The Benefit of Anti-Inflammatory and Renal-Protective Dietary Ingredients on the Biological Processes of Aging in the Kidney
Abstract
1. Introduction
2. Immune System
3. Inflammation
4. Body Composition
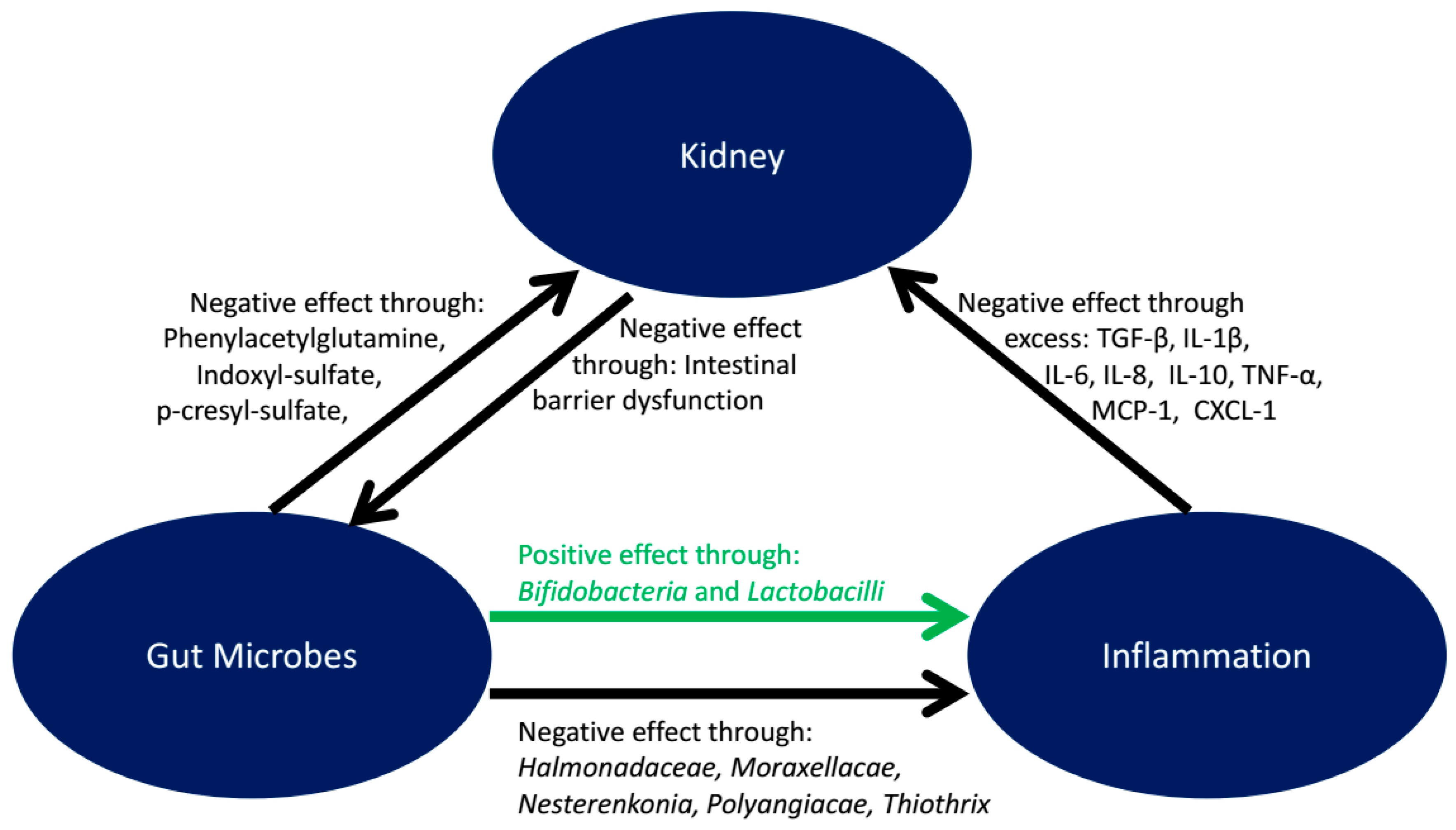
5. Microbiota
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kaysenm, G.A.; Myers, B.D. The aging kidney. Clin. Geriatr. Med. 1985, 1, 207–222. [Google Scholar] [CrossRef]
- Anderson, S.; Brenner, B.M. Effects of aging on the renal glomerulus. Am. J. Med. 1986, 80, 435–442. [Google Scholar] [CrossRef]
- Goyal, V.K. Changes with age in the human kidney. Exp. Gerontol. 1982, 17, 321–331. [Google Scholar] [CrossRef]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Yu, S.; Jewell, D.E. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, l-carnitine, and medium-chain triglycerides. Vet. J. 2014, 202, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Panickar, K.S.; Bobe, G.; Jewell, D.E. Nutritional interventions that slow the age-associated decline in renal function in a canine geriatric model for elderly humans. J. Nutr. Health Aging 2016, 20, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Solomon, C.; Shlipak, M.; Seliger, S.; Stehman-Breen, C.; Bleyer, A.J.; Chaves, P.; Furberg, C.; Kuller, L.; Newman, A. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J. Am. Soc. Nephrol. 2004, 15, 3184–3191. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Torreggiani, M.; Post, J.B.; Zheng, F.; Uribarri, J.; Striker, G.E. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. 2009, 76, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.; Pasternack, B.; Shah, H.; Gallo, G. Age-related incidence of sclerotic glomeruli in human kidneys. Am. J. Pathol. 1975, 80, 227–234. [Google Scholar] [PubMed]
- Hoy, W.E.; Douglas-Denton, R.N.; Hughson, M.D.; Cass, A.; Johnson, K.; Bertram, J.F. A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. Suppl. 2003, 63, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Amer, H.; Cornell, L.D.; Taler, S.J.; Cosio, F.G.; Kremers, W.K.; Textor, S.C.; Stegall, M.D. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 2010, 152, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and functional changes in human kidneys with healthy aging. J. Am. Soc. Nephrol. 2017, 10, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function-measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, R.C.R.M.; Burdmann, E.A.; Lebrão, M.L.; Duarte, Y.A.O.; Zanetta, D.M.T. Aging and decreased glomerular filtration rate: An elderly population-based study. PLoS ONE 2017, 12, e0189935. [Google Scholar] [CrossRef] [PubMed]
- Costello-White, R.; Ryff, C.D.; Coe, C.L. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age 2015, 37, 9808. [Google Scholar] [CrossRef] [PubMed]
- Schei, J.; Stefansson, V.T.; Eriksen, B.O.; Jenssen, T.G.; Solbu, M.D.; Wilsgaard, T.; Melsom, T. Association of TNF receptor 2 and CRP with GFR decline in the general nondiabetic population. Clin. J. Am. Soc. Nephrol. 2017, 12, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Nerpin, E.; Helmersson-Karlqvist, J.; Risérus, U.; Sundström, J.; Larsson, A.; Jobs, E.; Basu, S.; Ingelsson, E.; Arnlöv, J. Inflammation, oxidative stress, glomerular filtration rate, and albuminuria in elderly men: A cross-sectional study. BMC Res. Notes 2012, 5, 537. [Google Scholar] [CrossRef] [PubMed]
