The Multifaceted Importance of Amphibians: Ecological, Biomedical, and Socio-Economic Perspectives
Simple Summary
Abstract
1. Introduction
1.1. Global Patterns of Amphibian Diversity
1.2. Global Hotspots of Amphibian Diversity
1.3. Urgency of Conservation: Global Amphibian Decline and the Loss of Ecosystem Services
1.4. Methodological Framework of This Review
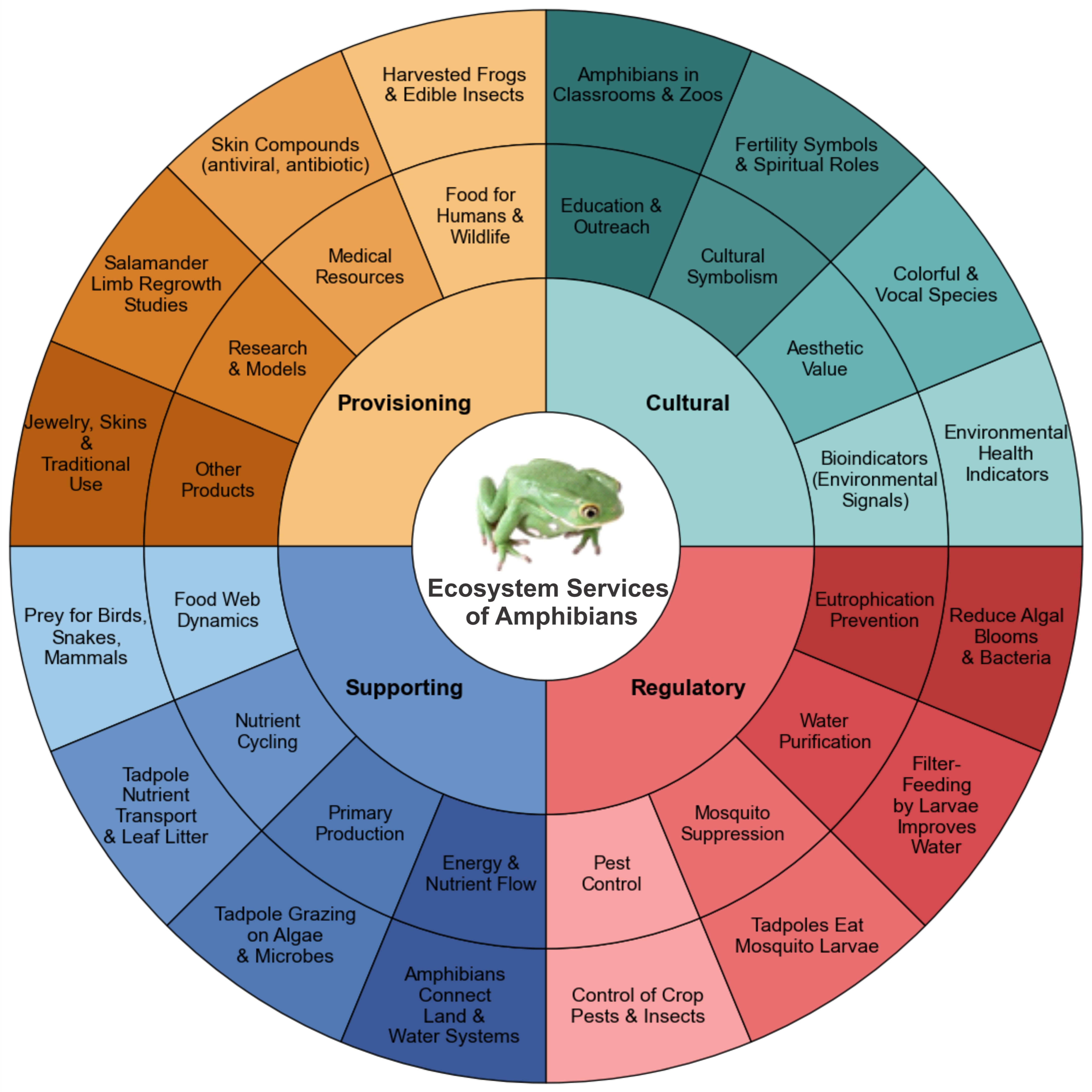
2. Amphibians as Key Players in Ecosystem Stability
2.1. Amphibians as Foundational Species
| Ecosystem Type | Region/ Country | Amphibian Biomass (kg/ha) | Bird Biomass (kg/ha) | Mammal Biomass (kg/ha) | Measurement Method | References |
|---|---|---|---|---|---|---|
| Temperate Forest | New Hampshire, USA | 3.7–4.6 | ~1.3 | ~0.3 | Pitfall traps, dry mass | [68] |
| Subtropical Wet Forest | Puerto Rico | 0.41–2.68 (wet weight) | NR | NR | Visual encounter, wet mass | [69,70] |
| Cloud Forest | Ecuador | 1.2 | 0.08 | 0.05 | Visual encounter surveys | [70,71] |
| Temperate Deciduous | Ontario, Canada | 1.0–2.8 | NR | NR | Quadrat dry mass sampling | [2,72] |
| Montane Stream Forest | Costa Rica | 5.6 | NR | NR | Visual counts, wet biomass | [73] |
| Tropical Forest | Puerto Rico | ~4.0 (C/N) | NR | NR | Nutrient cycling residue mass | [74,75] |
| Rainforest Streams | Panama | 0.15–3.6 μg NH4+/h | NR | NR | Nutrient excretion (lab) | [43,75] |
| Mediterranean Springs | Spain | High density reported | NR | NR | Breeding & larval density counts | [76] |
| Mountain Wetlands | Colombia | 20,000 individuals/ha | NR | NR | Density counts | [43] |
| Temperate Forest Streams | USA (Appalachians) | ~0.8–1.5 | ~1.0 | ~0.2 | Mark-recapture, dry mass | [77] |
| Lowland Rainforest | French Guiana | 2.4 | 0.5 | 0.6 | Pitfall and quadrat counts | [78] |
| Agricultural Wetland | India | High relative density | NR | NR | Quadrat surveys | [79] |
| Floodplain Forest | Peru | Not quantified | NR | NR | Vocalization and sighting counts | [67,80] |
| Temperate Swamp | Canada | ~1.5 | ~0.6 | ~0.4 | Pitfall and call index | [80] |
| Isolated Wetlands | Maine, USA | 3.2–6.4 (export) | NR | NR | Drift fences, pitfall traps | [81] |
2.2. Nutrient Cycling and Energy Flow Mediated by Amphibians
2.3. Trophic Contributions and Food Web Integration
2.4. Ecosystem Engineering
3. Biomedical Significance of Amphibians
3.1. Antimicrobial Peptides and Their Medical Applications
3.2. Regenerative Medicine
3.3. Potential Therapeutic Compounds
3.4. Current Biomedical Research and Future Applications
4. Amphibians as Bioindicators and Environmental Health Monitors
4.1. Sensitivity to Pollutants and Habitat Degradation
4.2. Bioaccumulation of Heavy Metals and Pesticides
4.3. The Role of Amphibians in Metabolomics and Early-Warning Systems
4.4. Indicators of Bioclimatic or Environmental Stress
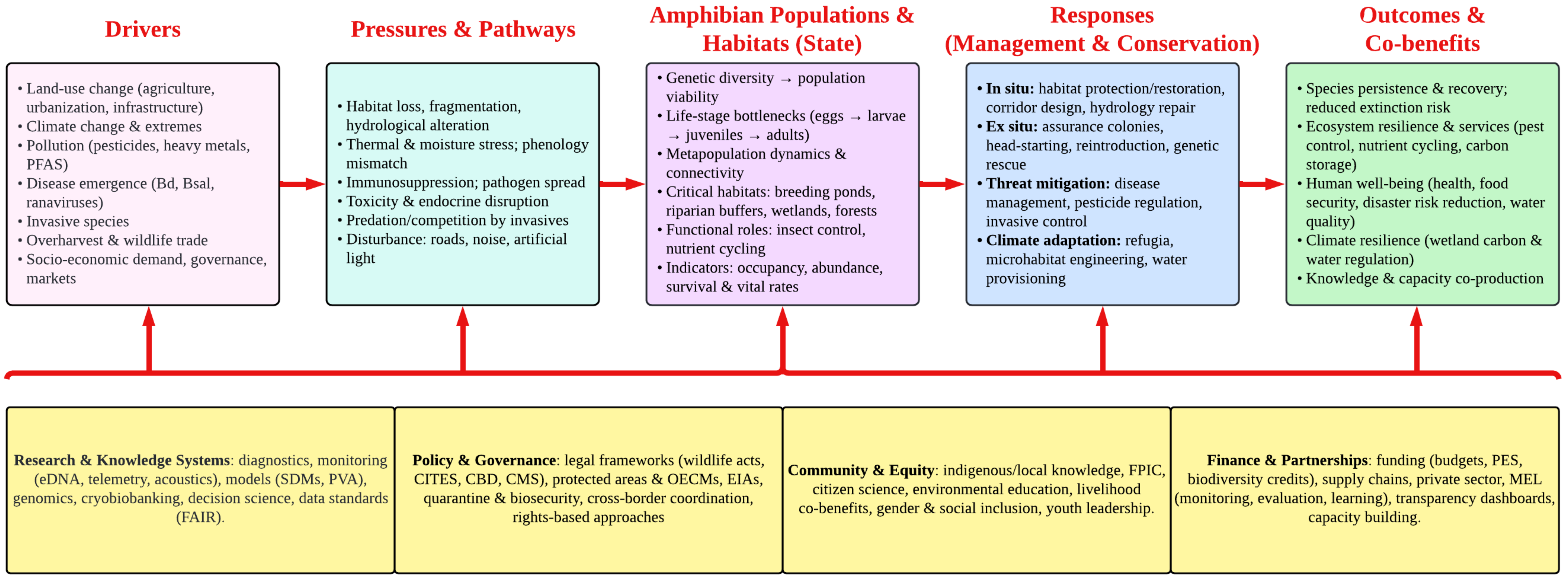
5. Challenges and Knowledge Gaps in Amphibian Conservation
5.1. Habitat Destruction and Fragmentation
5.2. Infectious Diseases and Pathogens
5.3. Climate Change
5.4. Lack of Local Engagement and Sociocultural Awareness
6. Conservation Strategies
7. Future Directions and Research Gaps
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing Declines for the World’s Amphibians in the Face of Emerging Threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J.; Hocking, D.J. Amphibian Contributions to Ecosystem Services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- Davic, R.D.; Welsh, H.H. On the Ecological Roles of Salamanders. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 405–434. [Google Scholar] [CrossRef]
- Button, S.; Borzée, A. An Integrative Synthesis to Global Amphibian Conservation Priorities. Glob. Chang. Biol. 2021, 27, 4516–4529. [Google Scholar] [CrossRef] [PubMed]
