Intraperitoneal Administration of Oxygen/Ozone to Rats Reduces the Pancreatic Damage Induced by Streptozotocin
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ozone and STZ Treatments
2.3. Experimental Groups
2.4. Tissue Sampling
2.5. Western Blotting Assay
2.6. Immunohistochemistry
2.7. Immunofluorescence
2.8. 4-Hydroxynonenal, Insulin and Leptin Levels
2.9. Statistical Analysis
3. Results
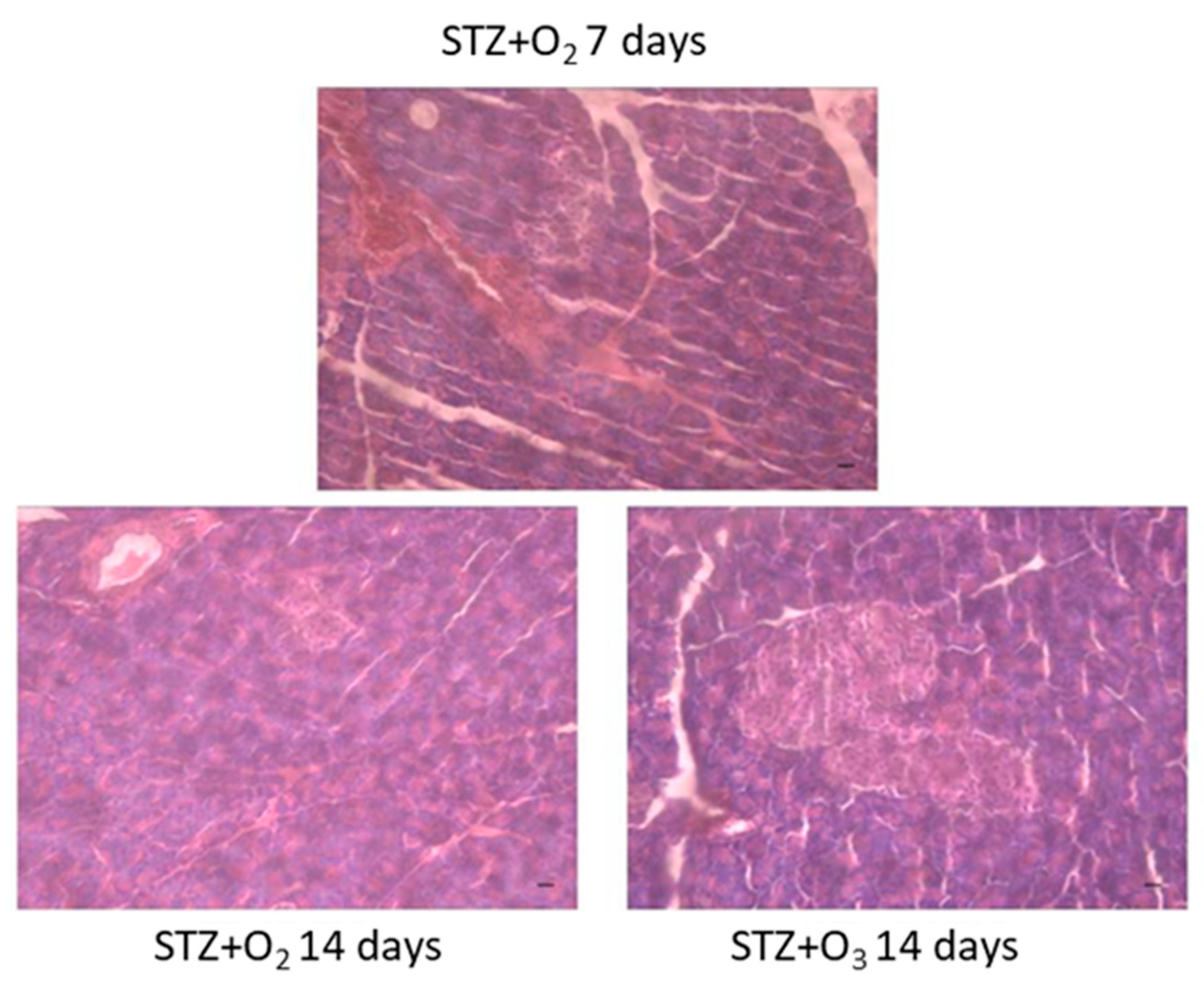
3.1. STZ Induces Pancreatic Tissue Damage
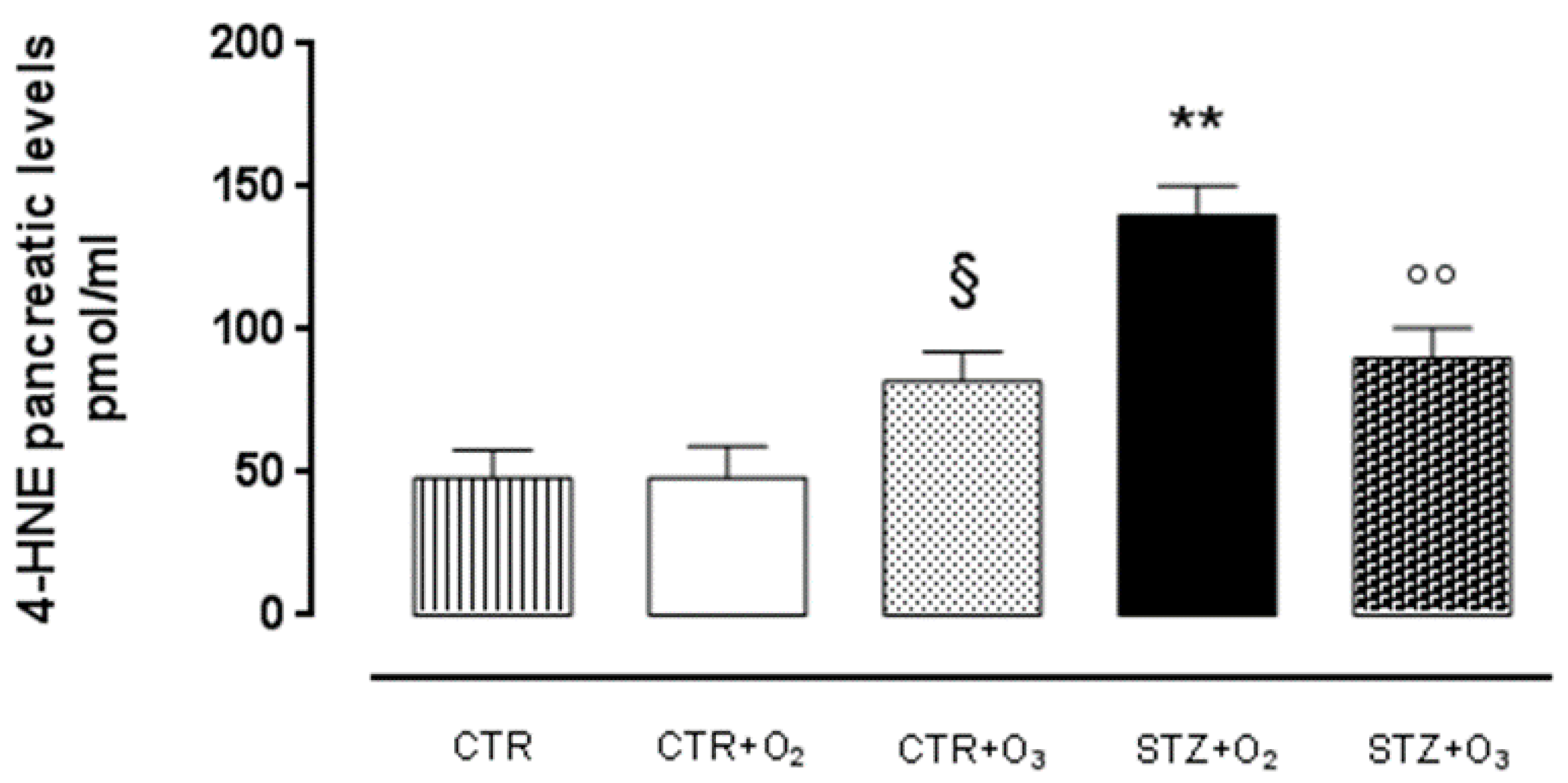
Ozone Reduces 4-HNE Adduct in STZ Rats
3.2. Ozone Treatment Reduces Pancreatic Tissue Damage in STZ Rats
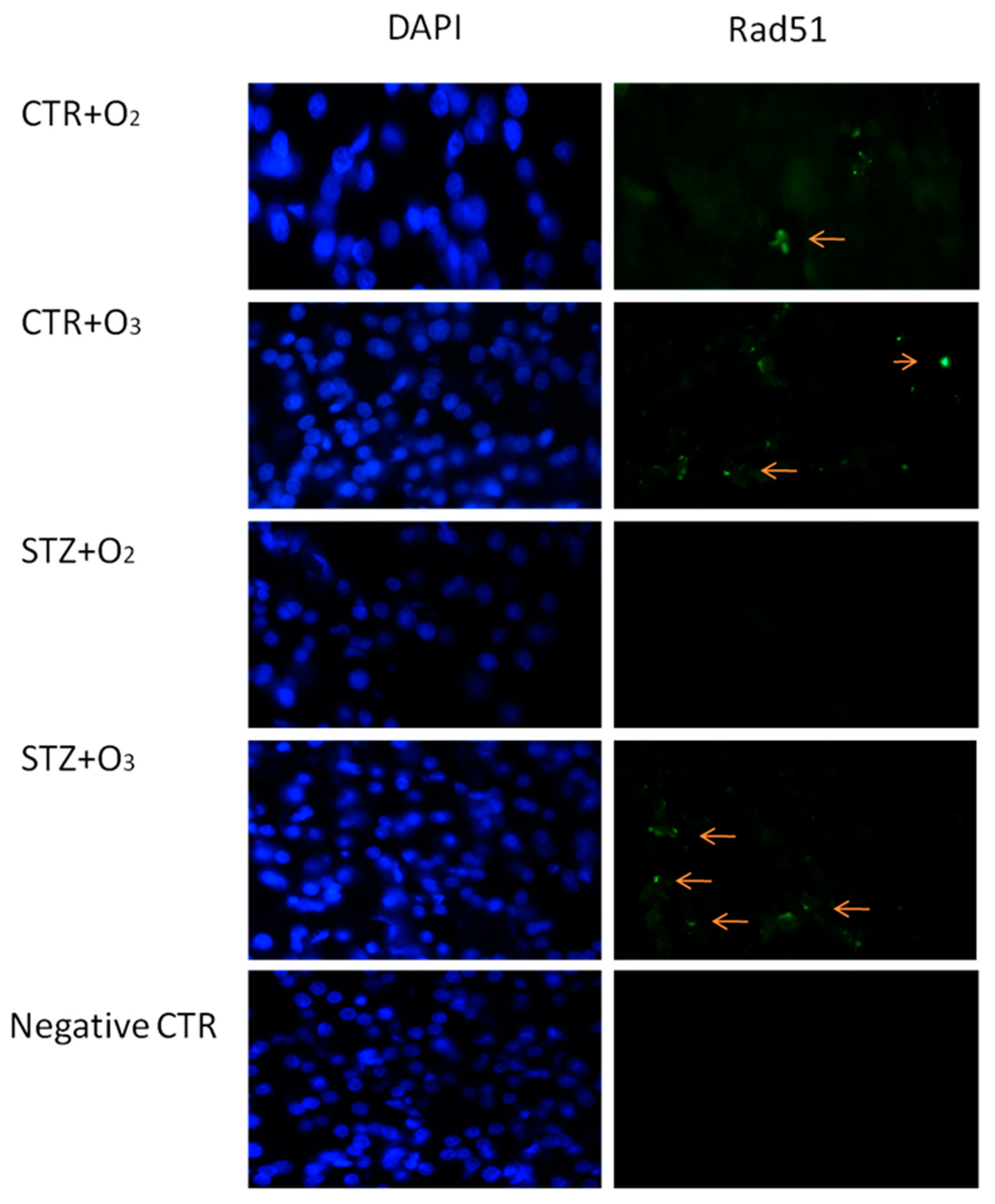
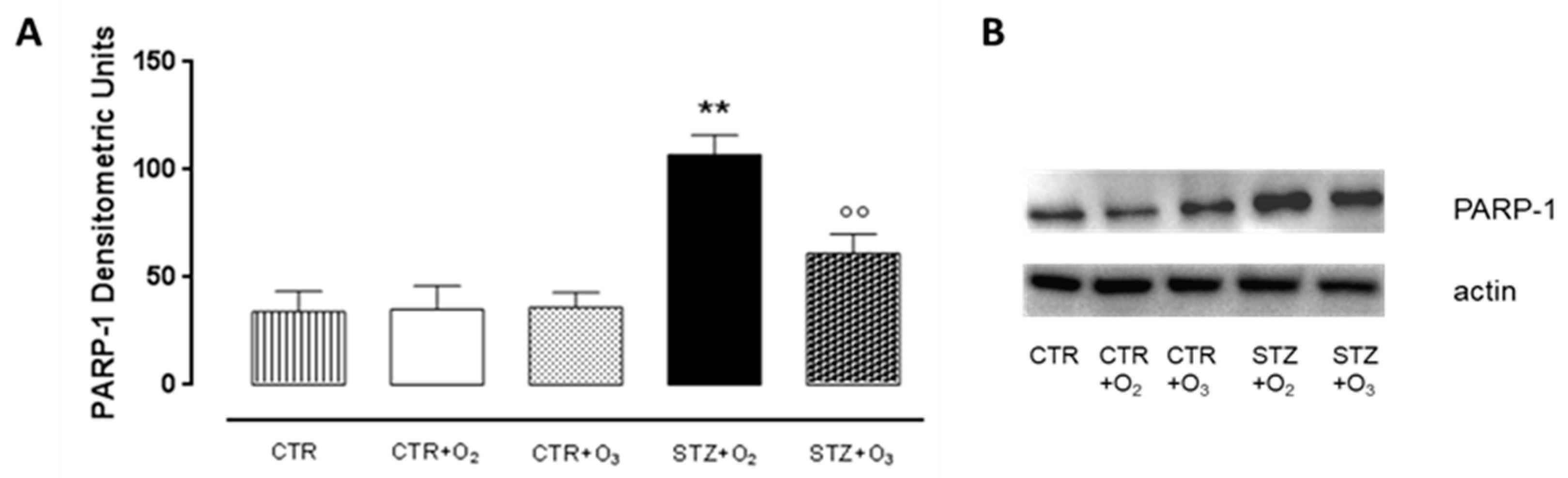
3.2.1. Ozone Treatment Reduces Beta Cells Death and DNA Damage in STZ Rats
3.2.2. Ozone Treatment Increases Nrf2 and GST Pancreatic Levels in STZ Rats
3.2.3. Ozone Reduces Glucagon and Increases Insulin Levels in Pancreas of STZ Rats
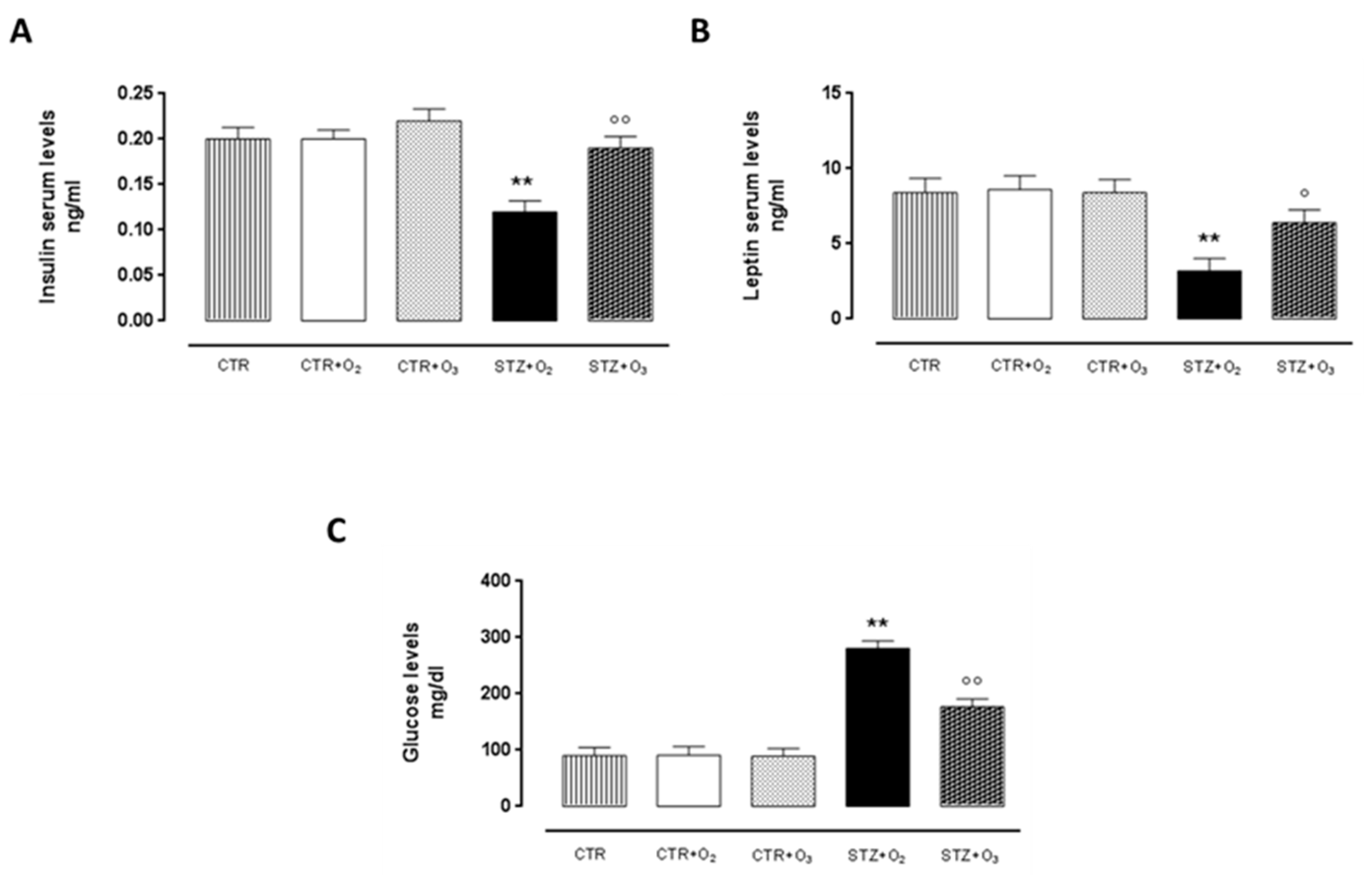
3.2.4. Ozone Treatment Improves Insulin and Leptin Serum Levels, Reducing Glycemia in STZ Rats
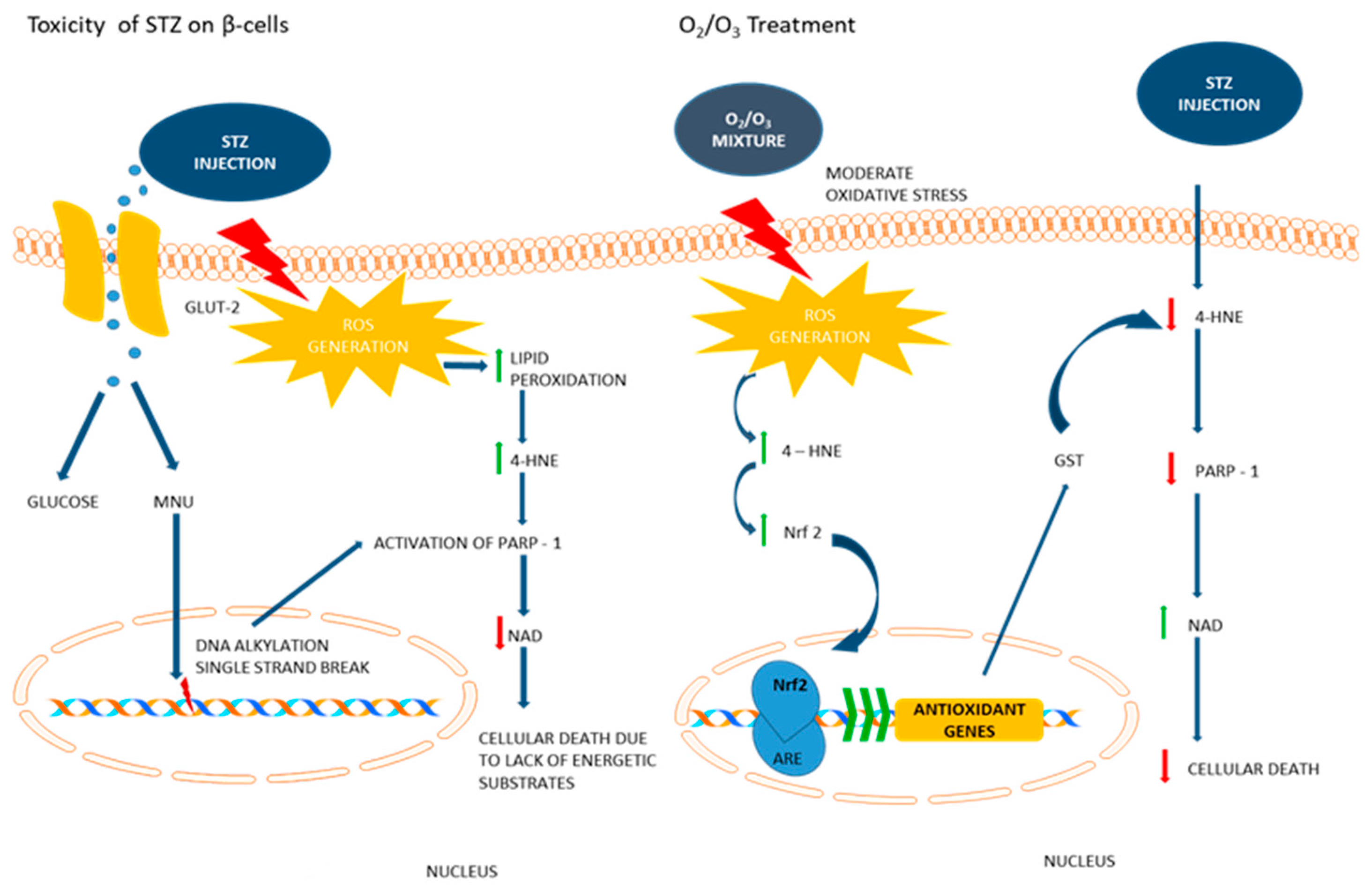
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Traverso, N.; Menini, S.; Odetti, P.; Pronzato, M.A.; Cottalasso, D.; Marinari, U.M. Diabetes impairs the enzymatic disposal of 4-hydroxynonenal in rat liver. Free Radic. Biol. Med. 2002, 32, 350–359. [Google Scholar] [CrossRef]
