Identification of Highly Specific scFvs against Total Adiponectin for Diagnostic Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Recombinant Adiponectin
2.2. Protein Extraction by Detergent-Based Cell Lysis
2.3. Purification of Soluble Proteins by Ni-NTA Purification
2.4. Phage Display
2.5. Enzyme-Linked Immuno Sorbent Assay (ELISA)
2.6. Analysis of Expressed scFvs by SDS-PAGE
2.7. ELISA Detection of Adiponectin—Binding Activity of scFvs
2.8. Purification of scFvs
2.9. Sandwich ELISA Assay
3. Results
3.1. Production of Recombinant Adiponectin
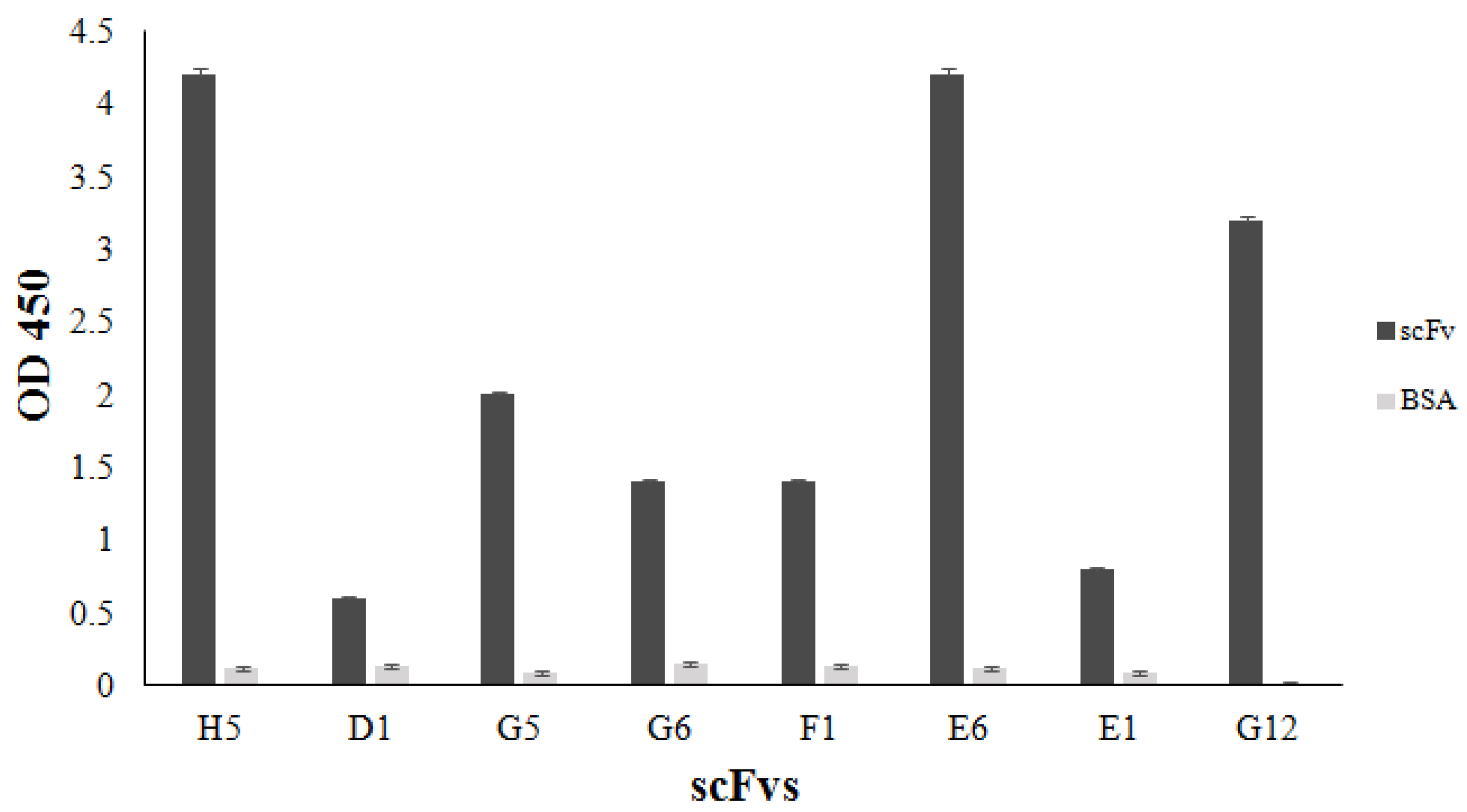
3.2. Bio-Panning
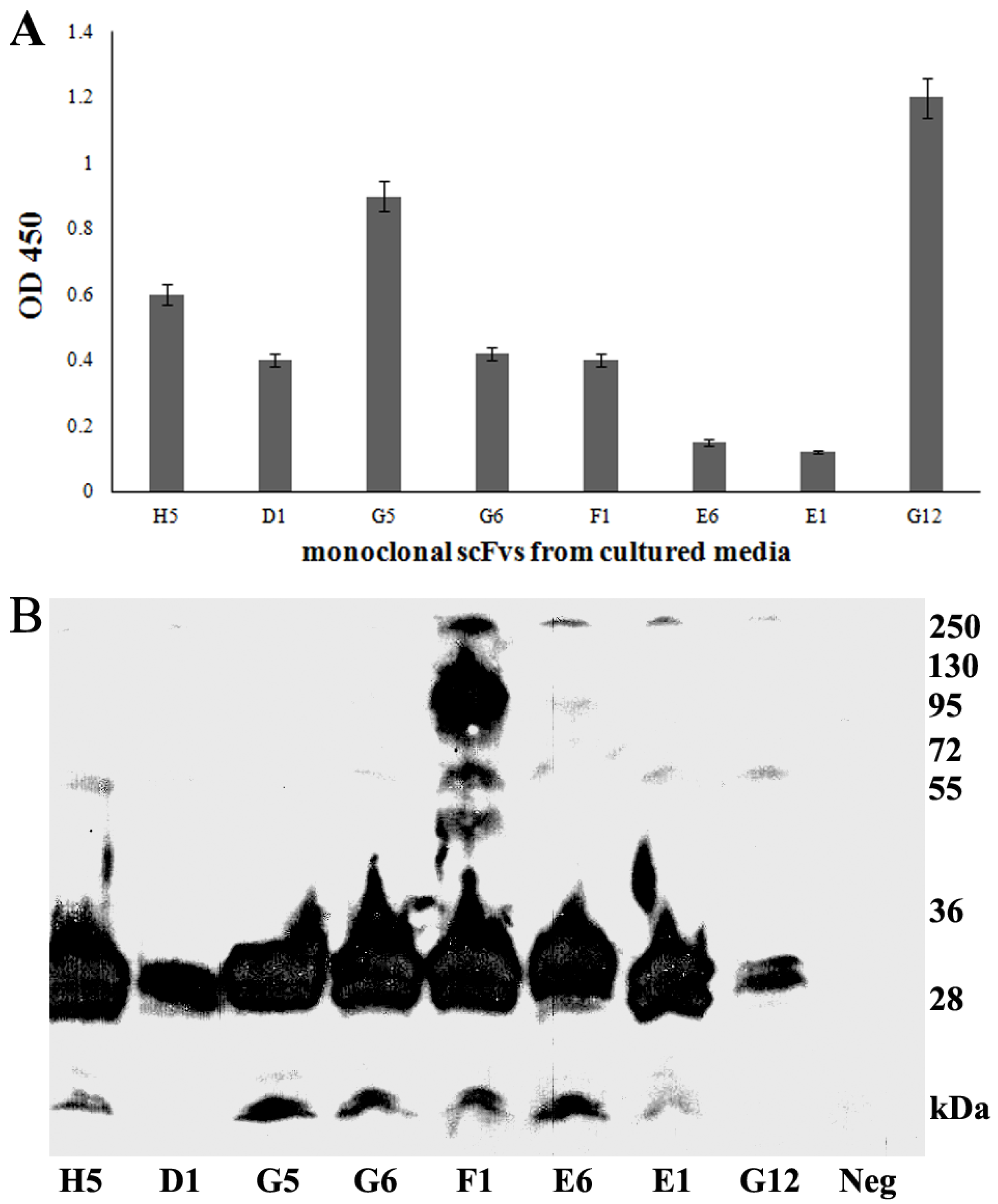
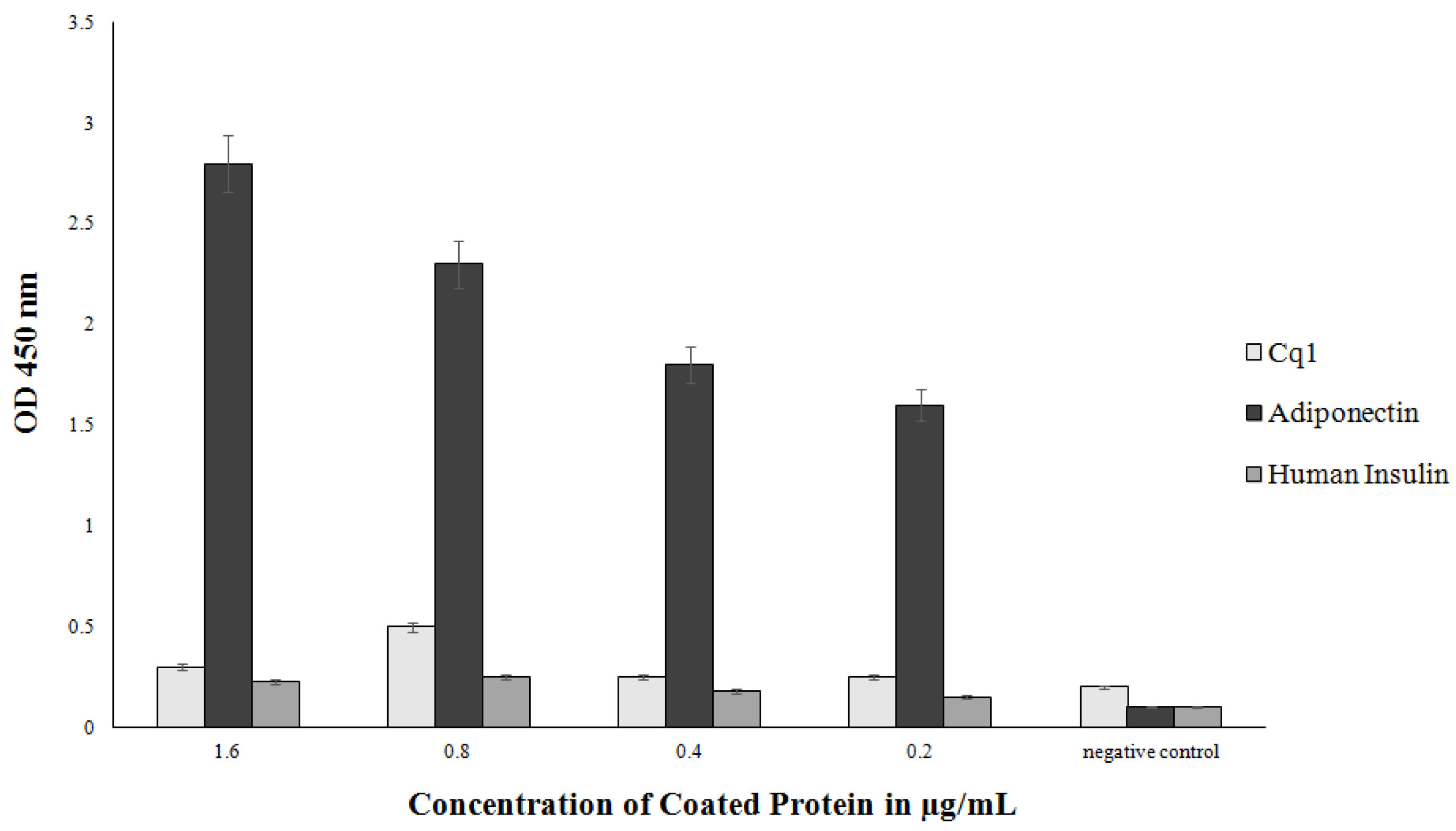
