Intraguild Predation Dynamics in a Lake Ecosystem Based on a Coupled Hydrodynamic-Ecological Model: The Example of Lake Kinneret (Israel)
Abstract
:1. Introduction
1.1. Intraguild Predation
1.2. Modeling Intraguild Predation
1.3. Intraguild Predation in Lake Kinneret
2. Materials and Methods
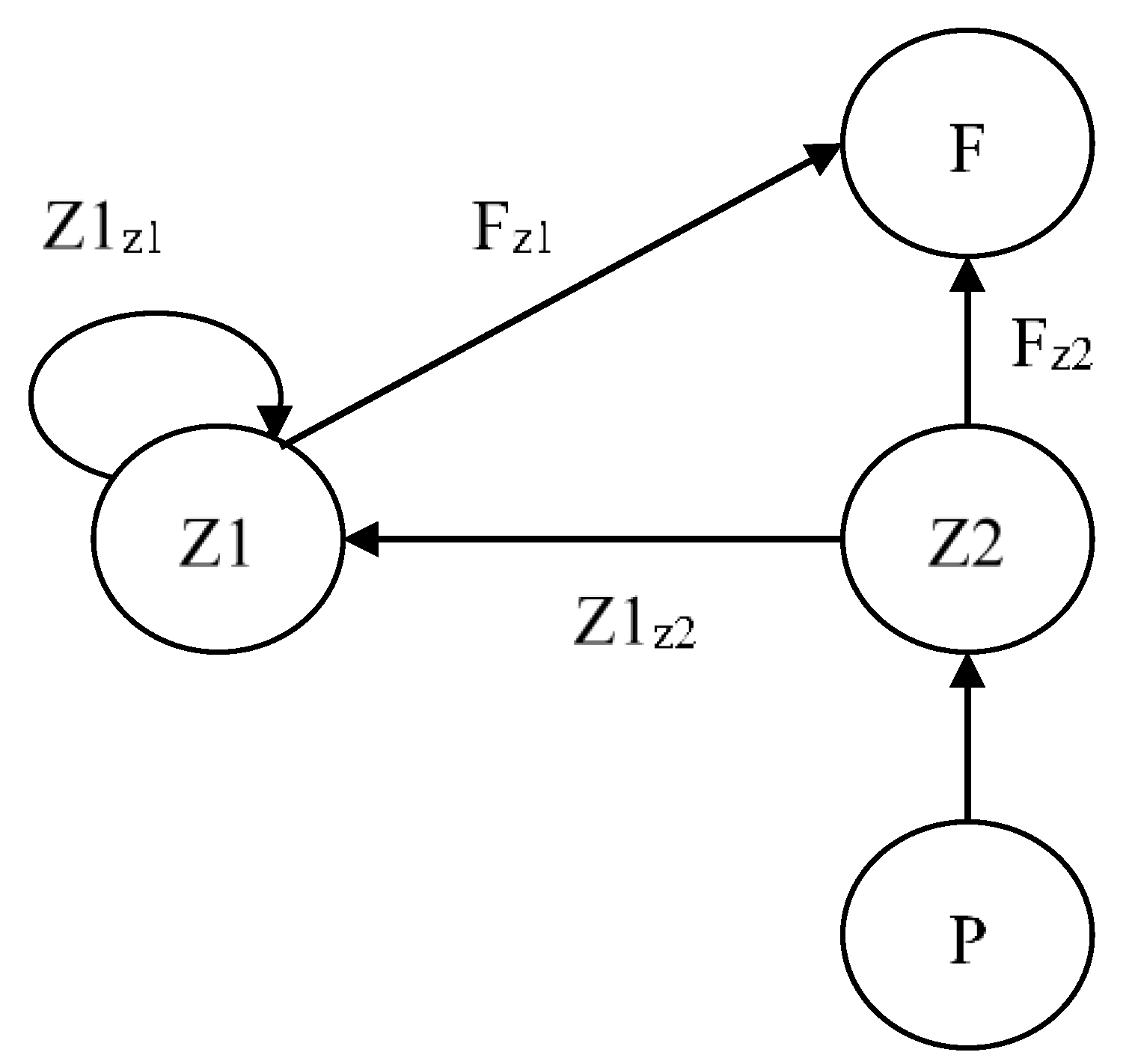
2.1. Ecological Configuration
2.2. Model Base-Case Simulations
2.3. IGP Scenarios and Sensitivity Analysis
2.4. Biomanipulation
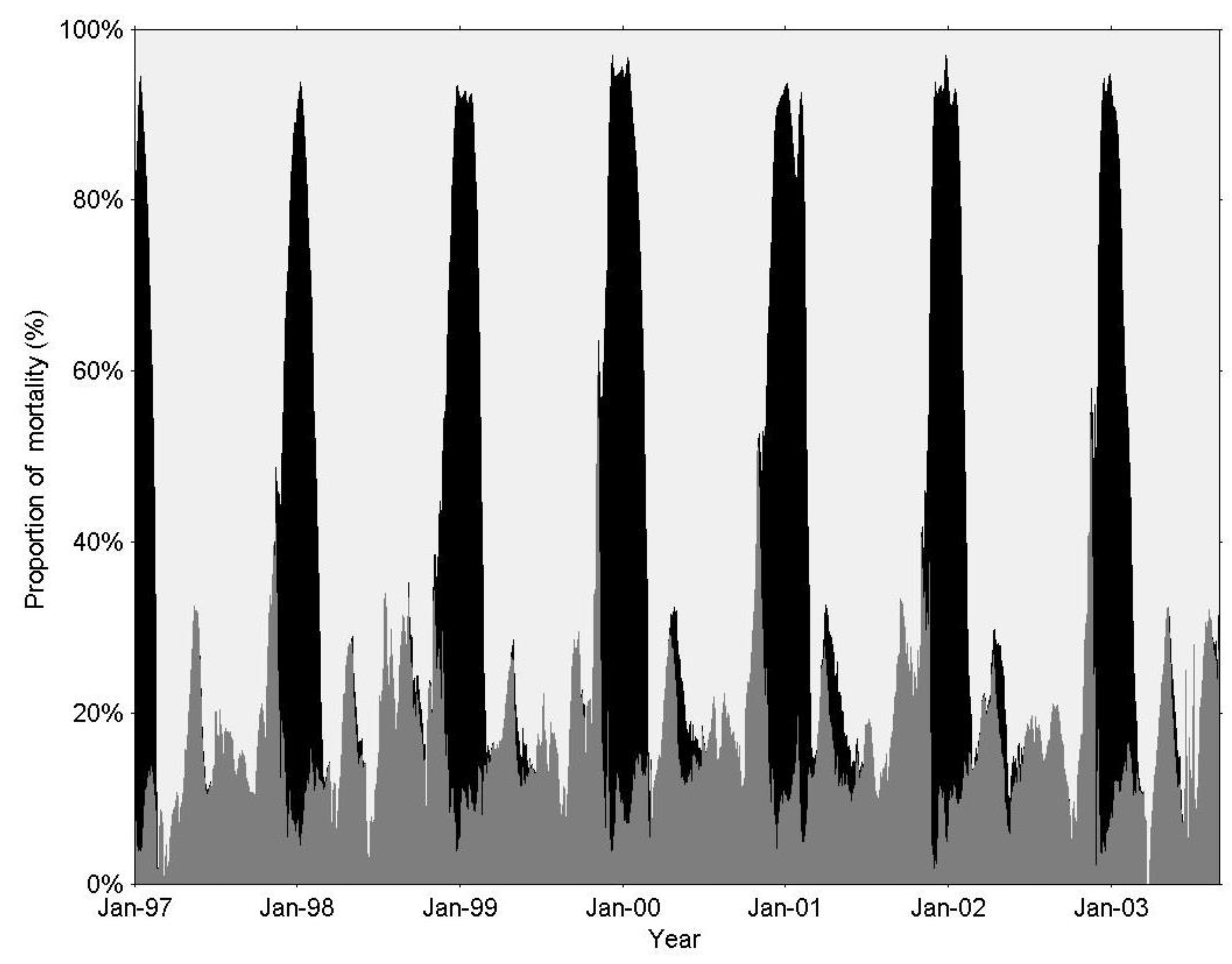
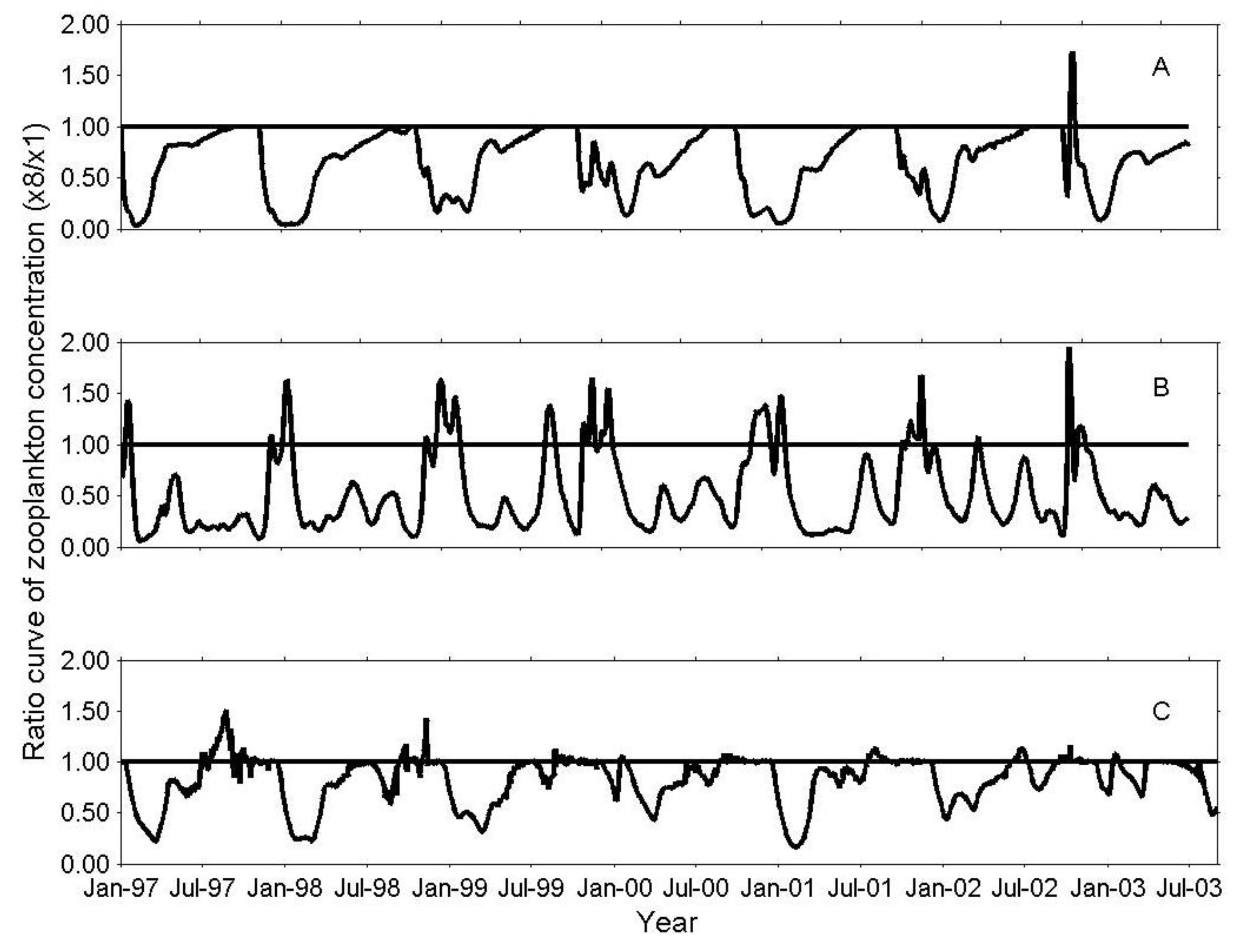
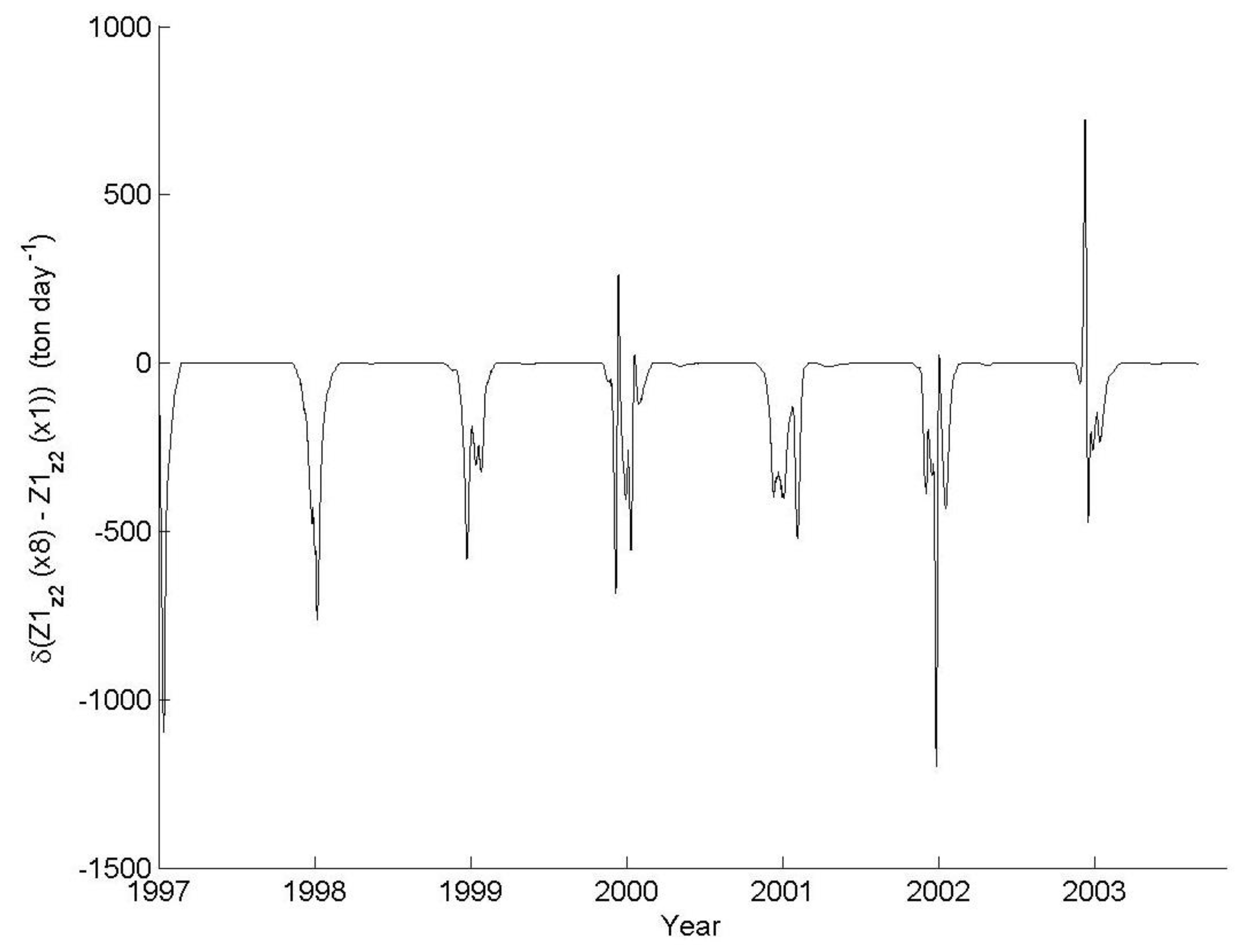
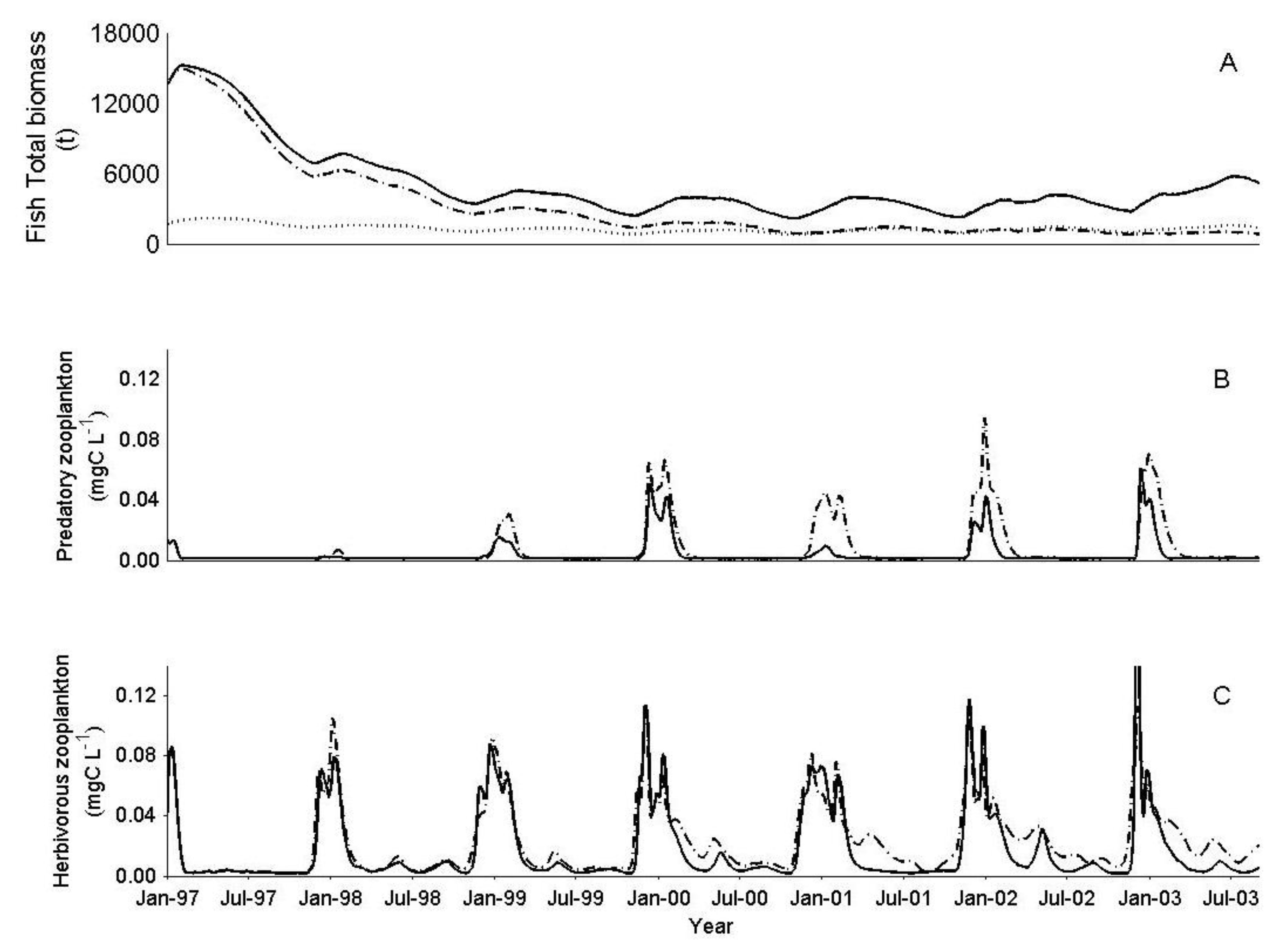
3. Results
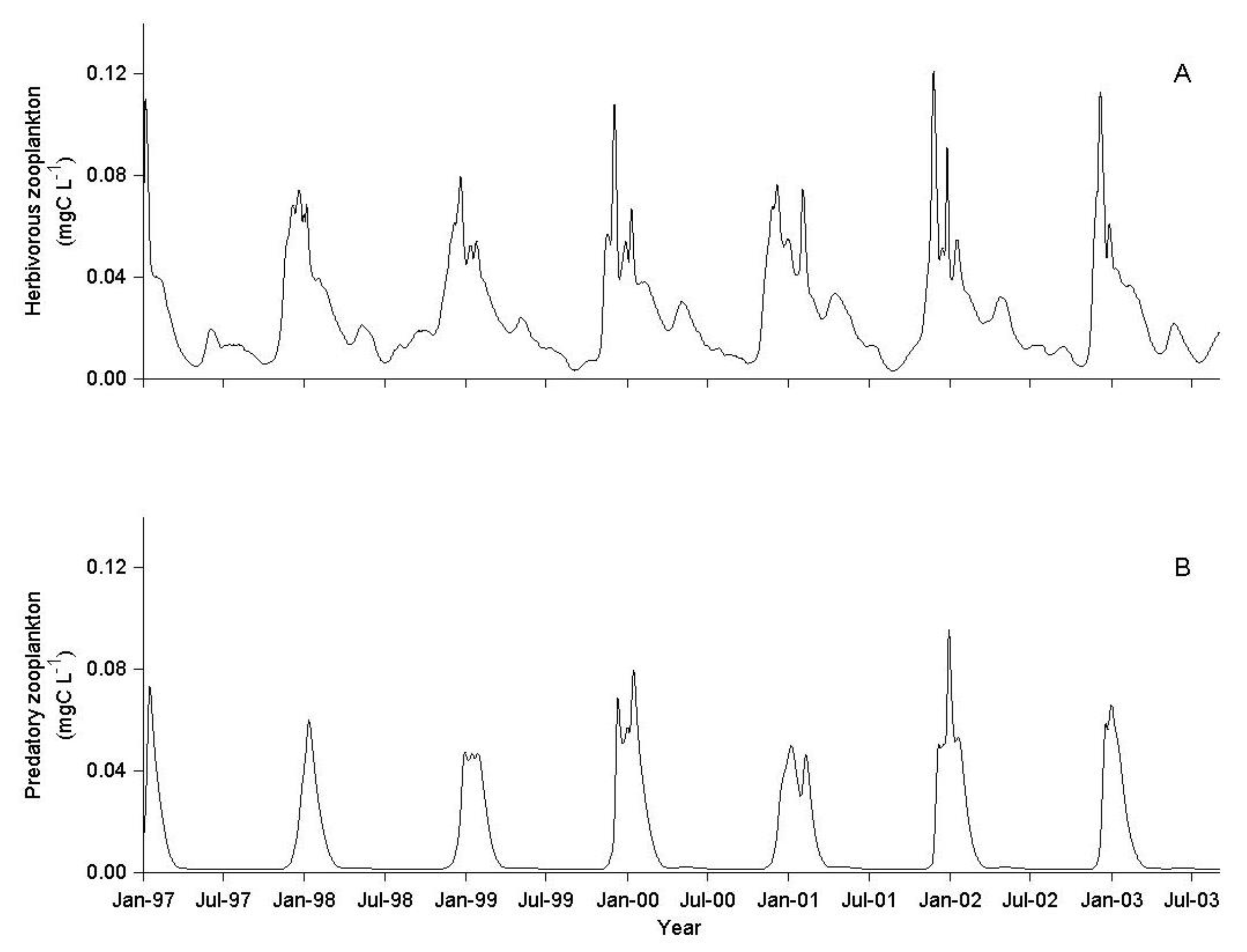
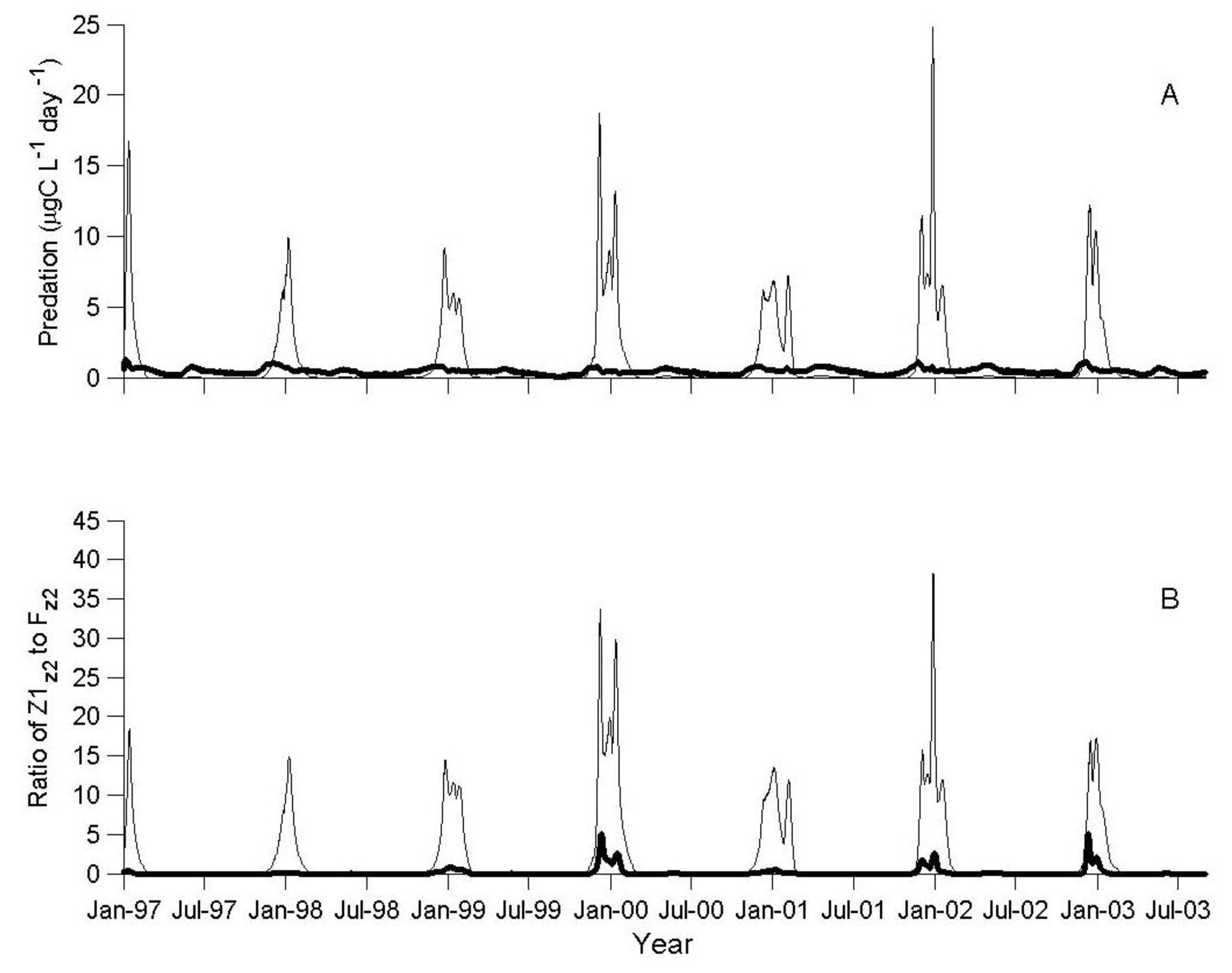
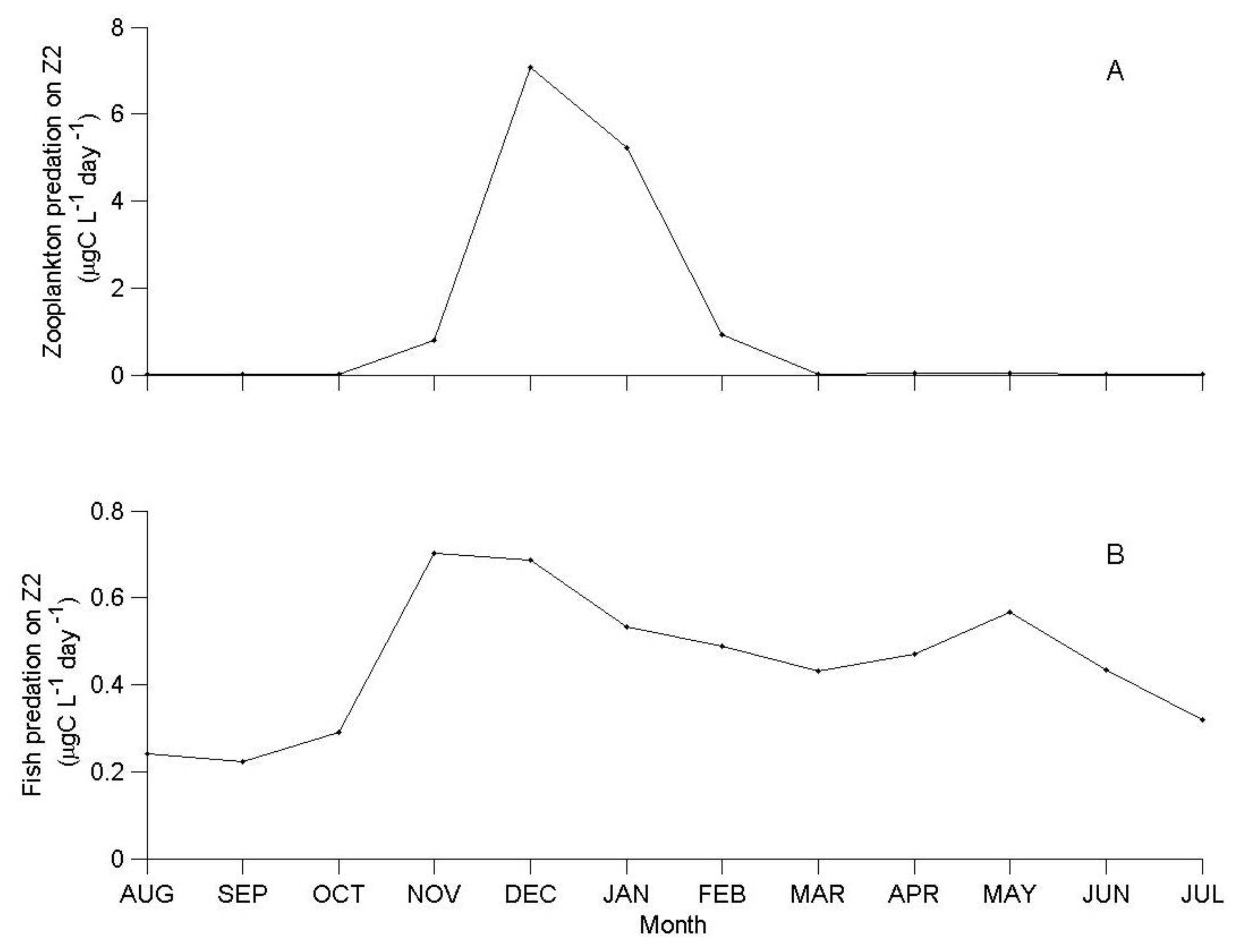
3.1. IGP Dynamics under Typical Conditions
3.2. IGP Dynamics during High Fish Abundance
3.3. The Effect of Biomanipulation
3.4. Sensitivity Analysis of the IGP Component
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holt, R.D.; Polis, G.A. A theoretical framework for intraguild predation. Am. Nat. 1997, 149, 745–764. [Google Scholar] [CrossRef]
- Mylius, S.D.; Klumpers, K.; de Roos, A.M.; Persson, L. Impact of intraguild predation and stage structure on simple communities along a productivity gradient. Am. Nat. 2001, 158, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Polis, G.A.; Meyers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation: Potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Flynn, K.; Moon, C.D. Effects of habitat complexity, prey type, and abundance on intraguild predation between larval odonates. Hydrobiologia 2011, 675, 97–104. [Google Scholar] [CrossRef]
- Morin, P. Productivity, intraguild predation, and population dynamics in experimental food webs. Ecology 1999, 80, 752–760. [Google Scholar]
- Blumenshine, S.C.; Hambright, K.D. Top-down control in pelagic systems: A role for invertebrate predation. Hydrobiologia 2003, 491, 347–356. [Google Scholar] [CrossRef]
