Phytoestrogens for Cancer Prevention and Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
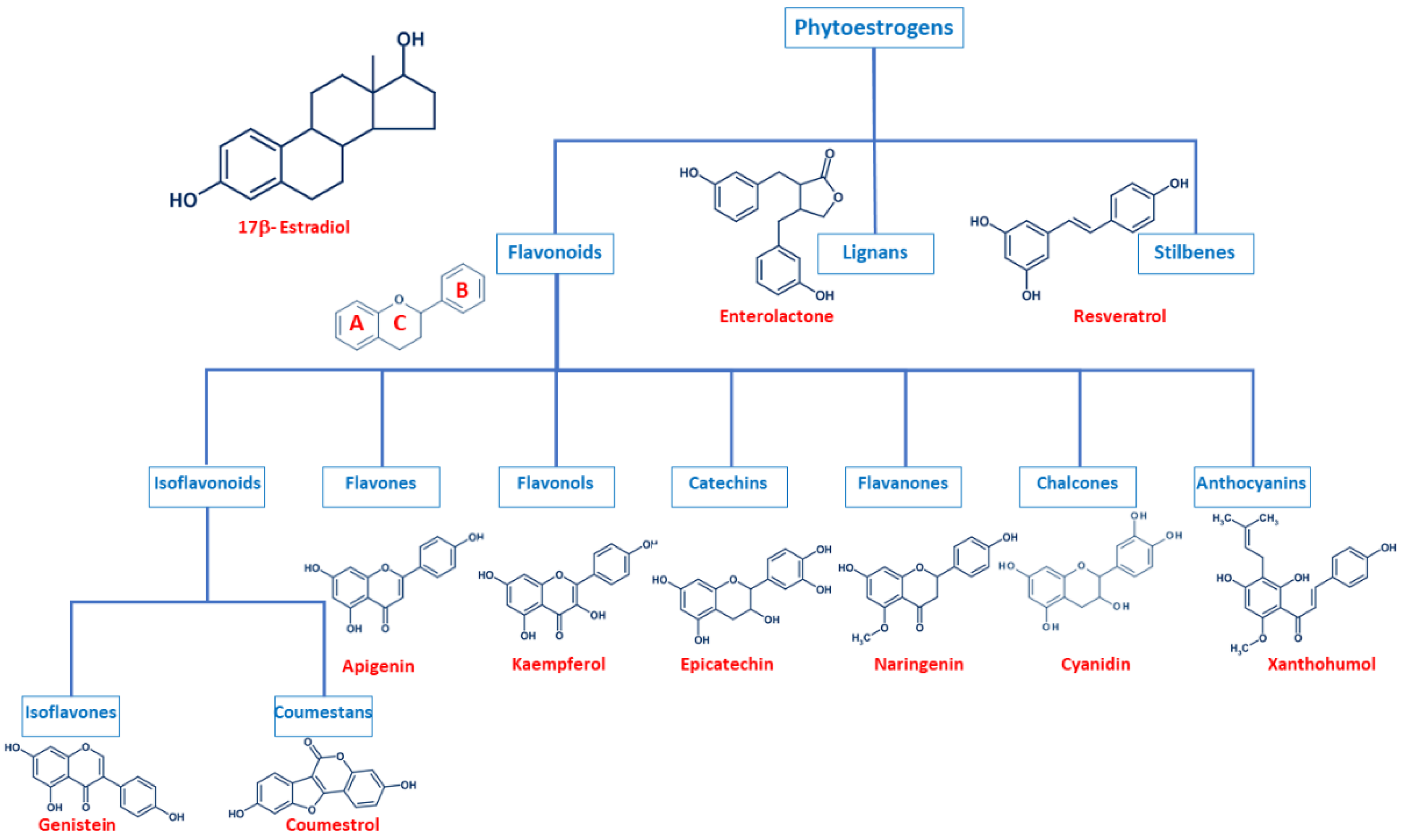
2. Phytoestrogen Structure and Classification
2.1. Flavonoids
2.2. Lignans
2.3. Stilbenes
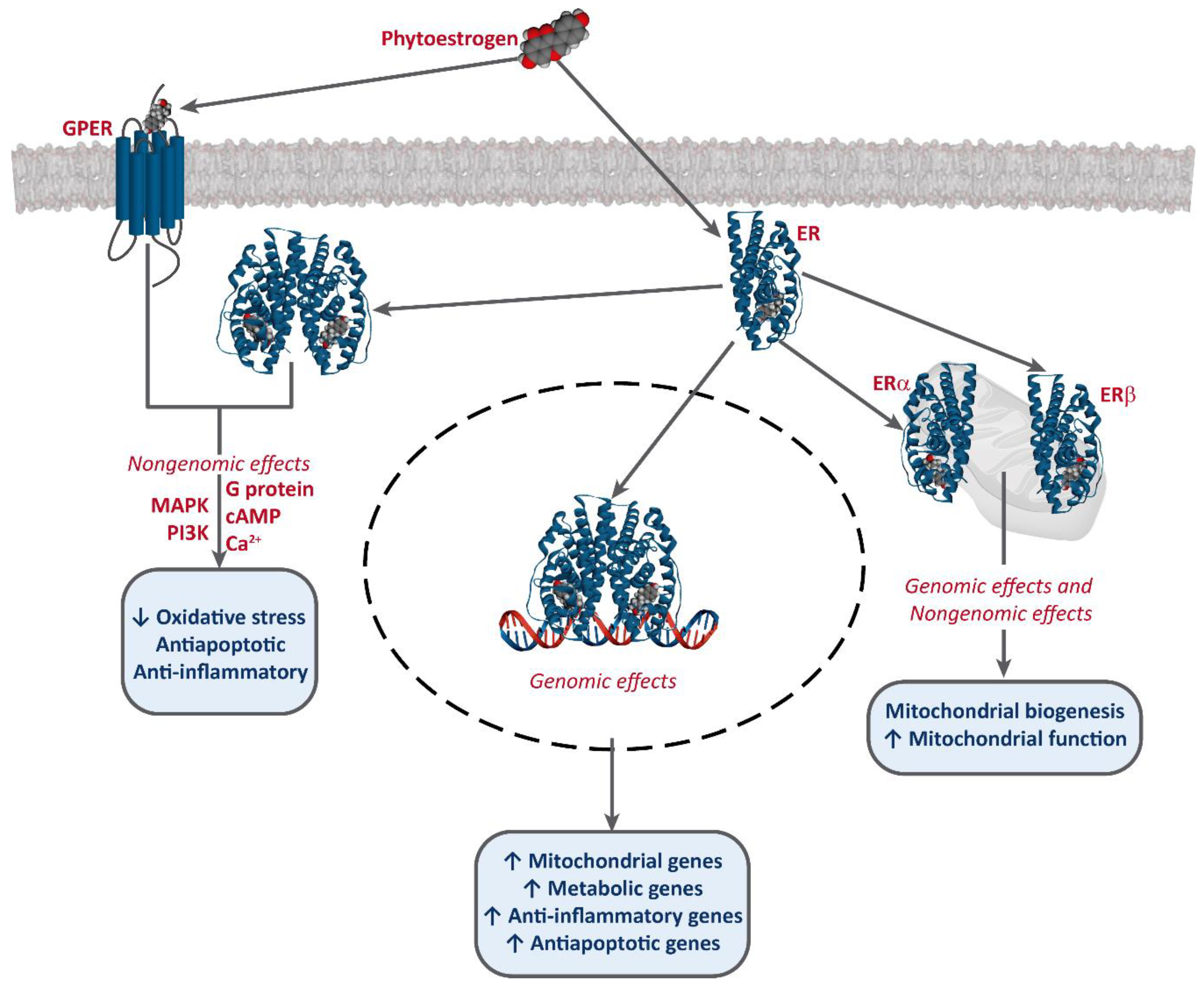
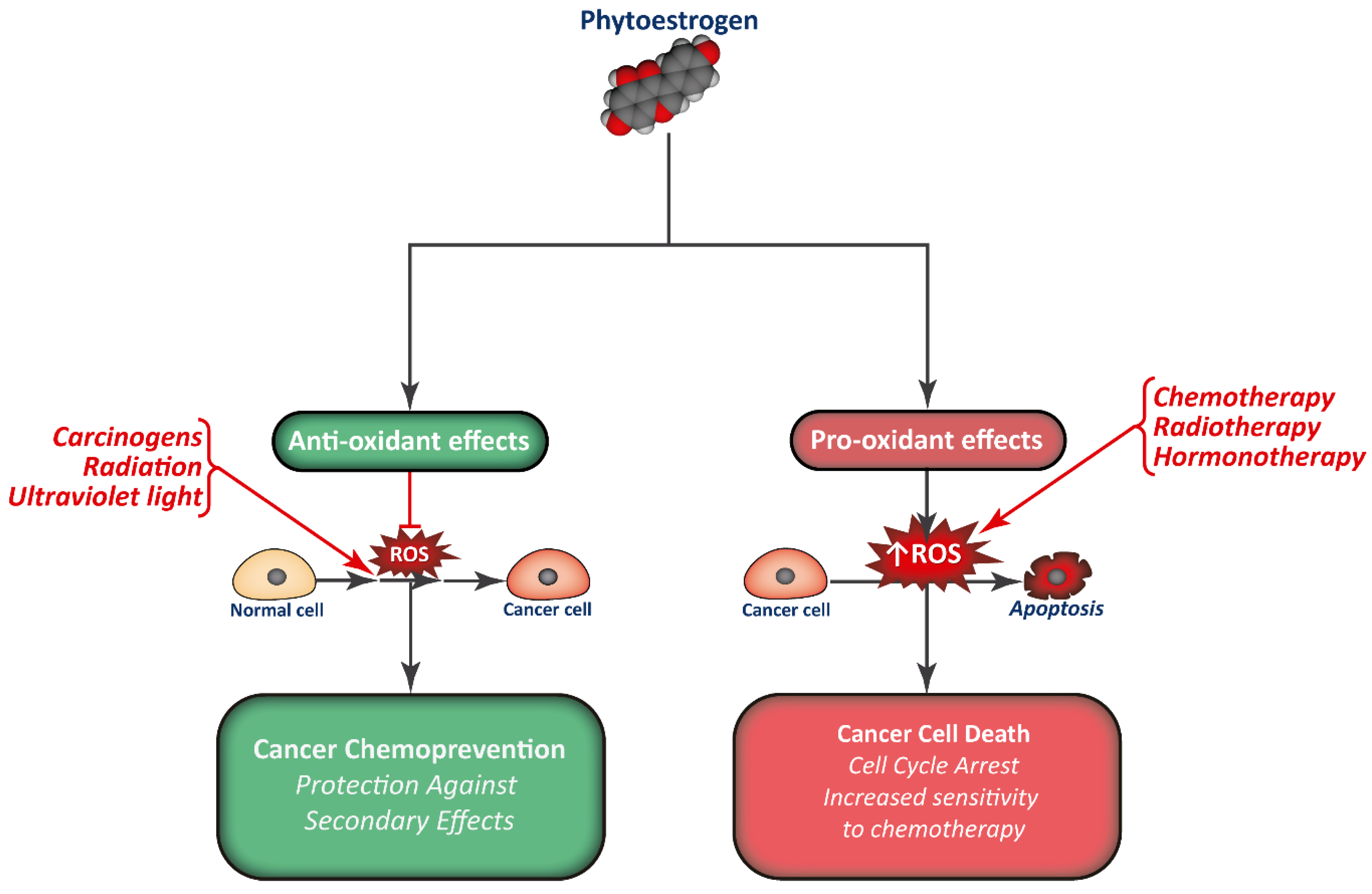
3. Mechanism of Action of Phytoestrogens and Cancer Prevention
4. Phytoestrogens as Cancer Treatment
4.1. Phytoestrogens and Hormonal Therapy
4.2. Phytoestrogens and Chemotherapy
4.3. Phytoestrogens and Radiotherapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roca, P.; Sastre-Serra, J.; Nadal-Serrano, M.; Pons, D.G.; Blanquer-Rossello, M.D.; Oliver, J. Phytoestrogens and Mitochondrial Biogenesis in Breast Cancer. Influence of Estrogen Receptors Ratio. Curr. Pharm. Des. 2014, 20, 5594–5618. [Google Scholar] [CrossRef]
- Senthilkumar, H.A.; Fata, J.E.; Kennelly, E. Phytoestrogens: The current state of research emphasizing breast pathophysiology. Phytotherapy Res. 2018, 32, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Fang, Y.; Zhang, M.; Zhang, Y. Phytoestrogen Intake and Risk of Ovarian Cancer: A Meta-Analysis of 10 Observational Studies. Asian Pac. J. Cancer Prev. 2014, 15, 9085–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonn, G.A.; Aronson, W.; Litwin, M.S. Impact of diet on prostate cancer: A review. Prostate Cancer Prostatic Dis. 2005, 8, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimazu, T.; Inoue, M.; Sasazuki, S.; Iwasaki, M.; Sawada, N.; Yamaji, T.; Tsugane, S. Plasma isoflavones and the risk of lung cancer in women: A nested case-control study in Japan. Cancer Epidemiol. Biomark. Prev. 2011, 20, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, L.; Na, R.; Xu, J.; Xiong, Z.; Zhang, N.; Dai, W.; Jiang, H.-W.; Ding, Q. Plasma genistein and risk of prostate cancer in Chinese population. Int. Urol. Nephrol. 2015, 47, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Ko, K.-P.; Yeo, Y.; Yoon, J.-H.; Kim, C.-S.; Tokudome, S.; Ngoan, L.T.; Koriyama, C.; Lim, Y.-K.; Chang, S.-H.; Shin, H.-R.; et al. Plasma phytoestrogens concentration and risk of colorectal cancer in two different Asian populations. Clin. Nutr. 2018, 37, 1675–1682. [Google Scholar] [CrossRef]
- Iwasaki, M.; Tsugane, S. Risk factors for breast cancer: Epidemiological evidence from Japanese studies. Cancer Sci. 2011, 102, 1607–1614. [Google Scholar] [CrossRef]
- Nikolic, I.; Savić-Gajić, I.; Tačić, A.; Savić, I. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent Developments. Planta Med. 2003, 69, 589–599. [Google Scholar]
- Wang, T.-Y.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnsworth, N.R.; Bingel, A.S.; Cordell, G.A.; Crane, F.A.; Fong, H.H.S. Potential Value of Plants as Sources of New Antifertility Agents II. J. Pharm. Sci. 1975, 64, 717–754. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, Flavones, Flavanones, and Human Health: Epidemiological Evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.M.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Konar, N. Non-isoflavone phytoestrogenic compound contents of various legumes. Eur. Food Res. Technol. 2013, 236, 523–530. [Google Scholar] [CrossRef]
- Blomquist, C.H.; Lima, P.H.; Hotchkiss, J.R. Inhibition of 3α-hydroxysteroid dehydrogenase (3α-HSD) activity of human lung microsomes by genistein, daidzein, coumestrol and C18-, C19- and C21-hydroxysteroids and ketosteroids. Steroids 2005, 70, 507–514. [Google Scholar] [CrossRef]
- Amin, A.; Buratovich, M. The Anti-Cancer Charm of Flavonoids: A Cup-of-Tea Will Do You Good! Front. Anti-Cancer Drug Discov. 2007, 2, 109–117. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; A Ralston, R.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.A.J.; Berghe, D.V. Structure–Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Onzalez-Mejia, M.E.; Voss, O.H.; Murnan, E.J.; Doseff, A.I. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein–27. Cell Death Dis. 2010, 1, e64. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules 2018, 23, 660. [Google Scholar] [CrossRef] [Green Version]