- Campbell Grant, E.H.; Amburgey, S.M.; Gratwicke, B.; Acosta-Chaves, V.; Belasen, A.M.; Bickford, D.; Brühl, C.A.; Calatayud, N.E.; Clemann, N.; Clulow, S.; et al. Priority Research Needs to Inform Amphibian Conservation in the Anthropocene. Conserv. Sci. Pract. 2023, 5, e12988. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference; Version 6.2; Electronic Database; American Museum of Natural History: New York, NY, USA, 2025; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 7 December 2025). [CrossRef]
- Janke, A. The Rise of Amphibians: 365 Million Years of Evolution. Syst. Biol. 2010, 59, 488–490. [Google Scholar] [CrossRef]
- Inger, R.F.; Szarski, H.; Kollros, J.J.; Duellman, W.E.; Trueb, L. Biology of Amphibians. Copeia 1986, 1986, 549. [Google Scholar] [CrossRef]
- Pabijan, M.; Palomar, G.; Antunes, B.; Antoł, W.; Zieliński, P.; Babik, W. Evolutionary Principles Guiding Amphibian Conservation. Evol. Appl. 2020, 13, 857–878. [Google Scholar] [CrossRef]
- West, M.; Hunter, D.; Scroggie, M.P.; Johnson, G.; Smith, S.; McCarthy, M.A.; Gillespie, G.R. Harnessing Historic Records and Long-Term Monitoring Data to Evaluate Amphibian Extinction Dynamics. Biol. Conserv. 2024, 292, 110477. [Google Scholar] [CrossRef]
- Duellman, W.E. Patterns of Distribution of Amphibians: A Global Perspective; The Johns Hopkins University Press: Baltimore, MD, USA; London, UK, 1999; 633p. [Google Scholar]
- Harvey Pough, F. Amphibian Biology and Husbandry. ILAR J. 2007, 48, 203–213. [Google Scholar] [CrossRef]
- Catenazzi, A.; Von May, R. Systematics and Conservation of Neotropical Amphibians and Reptiles. Diversity 2021, 13, 45. [Google Scholar] [CrossRef]
- Haddad, C.F.B.; Prado, C.P.A. Reproductive Modes in Frogs and Their Unexpected Diversity in the Atlantic Forest of Brazil. Bioscience 2005, 55, 207. [Google Scholar] [CrossRef]
- Penhacek, M.; Souza, T.; Santos, J.; Guerra, V.; Castro-Souza, R.; Rodrigues, D. Amazonian Amphibians: Diversity, Spatial Distribution Patterns, Conservation and Sampling Deficits. Biodivers. Data J. 2024, 12, e109785. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, J.E.; Piedade, M.T.F.; Schöngart, J.; Demarchi, L.O.; Quaresma, A.C.; Junk, W.J. A Review of the Ecological and Biogeographic Differences of Amazonian Floodplain Forests. Water 2022, 14, 3360. [Google Scholar] [CrossRef]
- Dahinten-Bailey, H.; Serrano, M.J.; Alonso-Ascencio, M.; Cruz-Font, J.J.; Rosito-Prado, I.; Ruiz-Villanueva, K.J.A.; Vásquez-Almazan, C.; Ariano-Sánchez, D. A New Species of Bolitoglossa (Caudata: Plethodontidae) of the Bolitoglossa Franklini Group from an Isolated Cloud Forest in Northern Guatemala. Zootaxa 2021, 4966, 202–214. [Google Scholar] [CrossRef]
- Savage, A.E.; Sredl, M.J.; Zamudio, K.R. Disease Dynamics Vary Spatially and Temporally in a North American Amphibian. Biol. Conserv. 2011, 144, 1910–1915. [Google Scholar] [CrossRef]
- Fouquet, A.; Noonan, B.P.; Rodrigues, M.T.; Pech, N.; Gilles, A.; Gemmell, N.J. Multiple Quaternary Refugia in the Eastern Guiana Shield Revealed by Comparative Phylogeography of 12 Frog Species. Syst. Biol. 2012, 61, 461. [Google Scholar] [CrossRef]
- Sutton, W.; Barrett, K.; Moody, A.; Loftin, C.; DeMaynadier, P.; Nanjappa, P. Predicted Changes in Climatic Niche and Climate Refugia of Conservation Priority Salamander Species in the Northeastern United States. Forests 2014, 6, 1–26. [Google Scholar] [CrossRef]
- Milanovich, J.R.; Peterman, W.E.; Nibbelink, N.P.; Maerz, J.C. Projected Loss of a Salamander Diversity Hotspot as a Consequence of Projected Global Climate Change. PLoS ONE 2010, 5, e12189. [Google Scholar] [CrossRef]
- Vieites, D.R.; Wollenberg, K.C.; Andreone, F.; Köhler, J.; Glaw, F.; Vences, M. Vast Underestimation of Madagascar’s Biodiversity Evidenced by an Integrative Amphibian Inventory. Proc. Natl. Acad. Sci. USA 2009, 106, 8267–8272. [Google Scholar] [CrossRef] [PubMed]
- Auteri, G.G.; Marchán-Rivadeneira, M.R.; Olson, D.H.; Knowles, L.L. Landscape Connectivity among Coastal Giant Salamander (Dicamptodon tenebrosus) Populations Shows No Association with Land Use, Fire Frequency, or River Drainage but Exhibits Genetic Signatures of Potential Conservation Concern. PLoS ONE 2022, 17, e0268882. [Google Scholar] [CrossRef]
