Changes in Membrane Protein Structural Biology
Abstract
Simple Summary
Abstract
1. Introduction
2. Recombinant Membrane Protein Production
2.1. Generating Constructs for Recombinant Protein Expression
2.2. Recombinant Membrane Protein Expression
2.3. Fusion Tags and Resins for MP Purification
3. Stabilisation of Membrane Proteins for Structural Studies
3.1. Use of Encapsulation Agents to Stabilise Membrane Proteins
3.2. Genetic Engineering of MPs to Improve Protein Stabilisation
3.3. Chaperones and Ligands to Aid MP Stabilisation
3.4. Assessing the Quality of Purified MPs
4. Advances in Sample Preparation and Data Collection
4.1. Membrane Protein Crystallisation and Data Collection
4.2. Preparation of Membrane Proteins for Cryo-EM and Data Collection
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Wallin, E.; von Heijne, G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998, 7, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Superti-Furga, G.; Lackner, D.; Wiedmer, T.; Ingles-Prieto, A.; Barbosa, B.; Girardi, E.; Goldmann, U.; Gurtl, B.; Klavins, K.; Klimek, C.; et al. The RESOLUTE consortium: Unlocking SLC transporters for drug discovery. Nat. Rev. Drug Discov. 2020, 19, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Membrane Proteins of Known 3D Structure Database. Available online: https://blanco.biomol.uci.edu/mpstruc/ (accessed on 30 September 2020).
- Carpenter, E.P.; Beis, K.; Cameron, A.D.; Iwata, S. Overcoming the challenges of membrane protein crystallography. Curr. Opin. Struct. Biol. 2008, 18, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Alianelli, L.; Burt, M.; Wagner, A.; Sawhney, K.J.S. Diamond Beamline 124: A Flexible Instrument for Macromolecular Micro-crystallography. Aip. Conf. Proc. 2007, 879, 836–839. [Google Scholar] [CrossRef]
- Grimes, J.M.; Hall, D.R.; Ashton, A.W.; Evans, G.; Owen, R.L.; Wagner, A.; McAuley, K.E.; von Delft, F.; Orville, A.M.; Sorensen, T.; et al. Where is crystallography going? Acta Crystallogr. Sect. D 2018, 74, 152–166. [Google Scholar] [CrossRef]
- Faruqi, A.R.; Cattermole, D.M.; Henderson, R.; Mikulec, B.; Raeburn, C. Evaluation of a hybrid pixel detector for electron microscopy. Ultramicroscopy 2003, 94, 263–276. [Google Scholar] [CrossRef]
- Egelman, E.H. The Current Revolution in Cryo-EM. Biophys. J. 2016, 110, 1008–1012. [Google Scholar] [CrossRef]
- Axford, D.; Foadi, J.; Hu, N.-J.; Choudhury, H.G.; Iwata, S.; Beis, K.; Evans, G.; Alguel, Y. Structure determination of an integral membrane protein at room temperature from crystals in situ. Acta Crystallogr. Sect. D 2015, 71, 1228–1237. [Google Scholar] [CrossRef]
- Axford, D.; Owen, R.L.; Aishima, J.; Foadi, J.; Morgan, A.W.; Robinson, J.I.; Nettleship, J.E.; Owens, R.J.; Moraes, I.; Fry, E.E.; et al. In situ macromolecular crystallography using microbeams. Acta Crystallogr. Sect. D 2012, 68, 592–600. [Google Scholar] [CrossRef]
- Bird, L.E.; Rada, H.; Verma, A.; Gasper, R.; Birch, J.; Jennions, M.; Löwe, J.; Moraes, I.; Owens, R.J. Green fluorescent protein-based expression screening of membrane proteins in Escherichia coli. J. Vis. Exp. 2015, 95, e52357. [Google Scholar] [CrossRef] [PubMed]
- Ishchenko, A.; Stauch, B.; Han, G.W.; Batyuk, A.; Shiriaeva, A.; Li, C.; Zatsepin, N.; Weierstall, U.; Liu, W.; Nango, E.; et al. Toward G protein-coupled receptor structure-based drug design using X-ray lasers. IUCrJ 2019, 6, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.O.C.; Reis, R.; Siligardi, G.; Hussain, R.; Cheruvara, H.; Moraes, I. Selection of Biophysical Methods for Characterisation of Membrane Proteins. Int. J. Mol. Sci. 2019, 20, 2605. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Weatherby, J.; Moraes, I. Crystal Dehydration in Membrane Protein Crystallography. Adv. Exp. Med. Biol. 2016, 922, 73–89. [Google Scholar] [CrossRef]
- Mylona, A.; Carr, S.; Aller, P.; Moraes, I.; Treisman, R.; Evans, G.; Foadi, J. A Novel Approach to Data Collection for Difficult Structures: Data Management for Large Numbers of Crystals with the BLEND Software. Crystals 2017, 7, 242. [Google Scholar] [CrossRef]
- Jormakka, M.; Yokoyama, K.; Yano, T.; Tamakoshi, M.; Akimoto, S.; Shimamura, T.; Curmi, P.; Iwata, S. Molecular mechanism of energy conservation in polysulfide respiration. Nat. Struct. Mol. Biol. 2008, 15, 730–737. [Google Scholar] [CrossRef]
- Drew, D.; Klepsch, M.M.; Newstead, S.; Flaig, R.; De Gier, J.W.; Iwata, S.; Beis, K. The structure of the efflux pump AcrB in complex with bile acid. Mol. Membr. Biol. 2008, 25, 677–682. [Google Scholar] [CrossRef]
- Weyand, S.; Shimamura, T.; Yajima, S.; Suzuki, S.i.; Mirza, O.; Krusong, K.; Carpenter, E.P.; Rutherford, N.G.; Hadden, J.M.; O’Reilly, J.; et al. Structure and Molecular Mechanism of a Nucleobase–Cation–Symport-1 Family Transporter. Science 2008, 322, 709–713. [Google Scholar] [CrossRef]
- Shimamura, T.; Weyand, S.; Beckstein, O.; Rutherford, N.G.; Hadden, J.M.; Sharples, D.; Sansom, M.S.P.; Iwata, S.; Henderson, P.J.F.; Cameron, A.D. Molecular Basis of Alternating Access Membrane Transport by the Sodium-Hydantoin Transporter Mhp1. Science 2010, 328, 470–473. [Google Scholar] [CrossRef]
- Newstead, S.; Drew, D.; Cameron, A.D.; Postis, V.L.G.; Xia, X.; Fowler, P.W.; Ingram, J.C.; Carpenter, E.P.; Sansom, M.S.P.; McPherson, M.J.; et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide–proton symporters, PepT1 and PepT2. Embo J. 2011, 30, 417–426. [Google Scholar] [CrossRef]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.-J.; Iwata, S.; Cameron, A.D.; Drew, D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature 2011, 478, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Rengachari, S.; Bezerra, G.A.; Riegler-Berket, L.; Gruber, C.C.; Sturm, C.; Taschler, U.; Boeszoermenyi, A.; Dreveny, I.; Zimmermann, R.; Gruber, K.; et al. The structure of monoacylglycerol lipase from Bacillus sp. H257 reveals unexpected conservation of the cap architecture between bacterial and human enzymes. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- McCusker, E.C.; Bagnéris, C.; Naylor, C.E.; Cole, A.R.; D’Avanzo, N.; Nichols, C.G.; Wallace, B.A. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat. Commun. 2012, 3, 1102. [Google Scholar] [CrossRef] [PubMed]
- Manolaridis, I.; Kulkarni, K.; Dodd, R.B.; Ogasawara, S.; Zhang, Z.; Bineva, G.; O’Reilly, N.; Hanrahan, S.J.; Thompson, A.J.; Cronin, N.; et al. Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature 2013, 504, 301–305. [Google Scholar] [CrossRef]
- Nogly, P.; Gushchin, I.; Remeeva, A.; Esteves, A.M.; Borges, N.; Ma, P.; Ishchenko, A.; Grudinin, S.; Round, E.; Moraes, I.; et al. X-ray structure of a CDP-alcohol phosphatidyltransferase membrane enzyme and insights into its catalytic mechanism. Nat. Commun. 2014, 5, 4169. [Google Scholar] [CrossRef]
- Anandan, A.; Evans, G.L.; Condic-Jurkic, K.; O’Mara, M.L.; John, C.M.; Phillips, N.J.; Jarvis, G.A.; Wills, S.S.; Stubbs, K.A.; Moraes, I.; et al. Structure of a lipid A phosphoethanolamine transferase suggests how conformational changes govern substrate binding. Proc. Natl. Acad. Sci. USA 2017, 114, 2218–2223. [Google Scholar] [CrossRef]
- Hakulinen, J.K.; Hering, J.; Brändén, G.; Chen, H.; Snijder, A.; Ek, M.; Johansson, P. MraY—Antibiotic complex reveals details of tunicamycin mode of action. Nat. Chem. Biol. 2017, 13, 265–267. [Google Scholar] [CrossRef]
- Wahlgren, W.Y.; Dunevall, E.; North, R.A.; Paz, A.; Scalise, M.; Bisignano, P.; Bengtsson-Palme, J.; Goyal, P.; Claesson, E.; Caing-Carlsson, R.; et al. Substrate-bound outward-open structure of a Na+-coupled sialic acid symporter reveals a new Na+ site. Nat. Commun. 2018, 9, 1753. [Google Scholar] [CrossRef]
- Moynié, L.; Milenkovic, S.; Mislin, G.L.A.; Gasser, V.; Malloci, G.; Baco, E.; McCaughan, R.P.; Page, M.G.P.; Schalk, I.J.; Ceccarelli, M.; et al. The complex of ferric-enterobactin with its transporter from Pseudomonas aeruginosa suggests a two-site model. Nat. Commun. 2019, 10, 3673. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Jones, D.T. The PSIPRED Protein Analysis Workbench: 20 Years on. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef] [PubMed]
