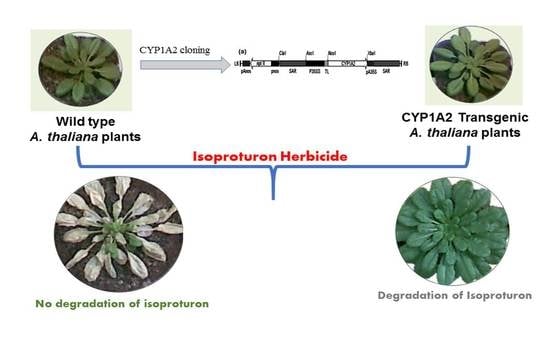
Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Constructs, Plant Transformation, and Growth Conditions
2.2. PCR and Western Blot
2.3. Evaluation of Isoproturon Phytotoxicity on Plant Growth
2.4. Effect of Isoproturon Treatment on Photosynthetic Pigments
2.5. Statistical Analysis
3. Results
3.1. Transgenic A. thaliana Plants Transformation and Selection
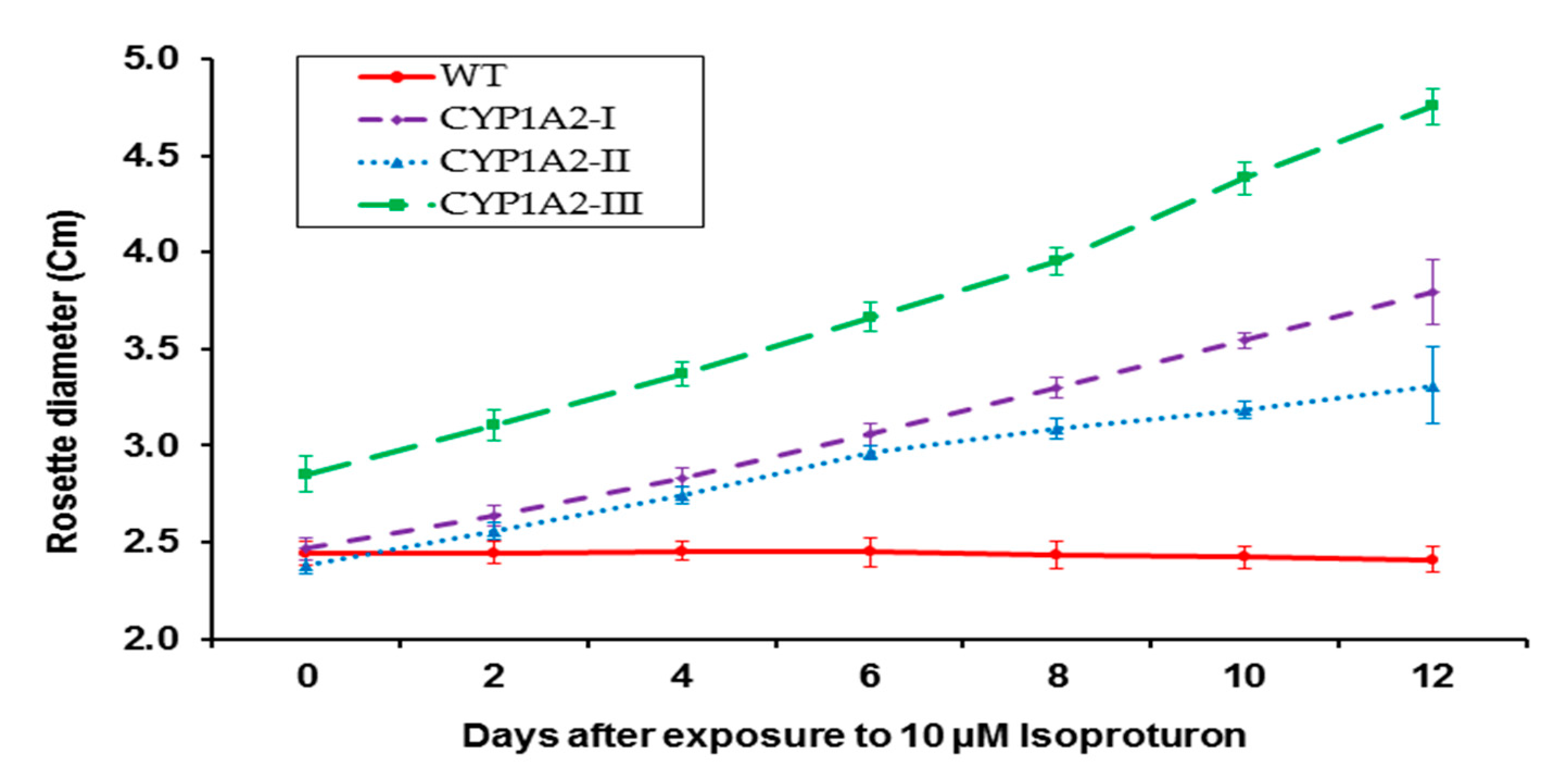
3.2. Effect of Isoproturon Treatments on A. thaliana Plant Primary Root Lengths and Rosette Diameter
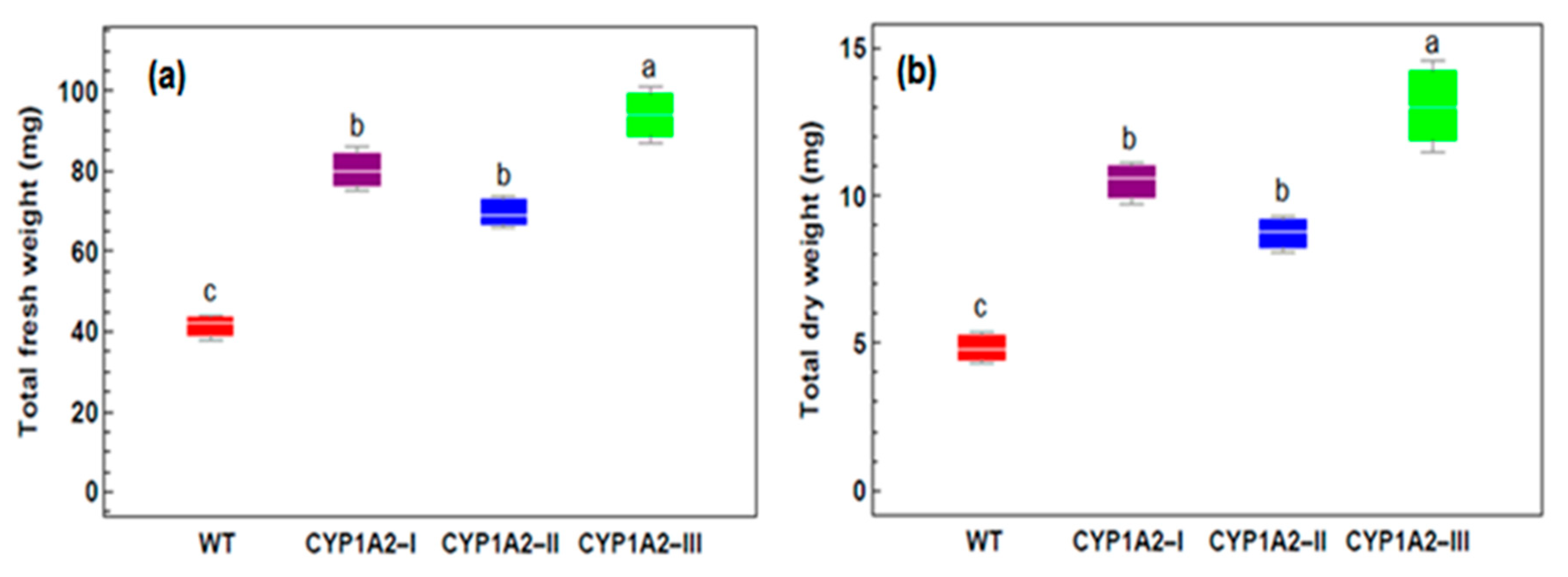
3.3. Effect of Isoproturon Treatments on the Total Plant Fresh and Dry Weight
3.4. Effect of Isoproturon Treatments on Rosette Diameter of Wild Type and CYP1A2 Transgenic Plants
3.5. Phenotype of Wild Type (WT) and CYP1A2 Transgenic A. thaliana Plants under Different Concentrations of Isoproturon
3.6. Chlorophyll Content of Wild and Transgenic Plants under Isoproturon Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Chapter 80—Phenylurea Herbicides. In Hayes’ Handbook of Pesticide Toxicology (Third Edition); Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1725–1731. [Google Scholar] [CrossRef]
- Yin, X.L.; Jiang, L.; Song, N.H.; Yang, H. Toxic reactivity of wheat (Triticum aestivum) plants to herbicide isoproturon. J. Agric. Food Chem. 2008, 56, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Lu, Y.C.; Zhang, S.; Luo, F.; Yang, H. Rice (Oryza sativa) laccases involved in modification and detoxification of herbicides atrazine and isoproturon residues in plants. J. Agric. Food Chem. 2016, 64, 6397–6406. [Google Scholar] [CrossRef] [PubMed]
- Lebailly, P.; Bouchart, V.; Baldi, I.; Lecluse, Y.; Heutte, N.; Gislard, A.; Malas, J.-P. Exposure to pesticides in open-field farming in France. Ann. Occup. Hyg. 2008, 53, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Jansen, M.; Altenburger, R. Toxic effects of isoproturon on periphyton communities—A microcosm study. Estuar. Coast. Shelf Sci. 2005, 62, 539–545. [Google Scholar] [CrossRef]
- Zeng, S.; Qin, X.; Xia, L. Degradation of the herbicide isoproturon by laccase-mediator systems. Biochem. Eng. J. 2017, 119, 92–100. [Google Scholar] [CrossRef]
- Sørensen, S.R.; Bending, G.D.; Jacobsen, C.S.; Walker, A.; Aamand, J. Microbial degradation of isoproturon and related phenylurea herbicides in and below agricultural fields. FEMS Microbiol. Ecol. 2003, 45, 1–11. [Google Scholar] [CrossRef]
