Glucose Favors Lipid Anabolic Metabolism in the Invasive Breast Cancer Cell Line MDA-MB-231
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Growth Curves
2.4. Cytotoxicity Assays
2.5. Extracellular Flux Analyzer Experiments
2.6. Analysis of Glucose and Palmitate Uptake
2.7. Lactate Production
2.8. Glutamine Uptake and Oxidation
2.9. RNA Isolation and Purification and cDNA Synthesis
2.10. qPCR
2.11. Western Blot
2.12. Statistical Analysis
3. Results and Discussion
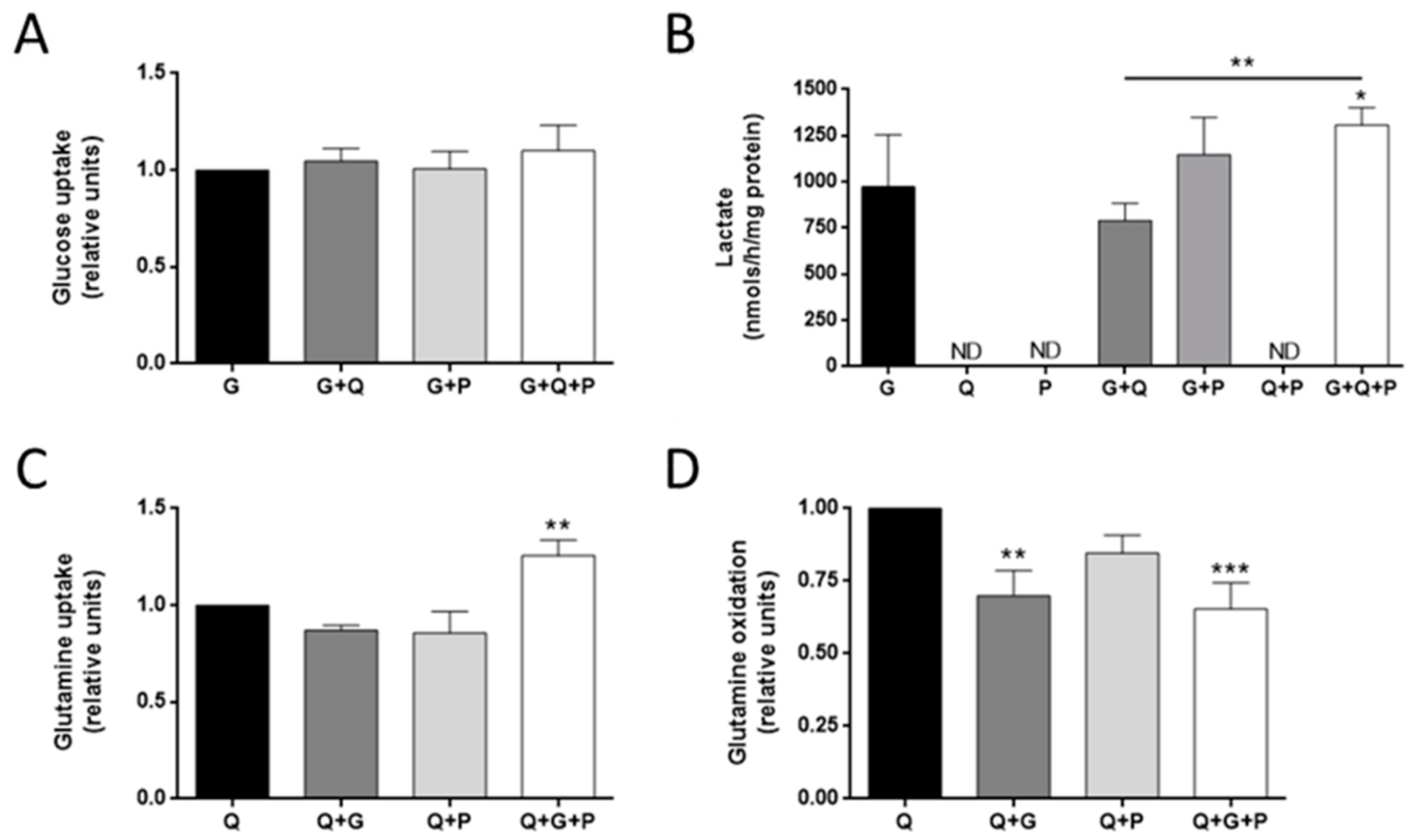
3.1. Glucose and Glutamine Are Essential for MDA-MB-231 Cells’ Proliferation
3.2. Effect of Different Metabolic Fuels on Glucose and Glutamine Metabolism
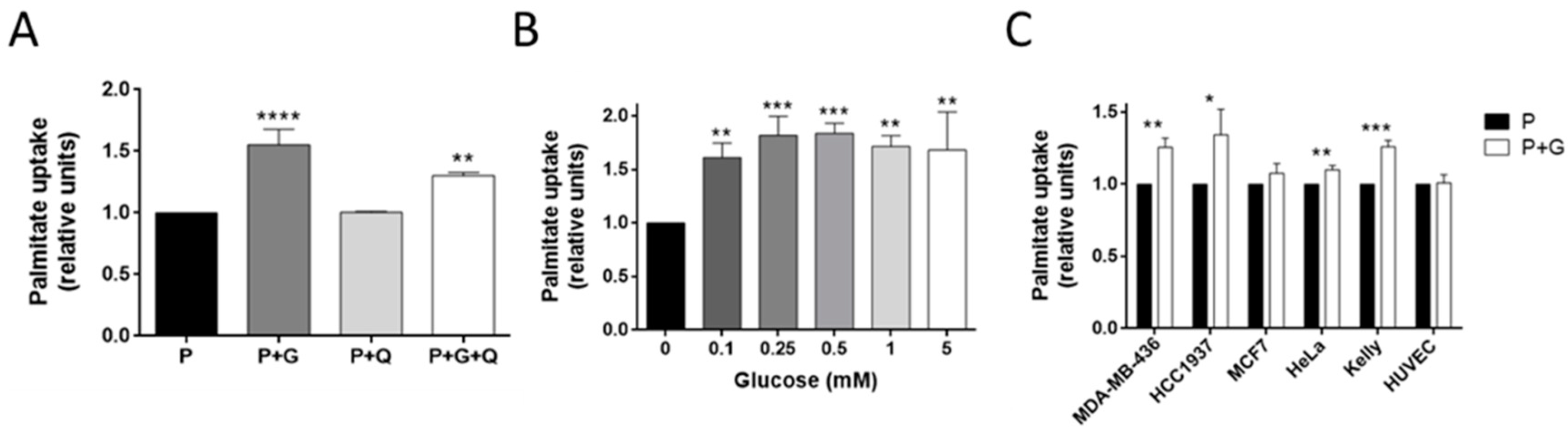
3.3. Glucose Increases Palmitate Uptake in MDA-MB-231 and Other Invasive Tumor Cell Lines
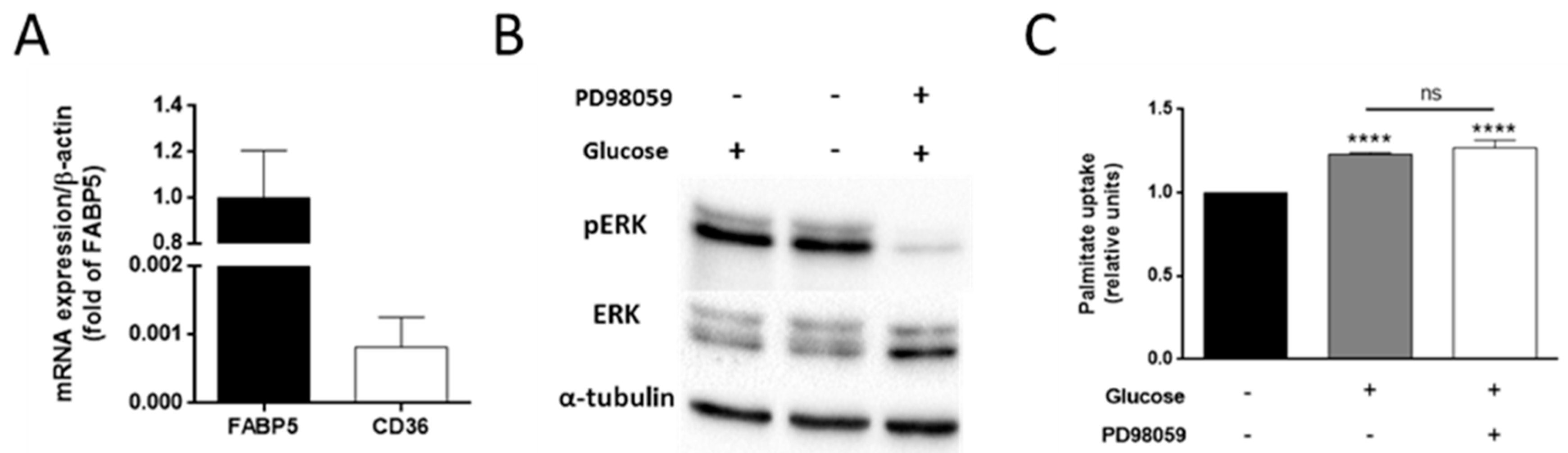
3.4. The Effect of Glucose on Palmitate Uptake Is Independent of the ERK Signaling Pathway
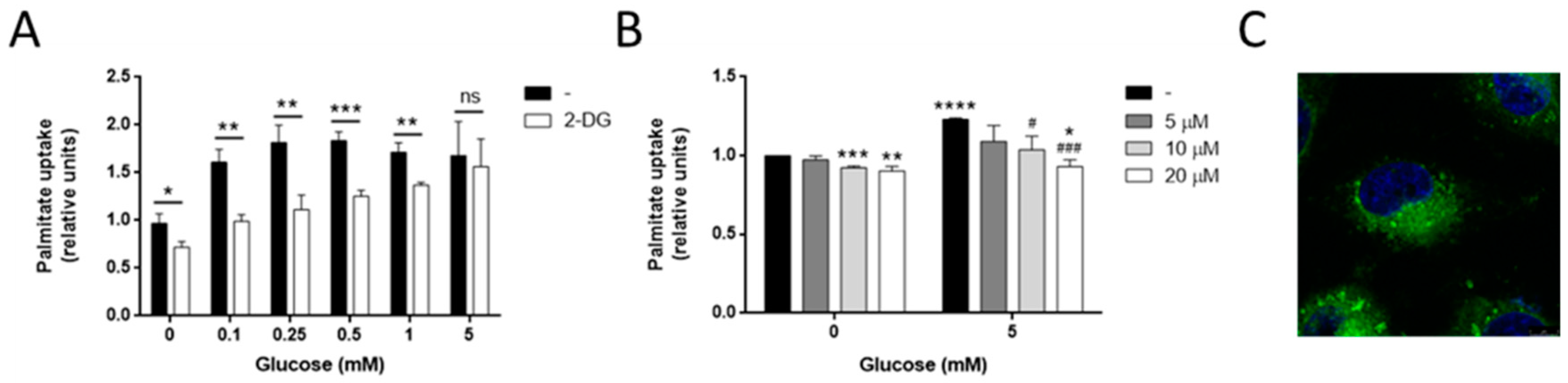
3.5. Glucose Is Needed for Palmitate Metabolism in MDA-MB-231 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburg, O. The metabolism of carcinoma cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.A. Metabolism within the tumor microenvironment and its implication on cancer progression: An ongoing therapeutic target. Med. Res. Rev. 2019, 39, 70–113. [Google Scholar] [CrossRef]
- Ruiz-Pérez, M.V.; Sánchez-Jimenez, F.; Alonso, F.J.; Segura, J.A.; Márquez, J.; Medina, M.Á. Glutamine, glucose and other fuels for cancer. Curr. Pharm. Des. 2014, 20, 2557–2579. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.; Thompson, C. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Desantis, C.E.; Fedewa, S.A.; Sauer, A.G.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J. Clin. 2016, 66, 31–42. [Google Scholar] [CrossRef]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef]
- Hardy, S.; El-Assaad, W.; Przybytkowski, E.; Joly, E.; Prentki, M.; Langelier, Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2003, 278, 31861–31870. [Google Scholar] [CrossRef]