- Lupachyk, S.; Shevalye, H.; Maksimchyk, Y.; Drel, V.R.; Obrosova, I.G. PARP inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: Correlation with peripheral nerve function. Free Radic. Biol. Med. 2011, 50, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Al-Dalain, S.M.; Martinez, G.; Candelano-Jali, E.; Menéndez, S.; Re, L.; Giuliani, A.; León, O.S. Ozone treatment reduces markers of oxidative and endothelial damage in an experimental diabetes model in rats. Pharmacol. Res. 2001, 44, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Martìnez, G.; Al-Dalain, S.M.; Menendez, S.; Giuliani, A.; Léon, O.S. Ozone treatment reduces blood oxidative stress and pancreas damage in streptozotocin induced diabetes model in rats. Acta Farm. Bonaer. 2005, 24, 491–497. [Google Scholar]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Re, L.; Martínez-Sánchez, G.; Bordicchia, M.; Malcangi, G.; Pocognoli, A.; Morales-Segura, M.A.; Rothchild, J.; Rojas, A. Is ozone pre-conditioning effect linked to Nrf2/EpRE activation pathway in vivo? A preliminary result. Eur. J. Pharmacol. 2014, 742, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, C.; Luongo, C.; Capodanno, P.; Giordano, C.; Scafuro, M.A.; Siniscalco, D.; Lettieri, B.; Rossi, F.; Maione, S.; Berrino, L. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur. J. Pharmacol. 2009, 603, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.; Wang, J.; Wong, G.T.; Cheung, C.W.; Zhang, L.; Chen, C.; Xia, Z.; Irwin, M.G. Susceptibility to myocardial ischemia reperfusion injury at early stage of type 1 diabetes in rats. Cardiovasc. Diabetol. 2013, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Maisto, R.; Gesualdo, C.; Trotta, M.C.; Ferraraccio, F.; Kaneva, M.K.; Getting, S.J.; Surace, E.; Testa, F.; Simonelli, F.; et al. Activation of Melanocortin Receptors MC1 and MC5 Attenuates Retinal Damage in Experimental Diabetic Retinopathy. Mediat. Inflamm. 2016. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Marfella, R.; Capodanno, P.; Ferraraccio, F.; Coppola, L.; Luongo, M.; Mascolo, L.; Luongo, C.; Capuano, A.; Rossi, F.; et al. Acute oxygen ozone administration to rats protects the heart from ischemia reperfusion infarct. Inflamm. Res. 2008, 57, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Trotta, M.C.; Maisto, R.; Siniscalco, D.; Luongo, M.; Mascolo, L.; Alfano, R.; Accardo, M.; Rossi, C.; Ferraraccio, F.; et al. Daily Oxygen/O3 Treatment Reduces Muscular Fatigue and Improves Cardiac Performance in Rats Subjected to Prolonged High Intensity Physical Exercise. