A Model In Vitro Study Using Hypericin: Tumor-Versus Necrosis-Targeting Property and Possible Mechanisms
Abstract
1. Introduction
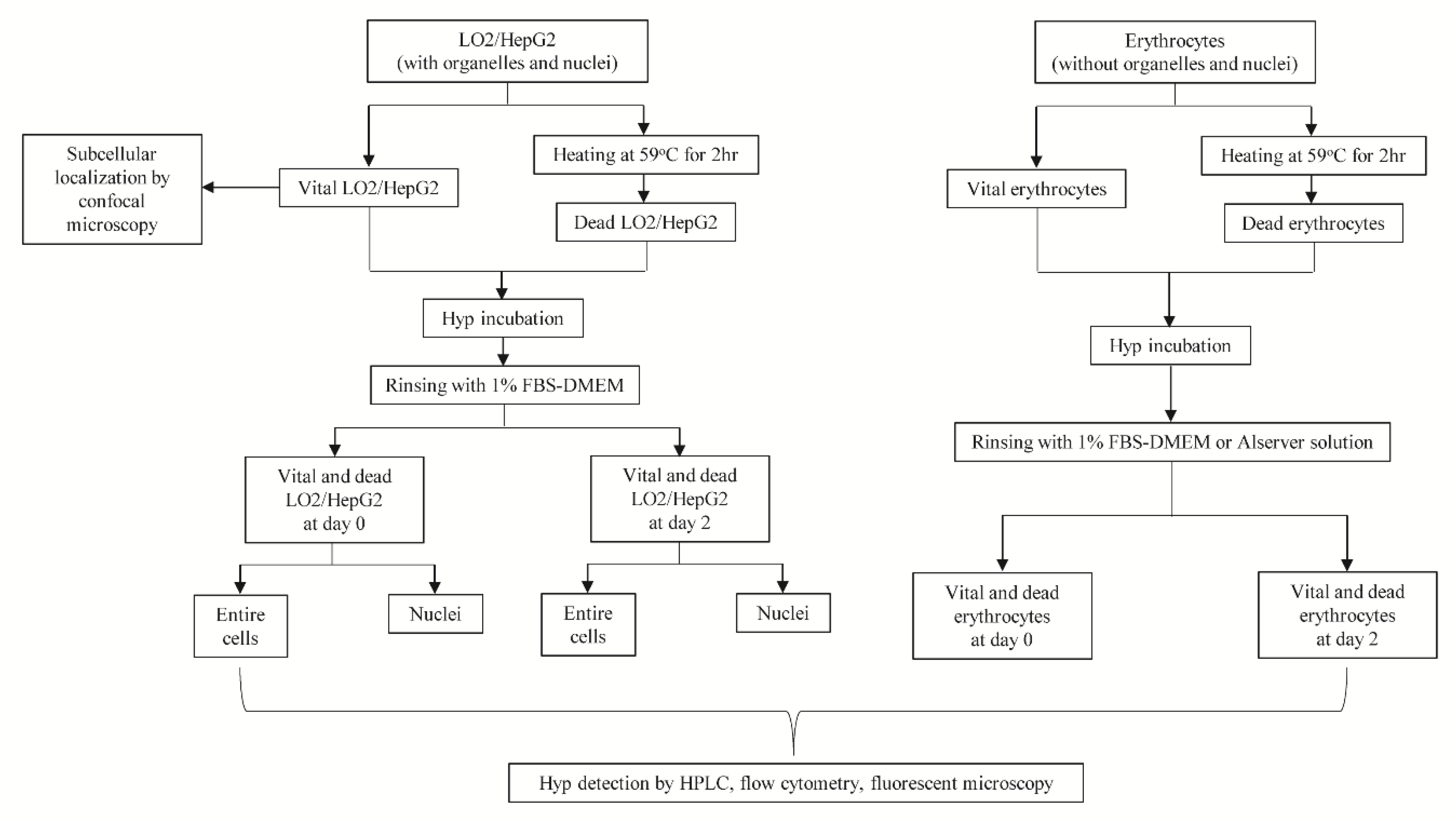
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.2.1. Intracellular Accumulation for Hyp
2.2.2. Fluorescent Microscopy
2.2.3. Flow Cytometry
2.2.4. HPLC
2.2.5. Co-Localization of Hyp and Organelles
2.2.6. Cell Fixation and Incubation with Hyp
2.2.7. Statistical Analyses
3. Results
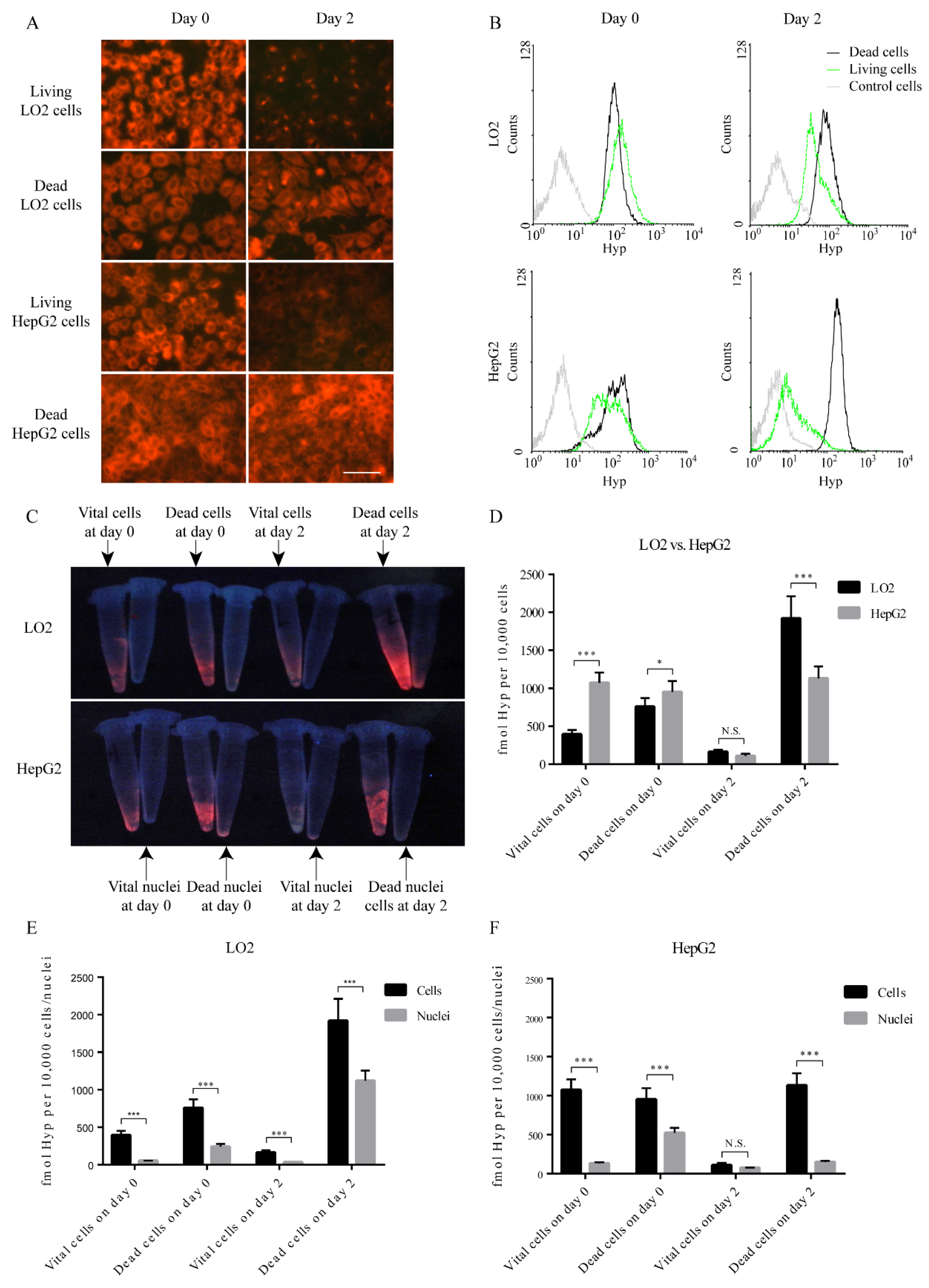
3.1. Accumulation of Hyp in Cells
3.2. Hyp Accumulation in Nuclei
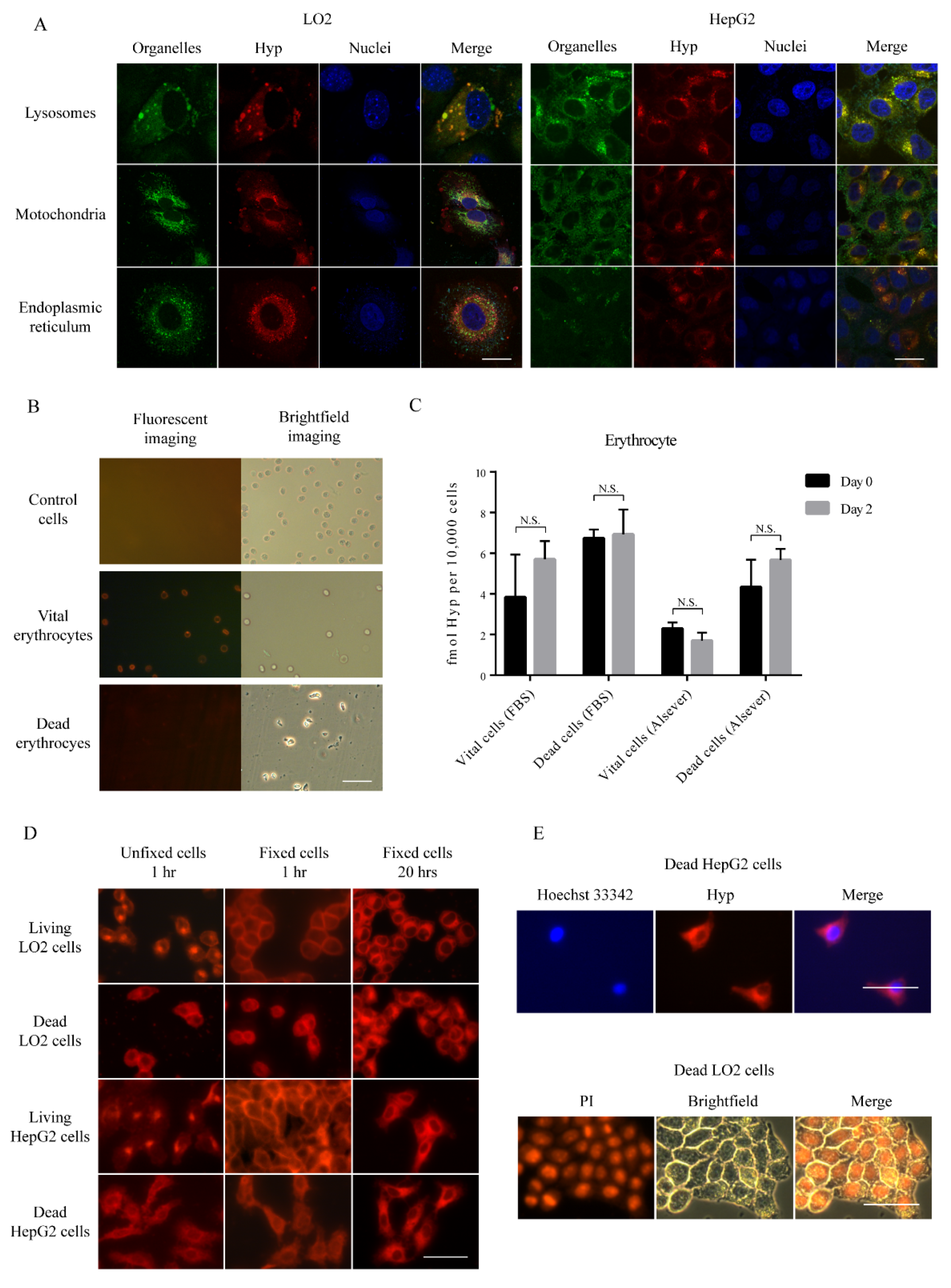
3.3. Subcellular Hyp Fluorescence Distribution
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Hallewin, M.A.; Kamuhabwa, A.R.; Roskams, T.; De Witte, P.A.; Baert, L. Hypericin-based fluorescence diagnosis of bladder carcinoma. BJU Int. 2002, 89, 760–763. [Google Scholar] [CrossRef]
- Olivo, M.; Lau, W.; Manivasager, V.; Tan, P.H.; Soo, K.C.; Cheng, C. Macro-microscopic fluorescence of human bladder cancer using hypericin fluorescence cystoscopy and laser confocal microscopy. Int. J. Oncol. 2003, 23, 983–990. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, S.; Jiang, C.; Li, J.; Wang, C.; Chen, L.; Jin, Q.; Song, S.; Feng, Y.; Ni, Y.; et al. Discovery of Radioiodinated Monomeric Anthraquinones as a Novel Class of Necrosis Avid Agents for Early Imaging of Necrotic Myocardium. Sci. Rep. 2016, 6, 21341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, D.; Ji, Y.; Jiang, C.; Li, Y.; Gao, M.; Yao, N.; Liu, X.; Shao, H.; Jing, S.; et al. Experimental evaluation of radioiodinated sennoside B as a necrosis-avid tracer agent. J. Drug Targets 2015, 23, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, D.; Lou, B.; Peng, F.; Wang, X.; Shan, X.; Jiang, C.; Gao, M.; Sun, Z.; et al. Evaluation of a metalloporphyrin (THPPMnCl) for necrosis-affinity in rat models of necrosis. J. Drug Targets 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yang, S.; Jiang, C.; Zhang, D.; Jin, Q.; Wang, Q.; Wang, C.; Ni, Y.; Yin, Z.; et al. Synthesis and Preclinical Evaluation of Radioiodinated Hypericin Dicarboxylic Acid as a Necrosis Avid Agent in Rat Models of Induced Hepatic, Muscular, and Myocardial Necroses. Mol. Pharm. 