Thermodynamic Stability of Psychrophilic and Mesophilic Pheromones of the Protozoan Ciliate Euplotes
Abstract
:1. Introduction
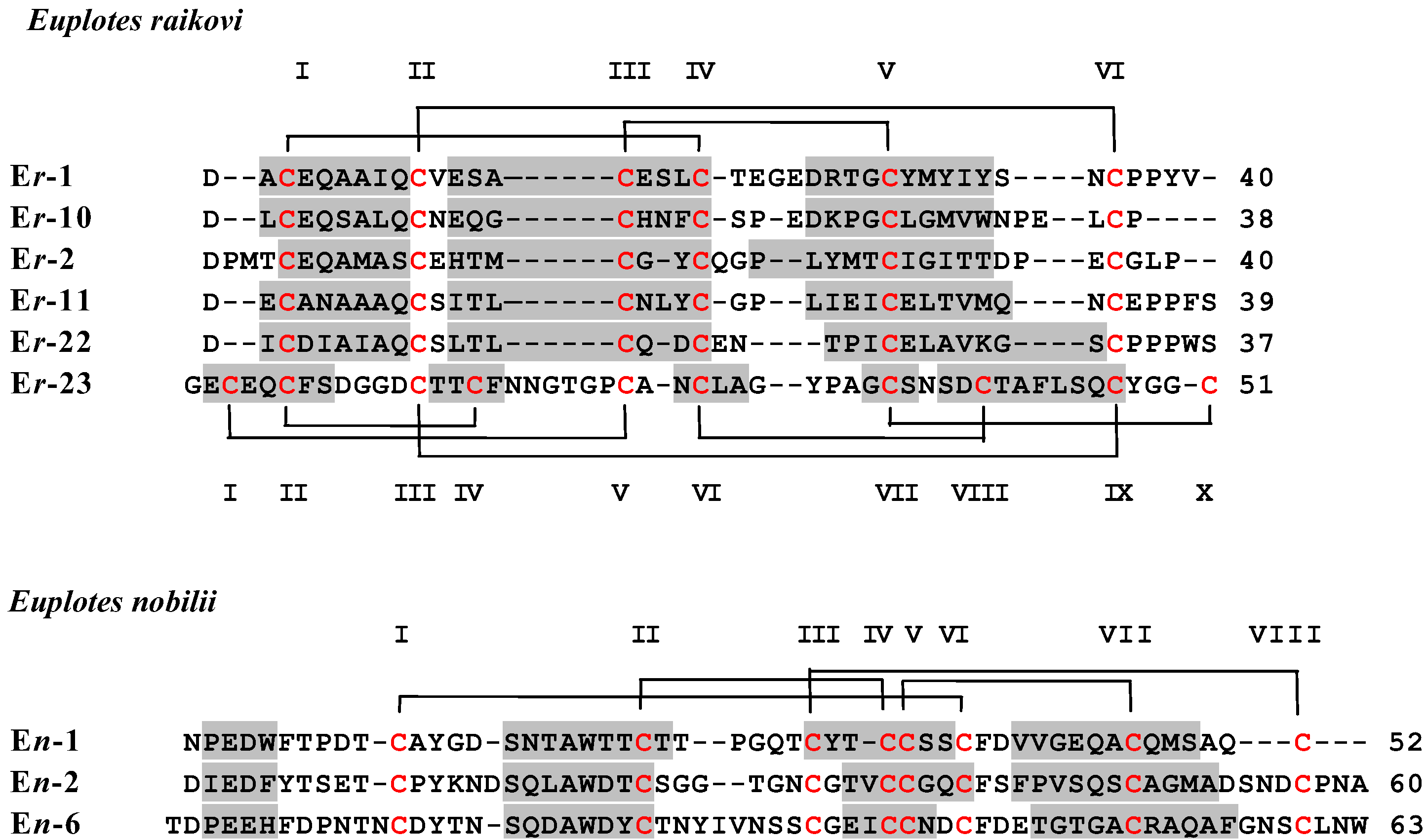
2. Materials and Methods
3. Results
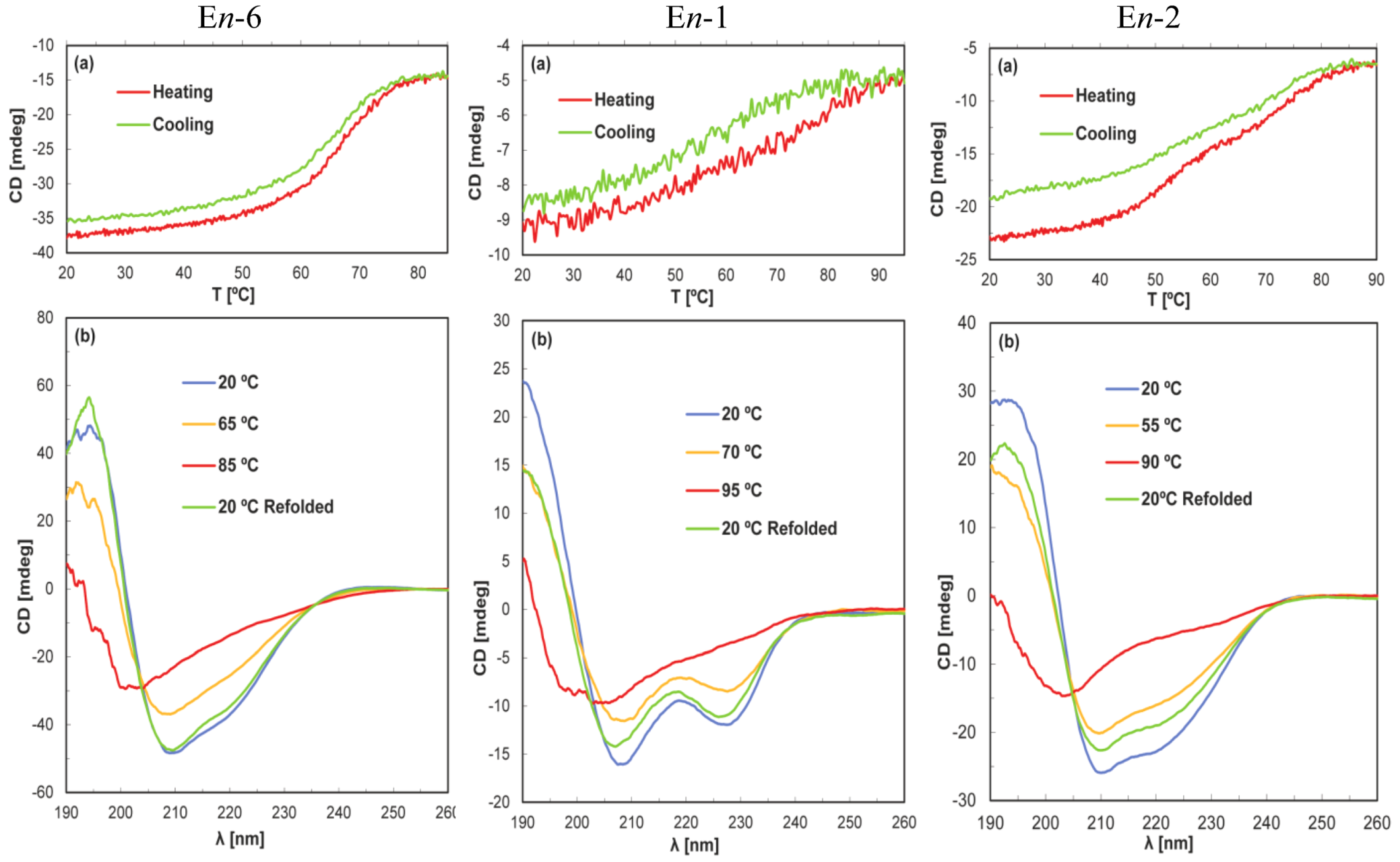
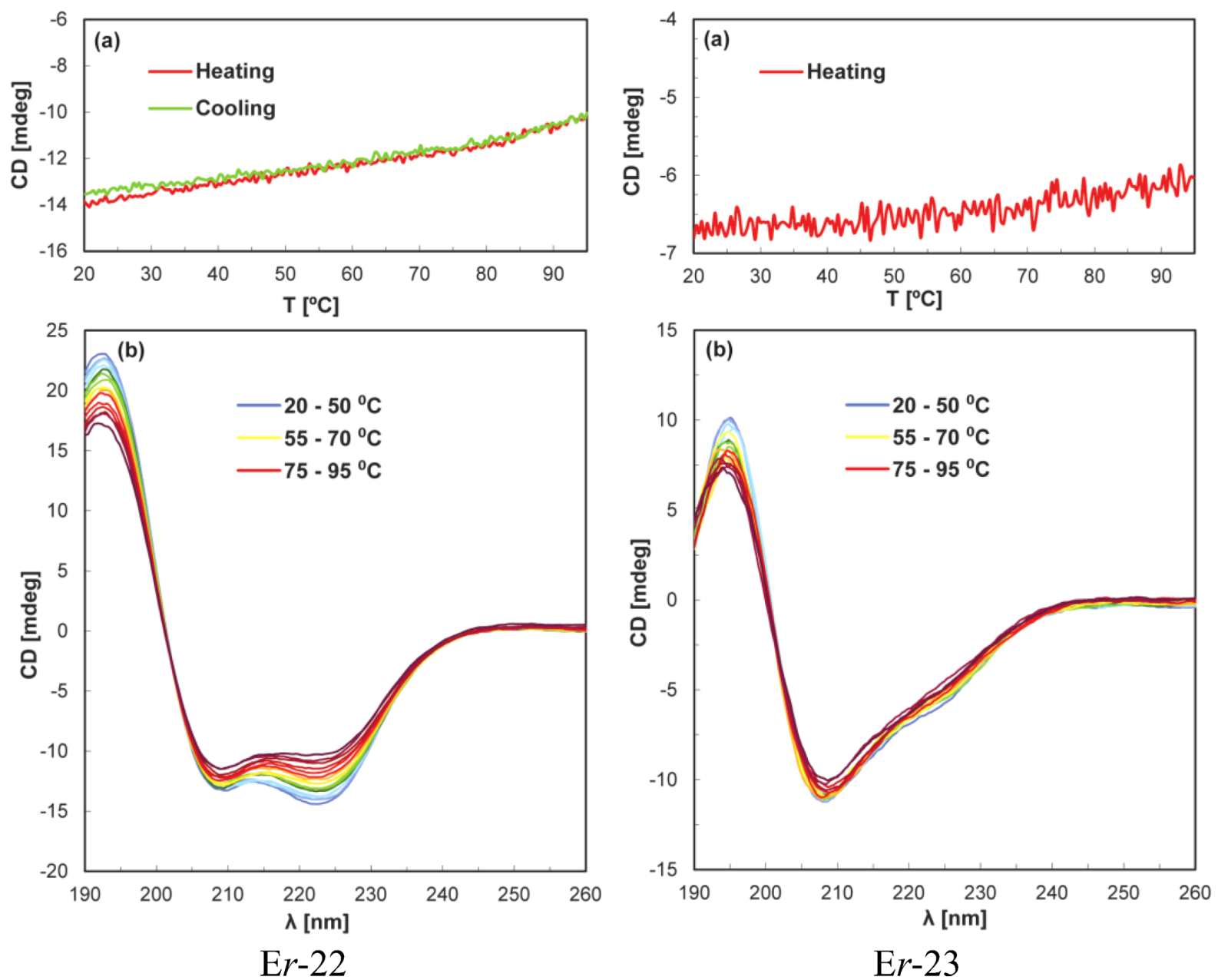
4. Discussion
5. Conclusions
Acknowledgments
References
- Petz, W. Ciliates. In Antarctic Marine Protists; Scott, F.J., Marchant, H.J., Eds.; Australian Biological Resources Study: Canberra and Australian Antarctic Division, Hobart, Australia, 2005; pp. 347–448. [Google Scholar]
- Petz, W.; Valbonesi, A.; Schiftner, U.; Quesada, A.; Cynan Ellis-Evans, J. Ciliate biogeography in Antarctic and Arctic freshwater ecosystems: endemism or global distribution of species? FEMS Microbiol. Ecol. 2007, 59, 396–408. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Erra, F.; Dini, F.; Alimenti, C.; Vallesi, A.; Pedrini, B.; Wüthrich, K.; Luporini, P. Antarctic and Arctic populations of the ciliate Euplotes nobilii show common pheromone-mediated cell-cell signaling and cross-mating. Proc. Natl. Acad. Sci. USA 2011, 108, 3181–3186. [Google Scholar]
- Luporini, P.; Alimenti, C.; Ortenzi, C.; Vallesi, A. Ciliate mating type and their specific protein pheromones. Acta Protozool. 2005, 44, 89–101. [Google Scholar]
- Alimenti, C.; Vallesi, A.; Federici, S.; di Giuseppe, G.; Dini, F.; Carratore, V.; Luporini, P. Isolation and structural characterization of two water-borne pheromones from Euplotes crassus, a ciliate commonly known to carry membrane-bound pheromones. J. Eukaryot. Microbiol. 2011, 58, 234–241. [Google Scholar] [CrossRef]
