High-Fat Diet Propelled AOM/DSS-Induced Colitis-Associated Colon Cancer Alleviated by Administration of Aster glehni via STAT3 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Animals and Diet
2.3. Preparation and Standardization of the Extract of AG
2.4. AOM/DSS-Induced CAC Model and Treatment
2.5. Histopathological Examination
2.6. Immunohistochemistry (IHC)
2.7. Western Blot Analysis
2.8. Statistical Analyses
3. Results
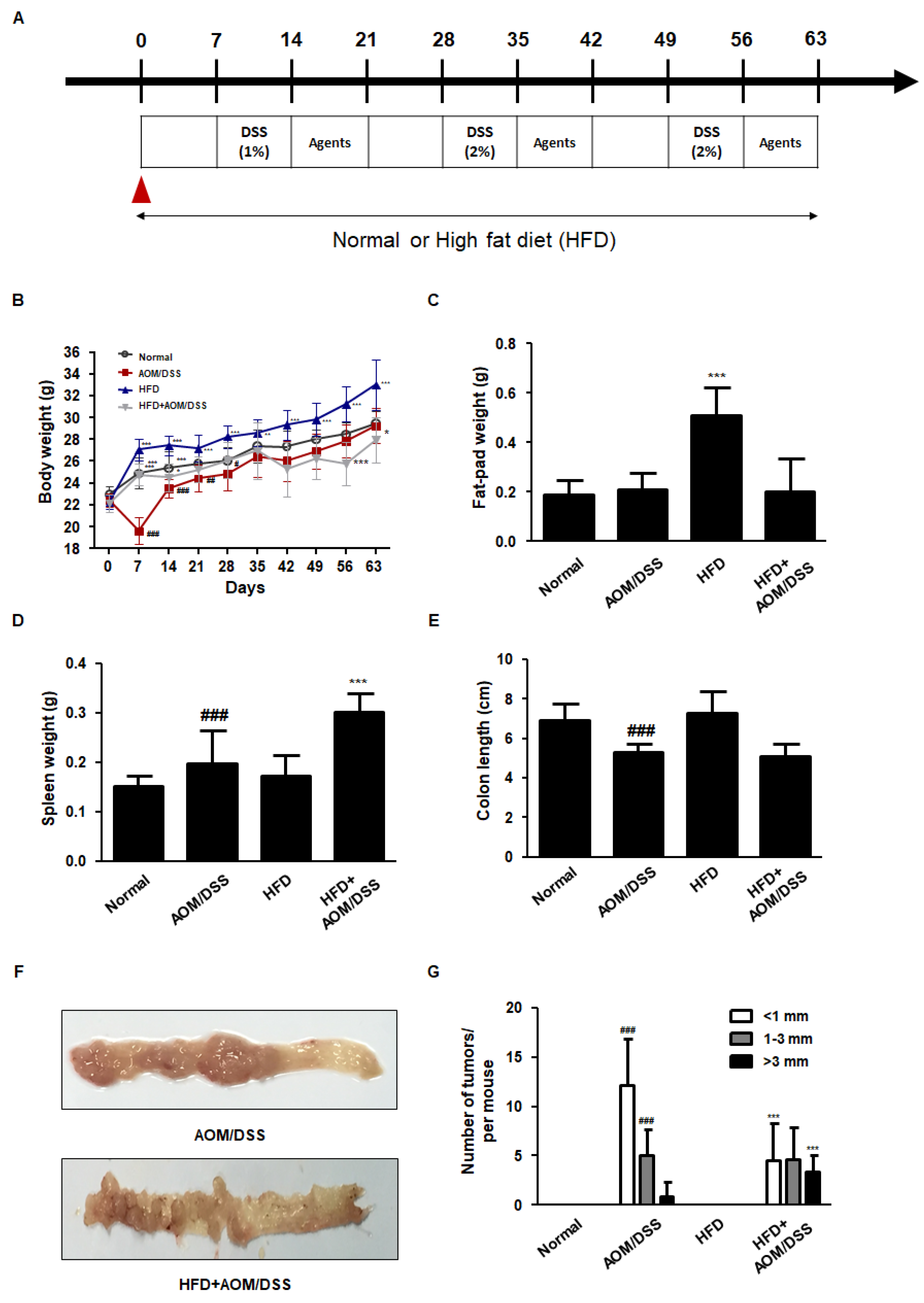
3.1. HFD Accelerated the Development of AOM/DSS-Induced CAC Mice Model
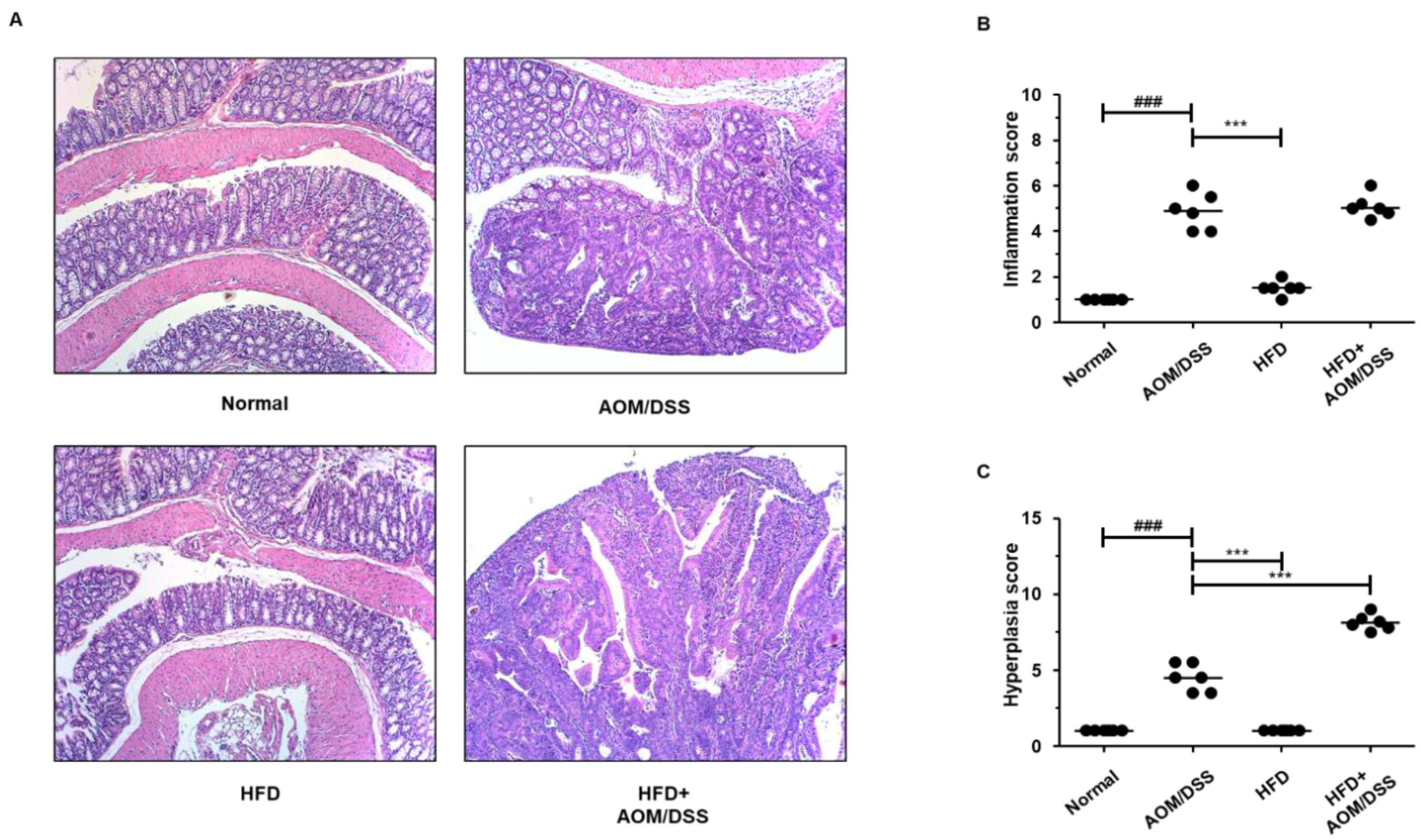
3.2. HFD Propelled Tumorigenesis and Tumor Progression in AOM/DSS-Induced CAC Mice Model
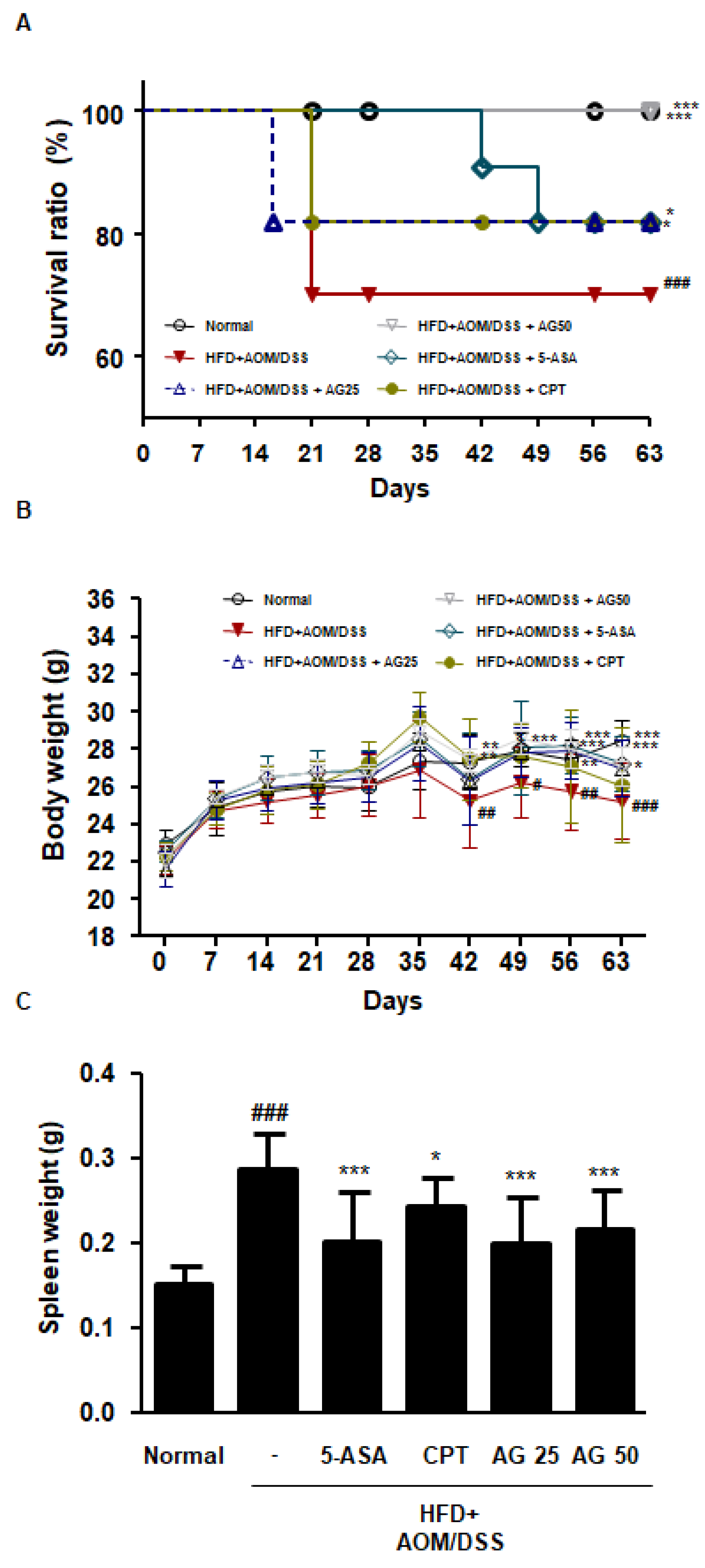
3.3. AG Ameliorated Survival and Pathological Symptoms in HFD-Propelled CAC Mice Model
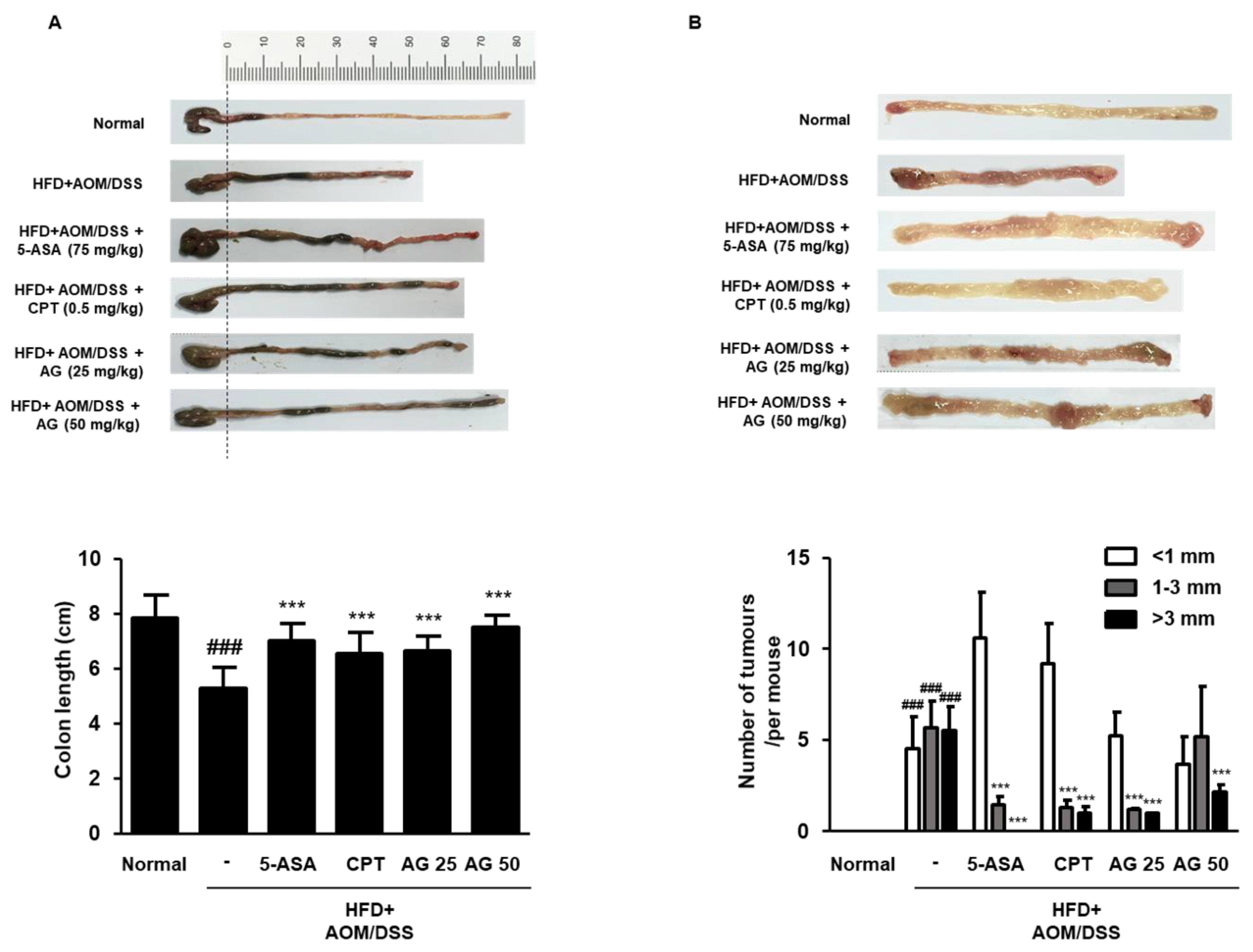
3.4. AG Suppressed the Development of Colonic Neoplasms in HFD-Propelled CAC Mice Model
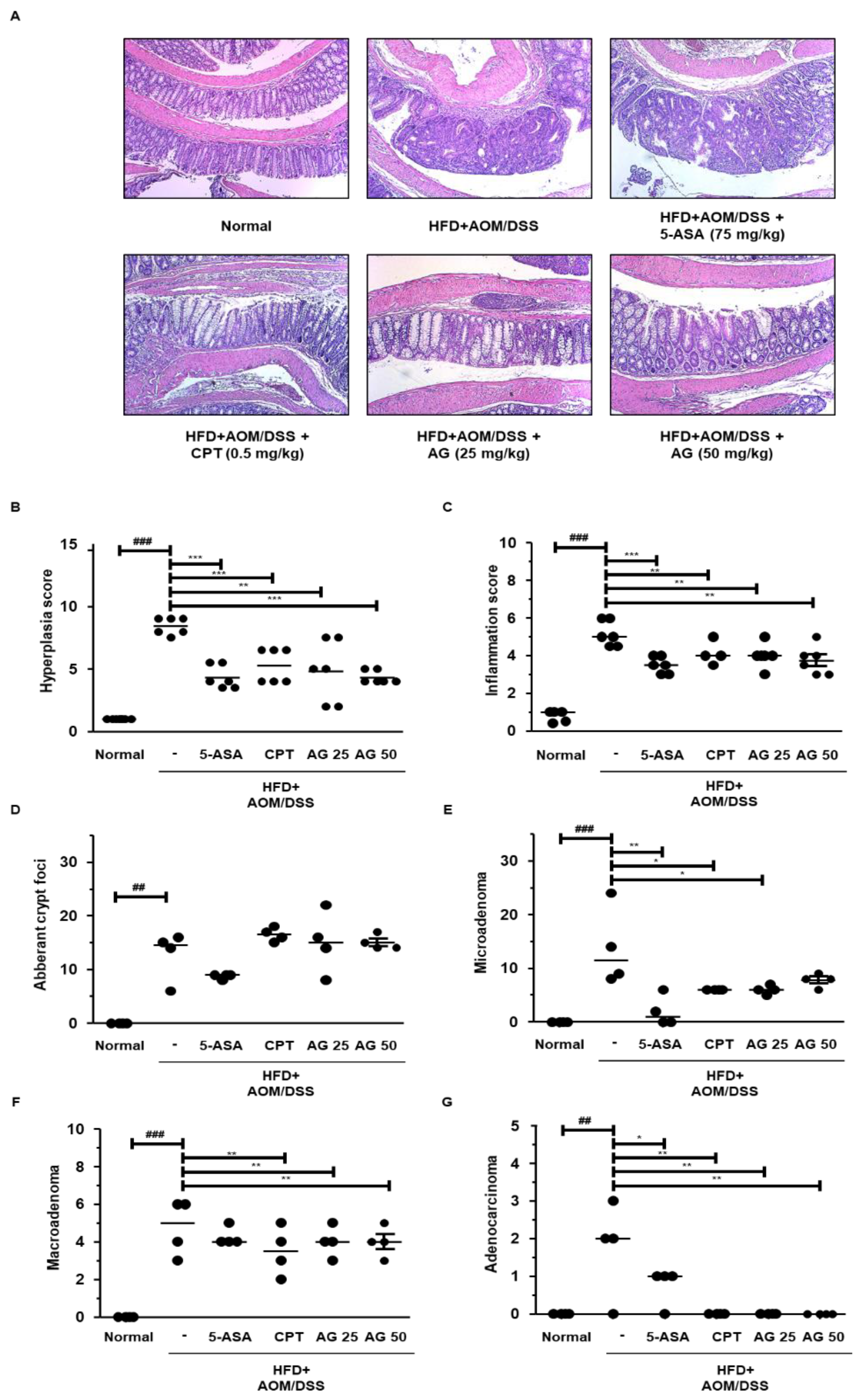