- Antalis, C.J.; Uchida, A.; Buhman, K.K.; Siddiqui, R.A. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin. Exp. Metastasis 2011, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, J.D.; Fernandes-Siqueira, L.O.; Carvalho, A.S.; Cararo-Lopes, E.; Dias, M.H.; Ketzer, L.A.; Galina, A.; Da Poian, A.T. Short-term starvation is a strategy to unravel the cellular capacity of oxidizing specific exogenous/endogenous substrates in mitochondria. J. Biol. Chem. 2017, 292, 14176–14187. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.R.; Vander Heiden, M.G. Famine versus feast: Understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 2015, 40, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Huynh, F.K.; Green, M.F.; Koves, T.R.; Hirschey, M.D. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 2014, 542, 391–405. [Google Scholar] [PubMed]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, P.; Martínez-Poveda, B.; Cheng-Sánchez, I.; Guerra, J.; Tobia, C.; López-Romero, J.M.; Sarabia, F.; Medina, M.A.; Quesada, A.R. Exploring the antiangiogenic potential of solomonamide A bioactive precursors: In vitro and in vivo evidences of the inhibitory activity of solo F-OH during angiogenesis. Mar. Drugs 2019, 17, 228. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.A. Highly glycolytic immortalized human dermal microvascular endothelial cells are able to grow in glucose-starved conditions. Biomolecules 2019, 9, 332. [Google Scholar] [CrossRef]
- Carrillo, P.; Martínez-Poveda, B.; Medina, M.A.; Quesada, A.R. The strigolactone analog GR-24 inhibits angiogenesis in vivo and in vitro by a mechanism involving cytoskeletal reorganization and VEGFR2 signalling. Biochem. Pharmacol. 2019, 168, 366–383. [Google Scholar] [CrossRef]
- Visagie, M.H.; Mqoco, T.V.; Liebenberg, L.; Mathews, E.H.; Mathews, G.E.; Joubert, A.M. Influence of partial and complete glutamine-and glucose deprivation of breast-and cervical tumorigenic cell lines. Cell Biosci. 2015, 5, 37. [Google Scholar] [CrossRef]
- Korangath, P.; Teo, W.W.; Sadik, H.; Han, L.; Mori, N.; Huijts, C.M.; Wildes, F.; Bharti, S.; Zhang, Z.; Santa-Maria, C.A.; et al. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin. Cancer Res. 2015, 21, 3263–3273. [Google Scholar] [CrossRef]