2016, 13, 232–240. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, M.; Li, Y.; Huang, D.; Yao, N.; Ji, Y.; Liu, X.; Zhang, D.; Wang, X.; Yin, Z.-Q.; et al. Exploring diagnostic Potentials of Radioiodinated Sennidin A in Rat model of Reperfused Myocardial Infarction. Int. J. Pharm. 2015, 495. [Google Scholar] [CrossRef]
- Kong, M.; Zhang, J.; Jiang, C.; Jiang, X.; Li, Y.; Gao, M.; Yao, N.; Huang, D.; Wang, X.; Fang, Z.; et al. Necrosis affinity evaluation of 131 I-hypericin in a rat model of induced necrosis. J. Drug Targets 2013, 21. [Google Scholar] [CrossRef]
- Qi, X.; Shao, H.; Zhang, J.; Sun, Z.; Ni, Y.; Xu, K. Radiopharmaceutical study on Iodine-131-labelled hypericin in a canine model of hepatic RFA-induced coagulative necrosis. La Radiol. Med. 2015, 120, 213–221. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Jiang, X.; Yao, N.; Gao, M.; Zhang, X.; Wang, J.; Wang, X.; Sun, Z.; Zhang, J.; et al. Hypericin as a marker for determination of myocardial viability in a rat model of myocardial infarction. Photochem. Photobiol. 2014, 90, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cona, M.M.; Vunckx, K.; Li, Y.; Chen, F.; Nuyts, J.; Gheysens, O.; Zhou, L.; Xie, Y.; Oyen, R.; et al. Detection and quantification of acute reperfused myocardial infarction in rabbits using DISA-SPECT/CT and 3.0 T cardiac MRI. Int. J. Cardiol. 2013, 168, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Van de Putte, M.; Marysael, T.; Fonge, H.; Roskams, T.; Cona, M.M.; Li, J.; Bormans, G.; Verbruggen, A.; Ni, Y.; de Witte, P.A. Radiolabeled iodohypericin as tumor necrosis avid tracer: Diagnostic and therapeutic potential. Int. J. Cancer 2012, 131, E129–E137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cona, M.M.; Chen, F.; Feng, Y.; Zhou, L.; Yu, J.; Nuyts, J.; de Witte, P.; Zhang, J.; Himmelreich, U.; et al. Exploring theranostic potentials of radioiodinated hypericin in rodent necrosis models. Theranostics 2012, 2, 1010–1019. [Google Scholar] [CrossRef]