- Milligan, S.; Kalita, J.; Pocock, V.; Heyerick, A.; De Cooman, L.; Rong, H.; De Keukeleire, D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction 2002, 123, 235–242. [Google Scholar] [CrossRef]
- Monteiro, R.; Becker, H.; Azevedo, I.; Calhau, C. Effect of Hop (Humulus lupulus L.) Flavonoids on Aromatase (Estrogen Synthase) Activity. J. Agric. Food Chem. 2006, 54, 2938–2943. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.; Navas, M.J.; Jiménez-Moreno, A.M.; Asuero, A.G. Anthocyanin Pigments: Importance, Sample Preparation and Extraction. In Phenolic Compounds—Natural Sources, Importance and Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satake, H.; Koyama, T.; Bahabadi, S.E.; Matsumoto, E.; Ono, E.; Murata, J. Essences in Metabolic Engineering of Lignan Biosynthesis. Metabolites 2015, 5, 270–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, L.A.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Cotterchio, M.; Boucher, B.; Kreiger, N.; Mills, C.A.; Thompson, L.U. Dietary phytoestrogen intake—Lignans and isoflavones—And breast cancer risk (Canada). Cancer Causes Control 2007, 19, 259–272. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry, and Applications; CRC, Taylor & Francis: Abingdon, UK, 2006. [Google Scholar]
- Peirotén, Á.; Bravo, D.; Landete, J.M. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit. Rev. Food Sci. Nutr. 2019, 60, 1922–1937. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Med. Cell. Longev. 2015, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; De Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Robb, E.L.; Stuart, J.A. Resveratrol interacts with estrogen receptor-β to inhibit cell replicative growth and enhance stress resistance by upregulating mitochondrial superoxide dismutase. Free Radic. Biol. Med. 2011, 50, 821–831. [Google Scholar] [CrossRef]
- Kampkötter, A.; Chovolou, Y.; Kulawik, A.; Röhrdanz, E.; Weber, N.; Proksch, P.; Wätjen, W. Isoflavone daidzein possesses no antioxidant activities in cell-free assays but induces the antioxidant enzyme catalase. Nutr. Res. 2008, 28, 620–628. [Google Scholar] [CrossRef]
- Surico, D.; Ercoli, A.; Farruggio, S.; Raina, G.; Filippini, D.; Mary, D.; Minisini, R.; Surico, N.; Pirisi, M.; Grossini, E. Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro. Cell. Physiol. Biochem. 2017, 42, 1051–1062. [Google Scholar] [CrossRef]
- Molina, L.; Bustamante, F.A.; Bhoola, K.D.; Figueroa, C.D.; Ehrenfeld, P. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin. Sci. 2018, 132, 2583–2598. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, V.; Miodini, P.; Di Fronzo, G.; Daidone, M.G. Modulation of estrogen receptor-β isoforms by phytoestrogens in breast cancer cells. Int. J. Oncol. 2006, 28, 1185–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, K.P.; Rajavel, T.; Daglia, M.; Nabavi, S.M.; Bishayee, A. Targeting miRNAs by polyphenols: Novel therapeutic strategy for cancer. Semin. Cancer Biol. 2017, 46, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bilal, I. Phytoestrogens and prevention of breast cancer: The contentious debate. World J. Clin. Oncol. 2014, 5, 705–712. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Hsu, Y.-L.; Huang, Y.-F.; Tsai, E.-M. Molecular Mechanisms of Anticancer Effects of Phytoestrogens in Breast Cancer. Curr. Protein Pept. Sci. 2018, 19, 323–332. [Google Scholar] [CrossRef]
- LeComte, S.; DeMay, F.; Ferrière, F.; Pakdel, F. Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef] [Green Version]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef]
- Susanikova, I.; Puchl’ová, M.; Lachová, V.; Švajdlenka, E.; Muèaji, P.; Smetana, K.; Gál, P. Genistein and selected phytoestrogen-containing extracts differently modulate antioxidant properties and cell differentiation: An in vitro study in NIH-3T3, HaCaT and MCF-7 cells. Folia Biol. 2019, 65, 24–35. [Google Scholar]