- McGuffin, L.J.; Jones, D.T. Improvement of the GenTHREADER method for genomic fold recognition. Bioinformatics 2003, 19, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T. GenTHREADER: An efficient and reliable protein fold recognition method for genomic sequences. J. Mol. Biol. 1999, 287, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999, 292, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Cozzetto, D. DISOPRED3: Precise disordered region predictions with annotated protein-binding activity. Bioinformatics 2015, 31, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 2007, 23, 538–544. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar] [CrossRef]
- Mauro, V.P.; Chappell, S.A. Considerations in the Use of Codon Optimization for Recombinant Protein Expression. In Recombinant Protein Expression in Mammalian Cells: Methods and Protocols; Hacker, D.L., Ed.; Springer: New York, NY, USA, 2018; pp. 275–288. [Google Scholar] [CrossRef]
- Berrow, N.S.; Alderton, D.; Sainsbury, S.; Nettleship, J.; Assenberg, R.; Rahman, N.; Stuart, D.I.; Owens, R.J. A versatile ligation-independent cloning method suitable for high-throughput expression screening applications. Nucleic Acids Res. 2007, 35, e45. [Google Scholar] [CrossRef]
- Bird, L.E.; Rada, H.; Flanagan, J.; Diprose, J.M.; Gilbert, R.J.C.; Owens, R.J. Application of In-Fusion™ Cloning for the Parallel Construction of E. coli Expression Vectors. In DNA Cloning and Assembly Methods; Valla, S., Lale, R., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 209–234. [Google Scholar] [CrossRef]
- Hartley, J.L. Use of the gateway system for protein expression in multiple hosts. Curr. Protoc. Protein Sci. 2003. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.E.; von Heijne, G.; Nordlund, P.; de Gier, J.W. Green fluorescent protein as an indicator to monitor membrane protein overexpression in Escherichia coli. FEBS Lett. 2001, 507, 220–224. [Google Scholar] [CrossRef]
- Newstead, S.; Kim, H.; von Heijne, G.; Iwata, S.; Drew, D. High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 13936–13941. [Google Scholar] [CrossRef] [PubMed]
- Bird, L.E. High throughput construction and small scale expression screening of multi-tag vectors in Escherichia coli. Methods 2011, 55, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.; Slotboom, D.J.; Poolman, B. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta 2003, 1610, 97–108. [Google Scholar] [CrossRef]
- Wagner, S.; Bader, M.L.; Drew, D.; de Gier, J.W. Rationalizing membrane protein overexpression. Trends Biotechnol. 2006, 24, 364–371. [Google Scholar] [CrossRef]
- Miroux, B.; Walker, J.E. Over-production of proteins in Escherichia coli: Mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 1996, 260, 289–298. [Google Scholar] [CrossRef]
- Angius, F.; Ilioaia, O.; Amrani, A.; Suisse, A.; Rosset, L.; Legrand, A.; Abou-Hamdan, A.; Uzan, M.; Zito, F.; Miroux, B. A novel regulation mechanism of the T7 RNA polymerase based expression system improves overproduction and folding of membrane proteins. Sci. Rep. 2018, 8, 8572. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Brooks, C.L.; Morrison, M.; Joanne Lemieux, M. Rapid expression screening of eukaryotic membrane proteins in Pichia pastoris. Protein Sci. 2013, 22, 425–433. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Method to increase the yield of eukaryotic membrane protein expression in Saccharomyces cerevisiae for structural and functional studies. Protein Sci. 2014, 23, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shaffer, P.L.; Huang, X.; Rose, P.E. Rapid screening of membrane protein expression in transiently transfected insect cells. Protein Expr. Purif. 2013, 88, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Manzan, M.A.; Peplinski, H.M.; Thiem, S.M. A new Trichoplusia ni cell line for membrane protein expression using a baculovirus expression vector system. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.J.; Rada, H.; Rahman, N.; Nettleship, J.E.; Bird, L.; Iwata, S.; Drew, D.; Cameron, A.D.; Owens, R.J. GFP-based expression screening of membrane proteins in insect cells using the baculovirus system. Methods Mol. Biol. 2015, 1261, 197–209. [Google Scholar] [CrossRef]
- Hanson, M.A.; Brooun, A.; Baker, K.A.; Jaakola, V.P.; Roth, C.; Chien, E.Y.; Alexandrov, A.; Velasquez, J.; Davis, L.; Griffith, M.; et al. Profiling of membrane protein variants in a baculovirus system by coupling cell-surface detection with small-scale parallel expression. Protein Expr. Purif. 2007, 56, 85–92. [Google Scholar] [CrossRef][Green Version]
- Berger, I.; Garzoni, F.; Chaillet, M.; Haffke, M.; Gupta, K.; Aubert, A. The multiBac protein complex production platform at the EMBL. J. Vis. Exp. 2013, 77, e50159. [Google Scholar] [CrossRef]
- Lemaitre, R.P.; Bogdanova, A.; Borgonovo, B.; Woodruff, J.B.; Drechsel, D.N. FlexiBAC: A versatile, open-source baculovirus vector system for protein expression, secretion, and proteolytic processing. BMC Biotechnol. 2019, 19, 20. [Google Scholar] [CrossRef]
- Hitchman, R.B.; Possee, R.D.; King, L.A. Baculovirus expression systems for recombinant protein production in insect cells. Recent Pat. Biotechnol. 2009, 3, 46–54. [Google Scholar] [CrossRef]
- Zhao, Y.; Chapman, D.A.; Jones, I.M. Improving baculovirus recombination. Nucleic Acids Res. 2003, 31, e6. [Google Scholar] [CrossRef]
- Thomas, J.A.; Tate, C.G. Quality Control in Eukaryotic Membrane Protein Overproduction. J. Mol. Biol. 2014, 426, 4139–4154. [Google Scholar] [CrossRef]
- Chaudhary, S.; Pak, J.E.; Pedersen, B.P.; Bang, L.J.; Zhang, L.B.; Ngaw, S.M.; Green, R.G.; Sharma, V.; Stroud, R.M. Efficient expression screening of human membrane proteins in transiently transfected Human Embryonic Kidney 293S cells. Methods 2011, 55, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Goehring, A.; Lee, C.H.; Wang, K.H.; Michel, J.C.; Claxton, D.P.; Baconguis, I.; Althoff, T.; Fischer, S.; Garcia, K.C.; Gouaux, E. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 2014, 9, 2574–2585. [Google Scholar] [CrossRef]
- O’Connor, S.; Li, E.; Majors, B.S.; He, L.; Placone, J.; Baycin, D.; Betenbaugh, M.J.; Hristova, K. Increased expression of the integral membrane protein ErbB2 in Chinese hamster ovary cells expressing the anti-apoptotic gene Bcl-xL. Protein Expr. Purif. 2009, 67, 41–47. [Google Scholar] [CrossRef]
- Krasnoselska, G.O.; Dumoux, M.; Gamage, N.; Cheruvara, H.; Birch, J.; Quigley, A.; Owens, R.J. Transient transfection and expression of eukaryotic membrane proteins in Expi293F cells and their screening on a small-scale: Application for structural studies. In Methods in Molecular Biology; Springer: Berlin, Germany, in press.
- Elegheert, J.; Behiels, E.; Bishop, B.; Scott, S.; Woolley, R.E.; Griffiths, S.C.; Byrne, E.F.X.; Chang, V.T.; Stuart, D.I.; Jones, E.Y.; et al. Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins. Nat. Protoc. 2018, 13, 2991–3017. [Google Scholar] [CrossRef] [PubMed]
- Grieben, M.; Pike, A.C.; Shintre, C.A.; Venturi, E.; El-Ajouz, S.; Tessitore, A.; Shrestha, L.; Mukhopadhyay, S.; Mahajan, P.; Chalk, R.; et al. Structure of the polycystic kidney disease TRP channel Polycystin-2 (PC2). Nat. Struct. Mol. Biol. 2017, 24, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chun, E.; Thompson, A.A.; Chubukov, P.; Xu, F.; Katritch, V.; Han, G.W.; Roth, C.B.; Heitman, L.H.; Ijzerman, A.P.; et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hresko, R.C.; Kraft, T.E.; Quigley, A.; Carpenter, E.P.; Hruz, P.W. Mammalian Glucose Transporter Activity Is Dependent upon Anionic and Conical Phospholipids. J. Biol. Chem. 2016, 291, 17271–17282. [Google Scholar] [CrossRef]
- Schmidt, T.G.M.; Skerra, A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat. Protoc. 2007, 2, 1528–1535. [Google Scholar] [CrossRef]