- Gal, G.; Rudstam, L.G.; Mills, E.L.; Lantry, J.R.; Johannsson, O.E.; Greene, C.H. Mysid and fish zooplanktivory in lake ontario: Quantification of direct and indirect effects. Can. J. Fish. Aquat. Sci. 2006, 63, 2734–2747. [Google Scholar] [CrossRef]
- Diehl, S.; Feissel, M. Effects of enrichment on three-level food chains with omnivory. Am. Nat. 2000, 155, 200–218. [Google Scholar] [PubMed]
- Manca, M. Invasions and re-emergences: An analysis of the success of bythotrephes in lago maggiore (Italy). J. Limnol. 2011, 70, 76–82. [Google Scholar] [CrossRef]
- Leoni, B. Zooplankton predators and preys: Body size and stable isotope to investigate the pelagic food web in a deep lake (Lake Iseo, northern Italy). J. Limnol. 2017, 76, 85–93. [Google Scholar] [CrossRef]
- Briggs, C.J.; Borer, E.T. Why short-term experiments may not allow long-term predictions about intraguild predation. Ecol. Appl. 2005, 15, 1111–1117. [Google Scholar] [CrossRef]
- Hastings, A. Transients: The key to long-term ecological understanding? Trends Ecol. Evol. 2004, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.R. Intraguild predation, invertebrate predators, and. Trophic cascades in lake food webs. J. Theor. Biol. 2002, 218, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Amarasekare, P. Coexistence of intraguild predators and prey in resource-rich environments. Ecology 2008, 89, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, E.; Schreiber, S.J. The dynamics of the schoener-polis-holt model of intra-guild predation. Math. Biosci. Eng. 2005, 2, 279–288. [Google Scholar] [PubMed]
- Gismervik, L.; Andersen, T. Prey switching by acartia clausi: Experimental evidence and implications of intraguild predation assessed by a model. Mar. Ecol. Prog. Ser. 1997, 157, 247–259. [Google Scholar] [CrossRef]
- Bettinetti, R.; Garibaldi, L.; Leoni, B.; Galassi, S. Zooplankton as an early warning system of persistent organic pollutants contamination in a deep lake (Lake Iseo, Northern Italy). J. Limnol. 2012, 71, 335–338. [Google Scholar]