3.3. Production of Soluble scFvs
3.4. Periplasmic Extraction of scFv
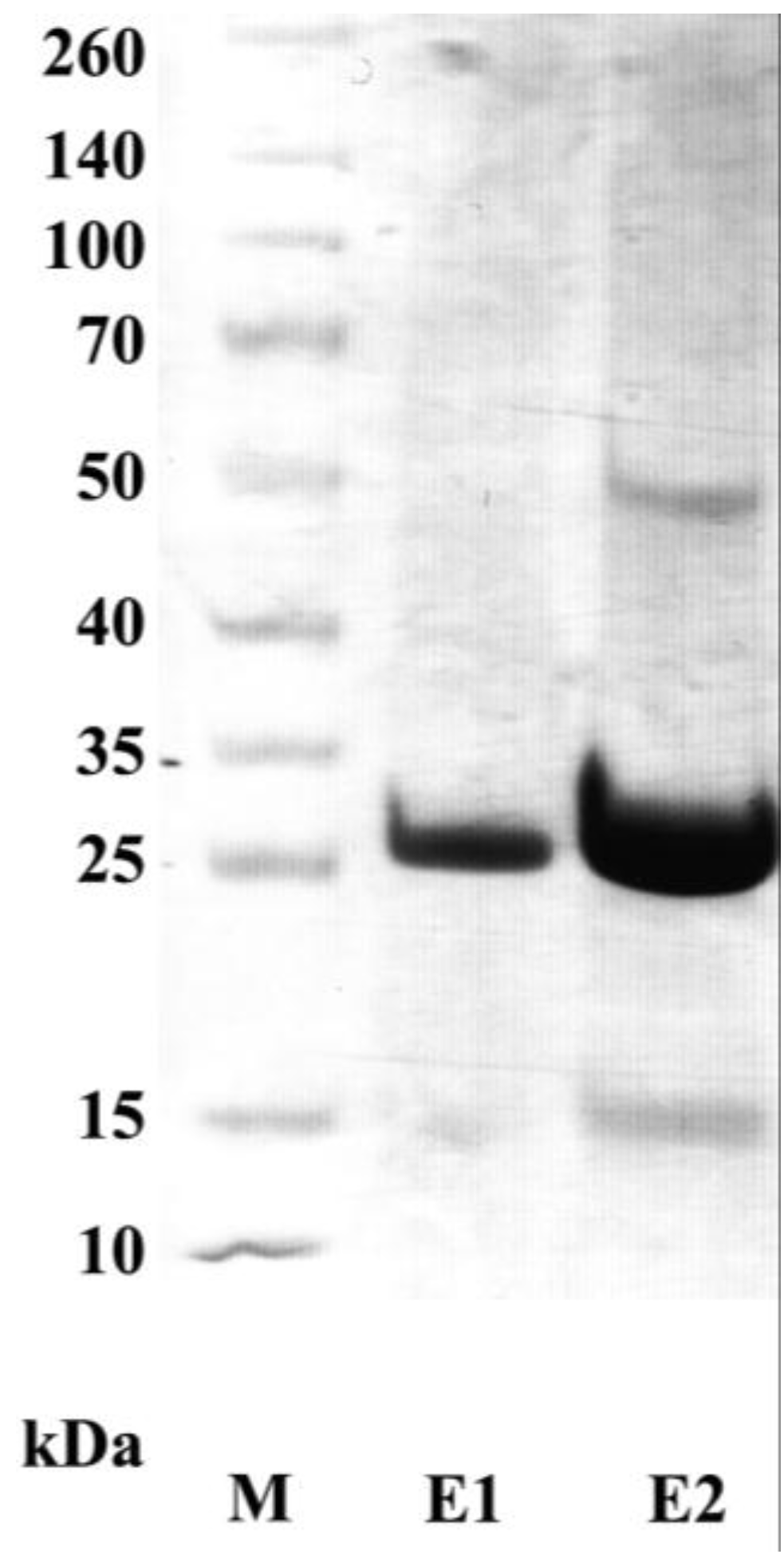
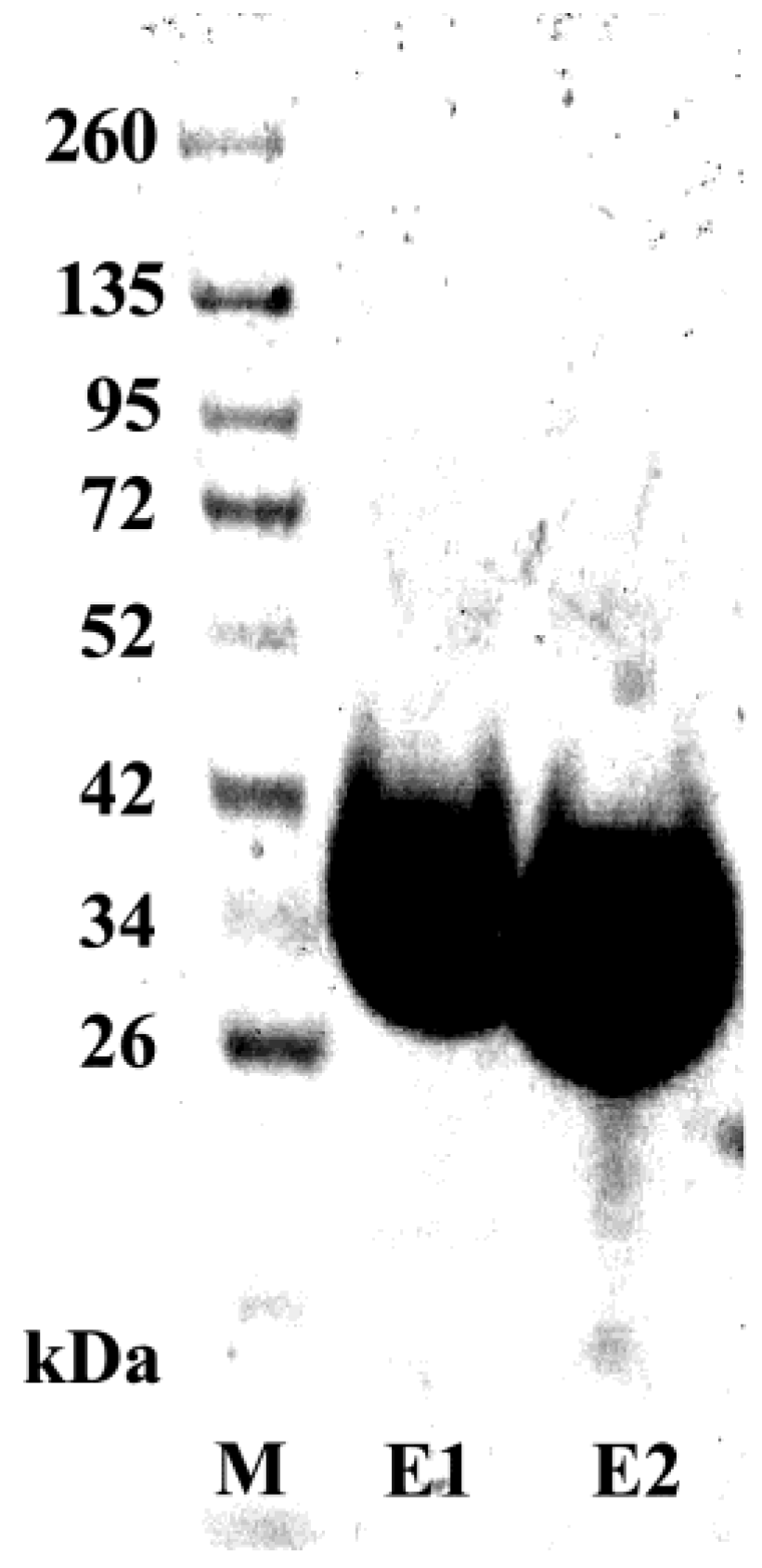
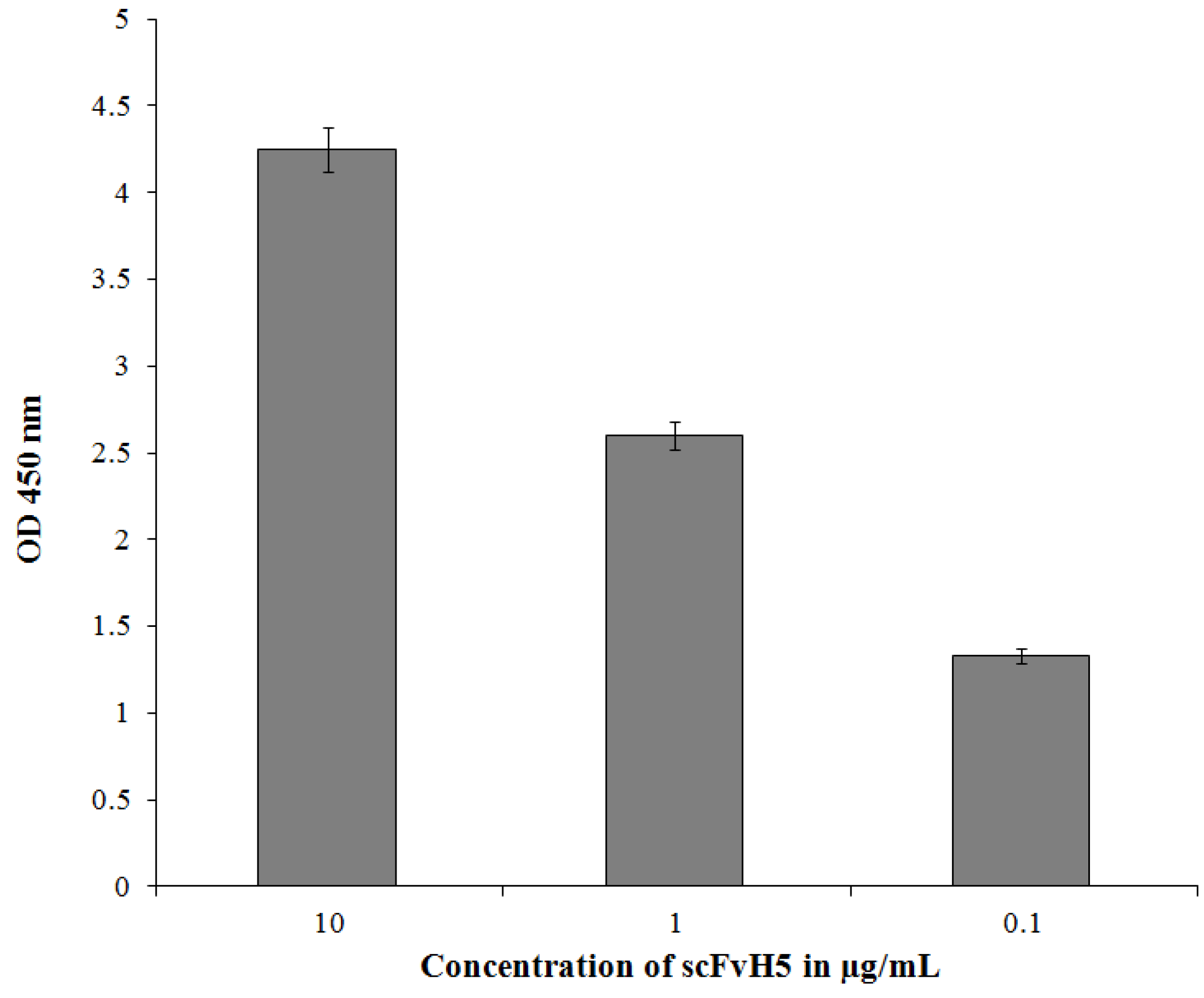
3.5. Purification and Characterization of the scFv Antibody
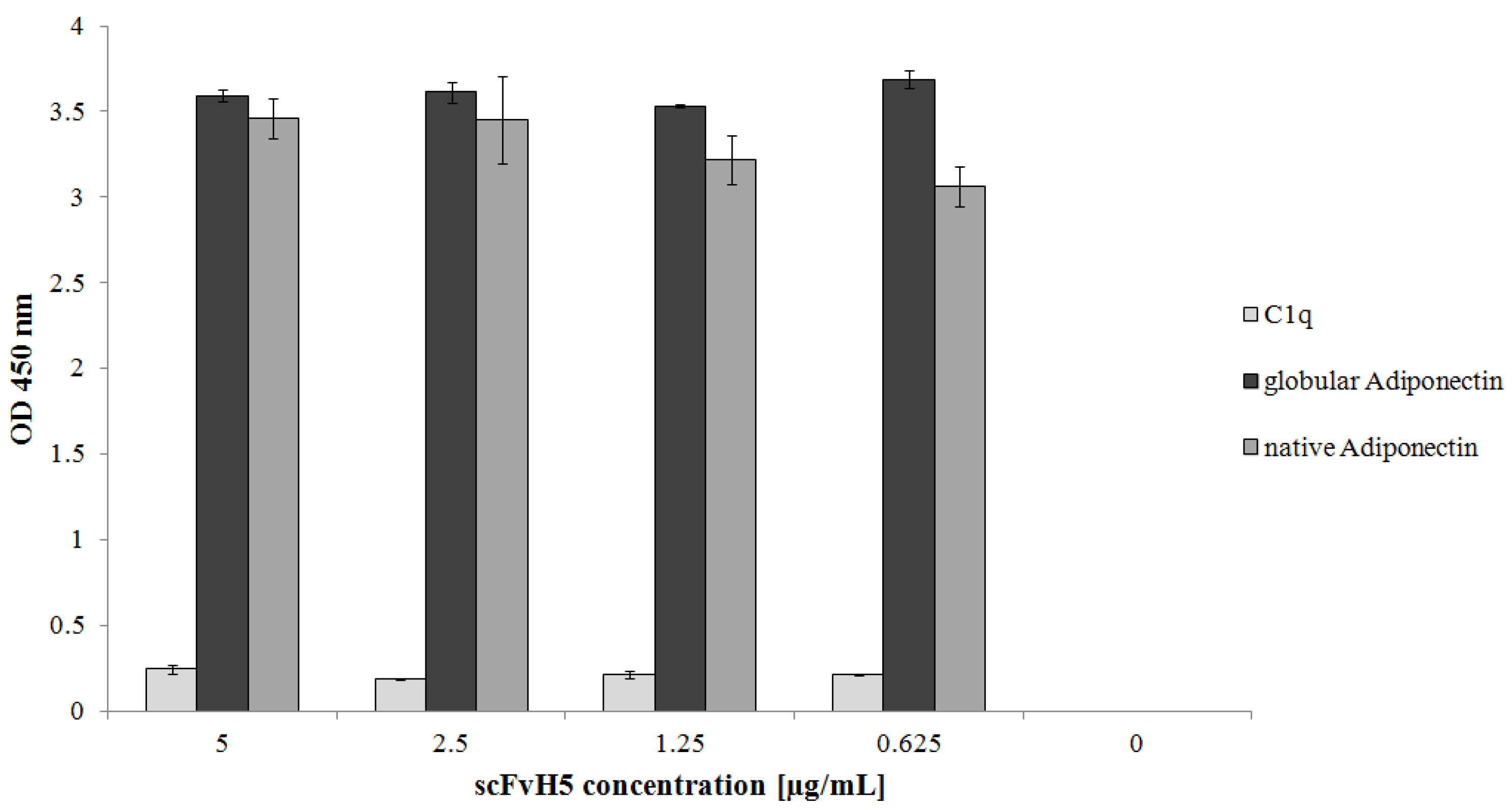
3.6. Binding of the Selected scFvH5 to Native and Globular Adiponectin
3.7. Binding of scFvH5 to Adiponectin in Human Plasma
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diez, J.J.; Iglesias, P. The Role of the Novel Adipocyte-Derived Hormone Adiponectin in Human Disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Schondorf, T.; Maiworm, A.; Emmison, N.; Forst, T.; Pfutzner, A. Biological