- Fu, Y.M.; Zhang, H.; Ding, M.; Li, Y.Q.; Fu, X.; Yu, Z.X.; Meadows, G.G. Specific amino acid restriction inhibits attachment and spreading of human melanoma via modulation of the integrin/focal adhesion kinase pathway and actin cytoskeleton remodeling. Clin. Exp. Metastasis 2004, 21, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.A.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Frankenthal, L.; Langan, J.; Morris, H.P.; Weinhouse, S. Fatty acid oxidation and ketogenesis in transplantable liver tumors. Cancer Res. 1965, 25, 732–736. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Thompson, C.B. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J. Biol. Chem. 2006, 281, 37372–37380. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Shrago, E. Energy substrate utilization in freshly isolated Morris hepatoma 7777 cells. Cancer Res. 1988, 48, 602–608. [Google Scholar]
- Arora, R.; Schmitt, D.; Karanam, B.; Tan, M.; Yates, C.; Dean-Colomb, W. Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget 2015, 6, 662–678. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Spector, A.A.; Steinberg, D. Relationship between fatty acid and glucose utilization in Ehrlich ascites tumor cells. J. Lipid Res. 1966, 7, 657–663. [Google Scholar]
- Binker-Cosen, M.J.; Richards, D.; Oliver, B.; Gaisano, H.Y.; Binker, M.G.; Cosen-Binker, L.I. Palmitic acid increases invasiveness of pancreatic cancer cells AsPC-1 through TLR4/ROS/NF-kB/MMP-9 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 484, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Li, I.; Roberts, L.R.; Chan, C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci. Rep. 2015, 5, 14752. [Google Scholar] [CrossRef] [PubMed]
- Al-Bakheit, A.; Traka, M.; Saha, S.; Mithen, R.; Melchini, A. Accumulation of palmitoylcarnitine and its effect on pro-inflammatory pathways and calcium influx in prostate cancer. Prostate 2016, 76, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; González-Mañan, D.; Ortiz, M.; Soto-Alarcón, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef]