- Okuyama, T. Intraguild predation in biological control: Consideration of multiple resource species. Biocontrol 2009, 54, 3–7. [Google Scholar] [CrossRef]
- Borer, E.T.; Briggs, C.J.; Holt, D. Predators, parasitoids, and pathogens: A cross-cutting examination of intraguild predation theory. Ecology 2007, 88, 2681–2688. [Google Scholar] [PubMed]
- Revilla, T. Effects of intraguild predation on resource competition. J. Theor. Biol. 2002, 214, 49–62. [Google Scholar] [PubMed]
- Venzon, M.; Janssen, A.; Sabelis, M.W. Prey preference, intraguild predation and population dynamics of an arthropod food web on plants. Exp. Appl. Acarol. 2001, 25, 785–808. [Google Scholar] [CrossRef] [PubMed]
- Rosenheim, J.A.; Harmon, J.P. Competition, Intraguild Predation, and Biological Control: A Reappraisal. In Proceedings of the IX International Congress of Ecology Montreal, Montreal, QC, Canada, 7–12 August 2005; p. 547. [Google Scholar]
- Kindlmann, P.; Houdková, K. Intraguild predation: Fiction or reality? Popul. Ecol. 2006, 48, 317–322. [Google Scholar]
- Borer, E.T. Does adding biological detail increase coexistence in an intraguild predation model? Ecol. Model. 2006, 196, 447–461. [Google Scholar] [CrossRef]
- Dunne, J.A.; Brose, U.; Williams, R.J.; Martinez, N.D. Modeling food-web structure and dynamics: Implications for complexity-stability. In Aquatic Food Webs: An Ecosystem Approach; Belgrano, A., Scharler, U., Dunne, J.A., Ulanowicz, R.E., Eds.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Holt, R.D.; Huxel, G.R. Alternative prey and the dynamics of intraguild predation: Theoretical perspectives. Ecology 2007, 88, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Steinitz, H. Acanthobrama terrae-sanctae sp. N. From Lake Tiberias, Israel. Ann. Mag. Nat. Hist. 1952, 12, 293–298. [Google Scholar] [CrossRef]