3.5. AG Alleviated Colonic Disease Progression and Tumorigenesis in HFD-Propelled CAC Mice Model
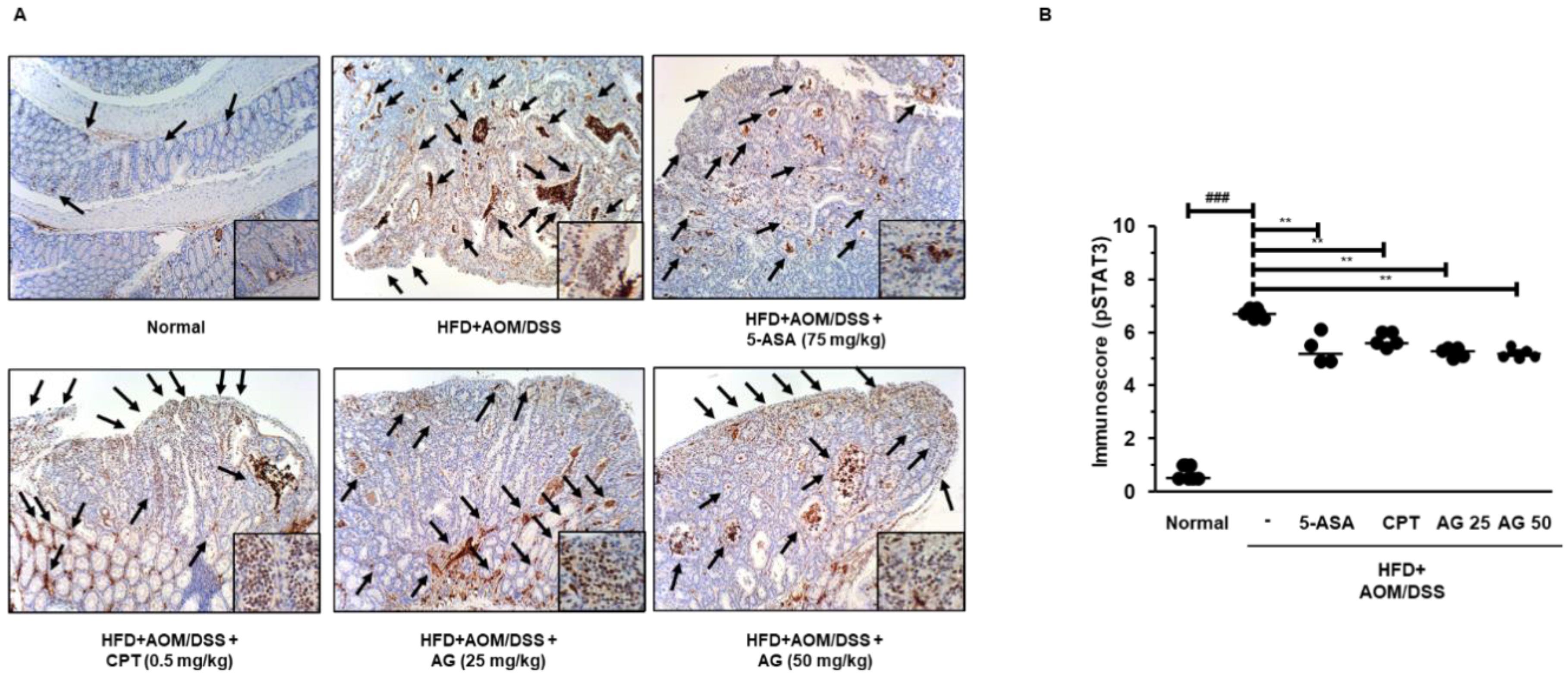
3.6. AG Inhibited STAT3 Activation and Expressions of STAT3-Related Proteins in HFD Propelled CAC Mice Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Sem. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer iv. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Laiyemo, A.O. The risk of colonic adenomas and colonic cancer in obesity. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 655–663. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- van der Logt, E.M.; Blokzijl, T.; van der Meer, R.; Faber, K.N.; Dijkstra, G. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. J. Nutr. Biochem. 2013, 24, 1159–1165. [Google Scholar] [CrossRef]
- Lee, H.; Pal, S.K.; Reckamp, K.; Figlin, R.A.; Yu, H. Stat3: A target to enhance antitumor immune response. Curr. Top. Microbiol. Immunol. 2011, 344, 41–59. [Google Scholar]