Background and Role of Adiponectin as Marker for Insulin Resistance and Cardiovascular Risk. Clin. Lab. 2005, 51, 489–494. [Google Scholar] [PubMed]
- Lara-Castro, C.; Fu, Y.; Chung, B.H.; Garvey, W.T. Adiponectin and the Metabolic Syndrome: Mechanisms Mediating Risk for Metabolic and Cardiovascular Disease. Curr. Opin. Lipidol. 2007, 18, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mamaeghani, M.; Mohammadi, S.; Arefhosseini, S.R.; Fallah, P.; Bazi, Z. Adiponectin as a Potential Biomarker of Vascular Disease. Vasc. Health Risk Manag. 2015, 11, 55–70. [Google Scholar] [PubMed]
- Forst, T.; Pfutzner, A. Current Laboratory Parameters in the Differential Diagnosis of Type 2 Diabetes Mellitus. Proinsulin, Adiponectin and Others. Dtsch. Med. Wochenschr. 2006, 131, S268–S273. [Google Scholar] [CrossRef] [PubMed]
- Pfutzner, A.; Weber, M.M.; Forst, T. A Biomarker Concept for Assessment of Insulin Resistance, Beta-Cell Function and Chronic Systemic Inflammation in Type 2 Diabetes Mellitus. Clin. Lab. 2008, 54, 485–490. [Google Scholar] [PubMed]
- Sente, T.; Gevaert, A.; Van Berendoncks, A.; Vrints, C.J.; Hoymans, V.Y. The Evolving Role of Adiponectin as an Additive Biomarker in Hfref. Heart Fail. Rev. 2016, 21, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Horikoshi, M.; Yamauchi, T.; Yago, H.; Miyazaki, O.; Ebinuma, H.; Imai, Y.; Nagai, R.; Kadowaki, T. Measurement