- Ostrovsky, I.; Goren, M.; Shapiro, J.; Snovsky, G.; Rynskiy, A. Fish biology and ecology. In Lake Kinneret—Ecology and Management; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Hambright, K.D.; Shapiro, J. The 1993 collapse of the lake kinneret commercial bleak fishery. Fish. Manag. Ecol. 1997, 4, 101–109. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I. Ecological impacts of excessive water level fluctuations in stratified freshwater lake. Inland Waters 2011, 1, 47–59. [Google Scholar] [CrossRef]
- Hambright, K.D. Long-term zooplankton body size and species changes in a subtropical lake: Implications for lake management. Fundam. Appl. Limnol. 2008, 173, 1–13. [Google Scholar] [CrossRef]
- Gal, G.; Anderson, W. A novel approach to detecting a regime shift in a lake ecosystem. Methods Ecol. Evol. 2010, 1, 45–52. [Google Scholar] [CrossRef]
- Gophen, M.; Serruya, S.; Spataru, P. Zooplankton community changes in Lake Kinneret (Israel) during 1969–1985. Hydrobiologia 1990, 191, 39–46. [Google Scholar]
- Gophen, M.; Serruya, S.; Threlkeld, S.T. Long term patterns in nutrients, phytoplankton and zooplankton of lake kinneret and future predictions for ecosystem structure. Hydrobiologie 1990, 118, 449–460. [Google Scholar]
- Hambright, K.D.; Parparov, A.; Berman, T. Indices of water quality for sustainable management and conservation of an arid region lake, lake kinneret (sea of galilee), Israel. Aquat. Conserv. Mar. Freshw. Ecosyst. 2000, 10, 393–406. [Google Scholar] [CrossRef]
- Hart, D.R.; Stone, L.; Berman, T. Seasonal food web dynamics of lake kinneret: The importance of the microbial loop. Limnol. Oceanogr. 2000, 45, 350–361. [Google Scholar] [CrossRef]
- Berman, T.; Jacobi, Y.Z.; Pollingher, U. Lake kinneret phytoplankton: Stability and variability during 20 years (1970–1989). Aquat. Sci. 1992, 52, 104–127. [Google Scholar]
- Berman, T.; Stone, L.; Yacobi, Y.Z.; Kaplan, B.; Schlichter, M.; Nishri, A.; Pollingher, U. Primary production and phytoplankton in lake kinneret: A long-term record (1972–1993). Limnol. Oceanogr. 1995, 40, 1064–1076. [Google Scholar] [CrossRef]