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Sapone, A.; Giordano, C.; Cirillo, A.; de Magistris, L.; Rossi, F.; Fasano, A.; Bradstreet, J.J.; Maione, S.; Antonucci, N. Cannabinoid receptor type 2, but not type 1, is up-regulated in peripheral blood mononuclear cells of children affected by autistic disorders. J. Autism Dev. Disord. 2013, 43, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced Diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, I.; Borrelli, E.; Valacchi, G.; Travagli, V.; Bocci, V. Ozone: A Multifaceted Molecule with Unexpected Therapeutic Activity. Curr. Med. Chem. 2016, 23, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.; Demirbag, S.; Poyrazoglu, Y.; Cayci, T.; Yesildaglar, N.; Guven, A.; Sürer, I.; Korkmaz, A. Medical ozone therapy decreases postoperative uterine adhesion formation in rats. Arch. Gynecol. Obstet. 2012, 286, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Bocci, V. Visual improvement following ozonetherapy in dry age related macular degeneration; a review. Med. Hypothesis Discov. Innov. Ophthalmol. 2013, 2, 47–51. [Google Scholar] [PubMed]
- Bocci, V.; Di Paolo, N. Oxygen-ozone therapy in medicine: An update. Blood Purif. 2009, 28, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.A.; Zanardi, I.; Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. How a calculated oxidative stress can yield multiple therapeutic effects. Free Radic. Res. 2012, 46, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Valacchi, G. Nrf2 activation as target to implement therapeutic treatments. Front. Chem. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Amarnath, V.; Pietenpol, J.A.; Marnett, L.J. 4-hydroxynonenal induces apoptosis via caspase-3 activation and cytochrome c release. Chem. Res. Toxicol. 2001, 14, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol. Sci. 2003, 71, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, A.; Erken, H.A.; Erken, G.; Dodurga, Y.; Yay, A.; Özçoban, Ö.; Şimşek, H.; Akçılar, A.; Koçak, F.E. The effects of ozone therapy on caspase pathways, TNF-α, and HIF-1α in diabetic nephropathy. Int. Urol. Nephrol. 2016, 48, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Uchigata, Y.; Okamoto, H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature 1981, 294, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Uchigata, Y.; Yamamoto, H.; Kawamura, A.; Okamoto, H. Protection by superoxide dismutase, catalase, and poly(ADPribose) synthetase inhibitors against alloxan- and streptozotocin