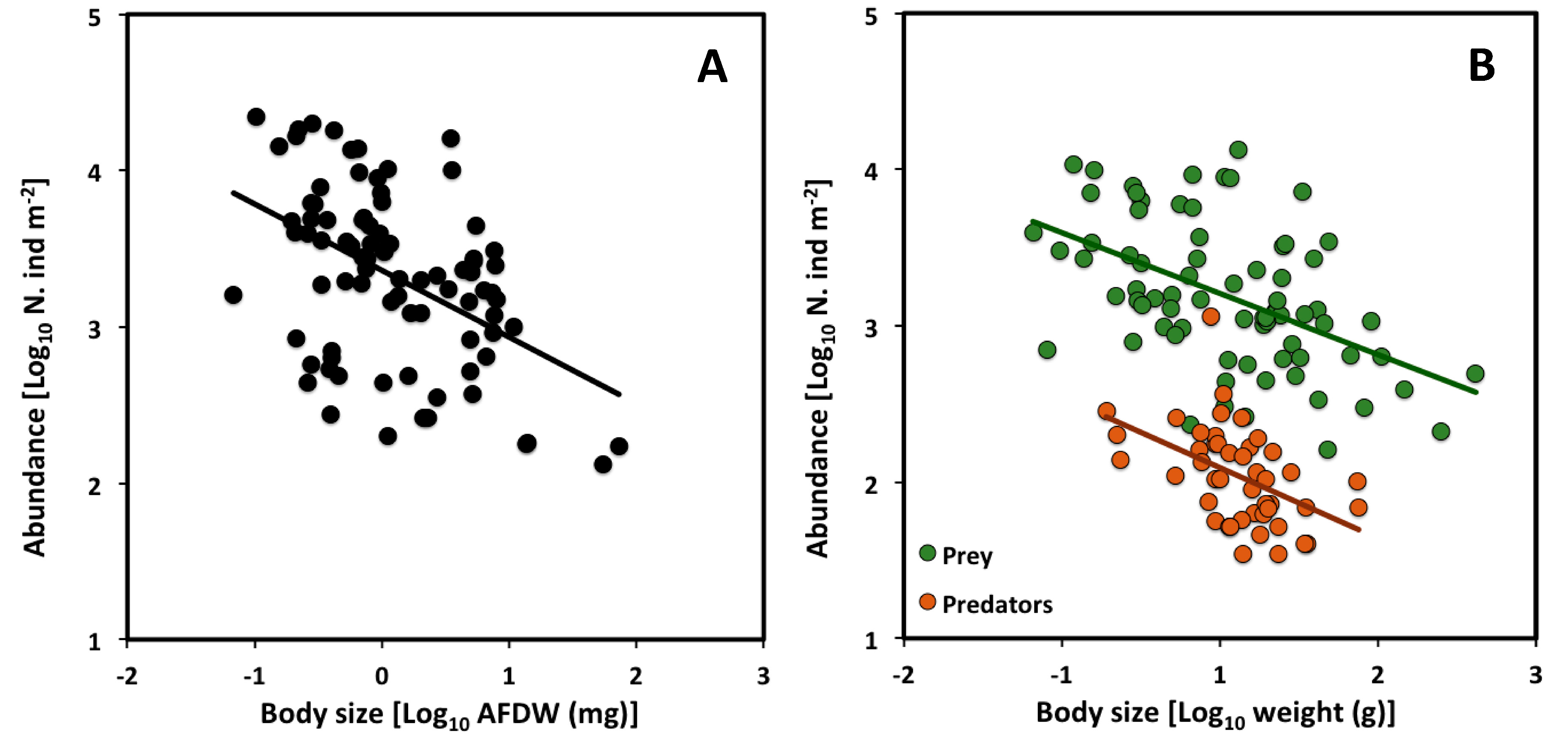
2.3. Across Trophic Levels
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
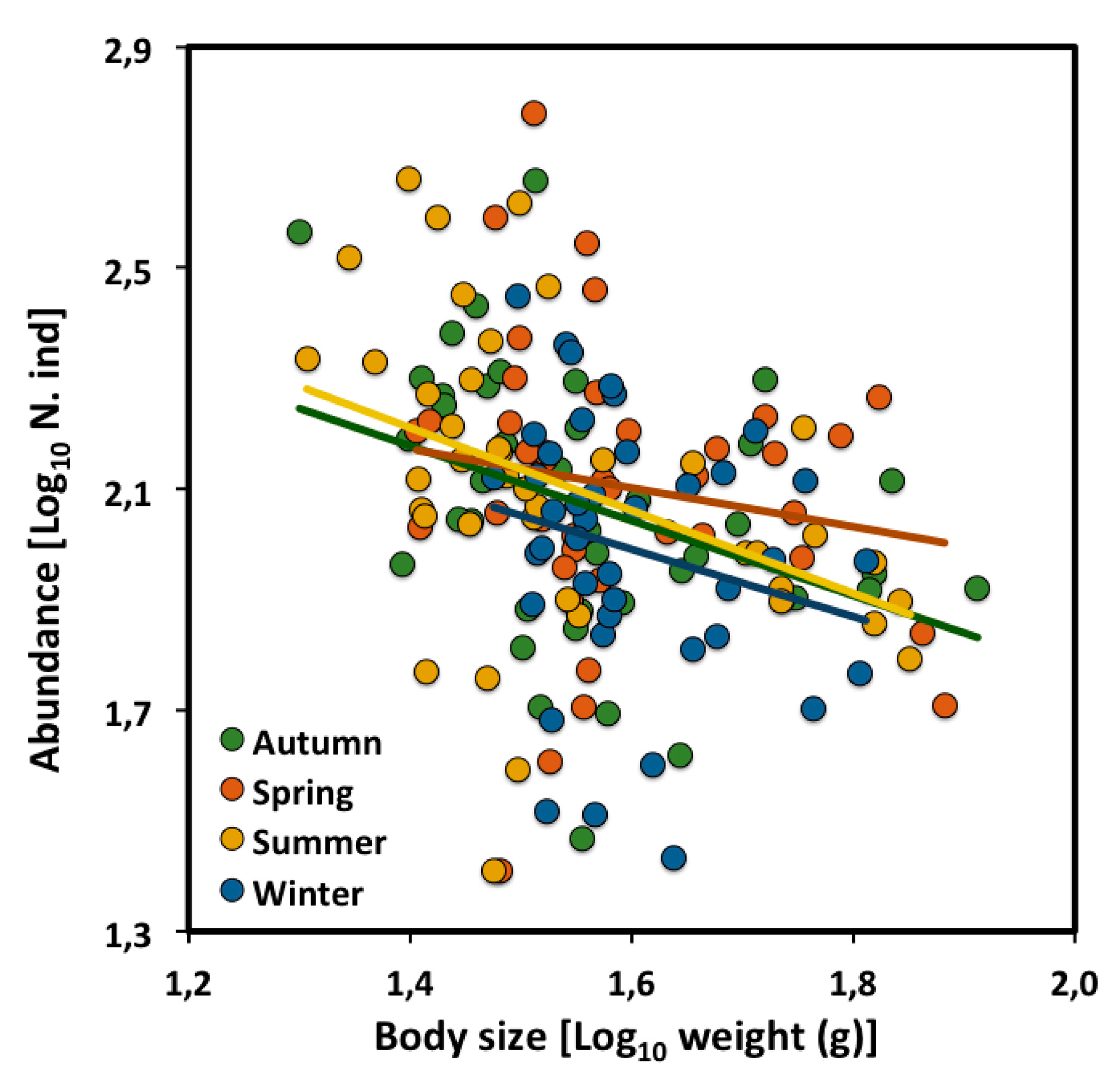
| CCSR | Slope | Intercept | 95% CI | n | r2 | p |
|---|---|---|---|---|---|---|
| Autumn | −0.71 | 3.18 | −1.27 to −0.15 | 41 | 0.15 | * |
| Spring | −0.35 | 2.67 | −1.05 to −0.34 | 41 | 0.03 | ns |
| Summer | −0.75 | 3.26 | −1.28 to −0.22 | 41 | 0.17 | ** |
| Winter | −0.61 | 2.97 | −1.47 to −0.25 | 41 | 0.05 | ns |
| Seasons | p Value for Slope a | p Value for Intercept b | ||||||
|---|---|---|---|---|---|---|---|---|
| AU | SP | SU | WI | AU | SP | SU | WI | |
| Autumn (AU) | - | ns | ns | ns | - | ns | ns | ns |
| Spring (SP) | ns | - | ns | ns | ns | - | ns | ns |
| Summer (SU) | ns | ns | - | ns | ns | ns | - | ns |
| Winter (WI) | ns | ns | ns | - | ns | ns | ns | - |
References
- Li, B.L.; Wu, H.I.; Zou, G. Self-thinning rule: A causal interpretation from ecological field theory. Ecol. Model. 2000, 132, 167–173. [Google Scholar] [CrossRef]
- Yoda, K. Self-thinning in overcrowded pure stands under cultivated and natural conditions (Intraspecific competition among higher plants. XI). J. Inst. Polytech. Osaka City Univ. 1963, 14, 107–129. [Google Scholar]
- Westoby, M. The place of the self-thinning rule in population dynamics. Am. Nat. 1981, 118, 581–587. [Google Scholar] [CrossRef]
- Belgrano, A.; Allen, A.P.; Enquist, B.J.; Gillooly, J.F. Allometric scaling of maximum population density: A common rule for marine phytoplankton and terrestrial plants. Ecol. Lett. 2002, 5, 611–613. [Google Scholar] [CrossRef]
- Begon, M.; Firbank, L.; Wall, R. Is there a self-thinning rule for animal populations? Oikos 1986, 46, 122–124. [Google Scholar] [CrossRef]
- Latto, J. Evidence for a self-thinning rule in animals. Oikos 1994, 69, 531–534. [Google Scholar] [CrossRef]
- Fréchette, M.; Lefaivre, D. On self-thinning in animals. Oikos 1995, 73, 425–428. [Google Scholar] [CrossRef]
- Branch, G.M. Mechanisms reducing intraspecific competition in Patella spp.: Migration, differentiation and territorial behaviour. J. Anim. Ecol. 1975, 44, 575–600. [Google Scholar] [CrossRef]