- Bulger, R.E.; Cronin, R.E.; Dobyan, D.C. Survey of the morphology of the dog kidney. Anat. Rec. 1979, 194, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Liu, H.H.; Syme, H.M.; Purcell, R.; Wheeler-Jones, C.P.D.; Elliott, J. The cat as a naturally occurring model of renal interstitial fibrosis: Characterisation of primary feline proximal tubular epithelial cells and comparative pro-fibrotic effects of TGF-β1. PLoS ONE 2018, 13, e0202577. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, J.M.; Bunch, D.R.; Hu, B.; Payto, D.; Reineks, E.Z.; Wang, S. Comparison of symmetric dimethylarginine with creatinine, cystatin C and their eGFR equations as markers of kidney function. Clin. Biochem. 2016, 49, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Vaske, H.H.; Armbrust, L.; Zicker, S.C.; Jewell, D.E.; Grauer, D.G.F. Assessment of renal function in hyperthyroid cats managed with a controlled iodine diet. Int. J. Appl. Res. Vet. M. 2016, 14, 38–48. [Google Scholar]
- Stevens, L.A.; Huang, C.; Levey, A.S. Measurement and estimation of kidney function. In Chronic Kidney Disease, Dialysis, and Transplantation, 3rd ed.; Elsevier: Saunders, PA, USA, 2010; pp. 22–38. [Google Scholar]
- Shankar, A.; Teppala, S. Relationship between body mass index and high cystatin levels among US adults. J. Clin. Hypertens. 2011, 13, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Takebe, N.; Nagasawa, K.; Todate, Y.; Nakagawa, R.; Nakano, R.; Hangai, M.; Hasegawa, Y.; Takahashi, Y.; Yoshioka, K.; et al. Association of epicardial adipose tissue with serum level of cystatin C in type 2 diabetes. PLoS ONE 2017, 12, e0184723. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Almes, K.; Jewell, D.E. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J. Vet. Intern. Med. 2016, 3, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Almes, K.; Jewell, D.E. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J. Vet. Intern. Med. 2014, 28, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Fritsch, D.A.; Yerramilili, M.; Obare, E.; Yerramilli, M.; Jewell, D.E. A longitudinal study on the acceptance and effects of a therapeutic renal food in pet dogs with IRIS-Stage 1 chronic kidney disease. J. Anim. Physiol. Anim. Nutr. 2018, 102, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Morimoto, S.; Okigaki, M.; Seo, M.; Someya, K.; Morita, T.; Matsubara, H.; Sugiura, T.; Iwasaka, T. Decreased plasma level of vitamin C in chronic kidney disease: Comparison between diabetic and non-diabetic patients. Nephrol. Dial. Transpl. 2011, 26, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Karamouzis, I.; Sarafidis, P.A.; Karamouzis, M.; Iliadis, S.; Haidich, A.B.; Sioulis, A.; Triantos, A.; Vavatsi-Christaki, N.; Grekas, D.M. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am. J. Nephrol. 2008, 28, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A. Oxidative stress and chronic kidney disease. Vet. Clin. North Am. Small Anim. Pract. 2008, 38, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.A.; Finco, D.R.; Crowell, W.A. Effect of dietary phosphorus restriction on the kidneys of cats with reduced renal mass. Am. J. Vet. Res. 1982, 43, 1023–1026. [Google Scholar] [PubMed]