- Gal, G.; Hipsey, M.R.; Parparov, A.; Wagner, U.; Makler, V.; Zohary, T. Implementation of ecological modeling as an effective management and investigation—Cast study of lake kinneret. Ecol. Model. 2009, 220, 1697–1718. [Google Scholar] [CrossRef]
- Ostrovsky, I.; Sukenik, A. Spatial heterogeneity of biogeochemical parameters in a subtropical lake. In Monitoring and Modeling Lakes and Coastal Environments; Mohanty, P.K., Ed.; Springer: Houten, The Netherlands, 2008; pp. 79–90. [Google Scholar]
- Zohary, T. Changes to the phytoplankton assemblage of lake kinneret after decades of a predictable, repetitive pattern. Freshw. Biol. 2004, 49, 1355–1371. [Google Scholar] [CrossRef]
- Roelke, D.L.; Zohary, T.; Hambright, K.D.; Montoya, J.V. Alternative states in the phytoplankton of lake kinneret, Israel (sea of galilee). Freshw. Biol. 2007, 52, 399–411. [Google Scholar] [CrossRef]
- Makler-Pick, V.; Gal, G.; Shapiro, J.; Hipsey, M.R. Exploring the role of fish in a lake ecosystem (Lake Kinneret, Israel) by coupling an individual-based fish population model to a dynamic ecosystem model. Can. J. Fish. Aquat. Sci. 2011, 68, 1265–1284. [Google Scholar] [CrossRef]
- Yeates, P.S.; Imberger, J. Pseudo two-dimensional simulations of internal and boundary fluxes in stratified lakes and reservoirs. Int. J. River Basin Manag. 2003, 1, 297–319. [Google Scholar] [CrossRef]
- Scheffer, M.; Baveco, J.; Deangelis, D.; Rose, K.; Van Nes, E. Super-individuals a simple solution for modelling large populations on an individual basis. Ecol. Model. 1995, 80, 161–170. [Google Scholar] [CrossRef]
- Easton, J.; Gophen, M. Trophic relations between zooplankton and bleaks (Acanthobrama spp.) in Lake Kinneret (Israel). Verh. Int. Ver. Limnol. 2002, 28, 228–231. [Google Scholar]
- Pastorok, R.A. Prey vulnerability and size selection by chaoborus larvae. Ecology 1981, 62, 1311–1324. [Google Scholar] [CrossRef]