- Li, J.; Cona, M.M.; Chen, F.; Feng, Y.; Zhou, L.; Zhang, G.; Nuyts, J.; de Witte, P.; Zhang, J.; Yu, J.; et al. Sequential systemic administrations of combretastatin A4 Phosphate and radioiodinated hypericin exert synergistic targeted theranostic effects with prolonged survival on SCID mice carrying bifocal tumor xenografts. Theranostics 2013, 3, 127–137. [Google Scholar] [CrossRef]
- Fonge, H.; Vunckx, K.; Wang, H.; Feng, Y.; Mortelmans, L.; Nuyts, J.; Bormans, G.; Verbruggen, A.; Ni, Y. Non-invasive detection and quantification of acute myocardial infarction in rabbits using mono-[123I]iodohypericin microSPECT. Eur. Heart J. 2008, 29, 260–269. [Google Scholar] [CrossRef]
- Li, J.; Sun, Z.; Zhang, J.; Shao, H.; Cona, M.M.; Wang, H.; Marysael, T.; Chen, F.; Prinsen, K.; Zhou, L.; et al. A Dual-targeting Anticancer Approach: Soil and Seed Principle. Radiology 2011, 260, 799–807. [Google Scholar] [CrossRef]
- Vuong, T.T.; Vever-Bizet, C.; Bonneau, S.; Bourg-Heckly, G. Hypericin incorporation and localization in fixed HeLa cells for various conditions of fixation and incubation. Photochem. Photobiol. Sci. 2011, 10, 561–568. [Google Scholar] [CrossRef]
- Verebova, V.; Belej, D.; Joniova, J.; Jurasekova, Z.; Miskovsky, P.; Kozar, T.; Horvath, D.; Stanicova, J.; Huntosova, V. Deeper insights into the drug defense of glioma cells against hydrophobic molecules. Int. J. Pharm. 2016, 503, 56–67. [Google Scholar] [CrossRef]
- Marysael, T.; Ni, Y.; Lerut, E.; de Witte, P. Influence of the vascular damaging agents DMXAA and ZD6126 on hypericin distribution and accumulation in RIF-1 tumors. J. Cancer Res. Clin. Oncol. 2011, 137, 1619–1627. [Google Scholar] [CrossRef]