- Vallesi, A.; Giuli, G.; Bradshaw, R.A.; Luporini, P. Autocrine mitogenic activity of pheromone produced by the protozoan ciliate Euplotes raikovi. Nature 1995, 376, 522–524. [Google Scholar] [CrossRef]
- Vallesi, A.; Ballarini, P.; di Pretoro, B.; Alimenti, C.; Miceli, C.; Luporini, P. Autocrine, mitogenic pheromone receptor loop of the ciliate Euplotes raikovi: Pheromone-induced receptor internalization. Eukaryot. Cell 2005, 4, 1221–1227. [Google Scholar] [CrossRef]
- La Terza, A.; Dobri, N.; Alimenti, C.; Vallesi, A.; Luporini, P. The water-borne protein signals (pheromones) of the Antarctic ciliated protozoan Euplotes nobilii: Structure of the gene coding for the En-6 pheromone. Can. J. Microbiol. 2009, 5, 57–62. [Google Scholar]
- Vallesi, A.; Alimenti, C.; La Terza, A.; di Giuseppe, G.; Dini, F.; Luporini, P. Characterization of the pheromone gene family of an Antarctic and Arctic protozoan ciliate, Euplotes nobilii. Mar. Genomics 2009, 2, 27–32. [Google Scholar] [CrossRef]
- Vallesi, A.; Alimenti, C.; Pedrini, B.; di Giuseppe, G.; Dini, F.; Wüthrich, K.; Luporini, P. Coding genes and molecular structures of the diffusible signalling proteins (pheromones) of the polar ciliate, Euplotes nobilii. Mar. Genomics 2012, 8, 9–13. [Google Scholar] [CrossRef]
- Brown, L.R.; Mronga, S.; Bradshaw, R.A.; Ortenzi, C.; Luporini, P.; Wüthrich, K. Nuclear magnetic resonance solution structure of the pheromone Er-10 from the ciliated protozoan Euplotes raikovi. J. Mol. Biol. 1993, 231, 800–816. [Google Scholar] [CrossRef]
- Mronga, S.; Luginbühl, P.; Brown, L.R.; Ortenzi, C.; Luporini, P.; Bradshaw, R.A.; Wüthrich, K. The NMR solution structure of the pheromone Er-1 from the ciliated protozoan Euplotes raikovi. Protein Sci. 1994, 3, 1527–1536. [Google Scholar] [CrossRef]