- Buettner, R.; Mora, L.B.; Jove, R. Activated stat signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 945–954. [Google Scholar]
- Pandurangan, A.K.; Esa, N.M. Signal transducer and activator of transcription 3—A promising target in colitis-associated cancer. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 551–560. [Google Scholar] [CrossRef]
- Li, Y.; de Haar, C.; Chen, M.; Deuring, J.; Gerrits, M.M.; Smits, R.; Xia, B.; Kuipers, E.J.; van der Woude, C.J. Disease-related expression of the il6/stat3/socs3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 2010, 59, 227–235. [Google Scholar] [CrossRef]
- Wang, S.W.; Sun, Y.M. The il-6/jak/stat3 pathway: Potential therapeutic strategies in treating colorectal cancer (review). Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Pappas, E.G.; Thomas, H.E.; Gough, D.J. The jak/stat pathway in obesity and diabetes. FEBS J. 2016, 283, 3002–3015. [Google Scholar] [CrossRef]
- Park, J.W.; Zhao, L.; Willingham, M.C.; Cheng, S.Y. Inhibition of stat3 signaling blocks obesity-induced mammary hyperplasia in a mouse model. Am. J. Cancer Res. 2017, 7, 727–739. [Google Scholar]
- Kim, T. Korean Plant Resources; Publishing Center of Seoul National University: Seoul, Korea, 1996; p. 270. [Google Scholar]
- Chung, K.S.; Cheon, S.Y.; Roh, S.S.; Lee, M.; An, H.J. Chemopreventive effect of aster glehni on inflammation-induced colorectal carcinogenesis in mice. Nutrients 2018, 10, 202. [Google Scholar] [CrossRef]
- Choi, J.H.; Chung, K.S.; Jin, B.R.; Cheon, S.Y.; Nugroho, A.; Roh, S.S.; An, H.J. Anti-inflammatory effects of an ethanol extract of aster glehni via inhibition of nf-kappab activation in mice with dss-induced colitis. Food Funct. 2017, 8, 2611–2620. [Google Scholar] [CrossRef]
- Lee, H.M.; Yang, G.; Ahn, T.G.; Kim, M.D.; Nugroho, A.; Park, H.J.; Lee, K.T.; Park, W.; An, H.J. Antiadipogenic effects of aster glehni extract: In vivo and in vitro