- Hogarth, P.J. Population Density, Mean Weight, and the Nature of the “Thinning Line” in Semibalanus balanoides (L.) (Cirripedia Thoracica). Crustaceana 1985, 49, 215–218. [Google Scholar] [CrossRef]
- Hughes, R.N.; Griffiths, C.L. Self-thinning in barnacles and mussels: The geometry of packing. Am. Nat. 1988, 132, 484–491. [Google Scholar] [CrossRef]
- Frechette, M.; Lefaivre, D. Discriminating between food and space limitation in benthic suspension feeders using self-thinning relationships. Mar. Ecol. Prog. Ser. 1990, 65, 15–23. [Google Scholar] [CrossRef]
- Petraitis, P.S. The role of growth in maintaining spatial dominance by mussels (Mytilus edulis). Ecology 1995, 76, 1337–1346. [Google Scholar] [CrossRef]
- Guinez, R.; Castilla, J.C. A tridimensional self-thinning model for multilayered intertidal mussels. Am. Nat. 1999, 154, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.W.; Kramer, D.L. Territory size as a predictor of the upper limit to population density of juvenile salmonids in streams. Can. J. Fish. Aquat. Sci. 1990, 47, 1724–1737. [Google Scholar] [CrossRef]
- Elliott, J.M. The self-thinning rule applied to juvenile sea trout, Salmo trutta. J. Anim. Ecol. 1993, 62, 371–379. [Google Scholar] [CrossRef]
- Bohlin, T.; Dellefors, C.; Faremo, U.; Johlander, A. The energetic equivalence hypothesis and the relation between population density and body size in stream-living salmonids. Am. Nat. 1994, 143, 478–493. [Google Scholar] [CrossRef]
- Armstrong, J.D.; Herbert, N.A. Homing movements of displaced stream-dwelling brown trout. J. Fish Biol. 1997, 50, 445–449. [Google Scholar] [CrossRef]
- Dunham, J.B.; Vinyard, G.L. Relationships between body mass, population density, and the self-thinning rule in stream-living salmonids. Can. J. Fish. Aquat. Sci. 1997, 54, 1025–1030. [Google Scholar] [CrossRef]
- White, E.P.; Ernest, S.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef]
- Damuth, J.D. Population density and body size in mammals. Nature 1981, 290, 699–700. [Google Scholar] [CrossRef]