- Ottiger, M.; Szyperski, T.; Luginbühl, P.; Ortenzi, C.; Luporini, P.; Bradshaw, R.A.; Wüthrich, K. The NMR solution structure of the pheromone Er-2 from the ciliated protozoan Euplotes raikovi. Protein Sci. 1994, 3, 1517–1526. [Google Scholar]
- Luginbühl, P.; Ottiger, M.; Mronga, S.; Wüthrich, K. Structure comparison of the NMR structures of the pheromones Er-1, Er-10, and Er-2 from Euplotes raikovi. Protein Sci. 1994, 3, 1537–1546. [Google Scholar] [CrossRef]
- Luginbühl, P.; Wu, J.; Zerbe, O.; Ortenzi, C.; Luporini, P.; Wüthrich, K. The NMR solution structure of the pheromone Er-11 from the ciliated protozoan Euplotes raikovi. Protein Sci. 1996, 5, 1512–1522. [Google Scholar] [CrossRef]
- Liu, A.; Luginbühl, P.; Zerbe, O.; Ortenzi, C.; Luporini, P.; Wüthrich, K. NMR structure of the pheromone Er-22 from Euplotes raikovi. J. Biomol. NMR 2001, 19, 75–78. [Google Scholar] [CrossRef]
- Zahn, R.; Damberger, F.; Ortenzi, C.; Luporini, P.; Wüthrich, K. NMR structure of the Euplotes raikovi pheromone Er-23 and identification of its five disulfide bonds. J. Mol. Biol. 2001, 313, 923–931. [Google Scholar] [CrossRef]
- Placzek, W.J.; Etezady-Esfarjani, T.; Herrmann, T.; Pedrini, B.; Peti, W.; Alimenti, C.; Luporini, P.; Wüthrich, K. Cold-adapted signal proteins: NMR structures of pheromones from the Antarctic ciliate Euplotes nobilii. IUBMB Life 2007, 59, 578–585. [Google Scholar] [CrossRef]
- Pedrini, B.; Placzek, W.J.; Koculi, E.; Alimenti, C.; La Terza, A.; Luporini, P.; Wüthrich, K. Cold-adaptation in sea-waterborne signal proteins: Sequence and NMR structure of the pheromone En-6 from the Antarctic ciliate Euplotes nobilii. J. Mol. Biol. 2007, 372, 277–286. [Google Scholar] [CrossRef]