- Gal, G.; Ofek, T.; Shapiro, J. The Connection between the Lavnun and Zooplankton and Its Implication on the Dilul Program; KLL: Kiryat Motzkin, Israel, 2010; pp. 105–109. [Google Scholar]
- Komarovsky, B. An analysis of the stomach content of Acanthobrama terrae-sanctae from Lake Tiberius. Bull. Sea Fish Res. Stn. Caesaria 1952, 4, 1–8. [Google Scholar]
- Beck, M.B. Water quality modeling: A review of the analysis of uncertainty. Water Resour. Res. 1987, 23, 1393–1442. [Google Scholar] [CrossRef]
- Gallagher, M.; Doherty, J. Parameter estimation and uncertainty analysis for a watershed model. Environ. Model. Softw. 2007, 22, 1000–1020. [Google Scholar] [CrossRef]
- Bar Massada, A.; Carmel, Y. Incorporating output variance in local sensitivity analysis for stochastic models. Ecol. Model. 2008, 213, 463–467. [Google Scholar] [CrossRef]
- Makler-Pick, V.; Gal, G.; Gorfine, M.; Hipsey, M.R.; Carmel, Y. Sensitivity analysis for complex ecological models e a new approach. Environ. Model. Softw. 2011, 26, 124–134. [Google Scholar] [CrossRef]
- Gal, G.; Makler-Pick, V.; Shachar, N. Dealing with uncertainty in ecosystem model scenarios: Application of single-model ensemble approach. Environ. Model. Softw. 2014, 61, 360–370. [Google Scholar]
- Zohary, T.; Gal, G.; Hambright, K.D. The pelagic food-web. In Lake Kinneret—Ecology and Management; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Gophen, M. Temperature effect on lifespan, metabolism & development time of Mesocyclops leuckarti (claus). Oecologia 1976, 25, 271–277. [Google Scholar] [PubMed]
- Makler-Pick, V. A Computer Ecosystem Model Applied to Studying the Food Web and Water Quality of Lake Kinneret, with Emphasis on Topdown Control; Technion: Haifa, Israel, 2010. [Google Scholar]