- Liu, J. CHAPTER 67—Phenylurea herbicides. In Handbook of Pesticide Toxicology (Second Edition); Krieger, R.I., Krieger, W.C., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 1521–1527. [Google Scholar] [CrossRef]
- Behera, B.C.; Bhunya, S.P. Genotoxic effect of isoproturon (herbicide) as revealed by three mammalian in vivo mutagenic bioassays. Indian J. Exp. Biol. 1990, 28, 862–867. [Google Scholar]
- Srivastava, M.K.; Raizada, R.B. Developmental toxicity of the substituted phenylurea herbicide isoproturon in rats. Vet. Hum. Toxicol. 1995, 37, 220–223. [Google Scholar]
- Authority, E.F.S. Conclusion on the peer review of the pesticide risk assessment of the active substance isoproturon. EFSA J. 2015, 13, 4206. [Google Scholar] [CrossRef]
- Disposal of waste. In Prudent Practices in the Laboratory: Handling and Disposal of Chemicals; Council, N.R., Ed.; The National Academies Press: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Scow, K.M.; Hicks, K.A. Natural attenuation and enhanced bioremediation of organic contaminants in groundwater. Curr. Opin. Biotechnol. 2005, 16, 246–253. [Google Scholar] [CrossRef]
- Kanissery, R.G.; Sims, G.K. Biostimulation for the enhanced degradation of herbicides in soil. Appl. Environ. Soil Sci. 2011, 2011, 843450. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O. Chapter 1—Phytoremediation: From Theory Toward Practice. In Phytomanagement of Polluted Sites; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–49. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Emam, M.H.; Lovett-Doust, L.; Azab, E.; El-Khatib, A.A. Response of duckweed to lead exposure: Phytomining, bioindicators and bioremediation. Desal. Water Treat. 2017, 70, 227–234. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A.K. Monitoring the efficiency of Rhazya stricta L. plants in phytoremediation of heavy metal-contaminated soil. Plants 2020, 9, 1057. [Google Scholar] [CrossRef]
- Peuke, A.D.; Rennenberg, H. Phytoremediation. EMBO Rep. 2005, 6, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Reynolds, C.M. Phytodegradation of organic compounds. Curr. Opin. Biotechnol. 2004, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.F.; Cavalieri, E.L.; Rogan, E.G. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16[alpha ]-hydroxylation of 17[beta ]-estradiol. Metab. Clin. Exp. 2001, 50, 1001–1003. [Google Scholar] [CrossRef]
- Bylund, J.; Kunz, T.; Valmsen, K.; Oliw, E.H. Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J. Pharmacol. Exp. Ther. 1998, 284, 51–60. [Google Scholar]