- Damuth, J.D. Of size and abundance. Nature 1991, 351, 268–269. [Google Scholar] [CrossRef]
- Damuth, J.D. Population ecology: Common rules for animals and plants. Nature 1998, 395, 115–116. [Google Scholar] [CrossRef]
- Nee, S.; Read, A.F.; Greenwood, J.J.; Harvey, P.H. The relationship between abundance and body size in British birds. Nature 1991, 351, 312–313. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- Li, W.K.W. Macroecological patterns of phytoplankton in the northwestern North Atlantic Ocean. Nature 2002, 419, 154–157. [Google Scholar] [CrossRef]
- Long, Z.T.; Morin, P.J. Effects of organism size and community composition on ecosystem functioning. Ecol. Lett. 2005, 8, 1271–1282. [Google Scholar] [CrossRef]
- Gjoni, V.; Cozzoli, F.; Rosati, I.; Basset, A. Size-density relationships: A cross-community approach to benthic macroinvertebrates in Mediterranean and Black Sea lagoons. Estuar. Coast. 2017, 40, 1142–1158. [Google Scholar] [CrossRef]
- Gjoni, V.; Basset, A. A cross-community approach to energy pathways across lagoon macroinvertebrate guilds. Estuar. Coast. 2018, 41, 2433–2446. [Google Scholar] [CrossRef]
- Gjoni, V.; Ghinis, S.; Pinna, M.; Mazzotta, L.; Marini, G.; Ciotti, M.; Rosati, I.; Vignes, F.; Arima, S.; Basset, A. Patterns of functional diversity of macroinvertebrates across three aquatic ecosystem types, NE Mediterranean. Mediterr. Mar. Sci. 2019, 20, 703–717. [Google Scholar] [CrossRef]
- Arim, M.; Berazategui, M.; Barreneche, J.M.; Ziegler, L.; Zarucki, M.; Abades, S.R. Determinants of density–body size scaling within food webs and tools for their detection. Adv. Ecol. Res. 2011, 45, 1–39. [Google Scholar]
- Meehan, T.D.; Jetz, W.; Brown, J.H. Energetic determinants of abundance in winter landbird communities. Ecol. Lett. 2004, 7, 532–537. [Google Scholar] [CrossRef]
- White, E.P.; Ernest, S.M.; Thibault, K.M. Trade-offs in community properties through time in a desert rodent community. Am. Nat. 2004, 164, 670–676. [Google Scholar] [CrossRef]
