Autoclaving Nest-Material Remains Influences the Probability of Ectoparasitism of Nestling Hoopoes (Upupa epops)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Experimental Design and Fieldwork
2.3. Laboratory Procedures
2.4. Statistical Analyses
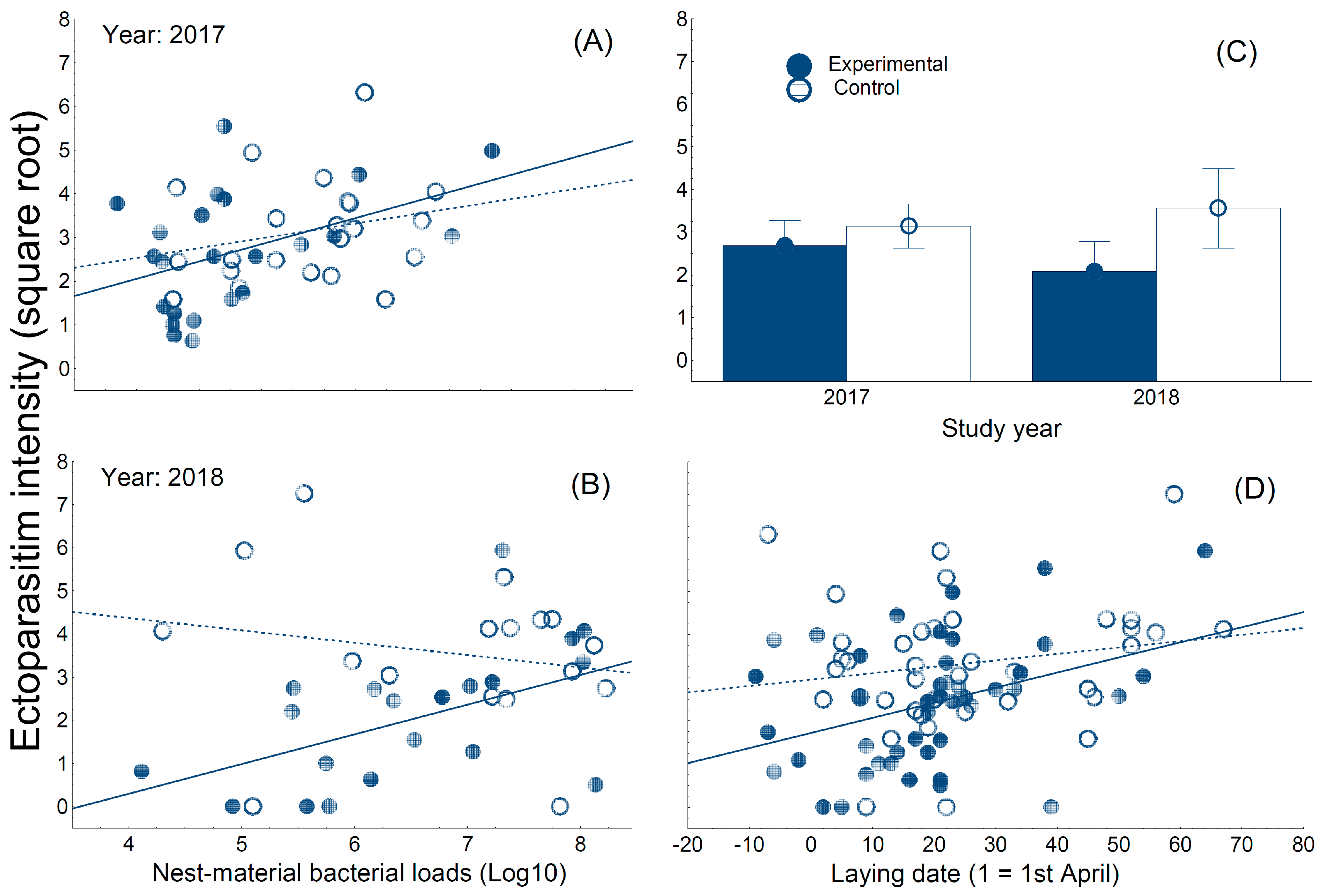
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Flórez, L.V.; Biedermann, P.H.W.; Engl, T.; Kaltenpoth, M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat. Prod. Rep. 2015, 32, 904–936. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; Giori, G.S.; de Sesma, F.; Sinderen, D.; van Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Org, E.; Parks, B.W.; Wha, J.; Joo, J.; Emert, B.; Schwartzman, W.; Kang, E.Y.; Mehrabian, M.; Pan, C.; Knight, R.; et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015, 25, 1558–1569. [Google Scholar] [CrossRef]
- Archie, E.A.; Theis, K.R. Animal behaviour meets microbial ecology. Anim. Behav. 2011, 82, 425–436. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef] [PubMed]
- Albone, E.S.; Gosden, P.E.; Ware, G.C.; Macdonald, D.W.; Hough, N.G. Bacterial action and chemical signalling in the Red Fox (Vulpes vulpes) and other mammals. Bact. Action Chem. Signal. 1978, 67, 78–91. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Williams, A.E. Microbes and animal olfactory communication: Where do we go from here? BioEssays 2014, 36, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Leclaire, S.; Jacob, S.; Greene, L.K.; Dubay, G.R.; Drea, C.M. Social odours covary with bacterial community in the anal secretions of wild meerkats. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Theis, K.R.; Venkataraman, A.; Dycus, J.A.; Koonter, K.D.; Schmitt-Matzen, E.N.; Wagner, A.P.; Holekamp, K.E.; Schmidt, T.M. Symbiotic bacteria appear to mediate hyena social odors. Proc. Natl. Acad. Sci. USA 2013, 110, 19832–19837. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Takken, W. Incubated human sweat but not fresh sweat attracts the malaria mosquito Anopheles gambiae sensu stricto. J. Chem. Ecol. 1999, 25, 663–672. [Google Scholar] [CrossRef]