- Chen, H.; Howald, W.N.; Juchau, M.R. Biosynthesis of all-trans-retinoic acid from all-trans-retinol: Catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metab. Dispos. Biol. Fate Chem. 2000, 28, 315–322. [Google Scholar]
- Lee, A.J.; Cai, M.X.; Thomas, P.E.; Conney, A.H.; Zhu, B.T. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology 2003, 144, 3382–3398. [Google Scholar] [CrossRef]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Engineering cytochrome P450 biocatalysts for biotechnology, medicine and bioremediation. Expert Opin. Drug Metab. Toxicol. 2010, 6, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jin, M.; Weemhoff, J.L. Cytochrome P450-mediated phytoremediation using transgenic plants: A need for engineered cytochrome P450 enzymes. J. Pet. Environ. Biotechnol. 2012, 3, 1000127. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; Bassard, J.-E.; Hamberger, B.; Werck-Reichhart, D. Cytochrome P450-mediated metabolic engineering: Current progress and future challenges. Curr. Opin. Plant Biol. 2014, 19, 27–34. [Google Scholar] [CrossRef]
- Kawahigashi, H.; Hirose, S.; Ohkawa, H.; Ohkawa, Y. Herbicide resistance of transgenic rice plants expressing human CYP1A1. Biotechnol. Adv. 2007, 25, 75–84. [Google Scholar] [CrossRef]
- Jhansi Rani, S.; Usha, R. Transgenic plants: Types, benefits, public concerns and future. J. Pharm. Res. 2013, 6, 879–883. [Google Scholar] [CrossRef]
- Key, S.; Ma, J.K.C.; Drake, P.M. Genetically modified plants and human health. J. R. Soc. Med. 2008, 101, 290–298. [Google Scholar] [CrossRef]
- James, C.A.; Strand, S.E. Phytoremediation of small organic contaminants using transgenic plants. Curr. Opin. Biotechnol. 2009, 20, 237–241. [Google Scholar] [CrossRef]
- Bevan, M.; Walsh, S. The Arabidopsis genome: A foundation for plant research. Genome Res. 2005, 15, 1632–1642. [Google Scholar] [CrossRef]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Azab, E.; Kebeish, R.; Hegazy, A.K. Expression of the human gene CYP1A2 enhances tolerance and detoxification of the phenylurea herbicide linuron in Arabidopsis thaliana plants and Escherichia coli. Environ. Pollut. 2018, 238, 281–290. [Google Scholar] [CrossRef]
- Kebeish, R.; Azab, E.; Peterhaensel, C.; El-Basheer, R. Engineering the metabolism of the phenylurea herbicide chlortoluron in genetically modified Arabidopsis thaliana plants expressing the mammalian cytochrome P450 enzyme CYP1A2. Environ. Sci. Pollut. Res. Int. 2014, 21, 8224–8232. [Google Scholar] [CrossRef] [PubMed]