- Alimenti, C.; Vallesi, A.; Pedrini, B.; Wüthrich, K.; Luporini, P. Molecular cold-adaptation: Comparative analysis of two homologous families of psychrophilic and mesophilic signal proteins of the protozoan ciliate Euplotes. IUBMB Life 2009, 61, 838–845. [Google Scholar] [CrossRef]
- Weiss, M.S.; Anderson, D.H.; Raffioni, S.; Bradshaw, R.A.; Ortenzi, C.; Luporini, P.; Eisenberg, D.A. Cooperative model for ligand recognition and cell adhesion: Evidence from the molecular packing in the 1.6 Å crystal structure of the pheromone Er-1 from the ciliate protozoan Euplotes raikovi. Proc. Natl. Acad. Sci. USA 1995, 92, 10172–10176. [Google Scholar]
- Concetti, A.; Raffioni, S.; Miceli, C.; Barra, D.; Luporini, P. Purification to apparent homogeneity of the mating pheromone of mat-1 Euplotes raikovi. J. Biol. Chem. 1986, 61, 10582–10587. [Google Scholar]
- Raffioni, S.; Miceli, C.; Vallesi, A.; Chowdhury, S.K.; Chait, B.T.; Luporini, P.; Bradshaw, R.A. Primary structure od the Euplotes raikovi pheromones: Comparison of five sequences of pheromones from cell with variable mating interactions. Proc. Natl. Acad. Sci. USA 1992, 89, 2071–2075. [Google Scholar]
- Alimenti, C.; Ortenzi, C.; Carratore, V.; Luporini, P. Structural characterization of protein pheromone from a cold-adapted (Antarctic) single-cell eukaryote, the ciliate Euplotes nobilii. FEBS Lett. 2002, 514, 329–332. [Google Scholar] [CrossRef]
- Alimenti, C.; Ortenzi, C.; Carratore, V.; Luporini, P. Structural characterization of En-1, a cold-adapted protein pheromone isolated from the Antarctic ciliate Euplotes nobilii. Biochem. Biophys. Acta 2003, 1621, 17–21. [Google Scholar] [CrossRef]