induced islet DNA strand breaks and against the inhibition of proinsulin synthesis. J. Biol. Chem. 1982, 257, 6084–6088. [Google Scholar] [PubMed]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Ali, A.M. Pathophysiological Role of Peroxynitrite Induced DNA Damage in Human Diseases: A Special Focus on Poly(ADP-ribose) Polymerase (PARP). Indian J. Clin. Biochem. 2015, 30, 368–385. [Google Scholar] [CrossRef] [PubMed]
- Burkart, V.; Wang, Z.Q.; Radons, J.; Heller, B.; Herceg, Z.; Stingl, L.; Wagner, E.F.; Kolb, H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat. Med. 1999, 5, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Masutani, M.; Suzuki, H.; Kamada, N.; Watanabe, M.; Ueda, O.; Nozaki, T.; Jishage, K.; Watanabe, T.; Sugimoto, T.; Nakagama, H.; et al. Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin induced diabetes. Proc. Natl. Acad. Sci. USA 1999, 96, 2301–2304. [Google Scholar] [CrossRef] [PubMed]
- Pieper, A.A.; Brat, D.J.; Krug, D.K.; Watkins, C.C.; Gupta, A.; Blackshaw, S.; Verma, A.; Wang, Z.Q.; Snyder, S.H. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin induced diabete. Proc. Natl. Acad. Sci. USA 1999, 96, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Devalaraja-Narashimha, K.; Padanilam, B.J. PARP-1 inhibits glycolysis in ischemic kidneys. J. Am. Soc. Nephrol. 