- Brown, S.A.; Crowell, W.A.; Barsanti, J.A.; White, J.V.; Finco, D.R. Beneficial effects of dietary mineral restriction in dogs with marked reduction of functional renal mass. J. Am. Soc. Nephrol. 1991, 1, 1169–1179. [Google Scholar] [PubMed]
- Martin, K.J.; González, E.A. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin. J. Am. Soc. Nephrol. 2011, 6, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Armstrong, L.E.; Rodriguez, N.R. Dietary protein intake and renal function. Nutr. Metab. 2005, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Metges, C.C.; Barth, C.A. Metabolic consequences of a high dietary-protein intake in adulthood: Assessment of the available evidence. J. Nutr. 2000, 130, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Kenner, C.H.; Evan, A.P.; Blomgren, P.; Aronoff, G.R.; Luft, F.C. Effect of protein intake on renal function and structure in partially nephrectomized rats. Kidney Int. 1985, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Lund, H.; Mattson, D.L. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 2011, 57, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Meek, R.L.; LeBoeuf, R.C.; Saha, S.A.; Alpers, C.E.; Hudkins, K.L.; Cooney, S.K.; Anderberg, R.J.; Tuttle, K.R. Glomerular cell death and inflammation with high-protein diet and diabetes. Nephrol. Dial. Transplant 2013, 28, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Melendez, L.D.; Jewell, D.E. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J. Vet. Intern. Med. 2015, 29, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Katz, R.; Murphy, R.; Newman, A.; Patel, K.; Ix, J.; Peralta, C.; Satterfield, S.; Fried, L.; Shlipak, M.; et al. Comparison between different measures of body fat with kidney function decline and incident CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Yokota, L.G.; Sampaio, B.M.; Rocha, E.P.; Balbi, A.L.; Sousa Prado, I.R.; Ponce, D. Acute kidney injury in elderly patients: Narrative review on incidence, risk factors, and mortality. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yanagita, M. Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Renal Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Cantley, L.G. The impact of aging on kidney repair. Am. J. Physiol. Renal Physiol. 2008, 294, F1265–F1272. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Nomellini, V.; Faunce, D.E.; Kovacs, E.J. Innate immunity and aging. Exp. Gerontol. 2008, 43, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.K.; Fialkow, L.; Chan, C.K.; Kishimoto, T.K.; Downey, G.P. Signaling functions of l-selectin. Enhancement of tyrosine phosphorylation and activation of MAP kinase. J. Biol. Chem. 1995, 270, 15403–15411. [Google Scholar] [CrossRef] [PubMed]
- Kettritz, R.; Gaido, M.L.; Haller, H.; Luft, F.C.; Jennette, C.J.; Falk, R.J. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha mediated apoptosis of human neutrophils. Kidney Int. 1998, 53, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.B.; Albright, B.N.; Caswell, J.L. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infect. Immun. 2003, 71, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Jozsef, L.; Khreiss, T.; El Kebir, D.; Filep, J.G. Activation of TLR-9 induces IL-8 secretion through peroxynitrite signaling in human neutrophils. J. Immunol. 