- Carey, A.F.; Wang, G.; Su, C.Y.; Zwiebel, L.J.; Carlson, J.R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 2010, 464, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.M.; Kännaste, A.; Knols, B.G.J.; Faye, I.; Borg-Karlson, A.K. Oviposition Responses of Anopheles gambiae s.s. (Diptera: Culicidae) and Identification of Volatiles from Bacteria-Containing Solutions. J. Med. Entomol. 2008, 45, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.O.; Takken, W.; Dicke, M.; Schraa, G.; Smallegange, R. Chemical ecology of interactions between human skin microbiota and mosquitoes. FEMS Microbiol. Ecol. 2010, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.O.; Andriessen, R.; Groenhagen, U.; Kiss, G.B.; Schulz, S.; Takken, W.; Loon, J.J.A.; van Schraa, G.; Smallegange, R.C. Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS ONE 2010, 5, e15829. [Google Scholar] [CrossRef] [PubMed]
- Busula, A.O.; Takken, W.; Boer, J.G.; de Mukabana, W.R.; Verhulst, N.O. Variation in host preferences of malaria mosquitoes is mediated by skin bacterial volatiles. Med. Vet. Entomol. 2017, 31, 320–326. [Google Scholar] [CrossRef]
- Verhulst, N.O.; Beijleveld, H.; Knols, B.G.; Takken, W.; Schraa, G.; Bouwmeester, H.J.; Smallegange, R.C. Cultured skin microbiota attracts malaria mosquitoes. Malar. J. 2009, 8, 1–12. [Google Scholar] [CrossRef]
- Verhulst, N.O.; Qiu, Y.T.; Beijleveld, H.; Maliepaard, C.; Knights, D.; Schulz, S.; Berg-Lyons, D.; Lauber, C.L.; Verduijn, W.; Haasnoot, G.W.; et al. Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS ONE 2011, 6, e28991. [Google Scholar] [CrossRef]
- González-Braojos, S.; Vela, A.I.; Ruiz-de-Castañeda, R.; Briones, V.; Moreno, J. Sources of variation in enterococci and enterobacteriaceae loads in nestlings of a hole-nesting Passerine. Ardea 2012, 100, 71–77. [Google Scholar] [CrossRef]
- Singleton, D.; Harper, R. Bacteria in old house wren nests (Bacterias en nidos usados por Troglodytes aedon). J. F. Ornithol. 1998, 71–74. Available online: https://www.jstor.org/stable/4514289 (accessed on 1 May 2020).