2009, 20, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shevalye, H.; Stavniichuk, R.; Xu, W.; Zhang, J.; Lupachyk, S.; Maksimchyk, Y.; Drel, V.R.; Floyd, E.Z.; Slusher, B.; Obrosova, I.G. Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model. Biochem. Pharmacol. 2010, 79, 1007–1014. [Google Scholar] [PubMed]
- Schein, P.S.; Cooney, D.A.; Vernon, M.L. The use of nicotinamide to modify the toxicity of streptozotocin diabetes without loss of antitumor activity. Cancer Res. 1967, 27, 2324–2332. [Google Scholar] [PubMed]
- Kuchmerovska, T.; Shymanskyy, I.; Bondarenko, L.; Klimenko, A. Effects of nicotinamide supplementation on liver and serum contents of amino acids in diabetic rats. Eur. J. Med. Res. 2008, 13, 275–280. [Google Scholar] [PubMed]
- Douglas, D.L.; Baines, C.P. PARP1-mediated necrosis is dependent on parallel JNK and Ca2+/calpain pathways. J. Cell Sci. 2014, 127, 4134–4145. [Google Scholar] [PubMed]
- Zhao, Y.; Scott, N.A.; Fynch, S.; Elkerbout, L.; Wong, W.W.; Mason, K.D.; Strasser, A.; Huang, D.C.; Kay, T.W.; Thomas, H.E. Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia 2015, 58, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.; Lopez, E.; Saleh-Gohari, N.; Helleday, T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003, 31, 4959–4964. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siniscalco, D.; Trotta, M.C.; Brigida, A.L.; Maisto, R.; Luongo, M.; Ferraraccio, F.; D’Amico, M.; Di Filippo, C. Intraperitoneal Administration of Oxygen/Ozone to Rats Reduces the Pancreatic Damage Induced by Streptozotocin. Biology 2018, 7, 10. https://doi.org/10.3390/biology7010010
Siniscalco D, Trotta MC, Brigida AL, Maisto R, Luongo M, Ferraraccio F, D’Amico M, Di Filippo C. Intraperitoneal Administration of Oxygen/Ozone to Rats Reduces the Pancreatic Damage Induced by Streptozotocin. Biology. 2018; 7(1):10. https://doi.org/10.3390/biology7010010
Chicago/Turabian StyleSiniscalco, Dario, Maria Consiglia Trotta, Anna Lisa Brigida, Rosa Maisto, Margherita Luongo, Franca Ferraraccio, Michele D’Amico, and Clara Di Filippo. 2018. "Intraperitoneal Administration of Oxygen/Ozone to Rats Reduces the Pancreatic Damage Induced by Streptozotocin" Biology 7, no. 1: 10. https://doi.org/10.3390/biology7010010
APA StyleSiniscalco, D., Trotta, M. C., Brigida, A. L., Maisto, R., Luongo, M., Ferraraccio, F., D’Amico, M., & Di Filippo, C. (2018). Intraperitoneal Administration of Oxygen/Ozone to Rats Reduces the Pancreatic Damage Induced by Streptozotocin. Biology, 7(1), 10. https://doi.org/10.3390/biology7010010