2006, 176, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Chinn, R.M.; Vorachek, W.R.; Gorman, M.E.; Jewell, D.E. Aged Beagle dogs have decreased neutrophil phagocytosis and neutrophil-related gene expression compared to younger dogs. Vet. Immunol. Immunopathol. 2010, 137, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C.; Uyemura, K.; Fulop, T.; Makinodan, T. Host resistance and immune responses in advanced age. Clin. Geriatr. Med. 2007, 23, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Mukherjee, M.; Go, A.; Barrows, I.R.; Ramezani, A.; Shoji, J.; Reilly, M.P.; Gnanaraj, J.; Deo, R.; Roas, S.; et al. Interleukin-6 is a risk factor for Atrial Fibrillation in chronic kidney disease: Findings from the CRIC Study. PLoS ONE 2016, 11, e0148189. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.; Akbulut, S.; Toka Ozer, T.; Kiyici, A.; Keles, M.; Baltaci, B.; Turk, S. Cytokines, adipocytokines and inflammatory markers in patients on continuous ambulatory peritoneal dialysis and hemodialysis. Ren. Fail. 2016, 38, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Wolkow, P.P.; Niewczas, M.A.; Perkins, B.; Ficociello, L.H.; Lipinski, B.; Warram, J.H.; Krolewski, A.S. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J. Am. Soc. Nephrol. 2008, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Habenicht, L.M.; Webb, T.L.; Clauss, L.A.; Dow, S.W.; Quimby, J.M. Urinary cytokine levels in apparently healthy cats and cats with chronic kidney disease. J. Feline Med. Surg. 2013, 15, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Green, D.R. Protease activation during apoptosis: Death by a thousand cuts? Cell 1995, 82, 349–352. [Google Scholar] [CrossRef]
- Alnemri, E.S.; Livingston, D.J.; Nicholson, D.W.; Salvesen, G.; Thornberry, N.A.; Wong, W.W.; Yuan, J.Y. Human ICE/CED-3 protease nomenclature. Cell 1996, 87, 171. [Google Scholar] [CrossRef]
- Black, R.A.; Kronheim, S.R.; Merriam, J.E.; March, C.J.; Hupp, T.P. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin1 beta. J. Biol. Chem. 1989, 264, 5323–5326. [Google Scholar] [PubMed]
- Tatsuta, T.; Cheng, J.; Mountz, J.D. Intracellular IL-1beta is an inhibitor of Fas-mediated apoptosis. J. Immunol. 1996, 157, 3949–3957. [Google Scholar] [PubMed]
- Dinarello, C.A. Biological basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [PubMed]
- Effros, R.B. Replicative senescence of CD8 T cells: Effect on human ageing. Exp. Gerontol. 2004, 39, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lara, M.A.; Carracedo, J.; Ramírez, R.; Martín-Malo, A.; Rodríguez, M.; Madueño, J.A.; Aljama, P. The imbalance in the ratio of Th1 and Th2 helper lymphocytes in uraemia is mediated by an increased apoptosis of Th1 subset. Nephrol. Dial. Transplant. 2004, 19, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Picton, R.A.; Finneran, P.S.; Bird, K.E.; Skinner, M.M.; Jewell, D.E.; Zicker, S. Dietary antioxidants and behavioral enrichment enhance neutrophil phagocytosis 1 in geriatric Beagles. Vet. Immunol. Immunopathol. 2006, 113, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Chinn, R.M.; Vorachek, W.R.; Gorman, M.E.; Greitl, J.L.; Joshi, D.K.; Jewell, D.E. Influence of dietary antioxidants and fatty acids on neutrophil mediated bacterial killing and gene expression in healthy Beagles. Vet. Immunol. Immunopathol. 2011, 139, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, J.; Sheng, L.; Zhang, D.; Chen, X.; Yang, J.; Wang, D. Total coumarins from Hydrangea paniculata show renal protective effects in lipopolysaccharide-induced acute kidney injury via anti-inflammatory and antioxidant activities. Front. Pharmacol. 2017, 8, 872. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Song, G.L.; Niu, Q.; Xu, S.Z.; Feng, G.L.; Wang, H.X.; Li, Y.; Li, S.G.; Li, F. Grape seed procyanidin extract reduces arsenic-induced renal inflammatory injury in male mice. Biomed. Environ. Sci. 2017, 30, 535–539. [Google Scholar] [PubMed]