- Azcárate-García, M.; Ruiz-Rodríguez, M.; Díaz-Lora, S.; Ruiz-Castellano, C.; Soler, J.J. Experimentally broken faecal sacs affect nest bacterial environment, development and survival of spotless starling nestlings. J. Avian Biol. 2019, 50, 1–10. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Valdivia, E.; Ruíz-Rodríguez, M.; Soler, J.J.; Martín-Vivaldi, M.; Maqueda, M.; Martínez-Bueno, M. Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10-3, isolated from the uropygial gland of the hoopoe (Upupa epops). Appl. Environ. Microbiol. 2006, 72, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, M.; Valdivia, E.; Soler, J.J.; Martín-Vivaldi, M.; Martín-Platero, A.M.; Martínez-Bueno, M. Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J. Exp. Biol. 2009, 212, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, M.; Valdivia, E.; Martín-Vivaldi, M.; Martín-Platero, A.M.; Martínez-Bueno, M.; Méndez, M.; Peralta-Sánchez, J.M.; Soler, J.J. Antimicrobial activity and genetic profile of enteroccoci isolated from hoopoes uropygial gland. PLoS ONE 2012, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, M.; Martínez-Bueno, M.; Martín-Vivaldi, M.; Valdivia, E.; Soler, J.J. Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microbiol. Ecol. 2013, 85, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vivaldi, M.; Peña, A.; Peralta-Sánchez, J.; Sánchez, L.; Ananou, S.; Ruiz-Rodríguez, M.; Soler, J. Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc. R. Soc. B Biol. Sci. 2010, 277, 123–130. [Google Scholar] [CrossRef]
- Díaz-Lora, S.; Martín-Vivaldi, M.; Juárez García-Pelayo, N.; Azcárate García, M.; Rodríguez-Ruano, S.M.; Martínez-Bueno, M.; Soler, J.J. Experimental old nest material predicts hoopoe Upupa epops eggshell and uropygial gland microbiota. J. Avian Biol. 2019, 50, 1–17. [Google Scholar] [CrossRef]
- Capelle, K.; Whitworth, T. The distribution and avian hosts of Carnus hemapterus (Diptera: Milichiidae) in north america. J. Med. Entomol. 1973, 10, 525–526. [Google Scholar] [CrossRef]
- Tomás, G.; Martín-Gálvez, D.; Ruiz-Castellano, C.; Ruiz-Rodríguez, M.; Peralta-Sánchez, J.M.; Martín-Vivaldi, M.; Soler, J.J. Ectoparasite activity during incubation increases microbial growth on avian eggs. Microb. Ecol. 2018, 76, 555–564. [Google Scholar] [CrossRef]
- Valera, F.; Casas-Crivillé, A.; Hoi, H. Interspecific parasite exchange in a mixed colony of birds. J. Parasitol. 2003, 89, 245–250. [Google Scholar] [CrossRef]
- Veiga, J.; Oña, P.; De Salazar, B.; Valera, F. Defining host range: Host-parasite compatibility during the non-infective phase of the parasite also matters. Parasitology 2019, 146, 234–240. [Google Scholar] [CrossRef]
- Martín-Vivaldi, M.; Ruiz-Rodríguez, M.; Méndez, M.; Soler, J.J. Relative importance of factors affecting nestling immune response differs between junior and senior nestlings within broods of hoopoes Upupa epops. J. Avian Biol. 2006, 37, 467–476. [Google Scholar] [CrossRef]
- Veiga, J.; Moreno, E.; Benzal, J.; Valera, F. Off-host longevity of the winged dispersal stage of Carnus hemapterus (Insecta: Diptera) modulated by gender, body size and food provisioning. Parasitology 2019, 146, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Liker, A.; Márkus, M.; Vozár, A.; Zemankovics, E.; Rózsa, L. Distribution of Carnus hemapterus in a starling colony. Can. J. Zool. 2001, 79, 574–580. [Google Scholar] [CrossRef]