- Gal, G.; Hambright, K.D. Metazoan zooplankton. In Lake Kinneret—Ecology and Management; Zohary, T., Sukenik, A., Berman, T., Nishri, A., Eds.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Gal, G.; Skerjanec, M.; Atanasova, N. Fluctuations in water level and the dynamics of zooplankton: A data-driven modelling approach. Freshw. Biol. 2013, 58, 800–816. [Google Scholar] [CrossRef]
- Ostrovsky, I.; Walline, P. Multiannual changes in the pelagic fish Acanthobrama terrae-sanctae in Lake Kinneret (Israel) in relation to food sources. Verh. Int. Ver. Limnol. 2001, 27, 2090–2094. [Google Scholar]
- Gal, G.; Chava, S.; Eston, J. Zooplankton Monitoring; KLL: Kiryat Motzkin, Isreal, 2005. [Google Scholar]
- Rudolf, V.H.W. The interaction of cannibalism and omnivory: Consequences for community dynamics. Ecology 2007, 88, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Montserrat, M.; HilleRisLambers, R.; de Roos, A.M.; Pallini, A.; Sabelis, M.W. Intraguild predation usually does not disrupt biological control. In Trophic and Guild Interactions in Biological Control; Brodeur, J., Boivin, G., Eds.; Springer: Houten, The Netherlands, 2006; pp. 21–44. [Google Scholar]
- Mooij, W.M.; Trolle, D.; Jeppesen, E.; Arhonditsis, G.; Belolipetsky, P.V.; Chitamwebwa, D.B.R.; Degermendzhy, A.G.; DeAngelis, D.L.; Domis, L.N.; Downing, A.S.; et al. Challenges and opportunities for integrating lake ecosystem modelling approaches. Aquat. Ecol. 2010, 44, 633–667. [Google Scholar] [CrossRef]
- Litchman, E.; Ohman, M.D.; Kiørboe, T. Trait-based approaches to zooplankton communities. J. Plankton Res. 2013, 35, 473–484. [Google Scholar] [CrossRef]
- Zhao, J.; Ramin, M.; Vincent, C.; Arhonditsis, G.B. Plankton community patterns across a trophic gradient: The role of zooplankton functional groups. Ecol. Model. 2008, 213, 417–436. [Google Scholar] [CrossRef]
| Variable | ×1 | ×2 | ×8 |
|---|---|---|---|
| Z1z1 | 12.62 | 6.89 (54%) | 1.17 (9%) |
| Fz1 | 2.37 | 3.48 (146%) | 5.01 (211%) |
| Z1z2 | 31.81 | 25.32 (79%) | 12.11 (38%) |
| Fz2 | 12.83 | 18.57 (144%) | 33.10 (258%) |
| Z1z3 | 0.59 | 0.45 (75%) | 0.16 (27%) |
| Fz3 | 0.08 | 0.13 (162%) | 0.35 (438%) |