- Azab, E.; Hegazy, A.K.; El-Sharnouby, M.E.; Abd Elsalam, H.E. Phytoremediation of the organic xenobiotic simazine by P450-1A2 transgenic Arabidopsis thaliana plants. Int. J. Phytoremed. 2016, 18, 738–746. [Google Scholar] [CrossRef]
- Jang, J.; Khanom, S.; Moon, Y.; Shin, S.; Lee, O.R. PgCYP76B93 docks on phenylurea herbicides and its expression enhances chlorotoluron tolerance in Arabidopsis. Appl. Biol. Chem. 2020, 63, 14. [Google Scholar] [CrossRef]
- Khanom, S.; Jang, J.; Lee, O.R. Overexpression of ginseng cytochrome P450 CYP736A12 alters plant growth and confers phenylurea herbicide tolerance in Arabidopsis. J. Ginseng Res. 2019, 43, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Höfer, R.; Boachon, B.; Renault, H.; Gavira, C.; Miesch, L.; Iglesias, J.; Ginglinger, J.-F.; Allouche, L.; Miesch, M.; Grec, S.; et al. Dual function of the cytochrome P450 CYP76 family from Arabidopsis thaliana in the metabolism of monoterpenols and phenylurea herbicides. Plant Physiol. 2014, 166, 1149–1161. [Google Scholar] [CrossRef]
- Siminszky, B.; Corbin, F.T.; Ward, E.R.; Fleischmann, T.J.; Dewey, R.E. Expression of a soybean cytochrome P450 monooxygenase cDNA in yeast and tobacco enhances the metabolism of phenylurea herbicides. Proc. Natl. Acad. Sci. USA 1999, 96, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Hediat, M.H.S.; Khedr, F.; Schäffer, A.; Azab, E. Phytoremediation of the herbicide simazine by p450 transgenic tobacco plants. Int. J. Curr. Res. 2014, 6, 5233–5240. [Google Scholar]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal. Biochem. 1995, 225, 163–164. [Google Scholar] [CrossRef]
- Niessen, M.; Thiruveedhi, K.; Rosenkranz, R.; Kebeish, R.; Hirsch, H.-J.; Kreuzaler, F.; Peterhansel, C. Mitochondrial glycolate oxidation contributes to photorespiration in higher plants. J. Exp. Bot. 2007, 58, 2709–2715. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Eperjesi, F.; Gilmartin, B. The application of analysis of variance (ANOVA) to different experimental designs in optometry. Ophthalmic Physiol. Opt. 2002, 22, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Bibi, Z. Role of cytochrome P450 in drug interactions. Nutr. Metab. 