- Jerine Peter, S.; Evan Prince, S. Diclofenac-induced renal toxicity in female Wistar albino rats is protected by the pre-treatment of aqueous leaves extract of Madhuca longifolia through suppression of inflammation, oxidative stress and cytokine formation. Biomed. Pharmacother. 2018, 98, 45–51. [Google Scholar]
- Sharma, S.; Joshi, A.; Hemalatha, S. Protective effect of Withania coagulans fruit extract on cisplatin-induced nephrotoxicity in rats. Pharmacogn. Res. 2017, 9, 354–361. [Google Scholar]
- Salimi, S.; Shardell, M.D.; Seliger, S.L.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Inflammation and trajectory of renal function in community-dwelling older adults. J. Am. Geriatr. Soc. 2018, 66, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.D.; German, A.J.; Noble, P.J. Metabolomics: An emerging post-genomic tool for nutrition. Br. J. Nutr. 2004, 92, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Kirwan, J.; Doherty, M.K.; Whitfield, P.D. Biomarker discovery in animal health and disease: The application of post-genomic technologies. Biomark. Insights 2007, 2, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jewell, D.E. Feeding healthy Beagles medium-chain triglycerides, fish oil, and carnitine offsets age-related changes in serum fatty acids and carnitine metabolites. PLoS ONE 2012, 7, e49510. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Yaqoob, P. Understanding omega-3 polyunsaturated fatty acids. Postgrad. Med. 2009, 121, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, W.D.; Jewell, D.E. Nutritional modification of inflammatory diseases. Semin. Vet. Med. Surg. 1997, 12, 212–222. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Kim, W.; Lupton, J.R.; McMurray, D.N. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.K.; Dodd, C.E.; Fritsch, D.A.; Allen, T.A.; Jewell, D.E. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, D.A.; Allen, T.A.; Dodd, C.E.; Jewell, D.E.; Sixby, K.A.; Leventhal, P.S.; Brejda, J.; Hahn, K.A. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 2010, 236, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, D.A.; Allen, T.A.; Dodd, C.E.; Jewell, D.E.; Sixby, K.A.; Leventhal, P.S.; Hahn, K.A. Dose-titration effects of fish oil in osteoarthritic dogs. J. Vet. Intern. Med. 2010, 24, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Brockman, J.A.; Jewell, D.E. Dietary fish oil alters the lysophospholipid metabolomic profile and decreases urinary 11-dehydro thromboxane B2 concentration in healthy Beagles. Vet. Immunol. Immunopathol. 2011, 144, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, C. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 2003, 41, S4–S12. [Google Scholar] [CrossRef]
- Allen, T.A.; Jewell, D.E. The effect of l-carnitine supplementation on body composition of obese-prone dogs. In Obesity—Weight Management in Cats and Dogs; Hill’s Pet Nutrition, Inc.: Topeka, KS, USA, 1999. [Google Scholar]