- Glazier, D.S. Beyond the ‘3/4−power law’: Variation in the intra−and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005, 80, 611–662. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 2010, 85, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Metabolic scaling in complex living systems. Systems 2014, 2, 451–540. [Google Scholar] [CrossRef]
- Glazier, D.S. Rediscovering and reviving old observations and explanations of metabolic scaling in living systems. Systems 2018, 6, 4. [Google Scholar] [CrossRef]
- Griffen, B.D.; Cannizzo, Z.J.; Gül, M.R. Ecological and evolutionary implications of allometric growth in stomach size of brachyuran crabs. PLoS ONE 2018, 13, e0207416. [Google Scholar] [CrossRef]
- Malerba, M.E.; Marshall, D.J. Size-abundance rules? Evolution changes scaling relationships between size, metabolism and demography. Ecol. Lett. 2019, 22, 1407–1416. [Google Scholar] [CrossRef]
- Agusti, S.; Kalff, J. The influence of growth conditions on the size dependence of maximal algal density and biomass. Limnol. Oceanogr. 1989, 34, 1104–1108. [Google Scholar] [CrossRef]
- Rodríguez, J. Some comments on the size-based structural analysis of the pelagic ecosystem. Sci. Mar. 1994, 58, 1–10. [Google Scholar]
- Li, W.K.W.; Glen Harrison, W.; Head, E.J. Coherent assembly of phytoplankton communities in diverse temperate ocean ecosystems. Proc. R. Soc. B−Biol. Sci. 2006, 273, 1953–1960. [Google Scholar] [CrossRef]
- Atkinson, D. Effects of temperature on the size of aquatic ectotherms: Exceptions to the general rule. J. Therm. Biol. 1995, 20, 61–74. [Google Scholar] [CrossRef]
- Atkinson, D.; Ciotti, B.J.; Montagnes, D.J.X. Protists decrease in size linearly with temperature: Ca. 2.5% C− 1. Proc. R. Soc. B−Biol. Sci. 2003, 270, 2605–2611. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; Li, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Kenagy, G.J.; Bartholomew, G.A. Seasonal reproductive patterns in five coexisting California desert rodent species. Ecol. Monogr. 1985, 55, 371–397. [Google Scholar] [CrossRef]
- Zeng, Z.; Brown, J.H. Population ecology of a desert rodent: Dipodomys merriami in the Chihuahuan desert. Ecology 1987, 68, 656–665. [Google Scholar] [CrossRef]