- Cronan, J.E., Jr. Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J. Biol. Chem. 1990, 265, 10327–10333. [Google Scholar] [PubMed]
- Fairhead, M.; Howarth, M. Site-Specific Biotinylation of Purified Proteins Using BirA. In Site-Specific Protein Labeling: Methods and Protocols; Gautier, A., Hinner, M.J., Eds.; Springer: New York, NY, USA, 2015; pp. 171–184. [Google Scholar] [CrossRef]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.F.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.J.; Kobilka, B.K.; Steyaert, J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Ding, Y.; Hattori, M. Structure-based engineering of anti-GFP nanobody tandems as ultra-high-affinity reagents for purification. Sci. Rep. 2020, 10, 6239. [Google Scholar] [CrossRef] [PubMed]
- Veesler, D.; Blangy, S.; Cambillau, C.; Sciara, G. There is a baby in the bath water: AcrB contamination is a major problem in membrane-protein crystallization. Acta Cryst. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.A.; Postis, V.L.; Charalambous, K.; Tzokov, S.B.; Booth, W.I.; Deacon, S.E.; Wallace, B.A.; Baldwin, S.A.; Bullough, P.A. AcrB contamination in 2-D crystallization of membrane proteins: Lessons from a sodium channel and a putative monovalent cation/proton antiporter. J. Struct. Biol. 2011, 176, 419–424. [Google Scholar] [CrossRef]
- Wiseman, B.; Kilburg, A.; Chaptal, V.; Reyes-Mejia, G.C.; Sarwan, J.; Falson, P.; Jault, J.-M. Stubborn Contaminants: Influence of Detergents on the Purity of the Multidrug ABC Transporter BmrA. PLoS ONE 2014, 9, e114864. [Google Scholar] [CrossRef] [PubMed]
- Psakis, G.; Polaczek, J.; Essen, L.O. AcrB et al.: Obstinate contaminants in a picogram scale. One more bottleneck in the membrane protein structure pipeline. J. Struct. Biol. 2009, 166, 107–111. [Google Scholar] [CrossRef]
- Abeyrathne, P.D.; Grigorieff, N. Expression, purification, and contaminant detection for structural studies of Ralstonia metallidurance ClC protein rm1. PLoS ONE 2017, 12, e0180163. [Google Scholar] [CrossRef]
- Pike, A.C.W.; Garman, E.F.; Krojer, T.; von Delft, F.; Carpenter, E.P. An overview of heavy-atom derivatization of protein crystals. Acta Crystallogr. Sect. D 2016, 72, 303–318. [Google Scholar] [CrossRef]
- Hofmann, S.; Januliene, D.; Mehdipour, A.R.; Thomas, C.; Stefan, E.; Brüchert, S.; Kuhn, B.T.; Geertsma, E.R.; Hummer, G.; Tampé, R.; et al. Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature 2019, 571, 580–583. [Google Scholar] [CrossRef]
- Savitsky, P.; Bray, J.; Cooper, C.D.; Marsden, B.D.; Mahajan, P.; Burgess-Brown, N.A.; Gileadi, O. High-throughput production of human proteins for crystallization: The SGC experience. J. Struct. Biol. 2010, 172, 3–13. [Google Scholar] [CrossRef]
- Chun, E.; Thompson, A.A.; Liu, W.; Roth, C.B.; Griffith, M.T.; Katritch, V.; Kunken, J.; Xu, F.; Cherezov, V.; Hanson, M.A.; et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 2012, 20, 967–976. [Google Scholar] [CrossRef]
- Magnani, F.; Serrano-Vega, M.J.; Shibata, Y.; Abdul-Hussein, S.; Lebon, G.; Miller-Gallacher, J.; Singhal, A.; Strege, A.; Thomas, J.A.; Tate, C.G. A mutagenesis and screening strategy to generate optimally thermostabilized membrane proteins for structural studies. Nat. Protoc. 2016, 11, 1554–1571. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Slotboom, D.J.; Friso, G.; Reda, T.; Genevaux, P.; Rapp, M.; Meindl-Beinker, N.M.; Lambert, W.; Lerch, M.; Daley, D.O.; et al. A scalable, GFP-based pipeline for membrane protein overexpression screening and purification. Protein Sci. 2005, 14, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.; Newstead, S.; Sonoda, Y.; Kim, H.; von Heijne, G.; Iwata, S. GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nat. Protoc. 2008, 3, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-C.; Yan, C.; Yang, G.; Lu, P.; Ma, D.; Sun, L.; Zhou, R.; Scheres, S.H.W.; Shi, Y. An atomic structure of human γ-secretase. Nature 2015, 525, 212–217. [Google Scholar] [CrossRef]
- Kawate, T.; Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 2006, 14, 673–681. [Google Scholar] [CrossRef]
- Kirchhofer, A.; Helma, J.; Schmidthals, K.; Frauer, C.; Cui, S.; Karcher, A.; Pellis, M.; Muyldermans, S.; Casas-Delucchi, C.S.; Cardoso, M.C.; et al. Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 2010, 17, 133–138. [Google Scholar] [CrossRef]
- Drew, D.; Lerch, M.; Kunji, E.; Slotboom, D.J.; de Gier, J.W. Optimization of membrane protein overexpression and purification using GFP fusions. Nat. Methods 2006, 3, 303–313. [Google Scholar] [CrossRef]
- Anandan, A.; Vrielink, A. Detergents in Membrane Protein Purification and Crystallisation. Adv. Exp. Med. Biol. 2016, 922, 13–28. [Google Scholar] [CrossRef]
- Stetsenko, A.; Guskov, A. An Overview of the Top Ten Detergents Used for Membrane Protein Crystallization. Crystals 2017, 7, 197. [Google Scholar] [CrossRef]
- Durand, G.; Abla, M.; Ebel, C.; Breyton, C. New Amphiphiles to Handle Membrane Proteins: “Ménage à Trois” between Chemistry, Physical Chemistry, and Biochemistry. In Membrane Proteins Production for Structural Analysis; Mus-Veteau, I., Ed.; Springer: New York, NY, USA, 2014; pp. 205–251. [Google Scholar] [CrossRef]
- Chae, P.S.; Rasmussen, S.G.; Rana, R.R.; Gotfryd, K.; Chandra, R.; Goren, M.A.; Kruse, A.C.; Nurva, S.; Loland, C.J.; Pierre, Y.; et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat. Methods 2010, 7, 1003–1008. [Google Scholar] [CrossRef]
- Chae, P.S.; Rasmussen, S.G.; Rana, R.R.; Gotfryd, K.; Kruse, A.C.; Manglik, A.; Cho, K.H.; Nurva, S.; Gether, U.; Guan, L.; et al. A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chemistry 2012, 18, 9485–9490. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Cryst. F Struct. Biol. Commun. 2015, 71, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hauer, F.; Gerle, C.; Fischer, N.; Oshima, A.; Shinzawa-Itoh, K.; Shimada, S.; Yokoyama, K.; Fujiyoshi, Y.; Stark, H. GraDeR: Membrane Protein Complex Preparation for Single-Particle Cryo-EM. Structure 2015, 23, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrand, D.; Diamanti, R.; Lundgren, C.A.K.; Wiseman, B.; Högbom, M. A rapid expression and purification condition screening protocol for membrane protein structural biology. Protein Sci. 2017, 26, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Vergis, J.M.; Purdy, M.D.; Wiener, M.C. A high-throughput differential filtration assay to screen and select detergents for membrane proteins. Anal. Biochem. 2010, 407, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotov, V.; Bartels, K.; Veith, K.; Josts, I.; Subhramanyam, U.K.T.; Gunther, C.; Labahn, J.; Marlovits, T.C.; Moraes, I.; Tidow, H.; et al. High-throughput stability screening for detergent-solubilized membrane proteins. Sci. Rep. 2019, 9, 10379. [Google Scholar] [CrossRef]
- Vergis, J.M.; Wiener, M.C. The variable detergent sensitivity of proteases that are utilized for recombinant protein affinity tag removal. Protein Expr. Purif. 2011, 78, 139–142. [Google Scholar] [CrossRef]
- Gewering, T.; Januliene, D.; Ries, A.B.; Moeller, A. Know your detergents: A case study on detergent background in negative stain electron microscopy. J. Struct. Biol. 2018, 203, 242–246. [Google Scholar] [CrossRef]
- Carlson, M.L.; Young, J.W.; Zhao, Z.; Fabre, L.; Jun, D.; Li, J.; Li, J.; Dhupar, H.S.; Wason, I.; Mills, A.T.; et al. The Peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution. eLife 2018, 7, e34085. [Google Scholar] [CrossRef]