- Grimaldi, D.A. The bird flies, genus Carnus: Species revision, generic relationships, and a fossil Meoneura in amber (Diptera, Carnidae). Am. Mus. Novit. 1997, 3190, 1–30. [Google Scholar]
- Roulin, A. Cycle de reproduction et abondance du diptère parasite Carnus hemapterus dans les nichées de chouettes effraies Tyto alba. Alauda (Dijon) 1998, 66, 265–272. [Google Scholar]
- Martín-Vivaldi, M.; Palomino, J.J.; Soler, M.; Soler, J.J. Determinants of reproductive success in the hoopoe upupa epops, a hole-nesting non-passerine bird with asynchronous hatching. Bird Study 1999, 46, 205–216. [Google Scholar] [CrossRef]
- Reichlin, T.S.; Hobson, K.A.; Wilgenburg, S.L.; Van Schaub, M.; Wassenaar, L.I.; Martín-Vivaldi, M.; Arlettaz, R.; Jenni, L. Conservation through connectivity: Can isotopic gradients in Africa reveal winter quarters of a migratory bird? Oecologia 2013, 171, 591–600. [Google Scholar] [CrossRef]
- Reichlin, T.S.; Schaub, M.; Menz, M.H.M.; Mermod, M.; Portner, P.; Arlettaz, R.; Jenni, L. Migration patterns of hoopoe Upupa epops and Wryneck Jynx torquilla: An analysis of European ring recoveries. J. Ornithol. 2009, 150, 393–400. [Google Scholar] [CrossRef]
- Martín-Vivaldi, M.; Doña, J.; Romero Masegosa, J.; Soto Cárdenas, M. Abubilla-Upupa Epops. En: Enciclopedia Virtual de los Vertebrados Españoles. 2014. Available online: http://www.vertebradosibericos.org/ (accessed on 1 May 2020).
- Podofillini, S.; Cecere, J.G.; Griggio, M.; Curcio, A.; Capua, E.L.; De Fulco, E.; Pirrello, S.; Saino, N.; Serra, L.; Visceglia, M.; et al. Home, dirty home: Effect of old nest material on nest-site selection and breeding performance in a cavity-nesting raptor. Curr. Zool. 2018, 64, 693–702. [Google Scholar] [CrossRef]
- Venables, W.; Ripley, B. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Takken, W.; Knols, B.G.J. Odor-mediated behavior of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef]
- Tomás, G.; Soler, J. Begging and ectoparasite attraction. Anim. Behav. 2016, 113, 93–98. [Google Scholar] [CrossRef]
- Whittaker, D.J.; Theis, K.R. Bacterial communities associated with Junco preen glands: Preliminary ramifications for chemical signaling. In Chemical Signals in Vertebrates 13; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 105–117. [Google Scholar] [CrossRef]
- Jacob, S.; Immer, A.; Leclaire, S.; Parthuisot, N.; Ducamp, C.; Espinasse, G.; Heeb, P. Uropygial gland size and composition varies according to experimentally modified microbiome in Great tits. BMC Evol. Biol. 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Álamo, J.D.; Rubio, E.; Soler, J.J. Evolution of nestling faeces removal in avian phylogeny. Anim. Behav. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Soler, J.J.; Ruiz-Castellano, C.; Figuerola, J.; Martín-Vivaldi, M.; Martínez-de la Puente, J.; Ruiz-Rodríguez, M.; Tomás, G. Telomere length and dynamics of spotless starling nestlings depend on nest-building materials used by parents. Anim. Behav. 2017, 126, 89–100. [Google Scholar] [CrossRef]