- Waser, P.M.; Jones, W.T. Survival and reproductive effort in banner-tailed kangaroo rats. Ecology 1991, 72, 771–777. [Google Scholar] [CrossRef]
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Odum, E.P. Energy flow in ecosystems: A historical review. Am. Zool. 1968, 8, 11–18. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W. Trophic Relationships of Macroinvertebrates. In Methods in Stream Ecology, 3rd ed.; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: London, UK, 2007; Volume 1, pp. 413–433. [Google Scholar]
- Cummins, K.W.; Merritt, R.W.; Andrade, P.C. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 69–89. [Google Scholar] [CrossRef]
- Carlier, A.; Riera, P.; Amouroux, J.M.; Bodiou, J.Y.; Escoubeyrou, K.; Desmalades, M.; Caparros, J.; Grémare, A. A seasonal survey of the food web in the Lapalme lagoon (northwestern Mediterranean) assessed by carbon and nitrogen stable isotope analysis. Estuar. Coast. Mar. Sci. 2007, 73, 299–315. [Google Scholar] [CrossRef]
- Cohen, J.E.; Jonsson, T.; Carpenter, S.R. Ecological community description using the food web, species abundance, and body size. Proc. Natl. Acad. Sci. USA 2003, 100, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Virnstein, R.W. The importance of predation by crabs and fishes on benthic infauna in Chesapeake Bay. Ecology 1977, 58, 1199–1217. [Google Scholar] [CrossRef]
- Peterson, C.H. Predation, Competitive Exclusion, and Diversity in the Soft Sediment Communities of Estuaries and Lagoon. In Ecological Processes in Coastal and Marine Systems; Livingston, R.J., Ed.; Plenum Publishing Co.: New York, NY, USA, 1979; pp. 233–264. [Google Scholar]
- Holland, A.F.; Mountford, N.K.; Hiegel, M.H.; Kaumeyer, K.R.; Mihursky, K.A. Influence of predation on infaunal abundance in upper Chesapeake Bay, USA. Mar. Biol. 1980, 57, 221–235. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Lipcius, R.N.; Hines, A.H. Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Mar. Ecol. Prog. Ser. 1992, 85, 55–68. [Google Scholar] [CrossRef]