- Park, C.; Cha, H.-J.; Lee, H.; Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.-W.; Han, M.H.; Choi, Y.H.; et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.-C.; Peng, S.-F.; Lai, K.; Liao, C.-L.; Huang, Y.-P.; Lin, C.-C.; Lin, M.-L.; Liu, K.-C.; Tsai, C.-C.; Ma, Y.-S.; et al. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol. 2019, 34, 443–456. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F.; Atashpour, S.; Haghighat, S. Effects of Genistein and Synergistic Action in Combination with Tamoxifen on the HepG2 Human Hepatocellular Carcinoma Cell Line. Asian Pac. J. Cancer Prev. 2017, 18, 2381–2385. [Google Scholar]
- Blanquer-Rosselló, M.D.M.; Hernández-López, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 431–440. [Google Scholar]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Blanquer-Rosselló, M.M.; Oliver, J.; Valle, A.; Roca, P. Effect of xanthohumol and 8-prenylnaringenin on MCF-7 breast cancer cells oxidative stress and mitochondrial complexes expression. J. Cell. Biochem. 2013, 114, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Serra, J.; Ahmiane, Y.; Roca, P.; Oliver, J.; Pons, D.G. Xanthohumol, a hop-derived prenylflavonoid present in beer, impairs mitochondrial functionality of SW620 colon cancer cells. Int. J. Food Sci. Nutr. 2018, 70, 396–404. [Google Scholar] [CrossRef]
- Zhang, B.; Chu, W.; Wei, P.; Liu, Y.; Wei, T. Xanthohumol induces generation of reactive oxygen species and triggers apoptosis through inhibition of mitochondrial electron transfer chain complex I. Free Radic. Biol. Med. 2015, 89, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Asslaber, D.; Grössinger, E.M.; Girbl, T.; Hofbauer, S.W.; Egle, A.; Weiss, L.; Greil, R.; Hartmann, T.N. Mimicking the microenvironment in chronic lymphocytic leukaemia—Where does the journey go? Br. J. Haematol. 2013, 160, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Yerer-Aycan, M.B. Natural Products for Cancer Prevention and Therapy; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Oliver, J.; Roca, P. The Phytoestrogen Genistein Affects Breast Cancer Cells Treatment Depending on the ERα/ERβ Ratio. J. Cell. Biochem. 2015, 117, 218–229. [Google Scholar] [CrossRef]
- Sotoca, A.; Ratman, D.; Van Der Saag, P.; Ström, A.; Gustafsson, J.; Vervoort, J.; Rietjens, I.M.; Murk, A. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERα/ERβ ratio. J. Steroid Biochem. Mol. Biol. 2008, 112, 171–178. [Google Scholar] [CrossRef]
- Pons, D.G.; Vilanova-Llompart, J.; Gaya-Bover, A.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. The phytoestrogen genistein affects inflammatory-related genes expression depending on the ERα/ERβ ratio in breast cancer cells. Int. J. Food Sci. Nutr. 2019, 70, 941–949. [Google Scholar] [CrossRef]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of Flavonoids and Lung Cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Closas, R.; Agudo, A.; González, C.A.; Riboli, E. Intake of specific carotenoids and flavonoids and the risk of lung cancer in women in Barcelona, Spain. Nutr. Cancer 1998, 32, 154–158. [Google Scholar] [CrossRef]
- Garcia-Closas, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control 1999, 10, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Zhang, Z.-F.; Yu, G.-P.; Lu, Q.-Y.; Li, Y.-L.; Lu, M.-L.; Wang, M.-R.; Guo, C.H.; Yu, S.-Z.; Kurtz, R.C.; et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. Int. J. Cancer 2001, 92, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Rau, K.-M.; Kang, H.-Y.; Cha, T.-L.; A Miller, S.; Hung, M.-C. The mechanisms and managements of hormone-therapy resistance in breast and prostate cancers. Endocr. Relat. Cancer 2005, 12, 511–532. [Google Scholar] [CrossRef] [Green Version]
- Van Duursen, M.B.M.; Nijmeijer, S.; De Morree, E.; De Jong, P.C.; Berg, M.V.D. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology 2011, 289, 67–73. [Google Scholar] [CrossRef]
- Constantinou, A.I.; White, B.E.; Tonetti, D.; Yang, Y.; Liang, W.; Li, W.; Van Breemen, R.B. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur. J. Cancer 2005, 41, 647–654. [Google Scholar] [CrossRef]
