Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations?
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Clinical Outcomes of the 1L-Treatment
2.3. Clinical Outcomes of the Second-Line Treatment
2.4. Clinical Outcomes of Patients with De Novo T790M Mutation in the Uncommon EGFR Mutation Group
2.5. Comparison between Common and Uncommon EGFR Mutation
3. Discussion
4. Materials and Methods
4.1. Study Subjects and Data Collection
4.2. Ethics Statement
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| EGFR | epidermal growth factor receptor |
| TKI | tyrosine kinase inhibitor |
| NGS | next-generation sequencing |
| NSCLC | non-small-cell lung cancer |
| OS | overall survival |
| PFS | progression-free survival |
References
- Yang, J.C.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Marten, A.; Kim, E.S. Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J. Thorac. Oncol. 2020, 15, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hochmair, M.J.; Morabito, A.; Hao, D.; Yang, C.T.; Soo, R.A.; Yang, J.C.; Gucalp, R.; Halmos, B.; Wang, L.; Marten, A.; et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: Updated analysis of the observational GioTag study. Future Oncol. 2019, 15, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Boggon, T.J.; Dayaram, T.; Janne, P.A.; Kocher, O.; Meyerson, M.; Johnson, B.E.; Eck, M.J.; Tenen, D.G.; Halmos, B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005, 352, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Arcila, M.E.; Sima, C.S.; Riely, G.J.; Chmielecki, J.; Kris, M.G.; Pao, W.; Ladanyi, M.; Miller, V.A. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin. Cancer Res. 2011, 17, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mao, S.; Li, X.; Zhao, C.; Liu, Q.; Yu, X.; Wang, Y.; Liu, Y.; Pan, Y.; Wang, C.; et al. Uncommon EGFR mutations associate with lower incidence of T790M mutation after EGFR-TKI treatment in patients with advanced NSCLC. Lung Cancer 2020, 139, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Sequist, L.V.; Geater, S.L.; Tsai, C.M.; Mok, T.S.; Schuler, M.; Yamamoto, N.; Yu, C.J.; Ou, S.H.; Zhou, C.; et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Cho, J.H.; Lim, S.H.; An, H.J.; Kim, K.H.; Park, K.U.; Kang, E.J.; Choi, Y.H.; Ahn, M.S.; Lee, M.H.; Sun, J.M.; et al. Osimertinib for Patients with Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J. Clin. Oncol. 2020, 38, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Bunn, P.A., Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009, 10, 432–433. [Google Scholar] [CrossRef]
- Angulo, B.; Conde, E.; Suarez-Gauthier, A.; Plaza, C.; Martinez, R.; Redondo, P.; Izquierdo, E.; Rubio-Viqueira, B.; Paz-Ares, L.; Hidalgo, M.; et al. A comparison of EGFR mutation testing methods in lung carcinoma: Direct sequencing, real-time PCR and immunohistochemistry. PLoS ONE 2012, 7, e43842. [Google Scholar] [CrossRef] [PubMed]
- Gristina, V.; Malapelle, U.; Galvano, A.; Pisapia, P.; Pepe, F.; Rolfo, C.; Tortorici, S.; Bazan, V.; Troncone, G.; Russo, A. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat. Rev. 2020, 85, 101994. [Google Scholar] [CrossRef] [PubMed]