- Glazier, D.S. Scaling of metabolic scaling within physical limits. Systems 2014, 2, 425–450. [Google Scholar] [CrossRef]
- Niklas, K.J.; Hammond, S.T. On the interpretation of the normalization constant in the scaling equation. Front. Ecol. Evol. 2018, 6, 212. [Google Scholar] [CrossRef]
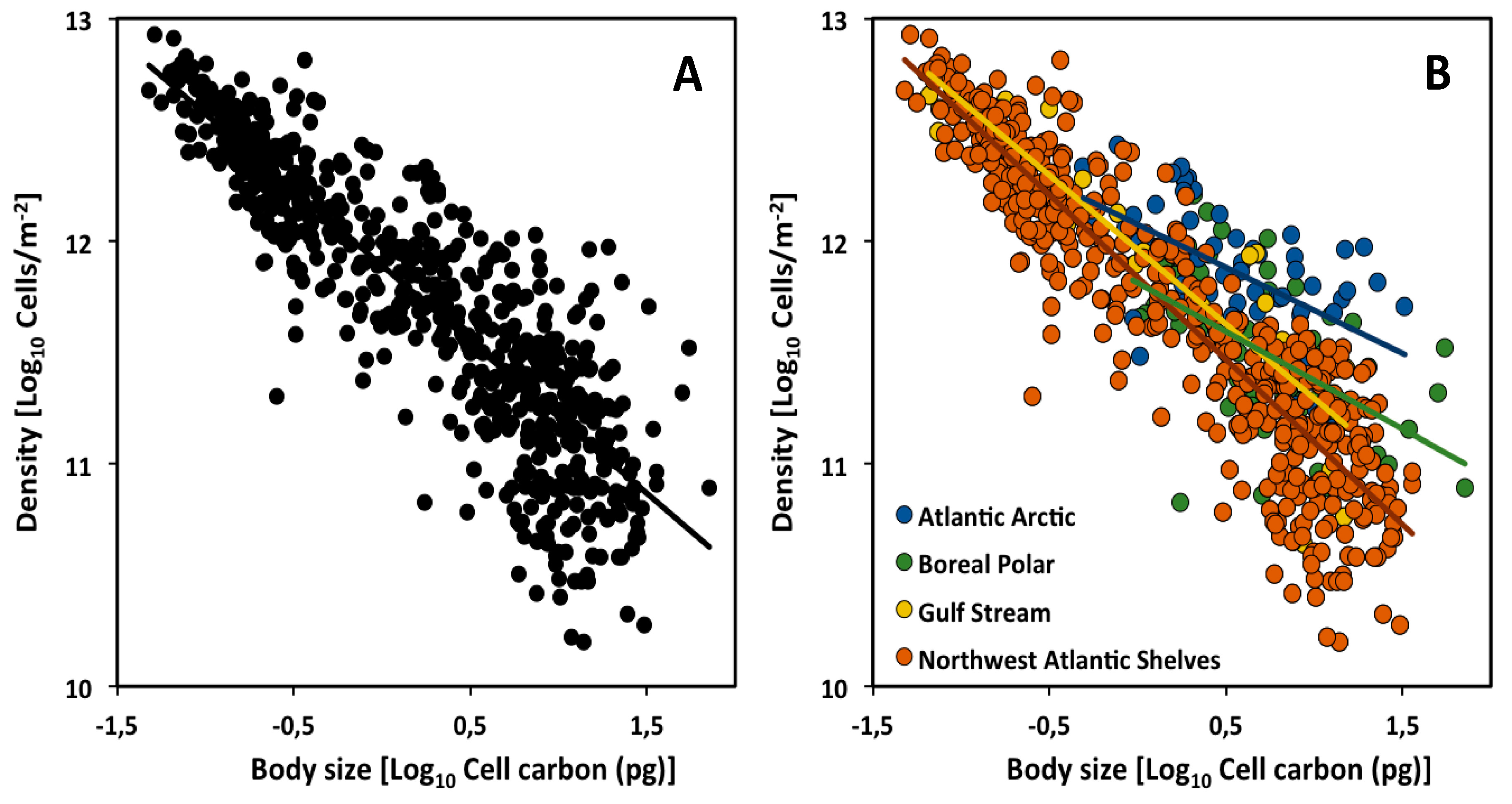
| CCSR | Slope | 95% CI | Intercept | n | r2 | p |
|---|---|---|---|---|---|---|
| All assemblages | −0.68 | −0.71 to −0.65 | 11.89 | 635 | 0.77 | *** |
| Atlantic Arctic | −0.39 | −0.53 to −0.24 | 12.07 | 59 | 0.33 | *** |
| Boreal Polar | −0.44 | −0.60 to −0.27 | 11.81 | 124 | 0.31 | *** |
| Gulf Stream | −0.67 | −0.77 to −0.71 | 11.96 | 31 | 0.77 | *** |
| NW Atlantic Shelves | −0.74 | −0.78 to −0.56 | 11.84 | 479 | 0.85 | *** |
| Climate Zone | p Value for Slope a | p Value for Intercept b | ||||||
|---|---|---|---|---|---|---|---|---|
| AA | BP | GS | NWAS | AA | BP | GS | NWAS | |
| Atlantic Arctic (AA) | - | ns | *** | *** | - | *** | - | - |
| Boreal Polar (BP) | ns | - | *** | *** | *** | - | - | - |
| Gulf Stream (GS) | *** | *** | - | ns | - | - | - | ** |
| NW Atlantic Shelves (NWAS) | *** | *** | ns | - | - | - | ** | - |
| CCSR | Slope | 95% CI | Intercept | n | r2 | p |
|---|---|---|---|---|---|---|
| All assemblages | −0.55 | −1.06 to −0.03 | 2.96 | 41 | 0.11 | * |
| Autumn | −0.77 | −1.24 to −0.31 | 3.32 | 38 | 0.25 | ** |
| Spring | −0.61 | −1.16 to −0.07 | 3.14 | 38 | 0.13 | * |
| Summer | −0.94 | −1.28 to −0.22 | 3.62 | 38 | 0.42 | *** |
| Winter | −0.79 | −1.47 to −0.25 | 3.30 | 38 | 0.12 | * |
| Seasons | p Value for Slope a | p Value for Intercept 1 b | ||||||
|---|---|---|---|---|---|---|---|---|
| AU | SP | SU | WI | AU | SP | SU | WI | |
| Autumn (AU) | - | ns | ns | ns | - | ns | ns | ns |
| Spring (SP) | ns | - | ns | ns | ns | - | ns | ** |
| Summer (SU) | ns | ns | - | ns | ns | ns | - | ns |
| Winter (WI) | ns | ns | ns | - | ns | ** | ns | - |
| CCSR | Slope | 95% CI | Intercept | n | r2 | p |
|---|---|---|---|---|---|---|
| All assemblages | −0.43 | −0.60 to −0.25 | 3.36 | 85 | 0.22 | *** |
| Prey | −0.39 | −0.53 to −0.24 | 3.40 | 75 | 0.25 | *** |