- Tonetti, D.A.; Zhang, Y.; Zhao, H.; Lim, S.-B.; Constantinou, A.I. The Effect of the Phytoestrogens Genistein, Daidzein, and Equol on the Growth of Tamoxifen-Resistant T47D/PKCα. Nutr. Cancer 2007, 58, 222–229. [Google Scholar] [CrossRef]
- Charalambous, C.; Constantinou, A.I. Abstract 577: Equol enhances tamoxifen’s antitumor effect by induction of caspase-mediated apoptosis in MCF-7 breast cancer cells. Prev. Res. 2012, 72, 577. [Google Scholar]
- Suh, Y.-A.; Jo, S.-Y.; Lee, H.-Y.; Lee, C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int. J. Oncol. 2014, 46, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cook, K.L.; Warri, A.; Cruz, I.M.; Rosim, M.; Helferich, W.; Doerge, D.; Clarke, R.; Hilakivi-clarke, L. Lifetime genistein intake increases the response of mammary tumors to tamoxifen in rats. Clin. Cancer Res. 2017, 23, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Caley, A.; Jones, R. The principles of cancer treatment by chemotherapy. Surgery 2012, 30, 186–190. [Google Scholar] [CrossRef]
- Huang, W.; Wan, C.; Luo, Q.-C.; Huang, Z.; Luo, Q. Genistein-Inhibited Cancer Stem Cell-Like Properties and Reduced Chemoresistance of Gastric Cancer. Int. J. Mol. Sci. 2014, 15, 3432–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ellis, K.-L.; Ali, S.; El-Rayes, B.F.; Nedeljkovic-Kurepa, A.; Kucuk, O.; Philip, P.A.; Sarkar, F.H. Apoptosis-Inducing Effect of Chemotherapeutic Agents Is Potentiated by Soy Isoflavone Genistein, a Natural Inhibitor of NF-kB in BxPC-3 Pancreatic Cancer Cell Line. Pancreas 2004, 28, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Belcher, S.M.; Burton, C.C.; Cookman, C.J.; Kirby, M.; Miranda, G.L.; Saeed, F.O.; Wray, K.E. Estrogen and soy isoflavonoids decrease sensitivity of medulloblastoma and central nervous system primitive neuroectodermal tumor cells to chemotherapeutic cytotoxicity. BMC Pharmacol. Toxicol. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-J.; Jiang, L.; Kluxen, F.M.; Diel, P. Genistein modulates the anti-tumor activity of cisplatin in MCF-7 breast and HT-29 colon cancer cells. Arch. Toxicol. 2014, 88, 625–635. [Google Scholar] [CrossRef]
- Aziz, N.; Froemming, G.; Kadir, S.; Ibrahim, M. Apigenin increases cisplatin inhibitory effects on the telomerase activity of triple negative breast cancer cells. J. Teknol. 2018, 80, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.; Natali, P.; Brunetti, M.; Aiello, F.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol Potentiates the Cytotoxic Oxidative Stress Induced by Chemotherapy in Human Colon Cancer Cells. Cell. Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- Kuhar, M.; Sen, S.; Singh, N. Role of mitochondria in quercetin-enhanced chemotherapeutic response in human non-small cell lung carcinoma H-520 cells. Anticancer Res. 2006, 26, 1297–1303. [Google Scholar] [PubMed]
- Sharma, H.; Sen, S.; Singh, N. Molecular pathways in the chemosensitization of cisplatin by quercetin in human head and neck cancer. Cancer Biol. Ther. 2005, 4, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Daddysman, M.K.; Rankin, G.O.; Jiang, B.-H.; Chen, Y.C. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell Int. 2010, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Thompson, E.W.; Quinn, M.A. Epithelial–Mesenchymal Interconversions in Normal Ovarian Surface Epithelium and Ovarian Carcinomas: An Exception to the Norm. J. Cell Physiol. 2007, 207, 581–588. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Kang, K.S.; Song, J.H.; Choi, Y.-K. Inhibition of Intracellular ROS Accumulation by Formononetin Attenuates Cisplatin-Mediated Apoptosis in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 813. [Google Scholar] [CrossRef]
- Suliman, F.A.; Khodeer, D.M.; Ibrahiem, A.; Mehanna, E.T.; El-Kherbetawy, M.K.; Mohammad, H.M.; Zaitone, S.A.; Moustafa, Y.M. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: Effect on inflammatory burden and p53 apoptosis. Int. Immunopharmacol. 2018, 61, 8–19. [Google Scholar] [CrossRef]