2008, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Schelstraete, W.; Clerck, L.D.; Govaert, E.; Millecam, J.; Devreese, M.; Deforce, D.; Bocxlaer, J.V.; Croubels, S. Characterization of porcine hepatic and intestinal drug metabolizing CYP450: Comparison with human orthologues from a quantitative, activity and selectivity perspective. Sci. Rep. 2019, 9, 9233. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Grenni, P.; Ciccoli, R.; Di Landa, G.; Cremisini, C. Simazine biodegradation in soil: Analysis of bacterial community structure by in situ hybridization. Pest. Manag. Sci. 2005, 61, 863–869. [Google Scholar] [CrossRef]
- Cherian, S.; Oliveira, M.M. Transgenic plants in phytoremediation: Recent advances and new possibilities. Environ. Sci. Technol. 2005, 39, 9377–9390. [Google Scholar] [CrossRef]
- Inui, H.; Shiota, N.; Motoi, Y.; Ido, Y.; Inoue, T.; Kodama, T.; Ohkawa, Y.; Ohkawa, H. Metabolism of herbicides and other chemicals in human cytochrome P450 species and in transgenic potato plants co-expressing human CYP1A1, CYP2B6 and CYP2C19. Nihon Noyaku Gakkaishi (J. Pest. Sci.) 2001, 26, 28–40. [Google Scholar] [CrossRef]
- Ohkawa, H.; Tsujii, H.; Ohkawa, Y. The use of cytochrome P450 genes to introduce herbicide tolerance in crops: A review. Pestic. Sci. 1999, 55, 867–874. [Google Scholar] [CrossRef]
- Nahar, N.; Rahman, A.; Nawani, N.; Ghosh, S.; Mandal, A. Phytoremediation of arsenic from the contaminated soil using transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana. J. Plant Physiol. 2017, 218. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bode, M.; Stoebe, P.; Thiede, B.; Schuphan, I.; Schmidt, B. Biotransformation of atrazine in transgenic tobacco cell culture expressing human P450. Pestic. Manag. Sci. 2003, 60, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Didierjean, L.; Gondet, L.; Perkins, R.; Lau, S.-M.C.; Schaller, H.; O’Keefe, D.P.; Werck-Reichhart, D. Engineering Herbicide Metabolism in Tobacco and Arabidopsis with CYP76B1, a Cytochrome P450 Enzyme from Jerusalem Artichoke. Plant Physiol. 2002, 130, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Burnet, M.W.M.; Loveys, B.R.; Holtum, J.A.M.; Powles, S.B. Increased detoxification is a mechanism of simazine resistance in Lolium rigidum. Pestic. Biochem. Physiol. 1993, 46, 207–218. [Google Scholar] [CrossRef]
- Javaid, M.M.; Zia, A.U.H.; Waheed, H.; Nargis, J.; Shahid, A.; Aziz, A.; Wasaya, A. Effect of isoproturon with and without adjuvants on photosynthetic attributes of wheat and its associated weeds. Planta Daninha 2020, 38. [Google Scholar]