of the High-Molecular Weight Form of Adiponectin in Plasma Is Useful for the Prediction of Insulin Resistance and Metabolic Syndrome. Diabetes Care 2006, 29, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Snehalatha, C.; Mukesh, B.; Simon, M.; Viswanathan, V.; Haffner, S.M.; Ramachandran, A. Plasma Adiponectin Is an Independent Predictor of Type 2 Diabetes in Asian Indians. Diabetes Care 2003, 26, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, Regulation and Association to Insulin Sensitivity. Obes. Rev. 2005, 6, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pfutzner, A.; Marx, N.; Lubben, G.; Langenfeld, M.; Walcher, D.; Konrad, T.; Forst, T. Improvement of Cardiovascular Risk Markers by Pioglitazone Is Independent from Glycemic Control: Results from the Pioneer Study. J. Am. Coll. Cardiol. 2005, 45, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Pfutzner, A.; Schondorf, T.; Seidel, D.; Winkler, K.; Matthaei, S.; Hamann, A.; Forst, T. Impact of Rosiglitazone on Beta-Cell Function, Insulin Resistance, and Adiponectin Concentrations: Results from a Double-Blind Oral Combination Study with Glimepiride. Metabolism 2006, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hust, M.; Dubel, S. Mating Antibody Phage Display with Proteomics. Trends Biotechnol. 2004, 22, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, C.E.; von Zur Muhlen, C.; von Elverfeldt, D.; Peter, K. Single-Chain Antibodies as Diagnostic Tools and Therapeutic Agents. Thromb. Haemost. 2009, 101, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.A.; Stephens, T.; Charlton, H.K.; Jones, A.; Macdonald, G.A.; Prins, J.B.; Whitehead, J.P. Adiponectin Multimerization Is Dependent on Conserved Lysines in the Collagenous Domain: Evidence for Regulation of Multimerization by Alterations in Posttranslational Modifications. Mol. Endocrinol. 2006, 20, 1673–1687. [Google Scholar] [PubMed]
- Boonrod, K.; RLP Agroscience GmbH, Alplanta Institute of Plant Research Neustadt an der Weinstraße, Neustadt, Germany. Data not shown, 2016.