- Jazirehi, A.R.; Bonavida, B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol. Cancer Ther. 2004, 3, 71–84. [Google Scholar]
- Öztürk, Y.; Günaydın, C.; Yalçın, F.; Nazıroğlu, M.; Braidy, N. Resveratrol Enhances Apoptotic and Oxidant Effects of Paclitaxel through TRPM2 Channel Activation in DBTRG Glioblastoma Cells. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Stearns, M.E.; Wang, M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (-)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef] [Green Version]
- Krajnović, T.T.; Kaluđerović, G.N.; Wessjohann, L.; Mijatovic, S.A.; Maksimovic-Ivanic, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Sun, Z.-J.; Yu, L.; Meng, K.-W.; Qin, X.-L.; Pan, C.-E. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J. Gastroenterol. 2004, 10, 3048–3052. [Google Scholar] [CrossRef]
- Lee, S.H.; Koo, B.S.; Park, S.Y.; Kim, Y.M. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol. Med. Rep. 2015, 12, 2777–2783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabinein vitroand in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, G.; Lazcano, E.; Li, H.; Mohamad, A.; DeMorrow, S. Resveratrol enhances the sensitivity of cholangiocarcinoma to chemotherapeutic agents. Lab. Investig. 2010, 90, 1325–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Shi, Z.-L.; Liu, B.; Yan, X.-B.; Feng, J.; Tao, H.-M. Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: The role of Akt and nuclear factor-κB. Anti-Cancer Drugs 2010, 21, 288–296. [Google Scholar] [CrossRef]
- Solomon, L.; Ali, S.; Banerjee, S.; Munkarah, A.; Morris, R.T.; Sarkar, F.H. Sensitization of ovarian cancer cells to cisplatin by genistein: The role of NF-kappaB. J. Ovarian Res. 2008, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Zhang, Y.; Ali, S.; Bhuiyan, M.; Wang, Z.; Chiao, P.J.; Philip, P.A.; Abbruzzese, J.; Sarkar, F.H. Molecular Evidence for Increased Antitumor Activity of Gemcitabine by GenisteinIn vitroandIn vivoUsing an Orthotopic Model of Pancreatic Cancer. Cancer Res. 2005, 65, 9064–9072. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Kang, Y.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar]
- Tang, S.-N.; Fu, J.; Shankar, S.; Srivastava, R. EGCG Enhances the Therapeutic Potential of Gemcitabine and CP690550 by Inhibiting STAT3 Signaling Pathway in Human Pancreatic Cancer. PLoS ONE 2012, 7, e31067. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Q.; Zhang, Y.; Sun, W.; Zhang, S.; Li, Y.; Wang, J.; Bao, Y. Functional daidzein enhances the anticancer effect of topotecan and reverses BCRP-mediated drug resistance in breast cancer. Pharmacol. Res. 2019, 147, 104387. [Google Scholar] [CrossRef] [PubMed]
- Rigalli, J.P.; Tocchetti, G.N.; Arana, M.R.; Villanueva, S.S.M.; Catania, V.A.; Theile, D.; Ruiz, M.L.; Weiss, J. The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer Lett. 2016, 376, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, S.; Khajoei, N.; Fazeli, M.; Askari, F. Doxorubicin cytotoxicity in combination with diadzein on MCF-7 Breast Cancer Cells. Malays. J. Nutr. 2015, 21, 67–73. [Google Scholar]
- Xue, J.-P.; Wang, G.; Zhao, Z.-B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Stearns, M.E.; Amatangelo, M.D.; Varma, D.; Sell, C.; Goodyear, S.M. Combination Therapy with Epigallocatechin-3-Gallate and Doxorubicin in Human Prostate Tumor Modeling Studies. Am. J. Pathol. 2010, 177, 3169–3179. [Google Scholar] [CrossRef]
- Reedijk, M.; Odorcic, S.; Zhang, H.; Chetty, R.; Tennert, C.; Dickson, B.C.; Lockwood, G.; Gallinger, S.; Egan, S.E. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int. J. Hyperth 2009, 33, 1223–1229. [Google Scholar]
- Du, G.; Song, Z.-H.; Lin, H.; Han, X.-F.; Zhang, S.; Yang, Y.-M. Luteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 372, 497–502. [Google Scholar] [CrossRef]
- Staedler, D.; Idrizi, E.; Kenzaoui, B.H.; Juillerat-Jeanneret, L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef] [Green Version]