- Turcotte, L.P.; Raney, M.A.; Todd, M.K. ERK1/2 inhibition prevents contraction-induced increase in plasma membrane FAT/CD36 content and FA uptake in rodent muscle. Acta Physiol. Scand. 2005, 184, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Guaita-Esteruelas, S.; Bosquet, A.; Saavedra, P.; Gumà, J.; Girona, J.; Lam, E.W.F.; Amillano, K.; Borràs, J.; Masana, L. Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol. Carcinog. 2017, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Uray, I.P.; Liang, Y.; Hyder, S.M. Estradiol down-regulates CD36 expression in human breast cancer cells. Cancer Lett. 2004, 207, 101–107. [Google Scholar] [CrossRef]
- Kannan-Thulasiraman, P.; Seachrist, D.D.; Mahabeleshwar, G.H.; Jain, M.K.; Noy, N. Fatty acid-binding protein 5 and PPARβ/δ are critical mediators of epidermal growth factor receptor-induced carcinoma cell growth. J. Biol. Chem. 2010, 285, 19106–19115. [Google Scholar] [CrossRef]
- Pellon-Maison, M.; Montanaro, M.A.; Lacunza, E.; Garcia-Fabiani, M.B.; Soler-Gerino, M.C.; Cattaneo, E.R.; Quiroga, I.Y.; Abba, M.C.; Coleman, R.A.; Gonzalez-Baro, M.R. Glycerol-3-phosphate acyltranferase-2 behaves as a cancer testis gene and promotes growth and tumorigenicity of the breast cancer MDA-MB-231 cell line. PLoS ONE 2014, 9, e100896. [Google Scholar] [CrossRef]
- Mráček, T.; Drahota, Z.; Houštěk, J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta 2013, 1827, 401–410. [Google Scholar] [CrossRef]
- Cattaneo, E.R.; Prieto, E.D.; Garcia-Fabiani, M.B.; Montanaro, M.A.; Guillou, H.; Gonzalez-Baro, M.R. Glycerol-3-phosphate acyltransferase 2 expression modulates cell roughness and membrane permeability: An atomic force microscopy study. PLoS ONE 2017, 12, e0189031. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Tan, J.; Wang, Y.; Yan, J.; Higashi, R.M.; Fan, T.W.M. Probing the metabolic phenotype of breast cancer cells by multiple tracer stable isotope resolved metabolomics. Metab. Eng. 2017, 43, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Balaban, S.; Lee, L.S.; Varney, B.; Aishah, A.; Gao, Q.; Shearer, R.F.; Saunders, D.N.; Grewal, T.; Hoy, A.J. Heterogeneity of fatty acid metabolism in breast cancer cells underlies differential sensitivity to palmitate-induced apoptosis. Mol. Oncol. 