- Frauenfeld, J.; Loving, R.; Armache, J.P.; Sonnen, A.F.; Guettou, F.; Moberg, P.; Zhu, L.; Jegerschold, C.; Flayhan, A.; Briggs, J.A.; et al. A saposin-lipoprotein nanoparticle system for membrane proteins. Nat. Methods 2016, 13, 345–351. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Self-Assembly of Discoidal Phospholipid Bilayer Nanoparticles with Membrane Scaffold Proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Tribet, C.; Audebert, R.; Popot, J.L. Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. USA 1996, 93, 15047–15050. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Knowles, T.J.; Postis, V.L.; Jamshad, M.; Parslow, R.A.; Lin, Y.P.; Goldman, A.; Sridhar, P.; Overduin, M.; Muench, S.P.; et al. A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat. Protoc. 2016, 11, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Sgro, G.G.; Costa, T.R.D. Cryo-EM Grid Preparation of Membrane Protein Samples for Single Particle Analysis. Front. Mol. Biosci. 2018, 5, 74. [Google Scholar] [CrossRef]
- Broecker, J.; Eger, B.T.; Ernst, O.P. Crystallogenesis of Membrane Proteins Mediated by Polymer-Bounded Lipid Nanodiscs. Structure 2017, 25, 384–392. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Sligar, S.G. Membrane protein assembly into Nanodiscs. FEBS Lett. 2010, 584, 1721–1727. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs for structural and functional studies of membrane proteins. Nat. Struct. Mol. Biol. 2016, 23, 481–486. [Google Scholar] [CrossRef]
- Nasr, M.L.; Baptista, D.; Strauss, M.; Sun, Z.J.; Grigoriu, S.; Huser, S.; Plückthun, A.; Hagn, F.; Walz, T.; Hogle, J.M.; et al. Covalently circularized nanodiscs for studying membrane proteins and viral entry. Nat. Methods 2017, 14, 49–52. [Google Scholar] [CrossRef]
- Nasr, M.L. Large nanodiscs going viral. Curr. Opin. Struct. Biol. 2020, 60, 150–156. [Google Scholar] [CrossRef]
- Padmanabha Das, K.M.; Shih, W.M.; Wagner, G.; Nasr, M.L. Large Nanodiscs: A Potential Game Changer in Structural Biology of Membrane Protein Complexes and Virus Entry. Front. Bioeng. Biotechnol. 2020, 8, 539. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, M.; Hogle, J.M.; Shih, W.M.; Wagner, G.; Nasr, M.L. DNA-Corralled Nanodiscs for the Structural and Functional Characterization of Membrane Proteins and Viral Entry. J. Am. Chem. Soc. 2018, 140, 10639–10643. [Google Scholar] [CrossRef] [PubMed]
- Lloris-Garcerá, P.; Klinter, S.; Chen, L.; Skynner, M.J.; Löving, R.; Frauenfeld, J. DirectMX—One-Step Reconstitution of Membrane Proteins From Crude Cell Membranes Into Salipro Nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Zoonens, M.; Popot, J.-L. Amphipols for each season. J. Membr. Biol. 2014, 247, 759–796. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, T.; Rappaport, F.; Popot, J.L. Amphipol-assisted folding of bacteriorhodopsin in the presence or absence of lipids: Functional consequences. Eur. Biophys. J. EBJ 2013, 42, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, J.H.; Popot, J.-L. Folding and stability of integral membrane proteins in amphipols. Arch. Biochem. Biophys. 2014, 564, 327–343. [Google Scholar] [CrossRef]
- Picard, M.; Dahmane, T.; Garrigos, M.; Gauron, C.; Giusti, F.; le Maire, M.; Popot, J.L.; Champeil, P. Protective and inhibitory effects of various types of amphipols on the Ca2+-ATPase from sarcoplasmic reticulum: A comparative study. Biochemistry 2006, 45, 1861–1869. [Google Scholar] [CrossRef]
- Huynh, K.W.; Cohen, M.R.; Moiseenkova-Bell, V.Y. Application of amphipols for structure-functional analysis of TRP channels. J. Membr. Biol. 2014, 247, 843–851. [Google Scholar] [CrossRef][Green Version]
- Klammt, C.; Perrin, M.H.; Maslennikov, I.; Renault, L.; Krupa, M.; Kwiatkowski, W.; Stahlberg, H.; Vale, W.; Choe, S. Polymer-based cell-free expression of ligand-binding family B G-protein coupled receptors without detergents. Protein Sci. 2011, 20, 1030–1041. [Google Scholar] [CrossRef]
- Prata, C.; Giusti, F.; Gohon, Y.; Pucci, B.; Popot, J.L.; Tribet, C. Nonionic amphiphilic polymers derived from Tris(hydroxymethyl)-acrylamidomethane keep membrane proteins soluble and native in the absence of detergent. Biopolymers 2000, 56, 77–84. [Google Scholar] [CrossRef]
- Huynh, K.W.; Jiang, J.; Abuladze, N.; Tsirulnikov, K.; Kao, L.; Shao, X.; Newman, D.; Azimov, R.; Pushkin, A.; Zhou, Z.H.; et al. CryoEM structure of the human SLC4A4 sodium-coupled acid-base transporter NBCe1. Nat. Commun. 2018, 9, 900. [Google Scholar] [CrossRef]
- Zubcevic, L.; Herzik, M.A.; Chung, B.C.; Liu, Z.; Lander, G.C.; Lee, S.-Y. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol. 2016, 23, 180–186. [Google Scholar] [CrossRef]
- Wang, K.; Preisler, S.S.; Zhang, L.; Cui, Y.; Missel, J.W.; Grønberg, C.; Gotfryd, K.; Lindahl, E.; Andersson, M.; Calloe, K.; et al. Structure of the human ClC-1 chloride channel. PLoS Biol. 2019, 17, e3000218. [Google Scholar] [CrossRef] [PubMed]
- Bazzacco, P.; Billon-Denis, E.; Sharma, K.S.; Catoire, L.J.; Mary, S.; Le Bon, C.; Point, E.; Banères, J.-L.; Durand, G.; Zito, F.; et al. Nonionic Homopolymeric Amphipols: Application to Membrane Protein Folding, Cell-Free Synthesis, and Solution Nuclear Magnetic Resonance. Biochemistry 2012, 51, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Polovinkin, V.; Gushchin, I.; Sintsov, M.; Round, E.; Balandin, T.; Chervakov, P.; Schevchenko, V.; Utrobin, P.; Popov, A.; Borshchevskiy, V.; et al. High-Resolution Structure of a Membrane Protein Transferred from Amphipol to a Lipidic Mesophase. J. Membr. Biol. 2014, 247, 997–1004. [Google Scholar] [CrossRef]
- Postis, V.; Rawson, S.; Mitchell, J.K.; Lee, S.C.; Parslow, R.A.; Dafforn, T.R.; Baldwin, S.A.; Muench, S.P. The use of SMALPs as a novel membrane protein scaffold for structure study by negative stain electron microscopy. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 496–501. [Google Scholar] [CrossRef]
- Simon, K.S.; Pollock, N.L.; Lee, S.C. Membrane protein nanoparticles: The shape of things to come. Biochem. Soc. Trans. 2018, 46, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Bada Juarez, J.F.; Muñoz-García, J.C.; Inácio dos Reis, R.; Henry, A.; McMillan, D.; Kriek, M.; Wood, M.; Vandenplas, C.; Sands, Z.; Castro, L.; et al. Detergent-free extraction of a functional low-expressing GPCR from a human cell line. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183152. [Google Scholar] [CrossRef]
- Swainsbury, D.J.K.; Scheidelaar, S.; Foster, N.; van Grondelle, R.; Killian, J.A.; Jones, M.R. The effectiveness of styrene-maleic acid (SMA) copolymers for solubilisation of integral membrane proteins from SMA-accessible and SMA-resistant membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 2133–2143. [Google Scholar] [CrossRef]
- Johnson, R.M.; Fais, C.; Parmar, M.; Cheruvara, H.; Marshall, R.L.; Hesketh, S.J.; Feasey, M.C.; Ruggerone, P.; Vargiu, A.V.; Postis, V.L.G.; et al. Cryo-EM Structure and Molecular Dynamics Analysis of the Fluoroquinolone Resistant Mutant of the AcrB Transporter from Salmonella. Microorganisms 2020, 8, 943. [Google Scholar] [CrossRef]
- Tascón, I.; Sousa, J.S.; Corey, R.A.; Mills, D.J.; Griwatz, D.; Aumüller, N.; Mikusevic, V.; Stansfeld, P.J.; Vonck, J.; Hänelt, I. Structural basis of proton-coupled potassium transport in the KUP family. Nat. Commun. 2020, 11, 626. [Google Scholar] [CrossRef]
- Harwood, C.R.; Sykes, D.A.; Hoare, B.; Heydenreich, F.M.; Uddin, R.; Poyner, D.R.; Briddon, S.J.; Veprintsev, D.B. Functional solubilisation of the β2-adrenoceptor (β2AR) using Diisobutylene maleic acid (DIBMA). bioRxiv 2020. [Google Scholar] [CrossRef]
- Long, A.R.; O’Brien, C.C.; Malhotra, K.; Schwall, C.T.; Albert, A.D.; Watts, A.; Alder, N.N. A detergent-free strategy for the reconstitution of active enzyme complexes from native biological membranes into nanoscale discs. BMC Biotechnol. 2013, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.A.; Sjöstrand, D.; Li, F.; Björck, M.; Schäfer, J.; Östbye, H.; Högbom, M.; von Ballmoos, C.; Lander, G.C.; Ädelroth, P.; et al. Isolation of yeast complex IV in native lipid nanodiscs. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.G.; Li, M.; Ma, Y.; Gumbart, J.C.; Bruce, B.D. Non-detergent isolation of a cyanobacterial photosystem I using styrene maleic acid alternating copolymers. RSC Adv. 2019, 9, 31781–31796. [Google Scholar] [CrossRef]