- Peralta-Sánchez, J.M.; Martín-Platero, A.M.; Wegener-Parfrey, L.; Martínez-Bueno, M.; Rodríguez-Ruano, S.; Navas-Molina, J.A.; Vázquez-Baeza, Y.; Martín-Gálvez, D.; Martín-Vivaldi, M.; Ibáñez-Álamo, J.D.; et al. Bacterial density rather than diversity correlates with hatching success across different avian species. FEMS Microbiol. Ecol. 2018, 94, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Parthuisot, N.; Vallat, A.; Ramon-Portugal, F.; Helfenstein, F.; Heeb, P. Microbiome affects egg carotenoid investment, nestling development and adult oxidative costs of reproduction in Great tits. Funct. Ecol. 2015, 29, 1048–1058. [Google Scholar] [CrossRef]
- Soler, J.J.; Martín-Vivaldi, M.; Ruiz-Rodríguez, M.; Valdivia, E.; Martín-Platero, A.M.; Martínez-Bueno, M.; Peralta-Sánchez, J.M.; Méndez, M. Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct. Ecol. 2008, 22, 864–871. [Google Scholar] [CrossRef]
| Predictors | Mean1 (SE) | Mean2 (SE) | β (SE) | Main Effects | Interaction with Experimental Treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | ||||
| Bacterial loads of nest-material (R2 = 0.49, F = 29.86, df = 3, 94; p < 0.001) | |||||||||
| Treatment | 5.57 (0.18) | 5.96 (0.17) | 3.31 | 1, 94 | 0.072 | ||||
| Year | 5.05 (0.09) | 6.68 (0.18) | 63.35 | 1, 94 | 0.001 | 0.23 | 1, 92 | 0.630 | |
| Laying Date | 0.01 (0.01) | 4.90 | 1, 94 | 0.029 | 0.94 | 1, 92 | 0.760 | ||
| Intensity of Ecto-parasitism (R2 = 0.20, F = 4.90, df = 4, 79, p = 0.001) | |||||||||
| Treatment | 2.39 (0.22) | 3.33 (0.23) | 4.77 | 1, 79 | 0.031 | ||||
| Year | 2.91 (0.18) | 2.75 (0.29) | 3.30 | 1, 79 | 0.073 | 8.31 | 1, 76 | 0.005 | |
| Laying Date | 0.02 (0.01) | 6.05 | 1, 79 | 0.016 | 0.78 | 1, 76 | 0.378 | ||
| Bact Loads | 0.24 (0.17) | 2.12 | 1, 79 | 0.149 | 5.21 | 1, 76 | 0.025 | ||
| Fledging Success (R2 = 0.06, F = 2.04, df = 5, 78, p = 0.083) | |||||||||
| Treatment | 70.25 (4.45) | 59.95 (4.90) | 0.55 | 1, 78 | 0.462 | ||||
| Year | 59.39 (4.49) | 72.92 (4.74) | 0.00 | 1, 78 | 0.962 | 0.96 | 1, 74 | 0.331 | |
| Laying Date | −0.20 (0.15) | 1.82 | 1, 78 | 0.181 | 1.66 | 1, 74 | 0.202 | ||
| Bact Load | 6.01 (2.53) | 5.63 | 1, 78 | 0.020 | 0.03 | 1, 74 | 0.855 | ||
| Int Parasitism | −0.89 (1.68) | 0.29 | 1, 78 | 0.596 | 0.46 | 1, 74 | 0.501 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazorra-Alonso, M.; Martín-Vivaldi, M.; Peralta-Sánchez, J.M.; Soler, J.J. Autoclaving Nest-Material Remains Influences the Probability of Ectoparasitism of Nestling Hoopoes (Upupa epops). Biology 2020, 9, 306. https://doi.org/10.3390/biology9100306
Mazorra-Alonso M, Martín-Vivaldi M, Peralta-Sánchez JM, Soler JJ. Autoclaving Nest-Material Remains Influences the Probability of Ectoparasitism of Nestling Hoopoes (Upupa epops). Biology. 2020; 9(10):306. https://doi.org/10.3390/biology9100306
Chicago/Turabian StyleMazorra-Alonso, Mónica, Manuel Martín-Vivaldi, Juan Manuel Peralta-Sánchez, and Juan José Soler. 2020. "Autoclaving Nest-Material Remains Influences the Probability of Ectoparasitism of Nestling Hoopoes (Upupa epops)" Biology 9, no. 10: 306. https://doi.org/10.3390/biology9100306
APA StyleMazorra-Alonso, M., Martín-Vivaldi, M., Peralta-Sánchez, J. M., & Soler, J. J. (2020). Autoclaving Nest-Material Remains Influences the Probability of Ectoparasitism of Nestling Hoopoes (Upupa epops). Biology, 9(10), 306. https://doi.org/10.3390/biology9100306