- Poynton, J.C. Afrotemperate Amphibians in Southern and Eastern Africa: A Critical Review. African J. Herpetol. 2013, 62, 5–20. [Google Scholar] [CrossRef]
- Dinesh, K.P. Past, Present and Future Trends of Amphibian Species Discovery Pattern in India. Rec. Zool. Surv. India 2025, 124, 303–314. [Google Scholar] [CrossRef]
- Biju, S.D.; Garg, S.; Mahony, S.; Wijayathilaka, N.; Senevirathne, G.; Meegaskumbura, M. DNA Barcoding, Phylogeny and Systematics of Golden-Backed Frogs (Hylarana, Ranidae) of the Western Ghats-Sri Lanka Biodiversity Hotspot, with the Description of Seven New Species. Contrib. Zool. 2014, 83, 269–335. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Bickford, D.; Howard, S.D.; Ng, D.J.J.; Sheridan, J.A. Impacts of Climate Change on the Amphibians and Reptiles of Southeast Asia. Biodivers. Conserv. 2010, 19, 1043–1062. [Google Scholar] [CrossRef]
- Riley, K.; Berry, O.F.; Roberts, J.D. Do Global Models Predicting Environmental Suitability for the Amphibian Fungus, Batrachochytrium Dendrobatidis, Have Local Value to Conservation Managers? J. Appl. Ecol. 2013, 50, 713–720. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are We in the Midst of the Sixth Mass Extinction? A View from the World of Amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Re:wild; Synchronicity Earth; IUCN SSC Amphibian Specialist Group. State of the World’s Amphibians: The Second Global Amphibian Assessment; Re:wild: Austin, TX, USA, 2023.
- Rondinini, C.; Di Marco, M.; Visconti, P.; Butchart, S.H.M.; Boitani, L. Update or Outdate: Long-Term Viability of the IUCN Red List. Conserv. Lett. 2014, 7, 126–130. [Google Scholar] [CrossRef]
- Betts, J.; Young, R.P.; Hilton-Taylor, C.; Hoffmann, M.; Rodríguez, J.P.; Stuart, S.N.; Milner-Gulland, E.J. A Framework for Evaluating the Impact of the IUCN Red List of Threatened Species. Conserv. Biol. 2020, 34, 632–643. [Google Scholar] [CrossRef]
- Sterrett, S.C.; Katz, R.A.; Brand, A.B.; Fields, W.R.; Dietrich, A.E.; Hocking, D.J.; Foreman, T.M.; Wiewel, A.N.M.; Campbell Grant, E.H. Proactive Management of Amphibians: Challenges and Opportunities. Biol. Conserv. 2019, 236, 404–410. [Google Scholar] [CrossRef]
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D.; et al. Global Protected Areas as Refuges for Amphibians and Reptiles under Climate Change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt-Amarante, S.; Abensur, R.; Furet, R.; Ragon, C.; Herrel, A. Do Human-Induced Habitat Changes Impact the Morphology of a Common Amphibian, Bufo bufo? Urban Ecosyst. 2025, 28, 87. [Google Scholar] [CrossRef]
- Tan, W.C.; Herrel, A.; Rödder, D. A Global Analysis of Habitat Fragmentation Research in Reptiles and Amphibians: What Have We Done so Far? Biodivers. Conserv. 2023, 32, 439–468. [Google Scholar] [CrossRef]
- Buck, J.C.; Hua, J.; Brogan, W.R.; Dang, T.D.; Urbina, J.; Bendis, R.J.; Stoler, A.B.; Blaustein, A.R.; Relyea, R.A. Effects of Pesticide Mixtures on Host-Pathogen Dynamics of the Amphibian Chytrid Fungus. PLoS ONE 2015, 10, e0132832. [Google Scholar] [CrossRef] [PubMed]
- Falaschi, M.; Melotto, A.; Manenti, R.; Ficetola, G.F. Invasive Species and Amphibian Conservation. Herpetologica 2020, 76, 216. [Google Scholar] [CrossRef]
- Whiles, M.R.; Lips, K.R.; Pringle, C.M.; Kilham, S.S.; Bixby, R.J.; Brenes, R.; Connelly, S.; Colon-Gaud, J.C.; Hunte-Brown, M.; Huryn, A.D.; et al. The Effects of Amphibian Population Declines on the Structure and Function of Neotropical Stream Ecosystems. Front. Ecol. Environ. 2006, 4, 27–34. [Google Scholar] [CrossRef]
- Hintz, W.D.; Relyea, R.A. A Review of the Species, Community, and Ecosystem Impacts of Road Salt Salinisation in Fresh Waters. Freshw. Biol. 2019, 64, 1081–1097. [Google Scholar] [CrossRef]
- West, J. Importance of Amphibians: A Synthesis of Their Environmental Functions, Benefits to Humans, and Need for Conservation. 2018. Available online: https://vc.bridgew.edu/honors_proj/261/ (accessed on 27 December 2025).