- Jamshad, M.; Charlton, J.; Lin, Y.-P.; Routledge, S.J.; Bawa, Z.; Knowles, T.J.; Overduin, M.; Dekker, N.; Dafforn, T.R.; Bill, R.M.; et al. G-protein coupled receptor solubilization and purification for biophysical analysis and functional studies, in the total absence of detergent. Biosci. Rep. 2015, 35, e00188. [Google Scholar] [CrossRef]
- Hall, S.C.L.; Tognoloni, C.; Charlton, J.; Bragginton, É.C.; Rothnie, A.J.; Sridhar, P.; Wheatley, M.; Knowles, T.J.; Arnold, T.; Edler, K.J.; et al. An acid-compatible co-polymer for the solubilization of membranes and proteins into lipid bilayer-containing nanoparticles. Nanoscale 2018, 10, 10609–10619. [Google Scholar] [CrossRef]
- Lindhoud, S.; Carvalho, V.; Pronk, J.W.; Aubin-Tam, M.-E. SMA-SH: Modified Styrene–Maleic Acid Copolymer for Functionalization of Lipid Nanodiscs. Biomacromolecules 2016, 17, 1516–1522. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Klingler, J.; Danielczak, B.; Babalola, J.O.; Vargas, C.; Pabst, G.; Keller, S. Formation of Lipid-Bilayer Nanodiscs by Diisobutylene/Maleic Acid (DIBMA) Copolymer. Langmuir 2017, 33, 14378–14388. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Danielczak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of Membrane Proteins into Functional Lipid-Bilayer Nanodiscs Using a Diisobutylene/Maleic Acid Copolymer. Angew. Chem. Int. Ed. 2017, 56, 1919–1924. [Google Scholar] [CrossRef]
- Gulamhussein, A.A.; Uddin, R.; Tighe, B.J.; Poyner, D.R.; Rothnie, A.J. A comparison of SMA (styrene maleic acid) and DIBMA (di-isobutylene maleic acid) for membrane protein purification. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183281. [Google Scholar] [CrossRef]
- Danielczak, B.; Meister, A.; Keller, S. Influence of Mg2+ and Ca2+ on nanodisc formation by diisobutylene/maleic acid (DIBMA) copolymer. Chem. Phys. Lipids 2019, 221, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.L.; Rai, M.; Simon, K.S.; Hesketh, S.J.; Teo, A.C.K.; Parmar, M.; Sridhar, P.; Collins, R.; Lee, S.C.; Stroud, Z.N.; et al. SMA-PAGE: A new method to examine complexes of membrane proteins using SMALP nano-encapsulation and native gel electrophoresis. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Angiulli, G.; Dhupar, H.S.; Suzuki, H.; Wason, I.S.; Duong Van Hoa, F.; Walz, T. New approach for membrane protein reconstitution into peptidiscs and basis for their adaptability to different proteins. eLife 2020, 9, e53530. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Warne, T.; Serrano-Vega, M.J.; Baker, J.G.; Moukhametzianov, R.; Edwards, P.C.; Henderson, R.; Leslie, A.G.W.; Tate, C.G.; Schertler, G.F.X. Structure of a β1-adrenergic G-protein-coupled receptor. Nature 2008, 454, 486–491. [Google Scholar] [CrossRef]
- Yao, H.; Cai, H.; Li, D. Thermostabilization of Membrane Proteins by Consensus Mutation: A Case Study for a Fungal Δ8-7 Sterol Isomerase. J. Mol. Biol. 2020, 432, 5162–5183. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.A.; Dodevski, I.; Kenig, M.; Dudli, S.; Mohr, A.; Hermans, E.; Plückthun, A. Directed evolution of a G protein-coupled receptor for expression, stability, and binding selectivity. Proc. Natl. Acad. Sci. USA 2008, 105, 14808–14813. [Google Scholar] [CrossRef]
- Nehmé, R.; Carpenter, B.; Singhal, A.; Strege, A.; Edwards, P.C.; White, C.F.; Du, H.; Grisshammer, R.; Tate, C.G. Mini-G proteins: Novel tools for studying GPCRs in their active conformation. PLoS ONE 2017, 12, e0175642. [Google Scholar] [CrossRef]
- Hunte, C.; Koepke, J.; Lange, C.; Rossmanith, T.; Michel, H. Structure at 2.3 A resolution of the cytochrome bc(1) complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure 2000, 8, 669–684. [Google Scholar] [CrossRef]
- Dutzler, R.; Campbell, E.B.; MacKinnon, R. Gating the selectivity filter in ClC chloride channels. Science 2003, 300, 108–112. [Google Scholar] [CrossRef]
- Iwata, S.; Ostermeier, C.; Ludwig, B.; Michel, H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 1995, 376, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Sennhauser, G.; Amstutz, P.; Briand, C.; Storchenegger, O.; Grütter, M.G. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol. 2007, 5, e7. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Chen, B.R.; Vibat, C.R.T.; Huang, N.; Lee, C.-W.; Chang, G. In vitro nanobody discovery for integral membrane protein targets. Sci. Rep. 2014, 4, 6760. [Google Scholar] [CrossRef]
- Romao, E.; Morales-Yanez, F.; Hu, Y.; Crauwels, M.; De Pauw, P.; Hassanzadeh, G.G.; Devoogdt, N.; Ackaert, C.; Vincke, C.; Muyldermans, S. Identification of Useful Nanobodies by Phage Display of Immune Single Domain Libraries Derived from Camelid Heavy Chain Antibodies. Curr. Pharm. Des. 2016, 22, 6500–6518. [Google Scholar] [CrossRef]
- Huo, J.; Le Bas, A.; Ruza, R.R.; Duyvesteyn, H.M.E.; Mikolajek, H.; Malinauskas, T.; Tan, T.K.; Rijal, P.; Dumoux, M.; Ward, P.N.; et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020, 27, 846–854. [Google Scholar] [CrossRef]
- Uchański, T.; Masiulis, S.; Fischer, B.; Kalichuk, V.; Wohlkönig, A.; Zögg, T.; Remaut, H.; Vranken, W.; Aricescu, A.R.; Pardon, E.; et al. Megabodies expand the nanobody toolkit for protein structure determination by single-particle cryo-EM. bioRxiv 2019, 812230. [Google Scholar] [CrossRef]
- Laverty, D.; Desai, R.; Uchański, T.; Masiulis, S.; Stec, W.J.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Steyaert, J.; Miller, K.W.; et al. Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Nature 2019, 565, 516–520. [Google Scholar] [CrossRef]
- Niesen, F.H.; Berglund, H.; Vedadi, M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2007, 2, 2212–2221. [Google Scholar] [CrossRef]
- Gao, K.; Oerlemans, R.; Groves, M.R. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys. Rev. 2020, 12, 85–104. [Google Scholar] [CrossRef]
- Alexandrov, A.I.; Mileni, M.; Chien, E.Y.T.; Hanson, M.A.; Stevens, R.C. Microscale Fluorescent Thermal Stability Assay for Membrane Proteins. Structure 2008, 16, 351–359. [Google Scholar] [CrossRef]
- Branigan, E.; Pliotas, C.; Hagelueken, G.; Naismith, J.H. Quantification of free cysteines in membrane and soluble proteins using a fluorescent dye and thermal unfolding. Nat. Protoc. 2013, 8, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Bergsdorf, C.; Fiez-Vandal, C.; Sykes, D.A.; Bernet, P.; Aussenac, S.; Charlton, S.J.; Schopfer, U.; Ottl, J.; Duckely, M. An Alternative Thiol-Reactive Dye to Analyze Ligand Interactions with the Chemokine Receptor CXCR2 Using a New Thermal Shift Assay Format. J. Biomol. Screen. 2015, 21, 243–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reis, R.I.; Moraes, I. Probing membrane protein assembly into nanodiscs by in situ dynamic light scattering: A2A receptor as a case study. Biology 2020, 9, 400. [Google Scholar] [CrossRef]
- Birch, J.; Axford, D.; Foadi, J.; Meyer, A.; Eckhardt, A.; Thielmann, Y.; Moraes, I. The fine art of integral membrane protein crystallisation. Methods 2018, 147, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Horne, R.W. A negative staining method for high resolution electron microscopy of viruses. Biochim. Biophys. Acta 1959, 34, 103–110. [Google Scholar] [CrossRef]
- Booth, D.S.; Avila-Sakar, A.; Cheng, Y. Visualizing proteins and macromolecular complexes by negative stain EM: From grid preparation to image acquisition. J. Vis. Exp. 2011, 58, e3227. [Google Scholar] [CrossRef] [PubMed]
- Scarff, C.A.; Fuller, M.J.G.; Thompson, R.F.; Iadaza, M.G. Variations on Negative Stain Electron Microscopy Methods: Tools for Tackling Challenging Systems. J. Vis. Exp. 2018, 132, e57199. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Javorfi, T.; Siligardi, G. Circular dichroism beamline B23 at the Diamond Light Source. J. Synchrotron Radiat. 2012, 19, 132–135. [Google Scholar] [CrossRef]
- Matsuo, K.; Gekko, K. Circular-Dichroism and Synchrotron-Radiation Circular-Dichroism Spectroscopy as Tools to Monitor Protein Structure in a Lipid Environment. In Lipid-Protein Interactions: Methods and Protocols; Kleinschmidt, J.H., Ed.; Springer: New York, NY, USA, 2019; pp. 253–279. [Google Scholar] [CrossRef]