- Lei, S.P.; Lin, H.C.; Wang, S.S.; Callaway, J.; Wilcox, G. Characterization of the Erwinia Carotovora Pelb Gene and Its Product Pectate Lyase. J. Bacteriol. 1987, 169, 4379–4383. [Google Scholar] [CrossRef] [PubMed]
- Arlaud, G.J.; Gaboriaud, C.; Thielens, N.M.; Rossi, V.; Bersch, B.; Hernandez, J.F.; Fontecilla-Camps, J.C. Structural Biology of C1: Dissection of a Complex Molecular Machinery. Immunol. Rev. 2001, 180, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, A.; Knight, C.; Xu, L.Y.; Cooper, G.J. Hydroxylation and Glycosylation of the Four Conserved Lysine Residues in the Collagenous Domain of Adiponectin. Potential Role in the Modulation of Its Insulin-Sensitizing Activity. J. Biol. Chem. 2002, 277, 19521–19529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Chan, L.; Chan, K.W.; Lam, J.B.; Lam, M.C.; Hoo, R.C.; Mak, W.W.; Cooper, G.J.; Xu, A. Post-Translational Modifications of the Four Conserved Lysine Residues within the Collagenous Domain of Adiponectin Are Required for the Formation of Its High Molecular Weight Oligomeric Complex. J. Biol. Chem. 2006, 281, 16391–16400. [Google Scholar] [CrossRef] [PubMed]
- Popplewell, A.G.; Sehdev, M.; Spitali, M.; Weir, A.N. Expression of Antibody Fragments by Periplasmic Secretion in Escherichia Coli. Methods Mol. Biol. 2005, 308, 17–30. [Google Scholar] [PubMed]
- Schirrmann, T.; Meyer, T.; Schutte, M.; Frenzel, A.; Hust, M. Phage Display for the Generation of Antibodies for Proteome Research, Diagnostics and Therapy. Molecules 2011, 16, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, T.; Kamiya, N.; Ueda, H.; Nagamune, T. Enzymatic Labeling of a Single Chain Variable Fragment of an Antibody with Alkaline Phosphatase by Microbial Transglutaminase. Biotechnol. Bioeng. 2004, 86, 399–404. [Google Scholar] [CrossRef] [PubMed]
| Plasmas | scFv H5 Sandwich-ELISA (µg/mL) | Reference Method TECO- Assay (µg/mL) | |
|---|---|---|---|
| Patient sample 1 | Value 1 | 15.23 | 16.95 |
| Value 2 | 15.09 | 15.80 | |
| Patient sample 2 | Value 1 | 2.06 | 1.67 |
| Value 2 | 2.06 | 1.67 | |
| Patient sample 3 | Value 1 | 2.80 | 3.19 |
| Value 2 | 2.80 | 3.19 | |
| Patient sample 4 | Value 1 | 7.11. | 4.08 |
| Value 2 | 6.81 | 3.82 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilton, P.; Steidel, M.; Krczal, G.; Hermanns, I.; Pfützner, A.; Konnerth, A.; Boonrod, K. Identification of Highly Specific scFvs against Total Adiponectin for Diagnostic Purposes. Biology 2017, 6, 26. https://doi.org/10.3390/biology6020026
Wilton P, Steidel M, Krczal G, Hermanns I, Pfützner A, Konnerth A, Boonrod K. Identification of Highly Specific scFvs against Total Adiponectin for Diagnostic Purposes. Biology. 2017; 6(2):26. https://doi.org/10.3390/biology6020026
Chicago/Turabian StyleWilton, Peter, Michael Steidel, Gabriele Krczal, Iris Hermanns, Andreas Pfützner, Alisa Konnerth, and Kajohn Boonrod. 2017. "Identification of Highly Specific scFvs against Total Adiponectin for Diagnostic Purposes" Biology 6, no. 2: 26. https://doi.org/10.3390/biology6020026
APA StyleWilton, P., Steidel, M., Krczal, G., Hermanns, I., Pfützner, A., Konnerth, A., & Boonrod, K. (2017). Identification of Highly Specific scFvs against Total Adiponectin for Diagnostic Purposes. Biology, 6(2), 26. https://doi.org/10.3390/biology6020026