- Jewell, D.E.; Toll, P.W. The effect of l-carnitine supplementation on body composition of obese-prone cats. In Obesity—Weight Management in Cats and Dogs; Hill’s Pet Nutrition, Inc.: Topeka, KS, USA, 1999. [Google Scholar]
- Sahebkar, A. Effect of l-carnitine supplementation on circulating c-reactive protein levels: A systematic review and meta-analysis. J. Med. Biochem. 2015, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Di Liberato, L.; Arduini, A.; Rossi, C.; Di Castelnuovo, A.; Posari, C.; Sacchetta, P.; Urbani, A.; Bonomini, M. l-Carnitine status in end-stage renal disease patients on automated peritoneal dialysis. J. Nephrol. 2014, 27, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, R.T.; van Schoor, N.M.; Lips, P. Changes in vitamin D endocrinology during aging in adults. Mol. Cell Endocrinol. 2017, 453, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Subih, H.S.; Behrens, J.; Burt, B.; Clement, L.; Pannell, R.; Macha, L.; Spallholz, J.; Boylan, M. 25 hydroxy vitamin D is higher when a renal multivitamin is given with cholecalciferol at hemodialysis. Asia Pac. J. Clin. Nutr. 2016, 25, 754–759. [Google Scholar] [PubMed]
- Mager, D.R.; Jackson, S.T.; Hoffmann, M.R.; Jindal, K.; Senior, P.A. Vitamin D3 supplementation, bone health and quality of life in adults with diabetes and chronic kidney disease: Results of an open label randomized clinical trial. Clin. Nutr. 2017, 36, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Georgianos, P.I. Con: Nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease. Nephrol. Dial. Transplant. 2016, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Duncan, E.L. Is there a role or target value for nutritional vitamin D in chronic kidney disease? Nephrology 2017, 22, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jin, J.; Li, X.; Zhao, Z.; Zhang, L.; Wang, Q.; Li, J.; Zhang, Q.; Xiang, S. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-l-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis 2018, 46, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hou, X.F.; Wang, G.; Zhong, Q.X.; Liu, Y.; Qiu, H.H.; Yang, N.; Gu, J.F.; Wang, C.F.; Zhang, L.; et al. Terpene glycoside component from Moutan Cortex ameliorates diabetic nephropathy by regulating endoplasmic reticulum stress-related inflammatory responses. J. Ethnopharmacol. 2016, 193, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhu, W.; Shao, Q.; Yan, X.; Jin, B.; Zhang, M.; Xu, B. Tanshinone IIA protects against folic acid-induced acute kidney injury. Am. J. Chin. Med. 2016, 44, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.T.; Huang, R.H.; Cheng, X.; Zhang, Z.H.; Yang, Y.J.; Lin, X. Tanshinone IIA attenuates renal fibrosis and inflammation via altering expression of TGF-β/Smad and NF-κB signaling pathway in 5/6 nephrectomized rats. Int. Immunopharmacol. 2015, 26, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.A.; Oliveira, D.; Barbosa, S.R.; Corrêa, J.; Colugnati, F.; Mansur, H.N.; Fernandes, N.; Bastos, M.G. .Sarcopenia in patients with chronic kidney disease not yet on dialysis: Analysis of the prevalence and associated factors. PLoS ONE 2017, 12, e0176230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hellberg, M.; Svensson, P.; Höglund, P.; Clyne, N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol. Dial. Transplant. 2018, 33, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Hawkins, M.; Abramowitz, M.K. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin. J. Am. Soc. Nephrol. 2014, 9, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Med. Sci. 2006, 61A, 1059–1064. [Google Scholar] [CrossRef]