- López, A.; Tarragó, T.; Vilaseca, M.; Giralt, E. Applications and future of ion mobility mass spectrometry in structural biology. New J. Chem. 2013, 37, 1283–1289. [Google Scholar] [CrossRef]
- Moraes, I.; Archer, M. Methods for the Successful Crystallization of Membrane Proteins. In Structural Proteomics: High-Throughput Methods; Owens, R.J., Ed.; Springer: New York, NY, USA, 2015; pp. 211–230. [Google Scholar] [CrossRef]
- Miercke, L.J.W.; Robbins, R.A.; Stroud, R.M. Tetra Detector Analysis of Membrane Proteins. Curr. Protoc. Protein Sci. 2014, 77, 29.10.1–29.10.30. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Warne, T. A colorimetric determination for glycosidic and bile salt-based detergents: Applications in membrane protein research. Anal. Biochem. 2005, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Nadler, T.; Strug, I. Detergent Analysis in Protein Samples Using Mid-Infrared (MIR) Spectroscopy. Curr. Protoc. Protein Sci. 2015, 81, 29.12.1–29.12.15. [Google Scholar] [CrossRef] [PubMed]
- Strug, I.; Utzat, C.; Cappione, A.; Gutierrez, S.; Amara, R.; Lento, J.; Capito, F.; Skudas, R.; Chernokalskaya, E.; Nadler, T. Development of a Univariate Membrane-Based Mid-Infrared Method for Protein Quantitation and Total Lipid Content Analysis of Biological Samples. J. Anal. Methods Chem. 2014, 2014, 657079. [Google Scholar] [CrossRef]
- Luft, J.R.; Newman, J.; Snell, E.H. Crystallization screening: The influence of history on current practice. Acta Crystallogr. Sect. F 2014, 70, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Newstead, S. Membrane Protein Crystallisation: Current Trends and Future Perspectives. In The Next Generation in Membrane Protein Structure Determination; Moraes, I., Ed.; Springer: Cham, Switzerland, 2016; pp. 61–72. [Google Scholar] [CrossRef]
- Ehsan, M.; Katsube, S.; Cecchetti, C.; Du, Y.; Mortensen, J.S.; Wang, H.; Nygaard, A.; Ghani, L.; Loland, C.J.; Kobilka, B.K.; et al. New Malonate-Derived Tetraglucoside Detergents for Membrane Protein Stability. ACS Chem. Biol. 2020, 15, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Arachea, B.T.; Sun, Z.; Potente, N.; Malik, R.; Isailovic, D.; Viola, R.E. Detergent selection for enhanced extraction of membrane proteins. Protein Expr. Purif. 2012, 86, 12–20. [Google Scholar] [CrossRef]
- Breibeck, J.; Rompel, A. Successful amphiphiles as the key to crystallization of membrane proteins: Bridging theory and practice. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 437–455. [Google Scholar] [CrossRef]
- Urner, L.H.; Liko, I.; Yen, H.-Y.; Hoi, K.-K.; Bolla, J.R.; Gault, J.; Almeida, F.G.; Schweder, M.-P.; Shutin, D.; Ehrmann, S.; et al. Modular detergents tailor the purification and structural analysis of membrane proteins including G-protein coupled receptors. Nat. Commun. 2020, 11, 564. [Google Scholar] [CrossRef]
- Gourdon, P.; Andersen, J.L.; Hein, K.L.; Bublitz, M.; Pedersen, B.P.; Liu, X.-Y.; Yatime, L.; Nyblom, M.; Nielsen, T.T.; Olesen, C.; et al. HiLiDe—Systematic Approach to Membrane Protein Crystallization in Lipid and Detergent. Cryst. Growth Des. 2011, 11, 2098–2106. [Google Scholar] [CrossRef]
- Sitsel, O.; Wang, K.; Liu, X.; Gourdon, P. Crystallization of P-type ATPases by the High Lipid-Detergent (HiLiDe) Method. Methods Mol. Biol. 2016, 1377, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Landau, E.M.; Rosenbusch, J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar] [CrossRef] [PubMed]
- Wadsten, P.; Wöhri, A.B.; Snijder, A.; Katona, G.; Gardiner, A.T.; Cogdell, R.J.; Neutze, R.; Engström, S. Lipidic Sponge Phase Crystallization of Membrane Proteins. J. Mol. Biol. 2006, 364, 44–53. [Google Scholar] [CrossRef]
- Ujwal, R.; Bowie, J.U. Crystallizing membrane proteins using lipidic bicelles. Methods 2011, 55, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Misquitta, Y.; Caffrey, M. Rational design of lipid molecular structure: A case study involving the C19:1c10 monoacylglycerol. Biophys. J. 2001, 81, 1047–1058. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Caffrey, M. Structure and Functional Characterization of Membrane Integral Proteins in the Lipid Cubic Phase. J. Mol. Biol. 2020, 432, 5104–5123. [Google Scholar] [CrossRef]
- Cherezov, V.; Liu, J.; Griffith, M.; Hanson, M.A.; Stevens, R.C. LCP-FRAP Assay for Pre-Screening Membrane Proteins for In Meso Crystallization. Cryst. Growth Des. 2008, 8, 4307–4315. [Google Scholar] [CrossRef]
- Xu, F.; Liu, W.; Hanson, M.A.; Stevens, R.C.; Cherezov, V. Development of an Automated High Throughput LCP-FRAP Assay to Guide Membrane Protein Crystallization in Lipid Mesophases. Cryst. Growth Des. 2011, 11, 1193–1201. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; Cherezov, V. Structural insights into melatonin receptors. FEBS J. 2020, 287, 1496–1510. [Google Scholar] [CrossRef]
- Nogly, P.; James, D.; Wang, D.; White, T.A.; Zatsepin, N.; Shilova, A.; Nelson, G.; Liu, H.; Johansson, L.; Heymann, M.; et al. Lipidic cubic phase serial millisecond crystallography using synchrotron radiation. IUCrJ 2015, 2, 168–176. [Google Scholar] [CrossRef]
- Cipriani, F.; Felisaz, F.; Launer, L.; Aksoy, J.-S.; Caserotto, H.; Cusack, S.; Dallery, M.; di-Chiaro, F.; Guijarro, M.; Huet, J.; et al. Automation of sample mounting for macromolecular crystallography. Acta Crystallogr. Sect. D 2006, 62, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.; Rower, M.; Landret, C.; Zander, U.; Felisaz, F.; Marquez, J.A. CrystalDirect: A new method for automated crystal harvesting based on laser-induced photoablation of thin films. Acta Crystallogr. Sect. D 2012, 68, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.D.; Collins, P.; Talon, R.; Nelson, E.; Koekemoer, L.; Ye, M.; Nowak, R.; Newman, J.; Ng, J.T.; Mitrovich, N.; et al. The Low-Cost, Semi-Automated Shifter Microscope Stage Transforms Speed and Robustness of Manual Protein Crystal Harvesting. bioRxiv 2019. [Google Scholar] [CrossRef]
- Huang, C.Y.; Olieric, V.; Ma, P.; Howe, N.; Vogeley, L.; Liu, X.; Warshamanage, R.; Weinert, T.; Panepucci, E.; Kobilka, B.; et al. In meso in situ serial X-ray crystallography of soluble and membrane proteins at cryogenic temperatures. Acta Cryst. D Struct. Biol. 2016, 72, 93–112. [Google Scholar] [CrossRef]
- Axford, D.; Aller, P.; Sanchez-Weatherby, J.; Sandy, J. Applications of thin-film sandwich crystallization platforms. Acta Cryst. F Struct Biol. Commun. 2016, 72, 313–319. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Olieric, V.; Howe, N.; Warshamanage, R.; Weinert, T.; Panepucci, E.; Vogeley, L.; Basu, S.; Diederichs, K.; Caffrey, M.; et al. In situ serial crystallography for rapid de novo membrane protein structure determination. Commun. Biol. 2018, 1, 124. [Google Scholar] [CrossRef]
- Le Maire, A.; Gelin, M.; Pochet, S.; Hoh, F.; Pirocchi, M.; Guichou, J.-F.; Ferrer, J.-L.; Labesse, G. In-plate protein crystallization, in situ ligand soaking and X-ray diffraction. Acta Crystallogr. Sect. D 2011, 67, 747–755. [Google Scholar] [CrossRef]
- Bingel-Erlenmeyer, R.; Olieric, V.; Grimshaw, J.P.A.; Gabadinho, J.; Wang, X.; Ebner, S.G.; Isenegger, A.; Schneider, R.; Schneider, J.; Glettig, W.; et al. SLS Crystallization Platform at Beamline X06DA—A Fully Automated Pipeline Enabling in Situ X-ray Diffraction Screening. Cryst. Growth Des. 2011, 11, 916–923. [Google Scholar] [CrossRef]
- Sanchez-Weatherby, J.; Sandy, J.; Mikolajek, H.; Lobley, C.M.C.; Mazzorana, M.; Kelly, J.; Preece, G.; Littlewood, R.; Sørensen, T.L.M. VMXi: A fully automated, fully remote, high-flux in situ macromolecular crystallography beamline. J. Synchrotron Radiat. 2019, 26, 291–301. [Google Scholar] [CrossRef]
- Evans, G.; Axford, D.; Owen, R.L. The design of macromolecular crystallography diffraction experiments. Acta Crystallogr. Sect. D 2011, 67, 261–270. [Google Scholar] [CrossRef]