- Devi, V.S.; Sprecher, C.B.; Hunziker, P.; Mittl, P.R.; Bosshard, H.R.; Jelesarov, I. Disulfide formation and stability of a cysteine-rich repeat protein from Helicobacter pylori. Biochemistry 2006, 45, 1599–1607. [Google Scholar]
- Trivedi, M.V.; Laurence, J.S.; Siahaan, T.J. The Role of thiols and disulfides on protein stability. Curr. Protein Pept. Sci. 2009, 10, 614–625. [Google Scholar] [CrossRef]
- Fass, D. Disulfide bonding in protein biophysics. In Annual Review of Biophysics; Rees, D.C., Ed.; Annual Reviews: Palo Alto, CA, USA, 2012; Volume 41, pp. 63–79. [Google Scholar]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Structures of the psychrophilic Alteromonas haloplanctis α-amylase give insights into cold adaptation at a molecular level. Structure 1998, 6, 1503–1516. [Google Scholar] [CrossRef]
- Bae, E.; Phillips, G.N., Jr. Structure and analysis of highly homologous psychrophilic, mesophilic, and thermophilic adenylate kinases. J. Biol. Chem. 2004, 279, 28202–28208. [Google Scholar] [CrossRef]
- Fedoy, A.E.; Yang, N.; Martinez, A.; Leiros, H.K.; Steen, I.H. Structural and functional properties of isocitrate dehydrogenase from the psychrophilic bacterium Desulfotalea psychrophila reveal a cold-active enzyme with an unusual high thermal stability. J. Mol. Biol. 2007, 372, 130–149. [Google Scholar] [CrossRef]
- Garcia-Arribas, O.; Mateo, R.; Melanie, M.; Tomczak, M.M.; Davies, P.L.; Mateu, M.G. Thermodynamic stability of a cold-adapted protein, type III antifreeze protein, and energetic contribution of salt bridges. Protein Sci. 2007, 16, 227–238. [Google Scholar]