- Duan, X.; Yin, Z.; Jiang, C.; Jin, Q.; Zhang, D.; Sun, Z.; Ye, W.; Zhang, J. Radioiodinated hypericin disulfonic acid sodium salts as a DNA-binding probe for early imaging of necrotic myocardium. Eur. J. Pharm. Biopharm. 2017, 117, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Smith, B.D. Biomarkers and molecular probes for cell death imaging and targeted therapeutics. Bioconj. Chem. 2012, 23, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xiong, C.; Zhou, M.; Lu, W.; Huang, Q.; Ku, G.; Zhao, J.; Flores, L.G., Jr.; Ni, Y.; Li, C. Small-animal PET of tumor damage induced by photothermal ablation with 64Cu-bis-DOTA-hypericin. J. Nucl. Med. 2011, 52, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, C.; Li, Y.; Liu, W.; Yao, N.; Gao, M.; Ji, Y.; Huang, D.; Yin, Z.; Sun, Z.; et al. Evaluation of hypericin: Effect of aggregation on targeting biodistribution. J. Pharm. Sci. 2015, 104, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Miranda Cona, M.; Liu, Y.-W.; Hubert, A.; Yin, T.; Feng, Y.-B.; de Witte, P.; Waelkens, E.; Jiang, Y.-S.; Zhang, J.; Mulier, S.; et al. Differential diagnosis of gallstones by using hypericin as a fluorescent optical imaging agent. World J. Gastroenterol. 2016, 22, 6690–6705. [Google Scholar] [CrossRef]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer‘s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Statistics. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 9 September 2019).
- Rybczynska, A.A.; Boersma, H.H.; de Jong, S.; Gietema, J.A.; Noordzij, W.; Dierckx, R.A.J.O.; Elsinga, P.H.; van Waarde, A. Avenues to molecular imaging of dying cells: Focus on cancer. Med. Res. Rev. 2018, 38, 1713–1768. [Google Scholar] [CrossRef]
- Huntosova, V.; Nadova, Z.; Dzurova, L.; Jakusova, V.; Sureau, F.; Miskovsky, P. Cell death response of U87 glioma cells on hypericin photoactivation is mediated by dynamics of hypericin subcellular distribution and its aggregation in cellular organelles. Photochem. Photobiol. Sci. 2012, 11, 1428–1436. [Google Scholar] [CrossRef]