- Trincao, J.; Warren, A.; Aller, P.; Duller, G.; Wilkinson, K.; Stallwood, A.; Laundy, D.; Allianeli, L.; Sahwney, K.; Rehm, G.; et al. VMXm: A new sub-micron beamline for macromolecular crystallography at Diamond Light Source. Acta Crystallogr. Sect. A 2015, 71, s191. [Google Scholar] [CrossRef]
- Sanishvili, R.; Nagarajan, V.; Yoder, D.; Becker, M.; Xu, S.; Corcoran, S.; Akey, D.L.; Smith, J.L.; Fischetti, R.F. A 7 microm mini-beam improves diffraction data from small or imperfect crystals of macromolecules. Acta Crystallogr. Sect. D 2008, 64, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Duman, R.; Henderson, K.; Mykhaylyk, V. In-vacuum long-wavelength macromolecular crystallography. Acta Crystallogr. Sect. D 2016, 72, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bountra, K.; Hagelueken, G.; Choudhury, H.G.; Corradi, V.; El Omari, K.; Wagner, A.; Mathavan, I.; Zirah, S.; Yuan Wahlgren, W.; Tieleman, D.P.; et al. Structural basis for antibacterial peptide self-immunity by the bacterial ABC transporter McjD. EMBO J. 2017, 36, 3062–3079. [Google Scholar] [CrossRef]
- Polsinelli, I.; Savko, M.; Rouanet-Mehouas, C.; Ciccone, L.; Nencetti, S.; Orlandini, E.; Stura, E.A.; Shepard, W. Comparison of helical scan and standard rotation methods in single-crystal X-ray data collection strategies. J. Synchrotron Radiat. 2017, 24, 42–52. [Google Scholar] [CrossRef]
- Quigley, A.; Dong, Y.Y.; Pike, A.C.; Dong, L.; Shrestha, L.; Berridge, G.; Stansfeld, P.J.; Sansom, M.S.; Edwards, A.M.; Bountra, C.; et al. The structural basis of ZMPSTE24-dependent laminopathies. Science 2013, 339, 1604–1607. [Google Scholar] [CrossRef]
- Cheng, Y. Membrane protein structural biology in the era of single particle cryo-EM. Curr. Opin. Struct. Biol. 2018, 52, 58–63. [Google Scholar] [CrossRef]
- Nygaard, R.; Kim, J.; Mancia, F. Cryo-electron microscopy analysis of small membrane proteins. Curr. Opin. Struct. Biol. 2020, 64, 26–33. [Google Scholar] [CrossRef]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef]
- Nakane, T.; Kotecha, A.; Sente, A.; McMullan, G.; Masiulis, S.; Brown, P.M.G.E.; Grigoras, I.T.; Malinauskaite, L.; Malinauskas, T.; Miehling, J.; et al. Single-particle cryo-EM at atomic resolution. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kühlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef]
- McMullan, G.; Faruqi, A.R.; Henderson, R. Direct Electron Detectors. Methods Enzymol. 2016, 579, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Calzada, A.; Carroni, M. Editorial: Technical Advances in Cryo-Electron Microscopy. Front. Mol. Biosci. 2019, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Fan, X. Challenges and opportunities in cryo-EM with phase plate. Curr. Opin. Struct. Biol. 2019, 58, 175–182. [Google Scholar] [CrossRef]
- Jain, T.; Sheehan, P.; Crum, J.; Carragher, B.; Potter, C.S. Spotiton: A prototype for an integrated inkjet dispense and vitrification system for cryo-TEM. J. Struct. Biol. 2012, 179, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.J.; Passmore, L.A. Electron microscopy: Ultrastable gold substrates for electron cryomicroscopy. Science 2014, 346, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- García-Nafría, J.; Tate, C.G. Cryo-Electron Microscopy: Moving Beyond X-ray Crystal Structures for Drug Receptors and Drug Development. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 51–71. [Google Scholar] [CrossRef]
- Johansson, L.C.; Stauch, B.; Ishchenko, A.; Cherezov, V. A Bright Future for Serial Femtosecond Crystallography with XFELs. Trends Biochem. Sci. 2017, 42, 749–762. [Google Scholar] [CrossRef]
- Weiss, M.S. Long-Wavelength X-ray Diffraction and Its Applications in Macromolecular Crystallography. In Protein Crystallography: Methods and Protocols; Wlodawer, A., Dauter, Z., Jaskolski, M., Eds.; Springer: New York, NY, USA, 2017; pp. 401–420. [Google Scholar] [CrossRef]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Acta Cryst. F Struct. Biol. Commun. 2014, 70, 2–20. [Google Scholar] [CrossRef]
- Ognjenović, J.; Grisshammer, R.; Subramaniam, S. Frontiers in Cryo Electron Microscopy of Complex Macromolecular Assemblies. Annu. Rev. Biomed. Eng. 2019, 21, 395–415. [Google Scholar] [CrossRef]
- Bloch, M.; Santiveri, M.; Taylor, N.M.I. Membrane Protein Cryo-EM: Cryo-Grid Optimization and Data Collection with Protein in Detergent. In Expression, Purification, and Structural Biology of Membrane Proteins; Perez, C., Maier, T., Eds.; Springer: New York, NY, USA, 2020; pp. 227–244. [Google Scholar] [CrossRef]
- Yao, X.; Fan, X.; Yan, N. Cryo-EM analysis of a membrane protein embedded in the liposome. Proc. Natl. Acad. Sci. USA 2020, 117, 18497–18503. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Gonen, T. Beyond protein structure determination with MicroED. Curr. Opin. Struct. Biol. 2020, 64, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F.; Walker, M.; Siebert, C.A.; Muench, S.P.; Ranson, N.A. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods 2016, 100, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Lyumkis, D. Challenges and opportunities in cryo-EM single-particle analysis. J. Biol. Chem. 2019, 294, 5181–5197. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. Sect. D 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zivanov, J.; Nakane, T.; Forsberg, B.O.; Kimanius, D.; Hagen, W.J.H.; Lindahl, E.; Scheres, S.H.W. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 2018, 7, e42166. [Google Scholar] [CrossRef] [PubMed]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef]
- Congreve, M.; Oswald, C.; Marshall, F.H. Applying Structure-Based Drug Design Approaches to Allosteric Modulators of GPCRs. Trends Pharmacol. Sci. 2017, 38, 837–847. [Google Scholar] [CrossRef]
- Scapin, G.; Potter, C.S.; Carragher, B. Cryo-EM for Small Molecules Discovery, Design, Understanding, and Application. Cell Chem. Biol. 2018, 25, 1318–1325. [Google Scholar] [CrossRef]
- Saur, M.; Hartshorn, M.J.; Dong, J.; Reeks, J.; Bunkoczi, G.; Jhoti, H.; Williams, P.A. Fragment-based drug discovery using cryo-EM. Drug Discov. Today 2020, 25, 485–490. [Google Scholar] [CrossRef]
- Marques, M.A.; Purdy, M.D.; Yeager, M. CryoEM maps are full of potential. Curr. Opin. Struct. Biol. 2019, 58, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Frank, J.; Moore, P.B. Identification of ions in experimental electrostatic potential maps. IUCrJ 2018, 5, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kosinska Eriksson, U.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom Resolution X-ray Structure Details Aquaporin-Water Interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef]
- Efremov, R.G.; Gatsogiannis, C.; Raunser, S. Lipid Nanodiscs as a Tool for High-Resolution Structure Determination of Membrane Proteins by Single-Particle Cryo-EM. Methods Enzymol. 2017, 594, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Paracini, N.; Hughes, A.V.; Lakey, J.H.; Steinke, N.-J.; Cooper, J.F.K.; Gavutis, M.; Skoda, M.W.A. Self-Assembled Fluid Phase Floating Membranes with Tunable Water Interlayers. Langmuir 2019, 35, 13735–13744. [Google Scholar] [CrossRef] [PubMed]
- Gabel, F. Applications of SANS to Study Membrane Protein Systems. In Biological Small Angle Scattering: Techniques, Strategies and Tips; Chaudhuri, B., Muñoz, I.G., Qian, S., Urban, V.S., Eds.; Springer: Singapore, 2017; pp. 201–214. [Google Scholar] [CrossRef]
- Josts, I.; Nitsche, J.; Maric, S.; Mertens, H.D.; Moulin, M.; Haertlein, M.; Prevost, S.; Svergun, D.I.; Busch, S.; Forsyth, V.T.; et al. Conformational States of ABC Transporter MsbA in a Lipid Environment Investigated by Small-Angle Scattering Using Stealth Carrier Nanodiscs. Structure 2018, 26, 1072–1079. [Google Scholar] [CrossRef]
- Semeraro, E.F.; Marx, L.; Frewein, M.P.K.; Pabst, G. Increasing complexity in small-angle X-ray and neutron scattering experiments: From biological membrane mimics to live cells. Soft Matter 2020. [Google Scholar] [CrossRef]