- Inui, H.; Ohkawa, H. Herbicide resistance in transgenic plants with mammalian P450 monooxygenase genes. Pestic. Manag. Sci. 2005, 61, 286–291. [Google Scholar] [CrossRef]
- Glassgen, W.E.; Komassa, D.; Bohnenkamper, O.; Haas, M.; Hertkorn, N.; May, R.G.; Szymczak, W.; Sandermann, H.J. Metabolism of the herbicide isoproturon in wheat and soybean cell suspension cultures. Pestic. Biochem. Physiol. 1999, 63, 97–113. [Google Scholar]



| Phytotoxicity of Isoproturon | ||||
|---|---|---|---|---|
| Plate Experiment | Pot Experiment | |||
| Parameters | Root Length and Rosette Diameter | Phenotype | Growth Rate | Fresh and Dry Weight |
| Tested stage | Seeds on MS medium | 7–8 weeks old | 4 weeks old | 6 weeks old |
| Exp. code | Ex1 | Ex2 | Ex3 | Ex4 |
| Isoproturon (µM) | 0, 0.2, 0.5, 1.0, 1.5, 2.0, and 2.5 | 0, 15, 50, 150, and 250 | 10 | 10 |
| Frequently | One time | Three times | One time | Three times |
| Days intervals | Once | 4 days | Once | 4 days |
| Duration | 28 days | 12 days | 12 days | 12 days |
| Obtained results | End of the experiment | Photo taken 2 days after last dose | Rosette diameter was recorded at 2 days intervals | Weight taken 14 days after last dose |
| A. thaliana Plants | WT | CYP1A2-I | CYP1A2-II | CYP1A2-III |
|---|---|---|---|---|
| Growth Rate (cm/day) | 0.00 | 0.08 | 0.16 | 0.14 |
| Without isoproturon fold increase in (RD) | 1.1 | 1.1 | 1.2 | 1.0 |
| With 10 μM isoproturon fold increase in (RD) | 0.00 | 0.54 | 0.39 | 0.67 |
| A. thaliana Plants | WT | CYP1A2-I | CYP1A2-II | CYP1A2-III |
|---|---|---|---|---|
| LD50 (growth medium) | 0.35 μM ± 0.05 | 1.90 μM ± 0.21 | 1.75 μM ± 0.33 | 2.4 μM ± 0.42 |
| LD50 (foliar application) | 28 μM ± 3 | 200 μMol ± 13 | 180 μMol ± 16 | 250 μMol ± 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, E.; Hegazy, A.K.; Gobouri, A.A.; Elkelish, A. Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2. Biology 2020, 9, 362. https://doi.org/10.3390/biology9110362
Azab E, Hegazy AK, Gobouri AA, Elkelish A. Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2. Biology. 2020; 9(11):362. https://doi.org/10.3390/biology9110362
Chicago/Turabian StyleAzab, Ehab, Ahmad K. Hegazy, Adil A. Gobouri, and Amr Elkelish. 2020. "Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2" Biology 9, no. 11: 362. https://doi.org/10.3390/biology9110362
APA StyleAzab, E., Hegazy, A. K., Gobouri, A. A., & Elkelish, A. (2020). Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2. Biology, 9(11), 362. https://doi.org/10.3390/biology9110362