- Misuth, M.; Joniova, J.; Horvath, D.; Dzurova, L.; Nichtova, Z.; Novotova, M.; Miskovsky, P.; Stroffekova, K.; Huntosova, V. The flashlights on a distinct role of protein kinase C delta: Phosphorylation of regulatory and catalytic domain upon oxidative stress in glioma cells. Cell Signal 2017, 34, 11–22. [Google Scholar] [CrossRef]
- Crnolatac, I.; Huygens, A.; Agostinis, P.; Kamuhabwa, A.R.; Maes, J.; van Aerschot, A.; De Witte, P.A. In vitro accumulation and permeation of hypericin and lipophilic analogues in 2-D and 3-D cellular systems. Int. J. Oncol. 2007, 30, 319–324. [Google Scholar] [PubMed]
- Kascakova, S.; Nadova, Z.; Mateasik, A.; Mikes, J.; Huntosova, V.; Refregiers, M.; Sureau, F.; Maurizot, J.C.; Miskovsky, P.; Jancura, D. High level of low-density lipoprotein receptors enhance hypericin uptake by U-87 MG cells in the presence of LDL. Photochem. Photobiol. 2008, 84, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Huntosova, V.; Alvarez, L.; Bryndzova, L.; Nadova, Z.; Jancura, D.; Buriankova, L.; Bonneau, S.; Brault, D.; Miskovsky, P.; Sureau, F. Interaction dynamics of hypericin with low-density lipoproteins and U87-MG cells. Int. J. Pharm. 2010, 389, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fonge, H.; Putte, M.V.D.; Huyghe, D.; Bormans, G.; Ni, Y.; Witte, P.D.; Verbruggen, A. Evaluation of tumor affinity of mono-[123I]iodohypericin and mono-[123I]iodoprotohypericin in a mouse model with a RIF-1 tumor. Contrast Media Mol. Imaging 2007, 2, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wolman, M.; Klatzo, I.; Chui, E.; Wilmes, F.; Nishimoto, K.; Fujiwara, K.; Spatz, M. Evaluation of the dye-protein tracers in pathophysiology of the blood-brain barrier. Acta Neuropathol. 1981, 54, 55–61. [Google Scholar] [CrossRef]
- Chen, B.; Xu, Y.; Roskams, T.; Delaey, E.; Agostinis, P.; Vandenheede, J.R.; de Witte, P. Efficacy of antitumoral photodynamic therapy with hypericin: Relationship between biodistribution and photodynamic effects in the RIF-1 mouse tumor model. Int. J. Cancer 2001, 93, 275–282. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S.; Anderson, R.G.; Russell, D.W.; Schneider, W.J. Receptor-mediated endocytosis: Concepts emerging from the LDL receptor system. Annu. Rev. Cell Biol. 1985, 1, 1–39. [Google Scholar] [CrossRef]