- Connelly, S.; Pringle, C.M.; Bixby, R.J.; Brenes, R.; Whiles, M.R.; Lips, K.R.; Kilham, S.; Huryn, A.D. Changes in Stream Primary Producer Communities Resulting from Large-Scale Catastrophic Amphibian Declines: Can Small-Scale Experiments Predict Effects of Tadpole Loss? Ecosystems 2008, 11, 1262–1276. [Google Scholar] [CrossRef]
- Springborn, M.R.; Weill, J.A.; Lips, K.R.; Ibáñez, R.; Ghosh, A. Amphibian Collapses Increased Malaria Incidence in Central America*. Environ. Res. Lett. 2022, 17, 104012. [Google Scholar] [CrossRef]
- Humphries, J.E.; Lanctôt, C.M.; McCallum, H.I.; Newell, D.A.; Grogan, L.F. Chytridiomycosis Causes High Amphibian Mortality Prior to the Completion of Metamorphosis. Environ. Res. 2024, 247, 118249. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Ghimire, S.; Paudel Khatiwada, S.; Paudel, B.; Bischof, R.; Jiang, J.; Haugaasen, T. Frogs as Potential Biological Control Agents in the Rice Fields of Chitwan, Nepal. Agric. Ecosyst. Environ. 2016, 230, 307–314. [Google Scholar] [CrossRef]
- Teng, Q.; Hu, X.-F.; Luo, F.; Cheng, C.; Ge, X.; Yang, M.; Liu, L. Influences of Introducing Frogs in the Paddy Fields on Soil Properties and Rice Growth. J. Soils Sediments 2016, 16, 51–61. [Google Scholar] [CrossRef]
- Zipkin, E.F.; DiRenzo, G.V.; Ray, J.M.; Rossman, S.; Lips, K.R. Tropical Snake Diversity Collapses after Widespread Amphibian Loss. Science 2020, 367, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Knapp, R.A.; Briggs, C.J. Declines and Extinctions of Mountain Yellow-legged Frogs Have Small Effects on Benthic Macroinvertebrate Communities. Ecosphere 2016, 7, e01327. [Google Scholar] [CrossRef]
- Chanda, A.K. Diversity, Ecology and Conservation of Anurans from Ramnagar, West Bengal, India. Zool Ecol. 2024, 34, 102–110. [Google Scholar] [CrossRef]
- Vidal, M.A.; Henríquez, N.; Torres-Díaz, C.; Collado, G.; Acuña-Rodríguez, I.S. Identifying Strategies for Effective Biodiversity Preservation and Species Status of Chilean Amphibians. Biology 2024, 13, 169. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the Brink as Indicators of Biological Annihilation and the Sixth Mass Extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef]
- Lourenço-de-Moraes, R.; Campos, F.S.; Ferreira, R.B.; Beard, K.H.; Solé, M.; Llorente, G.A.; Bastos, R.P. Functional Traits Explain Amphibian Distribution in the Brazilian Atlantic Forest. J. Biogeogr. 2020, 47, 275–287. [Google Scholar] [CrossRef]
- Olson, D.H.; Pilliod, D.S. Elevating Human Dimensions of Amphibian and Reptile Conservation, a USA Perspective. Conserv. Sci. Pract. 2022, 4, e12685. [Google Scholar] [CrossRef]
- Demartín, R.P.; Ghirardi, R.; López, J.A. Influence of Natural and Artificial Habitats and Microhabitats on Urban Amphibian Diversity and Behavior. Diversity 2025, 17, 292. [Google Scholar] [CrossRef]
- Akat Çömden, E.; Yenmiş, M.; Çakır, B. The Complex Bridge between Aquatic and Terrestrial Life: Skin Changes during Development of Amphibians. J. Dev. Biol. 2023, 11, 6. [Google Scholar] [CrossRef]
- Beard, K.H.; Eschtruth, A.K.; Vogt, K.A.; Vogt, D.J.; Scatena, F.N. The Effects of the Frog Eleutherodactylus coqui on Invertebrates and Ecosystem Processes at Two Scales in the Luquillo Experimental Forest, Puerto Rico. J. Trop. Ecol. 2003, 19, 607–617. [Google Scholar] [CrossRef]
- Grant, E.H.C.; Fleming, J.; Bastiaans, E.; Brand, A.B.; Brooks, J.L.; Devlin, C.; Epp, K.; Evans, M.; Fisher-Reid, M.C.; Gratwicke, B.; et al. Range-Wide Salamander Densities Reveal a Key Component of Terrestrial Vertebrate Biomass in Eastern North American Forests. Biol. Lett. 2024, 20, 20240033. [Google Scholar] [CrossRef]
- Mossman, A.; Lambert, M.R.; Ashton, M.S.; Wikle, J.; Duguid, M.C. Two Salamander Species Respond Differently to Timber Harvests in a Managed New England Forest. PeerJ 2019, 7, e7604. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, J. Reciprocal Role of Salamanders in Aquatic Energy Flow Pathways. Diversity 2020, 12, 32. [Google Scholar] [CrossRef]
- Vera Candioti, F.; Baldo, D.; Grosjean, S.; Pereyra, M.O.; Nori, J. Global Shortfalls of Knowledge on Anuran Tadpoles. NPJ Biodivers. 2023, 2, 22. [Google Scholar] [CrossRef]
- Ranvestel, A.W.; Lips, K.R.; Pringle, C.M.; Whiles, M.R.; Bixby, R.J. Neotropical Tadpoles Influence Stream Benthos: Evidence for the Ecological Consequences of Decline in Amphibian Populations. Freshw. Biol. 2004, 49, 274–285. [Google Scholar] [CrossRef]
- Jara, F.G.; Garcia, P.E.; Garcia, R.D.; Sganga, J.V.; Pueta, M. Which Variables Influence the Structure and Abundance of Aquatic Herbivorous Assemblages in Small Forested Patagonian Wetlands? N. Z. J. Mar. Freshw. Res. 2024, 58, 422–439. [Google Scholar] [CrossRef]
- Rossi, F.; Galassi, D.M.P.; Angeli, N.; Cantonati, M.; Di Cicco, M. The Effects of Cattle Grazing on the Composition of Diatom Assemblages in the Peatland Pools of the Southeastern Alps (Italy, Adamello-Brenta Nature Park). Land 2024, 13, 1780. [Google Scholar] [CrossRef]
- Dorgan, K.M.; Arwade, S.R. Material Properties of Sediments Steer Burrowers and Effect Bioturbation. J. Geophys. Res. Biogeosciences 2023, 128, e2022JG007269. [Google Scholar] [CrossRef]
- Flecker, A.S.; Feifarek, B.P.; Taylor, B.W. Ecosystem Engineering by a Tropical Tadpole: Density-Dependent Effects on Habitat Structure and Larval Growth Rates. Copeia 1999, 1999, 495. [Google Scholar] [CrossRef]
- Catenazzi, A. State of the World’s Amphibians. Annu. Rev. Environ. Resour. 2015, 40, 91–119. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Salamander Populations and Biomass in the Hubbard Brook Experimental Forest, New Hampshire. Copeia 1975, 1975, 541. [Google Scholar] [CrossRef]
- Woolbright, L.L. The Impact of Hurricane Hugo on Forest Frogs in Puerto Rico. Biotropica 1991, 23, 462. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J. Effects of Red-Backed Salamanders on Ecosystem Functions. PLoS ONE 2014, 9, e86854. [Google Scholar] [CrossRef]