- Kweon, S.H.; Song, J.H.; Kim, T.S. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Commun. 2010, 395, 104–110. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Zheng, J.-M.; Yan, X.-L.; Chen, H.-M.; Chen, J.-K.; Huang, H.-Q. Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. Int. J. Clin. Exp. Pathol. 2015, 8, 6434–6441. [Google Scholar] [PubMed]
- Hsu, Y.-N.; Shyu, H.-W.; Hu, T.-W.; Yeh, J.-P.; Lin, Y.-W.; Lee, L.-Y.; Yeh, Y.-T.; Dai, H.-Y.; Perng, D.-S.; Su, S.-H.; et al. Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem. Toxicol. 2018, 112, 194–204. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Formulation and Optimization of Doxorubicin and Biochanin A Combinational Liposomes for Reversal of Chemoresistance. AAPS PharmSciTech 2016, 18, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-L. A Potential Daidzein Derivative Enhances Cytotoxicity of Epirubicin on Human Colon Adenocarcinoma Caco-2 Cells. Int. J. Mol. Sci. 2012, 14, 158–176. [Google Scholar] [CrossRef] [Green Version]
- Somjen, D.; Katzburg, S.; Nevo, N.; Gayer, B.; Hodge, R.P.; Renevey, M.D.; Kalchenko, V.; Meshorer, A.; Stern, N.; Kohen, F. A daidzein–daunomycin conjugate improves the therapeutic response in an animal model of ovarian carcinoma. J. Steroid Biochem. Mol. Biol. 2008, 110, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Liu, Z.; Hou, X.; Fu, G.; An, N.; Wang, L. Trichostatin A potentiates genistein-induced apoptosis and reverses EMT in HEp2 cells. Mol. Med. Rep. 2016, 13, 5045–5052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiau, R.-J.; Chen, K.-Y.; Wen, Y.-D.; Chuang, C.-H.; Yeh, S.-L. Genistein and β-carotene enhance the growth-inhibitory effect of trichostatin A in A549 cells. Eur. J. Nutr. 2009, 49, 19–25. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, J.; Dai, W.; Zhang, Q.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W.; et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br. J. Cancer 2017, 117, 1518–1528. [Google Scholar] [CrossRef] [Green Version]
- Rigalli, J.P.; Ciriaci, N.; Arias, A.; Ceballos, M.P.; Villanueva, S.S.M.; Luquita, M.G.; Mottino, A.D.; Ghanem, C.I.; Catania, V.A.; Ruiz, M.L. Regulation of Multidrug Resistance Proteins by Genistein in a Hepatocarcinoma Cell Line: Impact on Sorafenib Cytotoxicity. PLoS ONE 2015, 10, e0119502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, D.; Zhelev, Z.; Semkova, S.; Aoki, I.; Bakalova, R. Resveratrol Modulates the Redox-status and Cytotoxicity of Anticancer Drugs by Sensitizing Leukemic Lymphocytes and Protecting Normal Lymphocytes. Anticancer Res. 2019, 39, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Bennett, L.L. Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomed. Pharmacother. 2016, 84, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.; Tolba, M.F.; Badawy, N.N.; Liu, A.W.; El-Ahwany, E.; Khalifa, A.E.; Zada, S.; Abdel-Naim, A.B. Novel combination of sorafenib and biochanin-A synergistically enhances the anti-proliferative and pro-apoptotic effects on hepatocellular carcinoma cells. Sci. Rep. 2016, 6, 30717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.Y.; Xu, H.; Wu, Z.F.; Chen, C.; Liu, J.Y.; Wu, G.N.; Yao, X.Q.; Liu, F.K.; Liang, S.; Shen, L. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget 2015, 6, 44563–44578. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin Sensitizes Colon Cancer Cells to Antitumor Activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanisic, D.; Costa, A.F.; Cruz, G.; Durán, N.; Tasic, L. Applications of Flavonoids, With an Emphasis on Hesperidin, as Anticancer Prodrugs: Phytotherapy as an Alternative to Chemotherapy. Stud. Nat. Prod. Chem. 2018, 58, 161–212. [Google Scholar]
- Singh, Y.; Palombo, M.; Sinko, P. Recent Trends in Targeted Anticancer Prodrug and Conjugate Design. Curr. Med. Chem. 2008, 15, 1802–1826. [Google Scholar] [CrossRef] [Green Version]
- Arroo, R.R.J.; Androutsopoulos, V.P.; Patel, A.; Surichan, S.; Wilsher, N.; Potter, G.A. Phytoestrogens as natural prodrugs in cancer prevention: A novel concept. Phytochem. Rev. 2008, 7, 431–443. [Google Scholar] [CrossRef]