- van den Boom, M.A.; Wassink, M.G.; Westerman, J.; de Fouw, N.J.; Roelofsen, B.; Op den Kamp, J.A.; van Deenen, L.L. In vivo turnover of phospholipids in rabbit erythrocytes. Biochim. Biophys. Acta 1994, 1215, 314–320. [Google Scholar] [CrossRef]

| LO2 | HepG2 | ||||||
|---|---|---|---|---|---|---|---|
| MFI | p Value | Ratio | MFI | p Value | Ratio | ||
| Day 0 | Control cells | 4.63 ± 0.2 | <0.001 | 0.71 | 5.1 ± 0.07 | <0.001 | 1.27 |
| Vital cells | 149.36 ± 5.59 | 93.3 ± 0.27 | |||||
| Dead cells | 106.78 ± 4.26 | 118.05 ± 0.5 | |||||
| Day 2 | Control cells | 4.72 ± 0.05 | <0.001 | 1.82 | 3.99 ± 0.03 | <0.001 | 14.21 |
| Vital cells | 46.59 ± 0.72 | 12.84 ± 0.16 | |||||
| Dead cells | 84.9 ± 0.48 | 182.51 ± 0.27 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, S.; Zhao, Y.; Saiyin, H.; He, X.; Zhao, J.; Li, L.; Talebi, A.; Huang, G.; Ni, Y. A Model In Vitro Study Using Hypericin: Tumor-Versus Necrosis-Targeting Property and Possible Mechanisms. Biology 2020, 9, 13. https://doi.org/10.3390/biology9010013
Li Y, Wang S, Zhao Y, Saiyin H, He X, Zhao J, Li L, Talebi A, Huang G, Ni Y. A Model In Vitro Study Using Hypericin: Tumor-Versus Necrosis-Targeting Property and Possible Mechanisms. Biology. 2020; 9(1):13. https://doi.org/10.3390/biology9010013
Chicago/Turabian StyleLi, Yue, Shuncong Wang, Yuanyu Zhao, Hexige Saiyin, Xiaoyan He, Juanzhi Zhao, Ling Li, Ali Talebi, Gang Huang, and Yicheng Ni. 2020. "A Model In Vitro Study Using Hypericin: Tumor-Versus Necrosis-Targeting Property and Possible Mechanisms" Biology 9, no. 1: 13. https://doi.org/10.3390/biology9010013
APA StyleLi, Y., Wang, S., Zhao, Y., Saiyin, H., He, X., Zhao, J., Li, L., Talebi, A., Huang, G., & Ni, Y. (2020). A Model In Vitro Study Using Hypericin: Tumor-Versus Necrosis-Targeting Property and Possible Mechanisms. Biology, 9(1), 13. https://doi.org/10.3390/biology9010013