- Whitfield, S.M.; Bell, K.E.; Philippi, T.; Sasa, M.; Bolaños, F.; Chaves, G.; Savage, J.M.; Donnelly, M.A. Amphibian and Reptile Declines over 35 Years at La Selva, Costa Rica. Proc. Natl. Acad. Sci. USA 2007, 104, 8352–8356. [Google Scholar] [CrossRef]
- Gibbon, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The Global Decline of Reptiles, Déjà Vu Amphibians. Bioscience 2000, 50, 653. [Google Scholar] [CrossRef]
- Donnelly, M.A.; Guyer, C. Patterns of Reproduction and Habitat Use in an Assemblage of Neotropical Hylid Frogs. Oecologia 1994, 98, 291–302. [Google Scholar] [CrossRef]
- Beard, K.H.; Vogt, K.A.; Kulmatiski, A. Top-down Effects of a Terrestrial Frog on Forest Nutrient Dynamics. Oecologia 2002, 133, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.M.; Ruiz-Agudelo, C.A.; Valencia-Aguilar, A.; Ladle, R.J. Ecological Functions of Neotropical Amphibians and Reptiles: A Review. Univ. Sci. 2014, 20, 229. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Barquín, J.; Bonada, N.; Cantonati, M.; Churro, C.; Corbera, J.; Delgado, C.; Dulsat-Masvidal, M.; Garcia, G.; Margalef, O.; et al. Mediterranean Springs: Keystone Ecosystems and Biodiversity Refugia Threatened by Global Change. Glob. Chang. Biol. 2024, 30, e16997. [Google Scholar] [CrossRef]
- Milanovich, J.R.; Maerz, J.C.; Rosemond, A.D. Stoichiometry and Estimates of Nutrient Standing Stocks of Larval Salamanders in Appalachian Headwater Streams. Freshw. Biol. 2015, 60, 1340–1353. [Google Scholar] [CrossRef]
- Ernst, R.; Rödel, M.-O. Anthropogenically Induced Changes of Predictability in Tropical Anuran Assemblages. Ecology 2005, 86, 3111–3118. [Google Scholar] [CrossRef]
- Machado, I.F.; Maltchik, L. Can Management Practices in Rice Fields Contribute to Amphibian Conservation in Southern Brazilian Wetlands? Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 39–46. [Google Scholar] [CrossRef]
- Bishop, C.A.; Ashpole, S.L.; Edwards, A.M.; Van Aggelen, G.; Elliott, J.E. Hatching Success and Pesticide Exposures in Amphibians Living in Agricultural Habitats of the South Okanagan Valley, British Columbia, Canada (2004–2006). Environ. Toxicol. Chem. 2010, 29, 1593–1603. [Google Scholar] [CrossRef]
- Earl, J.E.; Blomquist, S.M.; Harper, E.B.; Hocking, D.J.; Hunter, M.L.; Johnson, J.R.; Osbourn, M.S.; Patrick, D.A.; Popescu, V.D.; Rittenhouse, T.A.G.; et al. Amphibian Biomass Export from Geographically Isolated Wetlands: Temporal Variability, Species Composition, and Potential Implications for Terrestrial Ecosystems. Diversity 2022, 14, 163. [Google Scholar] [CrossRef]
- Regester, K.J.; Lips, K.R.; Whiles, M.R. Energy Flow and Subsidies Associated with the Complex Life Cycle of Ambystomatid Salamanders in Ponds and Adjacent Forest in Southern Illinois. Oecologia 2006, 147, 303–314. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; O’Donnell, K.M.; Thompson, F.R. Abundance, Biomass Production, Nutrient Content, and the Possible Role of Terrestrial Salamanders in Missouri Ozark Forest Ecosystems. Can. J. Zool. 2014, 92, 997–1004. [Google Scholar] [CrossRef]
- McCaffery, R.; Lips, K. Survival and Abundance in Males of the Glass Frog Espadarana (Centrolene) prosoblepon in Central Panama. J. Herpetol. 2013, 47, 162–168. [Google Scholar] [CrossRef]
- Stephens, J.P.; Altman, K.A.; Berven, K.A.; Tiegs, S.D.; Raffel, T.R. Bottom-up and Trait-mediated Effects of Resource Quality on Amphibian Parasitism. J. Anim. Ecol. 2017, 86, 305–315. [Google Scholar] [CrossRef]
- Montaña, C.G.; Silva, S.D.G.T.M.; Hagyari, D.; Wager, J.; Tiegs, L.; Sadeghian, C.; Schriever, T.A.; Schalk, C.M. Revisiting “What Do Tadpoles Really Eat?” A 10-year Perspective. Freshw. Biol. 2019, 64, 2269–2282. [Google Scholar] [CrossRef]
- Milanovich, J.R.; Peterman, W.E. Revisiting Burton and Likens (1975): Nutrient Standing Stock and Biomass of a Terrestrial Salamander in the Midwestern United States. Copeia 2016, 104, 165–171. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Energy Flow and Nutrient Cycling in Salamander Populations in the Hubbard Brook Experimental Forest, New Hampshire. Ecology 1975, 56, 1068–1080. [Google Scholar] [CrossRef]
- Cervantes-López, M.D.J.; Morante-Filho, J.C. A Global Meta-Analysis on Patterns of Amphibian and Reptile Diversity in Agroforestry Systems. Glob. Ecol. Conserv. 2024, 51, e02914. [Google Scholar] [CrossRef]
- Knapp, R.A.; Wilber, M.Q.; Joseph, M.B.; Smith, T.C.; Grasso, R.L. Reintroduction of Resistant Frogs Facilitates Landscape-Scale Recovery in the Presence of a Lethal Fungal Disease. Nat. Commun. 2024, 15, 9436. [Google Scholar] [CrossRef]
- Fritz, K.A.; Whiles, M.R. Amphibian-mediated Nutrient Fluxes across Aquatic–Terrestrial Boundaries of Temporary Wetlands. Freshw. Biol. 2018, 63, 1250–1259. [Google Scholar] [CrossRef]
- Seale, D.B. Influence of Amphibian Larvae on Primary Production, Nutrient Flux, and Competition in a Pond Ecosystem. Ecology 1980, 61, 1531–1550. [Google Scholar] [CrossRef]
- Michael Walton, B. Salamanders in Forest-Floor Food Webs: Environmental Heterogeneity Affects the Strength of Top-down Effects. Pedobiologia 2005, 49, 381–393. [Google Scholar] [CrossRef]
- Walton, B.M.; Steckler, S. Contrasting Effects of Salamanders on Forest-Floor Macro- and Mesofauna in Laboratory Microcosms. Pedobiologia 2005, 49, 51–60. [Google Scholar] [CrossRef]
- Wyman, R.L. Experimental Assessment of Salamanders as Predators of Detrital Food Webs: Effects on Invertebrates, Decomposition and the Carbon Cycle. Biodivers. Conserv. 1998, 7, 641–650. [Google Scholar] [CrossRef]