- Arroo, R.R.J.; Androutsopoulos, V.P.; Beresford, K.; Ruparelia, K.; Surichan, S.; Wilsher, N.; Potter, G.A. Phytoestrogens as natural prodrugs in cancer prevention: Dietary flavonoids. Phytochem. Rev. 2009, 8, 375–386. [Google Scholar] [CrossRef]
- Ruparelia, K.C.; Zeka, K.; Ijaz, T.; Ankrett, D.N.; Wilsher, N.E.; Butler, P.C.; Tan, H.L.; Lodhi, S.; Bhambra, A.S.; Potter, G.A.; et al. The Synthesis of Chalcones as Anticancer Prodrugs and their Bioactivation in CYP1 Expressing Breast Cancer Cells. Med. Chem. 2018, 14, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Zoratti, M. Prodrugs of quercetin and resveratrol: A strategy under development. Curr. Drug Metab. 2014, 15, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Mattarei, A.; Sassi, N.; Azzolini, M.; Romio, M.; Paradisi, C.; Zoratti, M. Improving the efficacy of plant polyphenols. Anti-Cancer Agents Med. Chem. 2014, 14, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Wigington, D.P.; Strugnell, S.A.; Knutson, J.C. Growth inhibition of cancer cells by an active metabolite of a novel vitamin D prodrug. Anticancer Res. 2005, 25, 4333–4339. [Google Scholar]
- Appel, E.; Rabinkov, A.; Neeman, M.; Kohen, F.; Mirelman, D. Conjugates of daidzein-alliinase as a targeted pro-drug enzyme system against ovarian carcinoma. J. Drug Target. 2010, 19, 326–335. [Google Scholar] [CrossRef]
- Di, Y.; Ji, S.; Wolf, P.; Krol, E.S.; Alcorn, J. Enterolactone glucuronide and β-glucuronidase in antibody directed enzyme prodrug therapy for targeted prostate cancer cell treatment. AAPS PharmSciTech 2017, 18, 2336–2345. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Ko, P.-W.; Chang, Y.-J.; Kapoor, M.; Liang, Y.-C.; Chu, H.-L.; Lin, H.-H.; Horng, J.-C.; Hsu, M.-H. Design and Synthesis of Benzimidazole-Chalcone Derivatives as Potential Anticancer Agents. Molecules 2019, 24, 3259. [Google Scholar] [CrossRef] [Green Version]
- El-Rayes, B.F.; Philip, P.A.; Sarkar, F.H.; Shields, A.F.; Ferris, A.M.; Hess, K.; Kaseb, A.O.; Javle, M.M.; Varadhachary, G.R.; Wolff, R.A.; et al. A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Investig. New Drugs 2010, 29, 694–699. [Google Scholar] [CrossRef]
- Löhr, J.-M.; Karimi, M.; Omazic, B.; Kartalis, N.; Verbeke, C.S.; Berkenstam, A.; Frödin, J.-E. A phase I dose escalation trial of AXP107-11, a novel multi-component crystalline form of genistein, in combination with gemcitabine in chemotherapy-naive patients with unresectable pancreatic cancer. Pancreatology 2016, 16, 640–645. [Google Scholar] [CrossRef]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Najafi, M.; Villa, V. Mechanisms of Radiation Bystander and Non-Targeted Effects: Implications to Radiation Carcinogenesis and Radiotherapy. Curr. Radiopharm. 2018, 11, 34–45. [Google Scholar] [CrossRef]
- Kim, I.G.; Kim, J.S.; Lee, J.H.; Cho, E.W. Genistein decreases cellular redox potential, partially suppresses cell growth in HL-60 leukemia cells and sensitizes cells to γ-radiation-induced cell death. Mol. Med. Rep. 2014, 10, 2786–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Ma, J.; Sun, J.; Yang, L.; Yang, F.; Zhang, W.; Li, R.; Wang, L.; Wang, Y.; Wang, H. Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol. Rep. 2018, 40, 579–588. [Google Scholar] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Che, M.; Knoll, Z.E.; Doerge, D.R.; Abrams, J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer 2007, 120, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.E.; Ivanov, V.N.; Hei, T.K. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 2008, 13, 790–802. [Google Scholar] [CrossRef]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef]
- Baatout, S.; Derradji, H.; Jacquet, P.; Ooms, D.; Michaux, A.; Mergeay, M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int. J. Mol. Med. 2004, 13, 895–902. [Google Scholar] [CrossRef]
- Zoberi, I.; Bradbury, C.; Curry, H.A.; Bisht, K.S.; Goswami, P.C.; Roti, J.L.R.; Gius, D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 2002, 175, 165–173. [Google Scholar] [CrossRef]