- Lockwood, B.L.; Somero, G.N. Functional determinants of temperature adaptation in enzymes of cold- versus warm-adapted mussels (genus Mytilus). Mol. Biol. Evol. 2012, 29, 3061–3070. [Google Scholar] [CrossRef]
- Vallesi, A.; di Giuseppe, G.; Dini, F.; Luporini, P. Pheromone evolution in the protozoan ciliate, Euplotes: The ability to synthesize diffusibile forms is ancestral and secondarily lost. Mol. Phylogenet. Evol. 2008, 47, 439–442. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Warren, A.; Al-Rasheid, K.A.; Song, W. Morphology and SSUrRNA gene-based phylogeny of two marine Euplotes species, E. orientalis spec. nov. and E. raikovi (Ciliophora, Euplotida). Eur. J. Protisotol. 2010, 46, 121–132. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Geralt, M.; Alimenti, C.; Vallesi, A.; Luporini, P.; Wüthrich, K. Thermodynamic Stability of Psychrophilic and Mesophilic Pheromones of the Protozoan Ciliate Euplotes. Biology 2013, 2, 142-150. https://doi.org/10.3390/biology2010142
Geralt M, Alimenti C, Vallesi A, Luporini P, Wüthrich K. Thermodynamic Stability of Psychrophilic and Mesophilic Pheromones of the Protozoan Ciliate Euplotes. Biology. 2013; 2(1):142-150. https://doi.org/10.3390/biology2010142
Chicago/Turabian StyleGeralt, Michael, Claudio Alimenti, Adriana Vallesi, Pierangelo Luporini, and Kurt Wüthrich. 2013. "Thermodynamic Stability of Psychrophilic and Mesophilic Pheromones of the Protozoan Ciliate Euplotes" Biology 2, no. 1: 142-150. https://doi.org/10.3390/biology2010142
APA StyleGeralt, M., Alimenti, C., Vallesi, A., Luporini, P., & Wüthrich, K. (2013). Thermodynamic Stability of Psychrophilic and Mesophilic Pheromones of the Protozoan Ciliate Euplotes. Biology, 2(1), 142-150. https://doi.org/10.3390/biology2010142