- Beard, K.H.; O’Neill, E.M. Infection of an Invasive Frog Eleutherodactylus coqui by the Chytrid Fungus Batrachochytrium dendrobatidis in Hawaii. Biol. Conserv. 2005, 126, 591–595. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Winne, C.T.; Scott, D.E.; Willson, J.D.; Glaudas, X.; Andrews, K.M.; Todd, B.D.; Fedewa, L.A.; Wilkinson, L.; Tsaliagos, R.N.; et al. Remarkable Amphibian Biomass and Abundance in an Isolated Wetland: Implications for Wetland Conservation. Conserv. Biol. 2006, 20, 1457–1465. [Google Scholar] [CrossRef]
- Aspey, E.W.P.; Lustick, S.I. Behavioral Energetics the Cost of Survival in Vertebrates; The Ohio State University Press: Columbus, OH, USA, 1983. [Google Scholar]
- Burrow, A.; Maerz, J. How Plants Affect Amphibian Populations. Biol. Rev. 2022, 97, 1749–1767. [Google Scholar] [CrossRef]
- Jansen, A.; Healey, M. Frog Communities and Wetland Condition: Relationships with Grazing by Domestic Livestock along an Australian Floodplain River. Biol. Conserv. 2003, 109, 207–219. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Fang, J.; Zheng, S.; Wang, Z.; Jiao, Y.; Xia, P.; Wu, H.; Ma, Z.; Hao, L. Peptides Isolated from Amphibian Skin Secretions with Emphasis on Antimicrobial Peptides. Toxins 2022, 14, 722. [Google Scholar] [CrossRef]
- Calhoun, D.M.; Woodhams, D.; Howard, C.; LaFonte, B.E.; Gregory, J.R.; Johnson, P.T.J. Role of Antimicrobial Peptides in Amphibian Defense Against Trematode Infection. Ecohealth 2016, 13, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kim, Y.H.; Dudley, S.C.; Choudhary, G.; Pfahnl, A.; Oshima, Y.; Daly, J.W. The Structure of Zetekitoxin AB, a Saxitoxin Analog from the Panamanian Golden Frog Atelopus zeteki: A Potent Sodium-Channel Blocker. Proc. Natl. Acad. Sci. USA 2004, 101, 4346–4351. [Google Scholar] [CrossRef]
- De Sousa, N.A.; Marani, M.M.; Lopes, A.L.F.; Silva, E.M.; Barbosa, E.A.; Vasconcelos, A.G.; Kuzniewski, F.T.B.; Lustosa, S.S.; Gomes, K.P.; Colugnati, D.B.; et al. BR-Bombesin: A Novel Bombesin-Related Peptide from the Skin Secretion of the Chaco Tree Frog (Boana raniceps) with Physiological Gastric Effects. Amino Acids 2022, 54, 733–747. [Google Scholar] [CrossRef]
- Carta, P.; Conlon, J.M.; Scorciapino, M.A. Conformational Change Following Conversion of Inactive Rhinophrynin-33 to Bioactive Rhinophrynin-27 in the Skin of the Frog Rhinophrynus dorsalis. Biochimie 2021, 181, 162–168. [Google Scholar] [CrossRef]
- Dourado, F.S.; Leite, J.R.S.A.; Silva, L.P.; Melo, J.A.T.; Bloch, C.; Schwartz, E.F. Antimicrobial Peptide from the Skin Secretion of the Frog Leptodactylus syphax. Toxicon 2007, 50, 572–580. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Liu, X.; Wu, J.; Liu, C.; Gong, W.; Zhao, Z.; Hong, J.; Lin, D.; Wang, Y.; et al. Antioxidant Peptidomics Reveals Novel Skin Antioxidant System. Mol. Cell. Proteomics 2009, 8, 571–583. [Google Scholar] [CrossRef]
- Wang, X.; Ren, S.; Guo, C.; Zhang, W.; Zhang, X.; Zhang, B.; Li, S.; Ren, J.; Hu, Y.; Wang, H. Identification and Functional Analyses of Novel Antioxidant Peptides and Antimicrobial Peptides from Skin Secretions of Four East Asian Frog Species. Acta Biochim. Biophys. Sin. 2017, 49, 550–559. [Google Scholar] [CrossRef]
- Popovic, S.; Urbán, E.; Lukic, M.; Conlon, J.M. Peptides with Antimicrobial and Anti-Inflammatory Activities That Have Therapeutic Potential for Treatment of Acne Vulgaris. Peptides 2012, 34, 275–282. [Google Scholar] [CrossRef]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stöckle, M. Antitumor Activity of the Antimicrobial Peptide Magainin II against Bladder Cancer Cell Lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef]
- Alfei, S.; Giannoni, P.; Signorello, M.G.; Torazza, C.; Zuccari, G.; Athanassopoulos, C.M.; Domenicotti, C.; Marengo, B. The Remarkable and Selective In Vitro Cytotoxicity of Synthesized Bola-Amphiphilic Nanovesicles on Etoposide-Sensitive and -Resistant Neuroblastoma Cells. Nanomaterials 2024, 14, 1505. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.A.; Reinoso, C.; Morales, F.; Pilaquinga, F.; Morán-Marcillo, G.; Proaño-Bolaños, C.; Blasco-Zúñiga, A.; Rivera, M.; Meneses, L. Novel Antimicrobial Cruzioseptin Peptides Extracted from the Splendid Leaf Frog, Cruziohyla calcarifer. Amino Acids 2021, 53, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The Frog Skin-Derived Antimicrobial Peptide Esculentin-1a(1-21)NH2 Promotes the Migration of Human HaCaT Keratinocytes in an EGF Receptor-Dependent Manner: A Novel Promoter of Human Skin Wound Healing? PLoS ONE 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.C.C.; Zanotta, L.C.; Kyaw, C.M.; Schwartz, E.N.F.; Schwartz, C.A.; Sebben, A.; Sousa, M.V.; Fontes, W.; Castro, M.S. Ocellatins: New Antimicrobial Peptides from the Skin Secretion of the South American Frog Leptodactylus ocellatus (Anura: Leptodactylidae). Protein J. 2004, 23, 501–508. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Power, G.J.; Flatt, P.R.; Woodhams, D.C.; Rollins-Smith, L.A.; Conlon, J.M. A Peptide of the Phylloseptin Family from the Skin of the Frog Hylomantis lemur (Phyllomedusinae) with Potent in Vitro and in Vivo Insulin-Releasing Activity. Peptides 2008, 29, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Leprince, J.; Vaudry, H.; Jouenne, T.; Condamine, E. A Glycine-Leucine-Rich Peptide Structurally Related to the Plasticins from Skin Secretions of the Frog Leptodactylus laticeps (Leptodactylidae). Peptides 2009, 30, 888–892. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An Antimicrobial Peptide, Magainin 2, Induced Rapid Flip-Flop of Phospholipids Coupled with Pore Formation and Peptide Translocation. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial CDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Gottler, L.M.; Ramamoorthy, A. Structure, Membrane Orientation, Mechanism, and Function of Pexiganan—A Highly Potent Antimicrobial Peptide Designed from Magainin. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; El Amri, C. The Dermaseptin Superfamily: A Gene-Based Combinatorial Library of Antimicrobial Peptides. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Pukala, T.L.; Bowie, J.H.; Maselli, V.M.; Musgrave, I.F.; Tyler, M.J. Host-Defence Peptides from the Glandular Secretions of Amphibians: Structure and Activity. Nat. Prod. Rep. 2006, 23, 368. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Wu, C.; Li, X.; Fu, Z.; Yang, M.; Bian, W.; Wang, S.; Song, Y.; Tang, J.; et al. Cathelicidin-OA1, a Novel Antioxidant Peptide Identified from an Amphibian, Accelerates Skin Wound Healing. Sci. Rep. 2018, 8, 943. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free Radical Scavenging Activity of a Novel Antioxidative Peptide Purified from Hydrolysate of Bullfrog Skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N.; Leprince, J.; Vaudry, H.; Coquet, L.; Jouenne, T.; King, J.D. Characterization of Antimicrobial Peptides from the Skin Secretions of the Malaysian Frogs, Odorrana hosii and Hylarana picturata (Anura:Ranidae). Toxicon 2008, 52, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Soltaninejad, H.; Zare-Zardini, H.; Ordooei, M.; Ghelmani, Y.; Ghadiri-Anari, A.; Mojahedi, S.; Hamidieh, A.A. Antimicrobial Peptides from Amphibian Innate Immune System as Potent Antidiabetic Agents: A Literature Review and Bioinformatics Analysis. J. Diabetes Res. 2021, 2021, 2894722. [Google Scholar] [CrossRef]
- Li, S.; Hao, L.; Bao, W.; Zhang, P.; Su, D.; Cheng, Y.; Nie, L.; Wang, G.; Hou, F.; Yang, Y. A Novel Short Anionic Antibacterial Peptide Isolated from the Skin of Xenopus laevis with Broad Antibacterial Activity and Inhibitory Activity against Breast Cancer Cell. Arch. Microbiol. 2016, 198, 473–482. [Google Scholar] [CrossRef]
- Marenah, L.; Flatt, P.R.; Orr, D.F.; McClean, S.; Shaw, C.; Abdel-Wahab, Y.H.A. Skin Secretion of the Toad Bombina variegata Contains Multiple Insulin-Releasing Peptides Including Bombesin and Entirely Novel Insulinotropic Structures. Biol. Chem. 2004, 385, 315–321. [Google Scholar] [CrossRef]
- Indriani, S.; Karnjanapratum, S.; Nirmal, N.P.; Nalinanon, S. Amphibian Skin and Skin Secretion: An Exotic Source of Bioactive Peptides and Its Application. Foods 2023, 12, 1282. [Google Scholar] [CrossRef]
- Renda, T.; D’Este, L.; Buffa, R.; Usellini, L.; Capella, C.; Vaccaro, R.; Melchiorri, P. Tryptophyllin-like Immunoreactivity in Rat Adenohypophysis. Peptides 1985, 6, 197–202. [Google Scholar] [CrossRef]
- Daly, J.W.; Myers, C.W.; Whittaker, N. Further Classification of Skin Alkaloids from Neotropical Poison Frogs (Dendrobatidae), with a General Survey of Toxic/Noxious Substances in the Amphibia. Toxicon 1987, 25, 1023–1095. [Google Scholar] [CrossRef]
- Hyung-Jin, P.; Yun-Yeol, L.; Hyuk-Il, K.; Won-Im, S.; Sang-Won, S. Isolation of Bombesin-Like Substances from the Skin of the Frog, Bombina orientalis: Its Molecular Heterogeneity and Biological Activity. Korean J. Physiol. 1989, 23, 79–87. [Google Scholar]
- Yin, S.; Wang, Y.; Liu, N.; Yang, M.; Hu, Y.; Li, X.; Fu, Y.; Luo, M.; Sun, J.; Yang, X. Potential Skin Protective Effects after UVB Irradiation Afforded by an Antioxidant Peptide from Odorrana andersonii. Biomed. Pharmacother. 2019, 120, 109535. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Yi, K.-S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure–Activity Analysis of Buforin II, a Histone H2A-Derived Antimicrobial Peptide: The Proline Hinge Is Responsible for the Cell-Penetrating Ability of Buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D.; Guerrini, R.; Marzola, E.; Salvadori, S. Synthesis and Antimicrobial Activity of Dermaseptin S1 Analogues. Bioorg. Med. Chem. 2008, 16, 8205–8209. [Google Scholar] [CrossRef]
- Owolabi, B.O.; Ojo, O.O.; Srinivasan, D.K.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. In Vitro and in Vivo Insulinotropic Properties of the Multifunctional Frog Skin Peptide Hymenochirin-1B: A Structure–Activity Study. Amino Acids 2016, 48, 535–547. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, H.; Yu, X.; Tan, L.; Ma, C.; Xi, X.; Li, L.; Wang, L.; Zhou, M.; Chen, T.; et al. Novel Frog Skin-Derived Peptide Dermaseptin-PP for Lung Cancer Treatment: In Vitro/Vivo Evaluation and Anti-Tumor Mechanisms Study. Front. Chem. 2020, 8, 476. [Google Scholar] [CrossRef]
- Amiche, M.; Seon, A.A.; Wroblewski, H.; Nicolas, P. Isolation of Dermatoxin from Frog Skin, an Antibacterial Peptide Encoded by a Novel Member of the Dermaseptin Genes Family. Eur. J. Biochem. 2000, 267, 4583–4592. [Google Scholar] [CrossRef] [PubMed]
- Mechkarska, M.; Attoub, S.; Sulaiman, S.; Pantic, J.; Lukic, M.L.; Michael Conlon, J. Anti-Cancer, Immunoregulatory, and Antimicrobial Activities of the Frog Skin Host-Defense Peptides Pseudhymenochirin-1Pb and Pseudhymenochirin-2Pa. Regul. Pept. 2014, 194–195, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Scorciapino, M.A.; Srinivasan, D.; Attoub, S.; Mangoni, M.L.; Rinaldi, A.C.; Casu, M.; Flatt, P.R.; Conlon, J.M. Conformational Analysis of the Host-Defense Peptides Pseudhymenochirin-1Pb and -2Pa and Design of Analogues with Insulin-Releasing Activities and Reduced Toxicities. J. Nat. Prod. 2015, 78, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Conlon, J.M. Insulinotropic Actions of the Frog Skin Host-Defense Peptide Alyteserin-2a: A Structure–Activity Study. Chem. Biol. Drug Des. 2013, 82, 196–204. [Google Scholar] [CrossRef]
- Mechkarska, M.; Ojo, O.O.; Meetani, M.A.; Coquet, L.; Jouenne, T.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; King, J.D.; Conlon, J.M. Peptidomic Analysis of Skin Secretions from the Bullfrog Lithobates catesbeianus (Ranidae) Identifies Multiple Peptides with Potent Insulin-Releasing Activity. Peptides 2011, 32, 203–208. [Google Scholar] [CrossRef]
- Zahid, O.K.; Mechkarska, M.; Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Meetani, M.A.; Conlon, J.M. Caerulein-and Xenopsin-Related Peptides with Insulin-Releasing Activities from Skin Secretions of the Clawed Frogs, Xenopus borealis and Xenopus amieti (Pipidae). Gen. Comp. Endocrinol. 2011, 172, 314–320. [Google Scholar] [CrossRef]