2018, 12, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Duncan, R.E.; Bazinet, R.P.; Archer, M.C. Fatty acid synthase plays a role in cancer metabolism beyond providing fatty acids for phospholipid synthesis or sustaining elevations in glycolytic activity. Exp. Cell Res. 2014, 320, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Przybytkowski, E.; Joly, É.; Nolan, C.J.; Hardy, S.; Francoeur, A.-M.; Langelier, Y.; Prentki, M. Upregulation of cellular triacylglycerol—Free fatty acid cycling by oleate is associated with long-term serum-free survival of human breast cancer cells. Biochem. Cell Biol. 2007, 85, 301–310. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers | Annealing Temperature (°C) | Amplicon Size (bp) |
|---|---|---|---|
| β-actin | Fw: GACGACATGGAGAAAATCTG Rv: ATGATCTGGGTCATCTTCTC | 60 | 131 |
| FABP5 | Fw: AAGATGGGAAATTAGTGGTG Rv: AACAGTATGGAGATTTGCTC | 60 | 153 |
| CD36 | Fw: AGCTTTCCAATGATTAGACG Rv: GTTTCTACAAGCTCTGGTTC | 60 | 111 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Glucose Favors Lipid Anabolic Metabolism in the Invasive Breast Cancer Cell Line MDA-MB-231. Biology 2020, 9, 16. https://doi.org/10.3390/biology9010016
Ocaña MC, Martínez-Poveda B, Quesada AR, Medina MÁ. Glucose Favors Lipid Anabolic Metabolism in the Invasive Breast Cancer Cell Line MDA-MB-231. Biology. 2020; 9(1):16. https://doi.org/10.3390/biology9010016
Chicago/Turabian StyleOcaña, Mª Carmen, Beatriz Martínez-Poveda, Ana R. Quesada, and Miguel Ángel Medina. 2020. "Glucose Favors Lipid Anabolic Metabolism in the Invasive Breast Cancer Cell Line MDA-MB-231" Biology 9, no. 1: 16. https://doi.org/10.3390/biology9010016
APA StyleOcaña, M. C., Martínez-Poveda, B., Quesada, A. R., & Medina, M. Á. (2020). Glucose Favors Lipid Anabolic Metabolism in the Invasive Breast Cancer Cell Line MDA-MB-231. Biology, 9(1), 16. https://doi.org/10.3390/biology9010016