| Total Zzi predation | 45.02 | 32.66 (76%) | 13.43 (30%) |
| Total Fzi predation | 15.28 | 22.19 (145%) | 38.45 (252%) |
| Total predation | 60.3 | 54.84 | 51.9 |
| Total predation on Z2 (Z1z2 + Fz2) | 44.64 | 43.89 | 45.21 |
| Z1z1/Fz1 | 5.32 | 1.98 | 0.23 |
| Z1z2/Fz2 | 2.48 | 1.36 | 0.37 |
| Z1z3/Fz3 | 7.7 | 3.4 | 0.45 |
| Parameter | Initial Parameter Value | −50% Change to Parameter Value | +50% Change to Parameter Value |
|---|---|---|---|
| Maximum predation rate of predatory zooplankton (gmax) | 3.03 | +27% | −10% |
| Preference of predatory zooplankton for predatory zooplankton—self limitation (Pzk1) * | 0.15 | −7% | +1% |
| Vulnerability of the predatory zooplankton (V11) | 0.4 | −8% | +1% |
| Vulnerability of the herbivorous zooplankton (V12) | 0.5 | +10% | −7% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makler-Pick, V.; Hipsey, M.R.; Zohary, T.; Carmel, Y.; Gal, G. Intraguild Predation Dynamics in a Lake Ecosystem Based on a Coupled Hydrodynamic-Ecological Model: The Example of Lake Kinneret (Israel). Biology 2017, 6, 22. https://doi.org/10.3390/biology6020022
Makler-Pick V, Hipsey MR, Zohary T, Carmel Y, Gal G. Intraguild Predation Dynamics in a Lake Ecosystem Based on a Coupled Hydrodynamic-Ecological Model: The Example of Lake Kinneret (Israel). Biology. 2017; 6(2):22. https://doi.org/10.3390/biology6020022
Chicago/Turabian StyleMakler-Pick, Vardit, Matthew R. Hipsey, Tamar Zohary, Yohay Carmel, and Gideon Gal. 2017. "Intraguild Predation Dynamics in a Lake Ecosystem Based on a Coupled Hydrodynamic-Ecological Model: The Example of Lake Kinneret (Israel)" Biology 6, no. 2: 22. https://doi.org/10.3390/biology6020022
APA StyleMakler-Pick, V., Hipsey, M. R., Zohary, T., Carmel, Y., & Gal, G. (2017). Intraguild Predation Dynamics in a Lake Ecosystem Based on a Coupled Hydrodynamic-Ecological Model: The Example of Lake Kinneret (Israel). Biology, 6(2), 22. https://doi.org/10.3390/biology6020022