- Hyun, Y.Y.; Lee, K.B.; Rhee, E.J.; Park, C.Y.; Chang, Y.; Ryu, S. Chronic kidney disease and high eGFR according to body composition phenotype in adults with normal BMI. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci. Rep. 2016, 6, 36618. [Google Scholar] [CrossRef] [PubMed]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, K.R.; Gallot, Y.S.; Sato, S.; Xiong, G.; Hindi, S.M.; Kumar, A. Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia. FASEB J. 2016, 30, 3053–3068. [Google Scholar] [CrossRef] [PubMed]
- Jheng, J.R.; Chen, Y.S.; Ao, U.I.; Chan, D.C.; Huang, J.W.; Hung, K.Y.; Tarng, D.C.; Chiang, C.K. The double-edged sword of endoplasmic reticulum stress in uremic sarcopenia through myogenesis perturbation. J. Cachexia Sarcopenia Muscle 2018. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Kagawa, S.; Arimitsu, J.; Nakanishi, M.; Sakashita, N.; Otsuka, S.; Yoshikawa, H.; Hagihara, K. Go-sha-jinki-Gan (GJG), a traditional Japanese herbal medicine, protects against sarcopenia in senescence-accelerated mice. Phytomedicine 2015, 22, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsuiji, K.; Li, B.; Tadakawa, M.; Yaegashi, N. Proliferative effect of Hachimijiogan, a Japanese herbal medicine, in C2C12 skeletal muscle cells. Clin. Interv. Aging 2015, 10, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, O.S. Jaeumganghwa-Tang, a traditional herbal formula, improves muscle function and attenuates muscle loss in aged mice. J. Exerc. Nutr. Biochem. 2017, 21, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Woodmansey, E.J. Intestinal bacteria and ageing. J. Appl. Microbiol. 2007, 102, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Armani, R.G.; Ramezani, A.; Yasir, A.; Sharama, S.; Canziani, M.E.F.; Raj, D.S. Gut microbiome in chronic kidney disease. Curr. Hypertens. Rep. 2017, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.N.; Wong, C.P.; Duyck, K.M.; Hord, N.; Ho, E.; Sharpton, T.J. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ 2016, 4, 1854. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T. Role of the gut microbiota in age-related chronic inflammation. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 361–367. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Simões-Silva, L.; Pestana, M.; Araujo, R.; Soares-Silva, I.J. The role of the gut microbiome on chronic kidney disease. Adv. Appl. Microbiol. 2016, 96, 65–94. [Google Scholar] [PubMed]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Nutrients turned into toxins: Microbiota modulation of nutrient properties in chronic kidney disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut-microbiota-metabolite axis in early renal function decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef] [PubMed]