effects. Evid. Based Complement. Altern. Med. eCAM 2013, 2013, 859624. [Google Scholar] [CrossRef]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic bacteroides fragilis, in wild-type c57bl/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, I.; Al-Rabadi, L.; Stavrakis, D.; Karagiannides, I.; Pothoulakis, C.; Bugni, J.M. Diet-induced obesity promotes colon tumor development in azoxymethane-treated mice. PLoS ONE 2013, 8, e60939. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The aom/dss murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [PubMed]
- Alrawi, S.J.; Schiff, M.; Carroll, R.E.; Dayton, M.; Gibbs, J.F.; Kulavlat, M.; Tan, D.; Berman, K.; Stoler, D.L.; Anderson, G.R. Aberrant crypt foci. Anticancer Res. 2006, 26, 107–119. [Google Scholar]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef]
- Corvinus, F.M.; Orth, C.; Moriggl, R.; Tsareva, S.A.; Wagner, S.; Pfitzner, E.B.; Baus, D.; Kaufmann, R.; Huber, L.A.; Zatloukal, K.; et al. Persistent stat3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 2005, 7, 545–555. [Google Scholar] [CrossRef]
- Lin, Q.; Lai, R.; Chirieac, L.R.; Li, C.; Thomazy, V.A.; Grammatikakis, I.; Rassidakis, G.Z.; Zhang, W.; Fujio, Y.; Kunisada, K.; et al. Constitutive activation of jak3/stat3 in colon carcinoma tumors and cell lines: Inhibition of jak3/stat3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am. J. Pathol. 2005, 167, 969–980. [Google Scholar] [CrossRef]

| Histological Parameters | Description | Score | |
|---|---|---|---|
| Mucosa | |||
| Non-dysplastic Epithelium | Mild (less than two-fold) crypt length | 1 | |
| Intense crypt length with hyperchromatic CEC | 2 | ||
| Dysplastic Epithelium | Dysplastic epithelial region (region < 20%) | 1 | |
| Dysplastic epithelial region (20% < region < 50%) | 2 | ||
| Dysplastic epithelial region (50% < region < 90%) | 4 | ||
| Histological Parameters | Description | Score |
|---|---|---|
| Mucosa | ||