- Manna, K.; Das, U.; Das, D.; Kesh, S.B.; Khan, A.; Chakraborty, A.; Dey, S. Naringin inhibits gamma radiation-induced oxidative DNA damage and inflammation, by modulating p53 and NF-κB signaling pathways in murine splenocytes. Free Radic. Res. 2015, 49, 422–439. [Google Scholar] [CrossRef]
| Phytoestrogen | Treatment Combination | Cancer Type | Effect | Reference |
|---|---|---|---|---|
| Apigenin | Tamoxifen | Ovarian Cancer | + | [84] |
| Ciaplatin | Breast and Ovarian Cancer, Melanoma | +/N.E. | [91,92,97] | |
| Navitoclax | Colon Cancer | + | [140] | |
| Biochanin A | Doxorubicin | Osteosarcoma, Colon Cancer | + | [126,127] |
| Sorafenib | Hepatocarcinoma | + | [138] | |
| Cisplatin | Kidney Cells | S.E. improvement | [99,100] | |
| Catechins | Doxorubicin | Hepatocarcinoma | + | [120] |
| Daidzein | TAM | Breast Cancer | − | [82] |
| Cisplatin | Medulloblastoma, Breast, and Colon Cancer | − | [89,90] | |
| Topotecan | Breast Cancer | + | [115] | |
| Epirubicin | Colon Cancer | + | [129] | |
| Daunomycin | Ovarian Cancer | +, S.E. improvement | [130] | |
| Radio | Prostate Cancer | + | [157] | |
| EGCG | Oxaliplatin | Ovarian Cancer | + | [98] |
| Docetaxel, paclitaxel | Prostate and Breast Cancer | + | [103,104] | |
| Gemcitabine | Pancreatic Cancer | + | [114] | |
| Doxorubicin | Prostate Cancer | + | [119] | |
| Equol | TAM | Breast Cancer | + | [83] |
| Formononetin | Sunitibib | Breast Cancer | + | [139] |
| Doxorubicin | Glioma | + | [125] | |
| Cisplatin | Kidney Cells | S.E. improvement | [99,100] | |
| Genistein | Tamoxifen | Breast Cancer | +/− | [72] |
| Fadrozole (Aromatase Inhibitor) | Breast Cancer | − | [80] | |
| Tamoxifen | Breast Cancer | +/− | [81,82] | |
| Cisplatin | Gastric and pancreatic Cancer | + | [87,88] | |
| Docetaxel | Pancreatic Cancer | + | [88] | |
| Cisplatin | Medulloblastoma, Breast, Ovarian, and Colon Cancer | −/N.E. | [89,90,97] | |
| Gemcitabine | Osteosarcoma, Ovarian and Pancreatic Cancer | + | [110,111,112] | |
| 5-FU | Pancreatic Cancer | + | [113] | |
| Doxorubicin | Breast Cancer | −/+/N.E. | [116,117,118] | |
| Doxorubicin | Prostate Cancer | + | [128] | |
| TSA | Lung and Laryngeal Carcinoma | + | [131,132] | |
| Sorafenib | Hepatocarcinoma | +/− | [134,135] | |
| Radio | Leukemia and Prostate Cancer | +, S.E. improvement | [155,156,157] | |
| Isoxanthohumol | Paclitaxel | Melanoma | + | [105] |
| Kaempferol | Cisplatin | Ovarian Cancer | + | [97] |
| Kaempferol | Doxorubicin | Glioblastoma | + | [123] |
| Luteolin | Doxorubicin | Breast Cancer | + | [121] |
| Naringin | Radiotherapy | Splenocytes | S.E. improvement | [162] |
| Quercetin | Doxorubicin | Breast Cancer | + | [122] |
| Cisplatin | Ovarian Cancer, Lung, and Laryngeal Carcinoma | + | [95,96,97] | |
| Resveratrol | Cisplatin | Colon Cancer | + | [93] |
| Etoposide | Colon Cancer | + | [93] | |
| Oxaliplatin | Colon Cancer | + | [94] | |
| 5FU | Colon and Liver Cancer, Melanoma, and Cholangiocarcinoma | + | [65,93,106,107,109] | |
| Paclitaxel | B-cell Malignancies, Glioblastoma | + | [101,102] | |
| Gemcitabine | Pancreatic Cancer and Cholangiocarcinoma | + | [108,109] | |
| Doxorubicin | Acute Myeloid Leukemia | +, S.E. improvement | [45,124] | |
| Barasertib | Leukemia | + | [136] | |
| Sorafenib | Breast Cancer | + | [137] | |
| Radiotherapy | Melanoma, Prostate, and Cervical Cancer | + | [158,159,160,161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrens-Mas, M.; Roca, P. Phytoestrogens for Cancer Prevention and Treatment. Biology 2020, 9, 427. https://doi.org/10.3390/biology9120427
Torrens-Mas M, Roca P. Phytoestrogens for Cancer Prevention and Treatment. Biology. 2020; 9(12):427. https://doi.org/10.3390/biology9120427
Chicago/Turabian StyleTorrens-Mas, Margalida, and Pilar Roca. 2020. "Phytoestrogens for Cancer Prevention and Treatment" Biology 9, no. 12: 427. https://doi.org/10.3390/biology9120427
APA StyleTorrens-Mas, M., & Roca, P. (2020). Phytoestrogens for Cancer Prevention and Treatment. Biology, 9(12), 427. https://doi.org/10.3390/biology9120427