- Chorev, D.S.; Baker, L.A.; Wu, D.; Beilsten-Edmands, V.; Rouse, S.L.; Zeev-Ben-Mordehai, T.; Jiko, C.; Samsudin, F.; Gerle, C.; Khalid, S.; et al. Protein assemblies ejected directly from native membranes yield complexes for mass spectrometry. Science 2018, 362, 829–834. [Google Scholar] [CrossRef]
- Hutchings, J.; Zanetti, G. Fine details in complex environments: The power of cryo-electron tomography. Biochem. Soc. Trans. 2018, 46, 807–816. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Rettberg, L.A.; Treuner-Lange, A.; Iwasa, J.; Søgaard-Andersen, L.; Jensen, G.J. Architecture of the type IVa pilus machine. Science 2016, 351, aad2001. [Google Scholar] [CrossRef]
- Cassidy, C.K.; Himes, B.A.; Alvarez, F.J.; Ma, J.; Zhao, G.; Perilla, J.R.; Schulten, K.; Zhang, P. CryoEM and computer simulations reveal a novel kinase conformational switch in bacterial chemotaxis signaling. eLife 2015, 4, e08419. [Google Scholar] [CrossRef] [PubMed]
- Kudryashev, M.; Castaño-Díez, D.; Deluz, C.; Hassaine, G.; Grasso, L.; Graf-Meyer, A.; Vogel, H.; Stahlberg, H. The Structure of the Mouse Serotonin 5-HT3 Receptor in Lipid Vesicles. Structure 2016, 24, 165–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuijtel, M.W.; Koster, A.J.; Jakobs, S.; Faas, F.G.A.; Sharp, T.H. Correlative cryo super-resolution light and electron microscopy on mammalian cells using fluorescent proteins. Sci. Rep. 2019, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
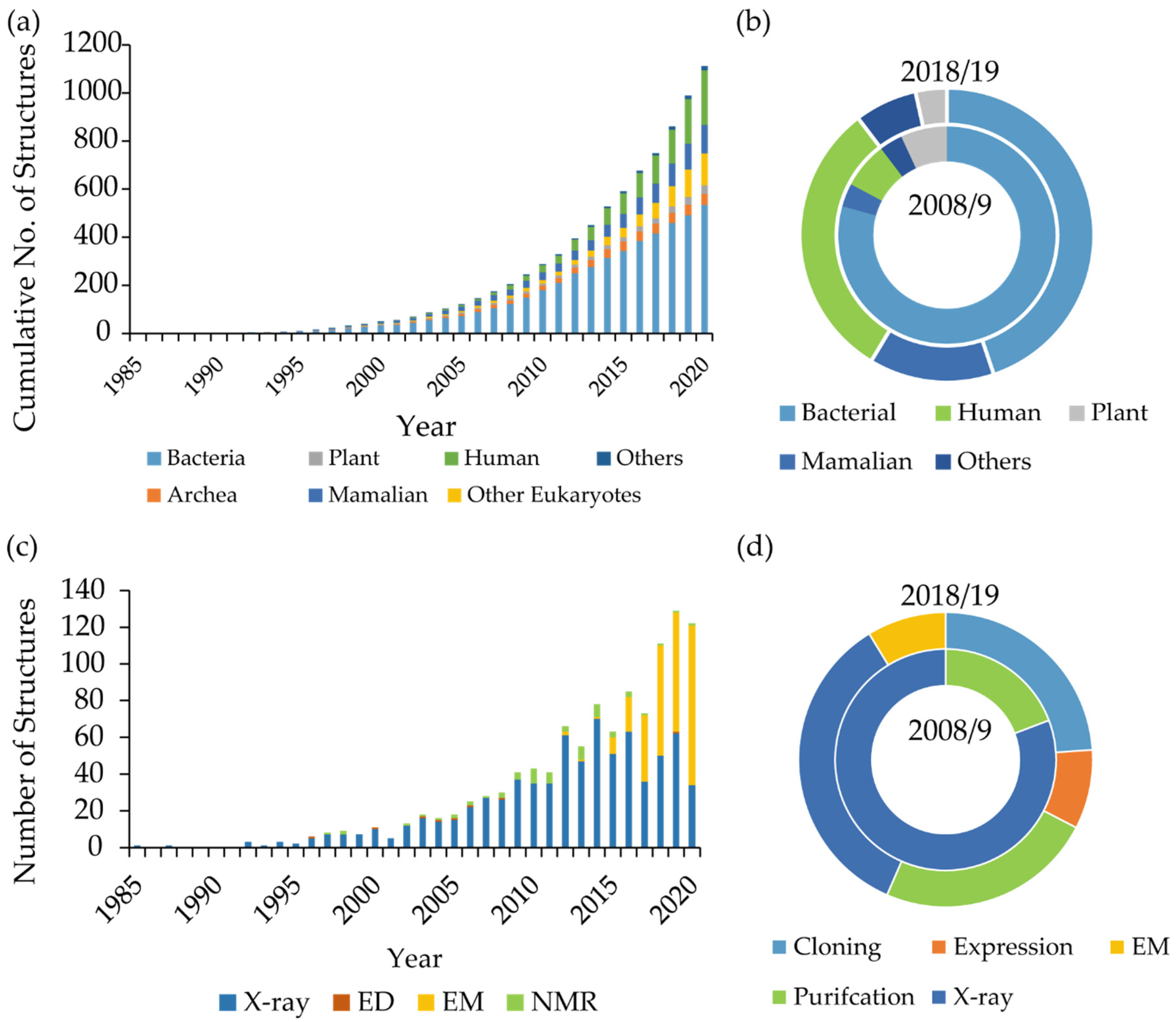

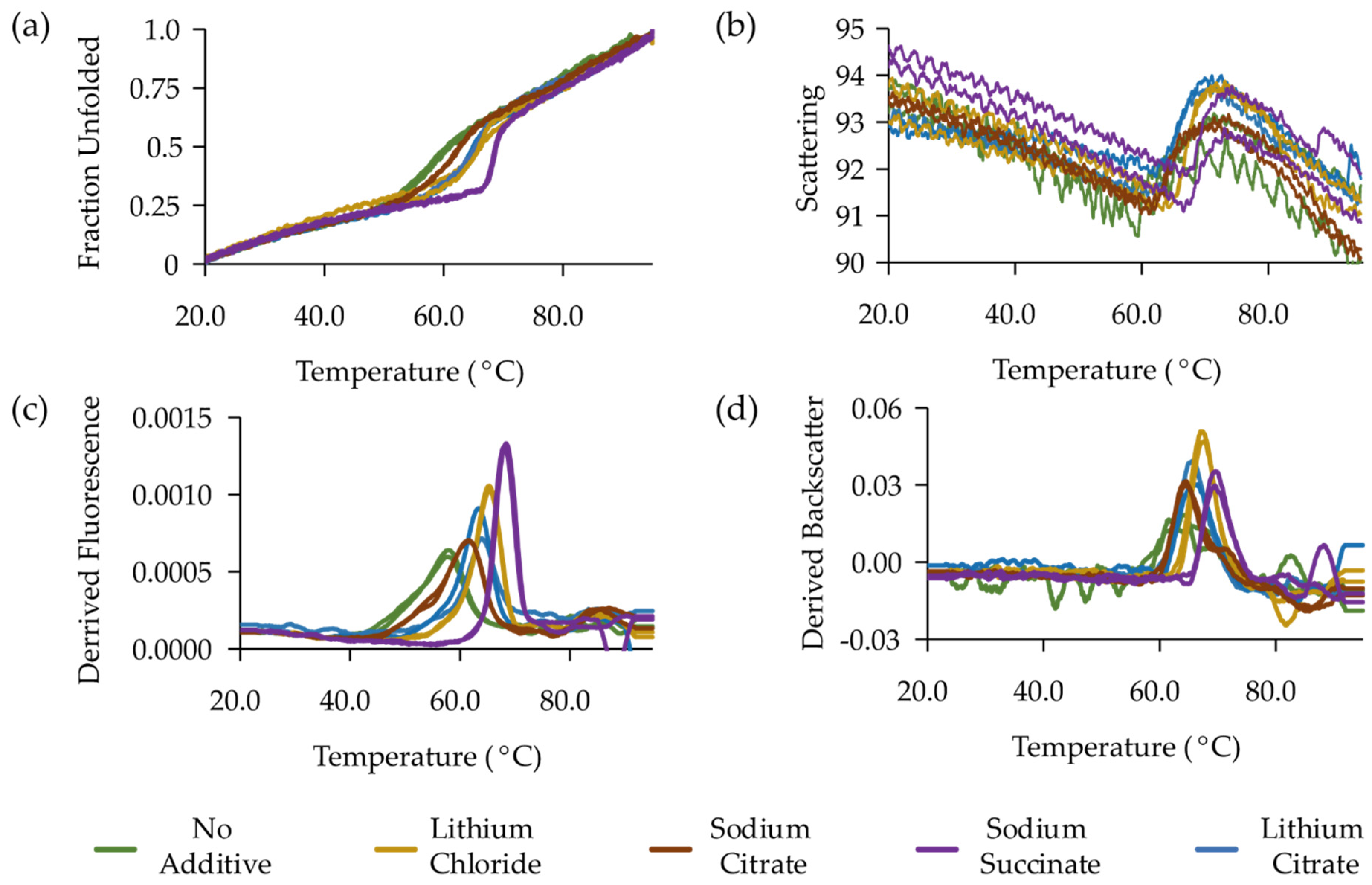
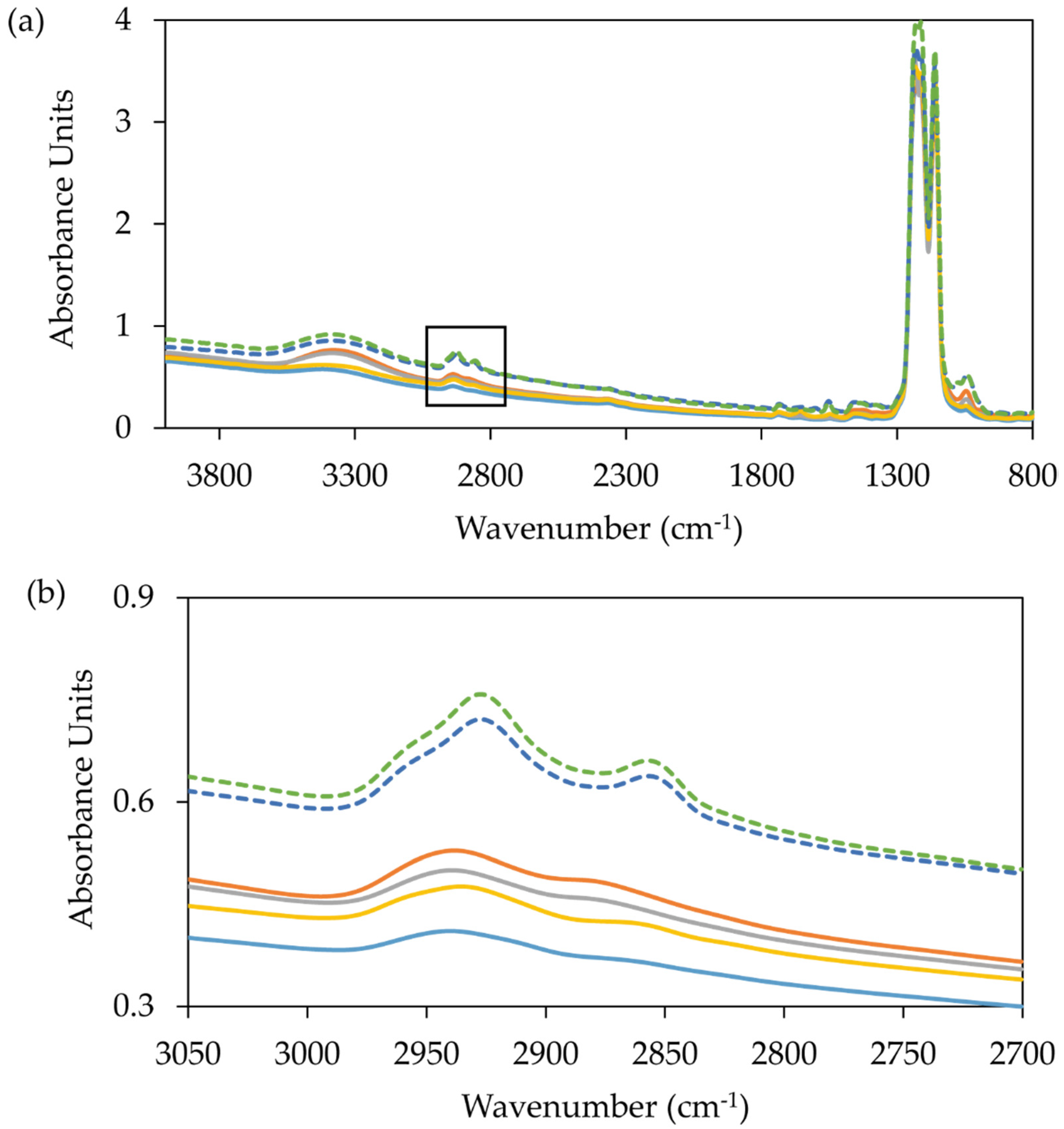
| MP Target | Target Origin | PDB Accession Code | Year | Publication |
|---|---|---|---|---|
| PsrABC | Bacterial | 2VPW, 2VPX, 2VPY, 2VPZ | 2008 | [17] |
| AcrB | Bacterial | 2W1B | 2008 | [18] |
| MHP1 | Bacterial | 2JLN, 2JLO, 2 × 79 | 2008/2010 | [19,20] |
| POT | Bacterial | 2XUT | 2010 | [21] |
| H1R | Human | 3RZE | 2011 | [22] |
| ASBT | Bacterial | 3ZUY, 3ZUX | 2011 | [23] |
| MGL | Bacterial | 3RM3, 3RLI | 2012 | [24] |
| NavAb | Bacterial | 4F4L | 2012 | [25] |
| RCE1 | Bacterial | 3VG9 | 2013 | [26] |
| IPCT-DIPPS | Bacterial | 4MND | 2014 | [27] |
| EptA | Bacterial | 5FGN | 2015 | [28] |
| MraY | Bacterial | 5JNQ | 2016 | [29] |
| SiaT | Bacterial | 5NV9, 5NVA | 2017 | [30] |
| PfeA | Bacterial | 6R1F, 6I2J | 2018 | [31] |
| AR3 | Archaea | 6S63, 6S6C, 6GUY, 6GUX, 6GUZ | 2019 | - |
| Vector | Selection Marker (E. coli/Host) | Tagged Terminus | Protease | Tags | Expression Host(s) | Reference |
|---|---|---|---|---|---|---|
| pWaldo GFP 8His 1 | Kan/Kan | C | TEV | GFP-8His | E | [45] |
| pDD-GFP2 1 | Amp/Uracil dropout | C | TEV | GFP-8His | S | [46] |
| pOPINEneo 3C-GFP 2 | Amp/Neo | C | 3C | GFP-8His | E, I, M | Addgene plasmid # 53534 |
| pOPINEneo TEV-GFP | Amp/Neo | C | 3C | GFP-8His | E, I, M | - |
| pOPINE-BAP 2 | Amp/- | C | - | Biotin | E, I, M | Unpublished |
| pOPINS3C 2 | Amp/- | N | 3C | SUMO | E, I, M | [47], Addgene plasmid # 41115 |
| pOPINF 2 | Amp/- | N | - | 6His | E, I, M | [41], Addgene plasmid # 26042 |
| Popinj 2 | Amp/- | N | 3C | 6His-GST | E, I, M | [41], Addgene plasmid # 26045 |
| pOPIN- HALO7 2 | Amp/- | N | 3C | 6His-HALO7 | E, I, M | As for pOPINS3C, Addgene plasmid # 41117 |
| pOPINE-3C-HALO7 2 | Amp/- | C | 3C | HALO7-6His | E, I, M | Addgene plasmid # 41126, unpublished |
| pOPINEneo-3C-2Strep 2 | Amp/Neo | C | 3C | 2StrepII-8His | E, I, M | Unpublished |
| pOPINEneo-3C-3FLAG 2 | Amp/Neo | C | 3C | 3FLAG-8His | E, I, M | Unpublished |
| X-ray Crystallography | Cryo-EM | |
|---|---|---|
| Protein size range | Average size of solved structures is ~100 kDa [7]. | Typically above 100 kDa. Volta Phase plates have been used to boost signal-to-noise for smaller proteins or binders such as Fabs and megabodies can be used to increase particle size [163,217]. |
| Sample heterogeneity | Usually a homogeneous sample is required [229]. | Can tolerate some sample heterogeneity but homogeneous samples lead more quickly to higher resolution structures [216]. |
| Sample concentration | Large quantities of pure protein [230]. Typically 100 to 200 µL at 5 to 40 mg mL−1. | Small quantities of pure protein [230]. Less than 10 to 100 µL at 0.5 to 5 mg mL−1. |
| Sample preparation | Relies on obtaining diffracting MP crystals which are difficult to obtain. MP must be removed from its native environment. Crystals grown in crystallisation trays, mounted in a loop and cryo-cooled in liquid nitrogen [171]. | MP blotted on to EM grids and vitrified in liquid ethane [110,231]. Single particle analysis can be carried out on proteoliposomes providing a more native environment [232]. |
| Screening throughput | High. Typically in 96 well plates, allowing 100s of conditions to be sampled simultaneously [171]. | Low. Each condition to be screened must be imaged individually. Negative stain can be used to narrow screening conditions [216]. |
| Collection method | X-ray diffraction of protein in crystalline lattice, typically using a synchrotron source [171]. Microcrystal electron diffraction is an area of increasing interest [233]. | Electron imaging in conjunction with a direct electron detector. Energy filters and phase plates may be helpful [234]. |
| Collection throughput | High. Typically, 15–30 crystals per hour [7]. | Low. Time taken several orders of magnitude behind X-ray crystallography [235]. |
| Data Analysis | Quick and highly automated. Complete datasets can be collected in seconds. Many synchrotrons have automated processing pipelines integrated into the data collection process [7]. Well established software suites such as CCP4i2 to aid the crystallographer [236]. | Slow. Reconstructions from 1000s of single images can take many days. Processing pipeline can be automated. Software packages to analyse data less established but constantly improving. Examples include RELION and cryoSPARC [237,238]. |
| Structure-based drug design | Routine, high resolution and high throughput. Well established for GPCRs [13,239]. | Currently lacks reproducibility, quality and throughput. Ideally requires protein structures at a resolution of less than 3 Å [240,241]. |
| MP conformational flexibility | Each crystal form relates to a single rigid MP conformation. | MP can be in different conformations, which can be identified during processing (but also impede processing) [216]. |
| Ion identification | Generally straightforward depending on resolution. Long-wavelength beamlines enables sodium ion to be distinguished from a potassium ions [228]. | Difficult to identify some anions ions in maps due to negative scattering factors [242]. Electrostatic potential maps may help to overcome this [243]. |
| Resolution | Typical range between 1.5 Å and 3.5 Å. For MPs crystallised in LCP Sub 2.5 Å are common. Highest resolution structure currently a yeast aquaporin at 0.88 Å, PDB: 3ZOJ [244]. | Typically, 2.5–4 Å are common including some smaller membrane proteins [217]. Highest resolution structure currently the β3 GABAA receptor homopentamer at 1.7 Å, PDB: 7A5V [219]. EM density maps can identify protein and ion charge states [242]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birch, J.; Cheruvara, H.; Gamage, N.; Harrison, P.J.; Lithgo, R.; Quigley, A. Changes in Membrane Protein Structural Biology. Biology 2020, 9, 401. https://doi.org/10.3390/biology9110401
Birch J, Cheruvara H, Gamage N, Harrison PJ, Lithgo R, Quigley A. Changes in Membrane Protein Structural Biology. Biology. 2020; 9(11):401. https://doi.org/10.3390/biology9110401
Chicago/Turabian StyleBirch, James, Harish Cheruvara, Nadisha Gamage, Peter J. Harrison, Ryan Lithgo, and Andrew Quigley. 2020. "Changes in Membrane Protein Structural Biology" Biology 9, no. 11: 401. https://doi.org/10.3390/biology9110401
APA StyleBirch, J., Cheruvara, H., Gamage, N., Harrison, P. J., Lithgo, R., & Quigley, A. (2020). Changes in Membrane Protein Structural Biology. Biology, 9(11), 401. https://doi.org/10.3390/biology9110401