- Brown, P. The Cobas(R) EGFR Mutation Test v2 assay. Future Oncol. 2016, 12, 451–452. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Number of Patient (%) Total n = 135 | |||
|---|---|---|---|---|
| Age | <60 years | 47 | 34.8% | |
| ≥60 years | 88 | 65.2% | ||
| Sex | Male | 64 | 47.4% | |
| Female | 71 | 52.6% | ||
| ECOG PS | 0 | 28 | 20.7% | |
| 1 | 96 | 71.1% | ||
| 2 | 11 | 8.1% | ||
| Smoking status | Never smoker | 75 | 55.6% | |
| Ex-smoker | 45 | 33.3% | ||
| Current smoker | 15 | 11.1% | ||
| History of curative thoracic surgery | Yes | 26 | 19.2% | |
| No | 109 | 80.7% | ||
| EGFR mutation type | G719X | 79 | 58.5% | |
| +Del 19 | 2 | |||
| +L858R | 3 | |||
| +S791I | 11 | |||
| +E709K | 1 | |||
| +L861Q | 1 | |||
| L861Q | 35 | 25.9% | ||
| +L858R | 16 | |||
| +G719X | 1 | |||
| S791I | 16 | 11.9% | ||
| +L858R | 5 | |||
| +G719X | 11 | |||
| T790M | 15 | 11.1% | ||
| +Del 19 | 4 | |||
| +L858R | 11 | |||
| L747S | 1 | 0.7% | ||
| H835Y | 1 | 0.7% | ||
| EGFR-TKI as the first-line treatment | Gefitinib | 53 | 39.3% | |
| Erlotinib | 21 | 15.6% | ||
| Afatinib | 61 | 45.2% | ||
| Uncommon EGFR Mutation | PFS | OS | |||
|---|---|---|---|---|---|
| 11.1 (7.2–15.0) | 25.6 (18.2–33.0) | ||||
| Median (95% CI) | p-Value | Median (95% CI) | p-Value | ||
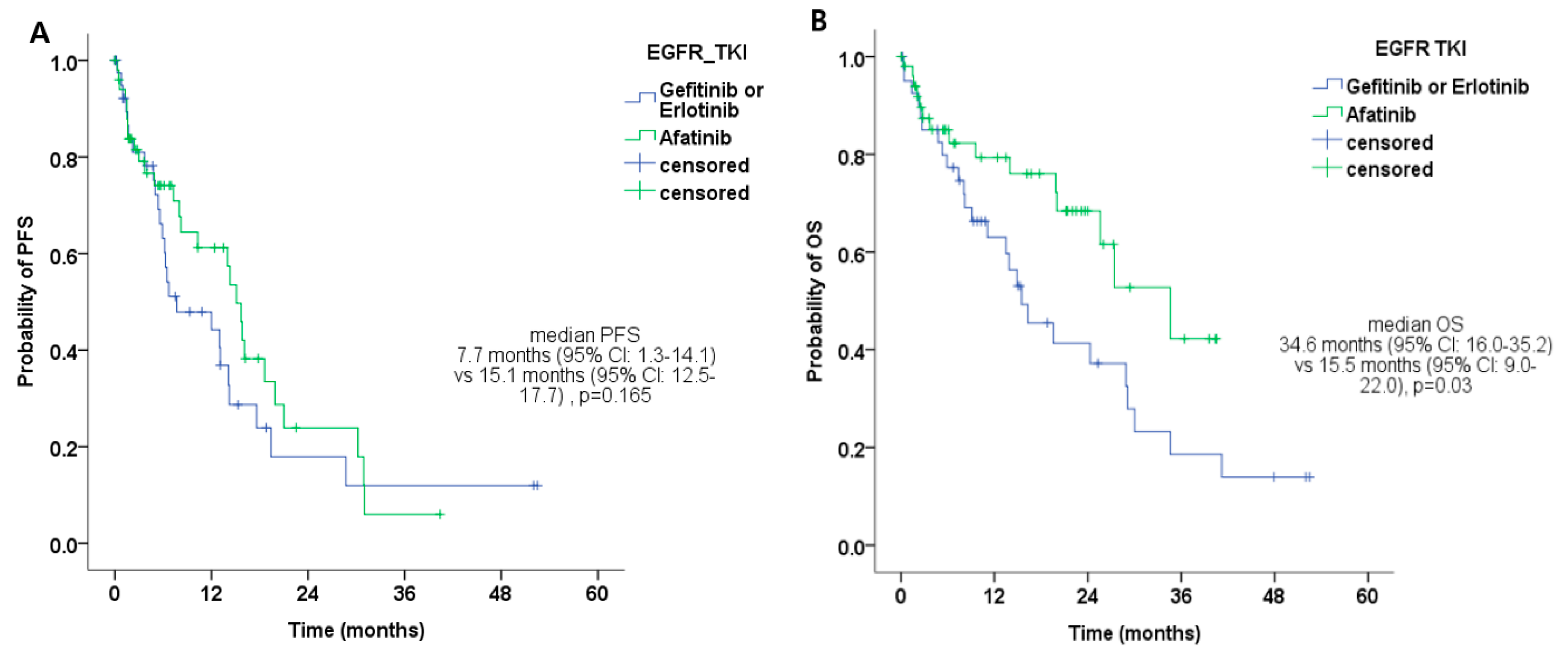
| EGFR-TKI * | Gefitinib or Erlotinib | 7.7 (1.3–14.1) | 0.165 | 15.5 (9.0–22.0) | 0.032 |
| Afatinib | 15.1 (12.5–17.7) | 34.6 (16.0–35.2) | |||
| Patient Characteristics | Uncommon Mutation (Except De Novo 790M) (n = 120) | ||||||
|---|---|---|---|---|---|---|---|
| No. of patients who experienced disease progression | 78 | ||||||
| Rate of re-biopsy after failing first-line EGFR-TKIs | 35 (44.9%, 35/78) | ||||||
| Detection rate of T790M | 10 (28.6%, 10/35) | ||||||
| Sequential Treatment | Number of Patient (%) | PFS2 | OS2 | OS | |||
| Median PFS2 (95% CI) | p-value | Median OS2 (95% CI) | p-value | Median OS (95% CI) | p-value | ||
| 3G EGFR-TKIs | 17 (21.8%) | 8.9 (8.0–9.8) | 0.055 | 15.1 (9.0–21.2) | 0.104 | 34.6 (29.8–39.4) | <0.001 |
| Cytotoxic chemotherapy | 41 (52.6%) | 4.2 (2.3–6.2) | 11.0 (5.1–16.9) | 24.4 (17.4–31.4) | |||
| No sequential treatment | 20 (25.6%) | 5.3 (2.9–7.7) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.A.; Park, S.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Ahn, M.-J.; Park, K. Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations? Biology 2020, 9, 326. https://doi.org/10.3390/biology9100326
Jung HA, Park S, Sun J-M, Lee S-H, Ahn JS, Ahn M-J, Park K. Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations? Biology. 2020; 9(10):326. https://doi.org/10.3390/biology9100326
Chicago/Turabian StyleJung, Hyun Ae, Sehhoon Park, Jong-Mu Sun, Se-Hoon Lee, Jin Seok Ahn, Myung-Ju Ahn, and Keunchil Park. 2020. "Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations?" Biology 9, no. 10: 326. https://doi.org/10.3390/biology9100326
APA StyleJung, H. A., Park, S., Sun, J.-M., Lee, S.-H., Ahn, J. S., Ahn, M.-J., & Park, K. (2020). Treatment and Outcomes of Metastatic Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: Are They Different from Those with Common EGFR Mutations? Biology, 9(10), 326. https://doi.org/10.3390/biology9100326