| Epithelial cell | Prolonged epithelial cell or crypt | 1 |
| Destruction of barrier | 2 | |
| Ulcer (30% < loss < 60%) | 3 | |
| Ulcer (loss > 60%) | 4 | |
| Immune cell | Infiltration (mild) | 1 |
| Infiltration (moderate) | 2 | |
| Infiltration (severe) | 3 | |
| Submucosa | ||
| Immune cell | Infiltration (mild) | 1 |
| Infiltration (moderate) | 2 | |
| Infiltration (severe) | 3 |
| Group | ACF (%) | Microadenoma (%) | Low-Grade Macroadenoma (%) | High-Grade Macroadenoma (%) | Adenocarcinoma (%) | Neoplasm-Associated Lesions (N) |
|---|---|---|---|---|---|---|
| Normal | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0 |
| AOM/DSS | 33.33% | 26.19% | 19.05% | 7.14% | 2.38% | 21 |
| HFD | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0 |
| HFD+AOM/DSS | 35.00% | 22.50% | 12.50% | 20.00% | 5.00% | 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.-R.; Chung, K.-S.; Lee, M.; An, H.-J. High-Fat Diet Propelled AOM/DSS-Induced Colitis-Associated Colon Cancer Alleviated by Administration of Aster glehni via STAT3 Signaling Pathway. Biology 2020, 9, 24. https://doi.org/10.3390/biology9020024
Jin B-R, Chung K-S, Lee M, An H-J. High-Fat Diet Propelled AOM/DSS-Induced Colitis-Associated Colon Cancer Alleviated by Administration of Aster glehni via STAT3 Signaling Pathway. Biology. 2020; 9(2):24. https://doi.org/10.3390/biology9020024
Chicago/Turabian StyleJin, Bo-Ram, Kyung-Sook Chung, Minho Lee, and Hyo-Jin An. 2020. "High-Fat Diet Propelled AOM/DSS-Induced Colitis-Associated Colon Cancer Alleviated by Administration of Aster glehni via STAT3 Signaling Pathway" Biology 9, no. 2: 24. https://doi.org/10.3390/biology9020024
APA StyleJin, B.-R., Chung, K.-S., Lee, M., & An, H.-J. (2020). High-Fat Diet Propelled AOM/DSS-Induced Colitis-Associated Colon Cancer Alleviated by Administration of Aster glehni via STAT3 Signaling Pathway. Biology, 9(2), 24. https://doi.org/10.3390/biology9020024