- Black, A.P.; Anjos, J.S.; Cardozo, L.; Carmo, F.L.; Dolenga, C.J.; Nakao, L.S.; de Carvalho Ferreira, D.; Rosado, A.; Carraro Eduardo, J.C.; Mafra, D. Does low-protein diet influence the uremic toxin serum levels from the gut microbiota in nondialysis chronic kidney disease patients? J. Ren. Nutr. 2018, 28, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Grieshop, C.M.; Clapper, G.M.; Shields, R.G.; Belay, T.; Merchen, N.R.; Fahey, G.C. Fruit and vegetable fiber fermentation by gut microflora from canines. J. Anim. Sci. 2001, 79, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Buddington, K.K.; Sunvold, G.D. Influence of fermentable fiber on small intestinal dimensions and transport of glucose and proline in dogs. Am. J. Vet. Res. 1999, 60, 354–358. [Google Scholar] [PubMed]
- Hall, J.A.; MacLeay, J.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Schiefelbein, H.; Paetau-Robinson, I.; Jewell, D.E. Positive impact of nutritional interventions on serum symmetric dimethylarginine and creatinine concentrations in client-owned geriatric dogs. PLoS ONE 2016, 11, e0153653. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S.; Jackson, M.I.; Gao, X.; Yerramilli, M.; Jewell, D.E. Improvement in circulating markers of health in aging dogs with diet supplemented with anti-inflammatory and antioxidant ingredients. J. Vet. Intern. Med. 2014, 28, 1088. [Google Scholar]
- Jewell, D.E.; Jackson, M.I.; Hall, J.A. Feline foods enhanced with bioactive ingredients including fish oil increase omega 3 fatty acids and reduce prostaglandin E2 and enhance body lean. In Proceedings of the 27th ECVIM-CA Annual Congress, St. Julians, Malta, 14–16 September 2017. [Google Scholar]
- Hall, J.A.; MacLeay, J.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Schiefelbein, H.; Paetau-Robinson, I.; Jewell, D.E. Positive impact of nutritional interventions on serum symmetric dimethylarginine and creatinine concentrations in client-owned geriatric cats. PLoS ONE 2016, 11, e0153654. [Google Scholar] [CrossRef] [PubMed]
- Gebreselassie, E.E.; Jackson, M.I.; Jewell, D.E. Dietary fiber from fruits and vegetables enhances canine and feline health by modulating microbiota and metabolite profiles. In Proceedings of the 11th Vahouny Fiber Symposium, Bethesda, MD, USA, 15–16 June 2017. [Google Scholar]
- Gebreselassie, E.E.; Jackson, M.I.; Jewell, D.E. Fermentable fibers influence markers of aging in senior dogs and cats. In Proceedings of the International Scientific Association for Probiotics and Prebiotics, Chicago, IL, USA, 27–29 June 2017. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panickar, K.S.; Jewell, D.E. The Benefit of Anti-Inflammatory and Renal-Protective Dietary Ingredients on the Biological Processes of Aging in the Kidney. Biology 2018, 7, 45. https://doi.org/10.3390/biology7040045
Panickar KS, Jewell DE. The Benefit of Anti-Inflammatory and Renal-Protective Dietary Ingredients on the Biological Processes of Aging in the Kidney. Biology. 2018; 7(4):45. https://doi.org/10.3390/biology7040045
Chicago/Turabian StylePanickar, Kiran S., and Dennis E. Jewell. 2018. "The Benefit of Anti-Inflammatory and Renal-Protective Dietary Ingredients on the Biological Processes of Aging in the Kidney" Biology 7, no. 4: 45. https://doi.org/10.3390/biology7040045
APA StylePanickar, K. S., & Jewell, D. E. (2018). The Benefit of Anti-Inflammatory and Renal-Protective Dietary Ingredients on the Biological Processes of Aging in the Kidney. Biology, 7(4), 45. https://doi.org/10.3390/biology7040045