| Predators | −0.45 | −0.78 to −0.24 | 2.32 | 45 | 0.23 | *** |
| Trophic Level | p Value for Slope a | p Value for Intercept b | ||
|---|---|---|---|---|
| Prey | Predators | Prey | Predators | |
| Prey | - | ns | - | *** |
| Predators | ns | - | *** | - |
| Assemblages | N | Slope | 95% CI | Deviation from −3/4 | Reference |
|---|---|---|---|---|---|
| Phytoplankton | 656 | −0.78 | −0.74 to −0.811 | = | [1] |
| Algae, bacteria & protozoa | 20 | −0.35 | −0.01 to −0.71 1 | > | [27] |
| 20 | 0.36 | 0.00 to −0.72 2 | > | ||
| 20 | −1.15 | −0.96 to −1.34 3 | < | ||
| 20 | −1.34 | −1.01 to−1.67 4 | < | ||
| 20 | −1.05 | −1.21 to −0.89 5 | < | ||
| 20 | −1.02 | −1.16 to −0.88 6 | < | ||
| Macroinvertebrates | 158 | −0.27 | −0.41 to −0.131 | > | [28] |
| Macroinvertebrates | 75 | −0.35 | −0.55 to −0.23 7 | > | [29] |
| 68 | −0.35 | −0.62 to −0.08 8 | > | ||
| 65 | −0.58 | −0.78 to −0.37 9 | > | ||
| 64 | −0.44 | −0.58 to 0.31 10 | > | ||
| 45 | −0.45 | −0.67 to−0.24 11 | > | ||
| Macroinvertebrates | 32 | −0.36 | −0.67 to −0.06 | > | [30] |
| Amphibians, fishes | 18 | −0.64 | −1.00 to −0.28 | = | [31] |
| & macroinvertebrates | |||||
| Winter land birds | 285 | −1.00 | −1.43 to −0.57 | < | [32] |
| Desert rodents | 25 | −0.57 | −0.97 to −0.18 | = | [33] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gjoni, V.; Glazier, D.S. A Perspective on Body Size and Abundance Relationships across Ecological Communities. Biology 2020, 9, 42. https://doi.org/10.3390/biology9030042
Gjoni V, Glazier DS. A Perspective on Body Size and Abundance Relationships across Ecological Communities. Biology. 2020; 9(3):42. https://doi.org/10.3390/biology9030042
Chicago/Turabian StyleGjoni, Vojsava, and Douglas Stewart Glazier. 2020. "A Perspective on Body Size and Abundance Relationships across Ecological Communities" Biology 9, no. 3: 42. https://doi.org/10.3390/biology9030042
APA StyleGjoni, V., & Glazier, D. S. (2020). A Perspective on Body Size and Abundance Relationships across Ecological Communities. Biology, 9(3), 42. https://doi.org/10.3390/biology9030042