- Ojo, O.O.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Frog Skin Peptides (Tigerinin-1R, Magainin-AM1, -AM2, CPF-AM1, and PGla-AM1) Stimulate Secretion of Glucagon-like Peptide 1 (GLP-1) by GLUTag Cells. Biochem. Biophys. Res. Commun. 2013, 431, 14–18. [Google Scholar] [CrossRef]
- Brand, G.D.; Leite, J.R.S.A.; Silva, L.P.; Albuquerque, S.; Prates, M.V.; Azevedo, R.B.; Carregaro, V.; Silva, J.S.; Sá, V.C.L.; Brandão, R.A.; et al. Dermaseptins from Phyllomedusa oreades And Phyllomedusa distincta. J. Biol. Chem. 2002, 277, 49332–49340. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.R.S.A.; Silva, L.P.; Rodrigues, M.I.S.; Prates, M.V.; Brand, G.D.; Lacava, B.M.; Azevedo, R.B.; Bocca, A.L.; Albuquerque, S.; Bloch, C. Phylloseptins: A Novel Class of Anti-Bacterial and Anti-Protozoan Peptides from the Phyllomedusa Genus. Peptides 2005, 26, 565–573. [Google Scholar] [CrossRef]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Vasu, S.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Esculentin-2CHa-Related Peptides Modulate Islet Cell Function and Improve Glucose Tolerance in Mice with Diet-Induced Obesity and Insulin Resistance. PLoS ONE 2015, 10, e0141549. [Google Scholar] [CrossRef]
- Zhou, M.; Ren, G.; Zhang, B.; Ma, F.; Fan, J.; Qiu, Z. Screening and Identification of a Novel Antidiabetic Peptide from Collagen Hydrolysates of Chinese Giant Salamander Skin: Network Pharmacology, Inhibition Kinetics and Protection of IR-HepG2 Cells. Food Funct. 2022, 13, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.R.; Shahani, S.K.; Meherji, P.K. Spermicidal Activity of Magainins: In Vitro and in Vivo Studies. Contraception 1996, 53, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.; Berto, R.F.; Gois, E.A.; Fontenele-Cardi, N.C.; Honório-Júnior, J.E.R.; Konno, K.; Richardson, M.; Rocha, M.F.G.; Camargo, A.A.C.M.; Pimenta, D.C.; et al. Leptoglycin: A New Glycine/Leucine-Rich Antimicrobial Peptide Isolated from the Skin Secretion of the South American Frog Leptodactylus pentadactylus (Leptodactylidae). Toxicon 2009, 54, 23–32. [Google Scholar] [CrossRef]
- McMillan, K.A.M.; Coombs, M.R.P. Review: Examining the Natural Role of Amphibian Antimicrobial Peptide Magainin. Molecules 2020, 25, 5436. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Flatt, P.R.; Conlon, J.M. Insulin Releasing Properties of the Temporin Family of Antimicrobial Peptides. Protein Pept. Lett. 2007, 14, 702–707. [Google Scholar] [CrossRef]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Beneficial Effects of Tigerinin-1R on Glucose Homeostasis and Beta Cell Function in Mice with Diet-Induced Obesity-Diabetes. Biochimie 2015, 109, 18–26. [Google Scholar] [CrossRef]
- Liu, H.; Mu, L.; Tang, J.; Shen, C.; Gao, C.; Rong, M.; Zhang, Z.; Liu, J.; Wu, X.; Yu, H.; et al. A Potential Wound Healing-Promoting Peptide from Frog Skin. Int. J. Biochem. Cell Biol. 2014, 49, 32–41. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Y.; Wang, C.; Wu, G.; Wang, Z. Purification, Molecular Cloning, and Antimicrobial Activity of Peptides from the Skin Secretion of the Black-Spotted Frog, Rana nigromaculata. World J. Microbiol. Biotechnol. 2013, 29, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Morton, L.H.; Harris, F.; Phoenix, D.A. Low PH Enhances the Action of Maximin H5 against Staphylococcus aureus and Helps Mediate Lysylated Phosphatidylglycerol-Induced Resistance. Biochemistry 2016, 55, 3735–3751. [Google Scholar] [CrossRef]
- Conlon, J.M.; Leprince, J.; Vaudry, H.; Jiansheng, H.; Nielsen, P.F. A Family of Antimicrobial Peptides Related to Japonicin-2 Isolated from the Skin of the Chaochiao Brown Frog Rana chaochiaoensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 101–105. [Google Scholar] [CrossRef]
- Van Zoggel, H.; Hamma-Kourbali, Y.; Galanth, C.; Ladram, A.; Nicolas, P.; Courty, J.; Amiche, M.; Delbé, J. Antitumor and Angiostatic Peptides from Frog Skin Secretions. Amino Acids 2012, 42, 385–395. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.-B.; Li, S.; Tian, L.-L.; Shang, D.-J. Antitumor Effects and Cell Selectivity of Temporin-1CEa, an Antimicrobial Peptide from the Skin Secretions of the Chinese Brown Frog (Rana chensinensis). Biochimie 2012, 94, 434–441. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Orr, D.F.; Shaw, C.; Flatt, P.R. Isolation and Structural Characterisation of a Novel 13-Amino Acid Insulin-Releasing Peptide from the Skin Secretion of Agalychnis calcarifer. Biol. Chem. 2005, 386, 581–587. [Google Scholar] [CrossRef]
- Yun, M.H. Salamander Insights Into Ageing and Rejuvenation. Front. Cell Dev. Biol. 2021, 9, 689062. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, B.; Stocum, D.L. Amphibians as Research Models for Regenerative Medicine. Organogenesis 2010, 6, 141–150. [Google Scholar] [CrossRef]
- Min, S.; Whited, J.L. Limb Blastema Formation: How Much Do We Know at a Genetic and Epigenetic Level? J. Biol. Chem. 2023, 299, 102858. [Google Scholar] [CrossRef]
- Bassat, E.; Tanaka, E.M. The Cellular and Signaling Dynamics of Salamander Limb Regeneration. Curr. Opin. Cell Biol. 2021, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Muneoka, K.; Fox, W.F.; Bryant, S. V Cellular Contribution from Dermis and Cartilage to the Regenerating Limb Blastema in Axolotls. Dev. Biol. 1986, 116, 256–260. [Google Scholar] [CrossRef]
- Odelberg, S.J.; Kollhoff, A.; Keating, M.T. Dedifferentiation of Mammalian Myotubes Induced by Msx1. Cell 2000, 103, 1099–1109. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Endo, T.; Bryant, S.V. The Molecular Basis of Amphibian Limb Regeneration: Integrating the Old with the New. Semin. Cell Dev. Biol. 2002, 13, 345–352. [Google Scholar] [CrossRef]
- Nowoshilow, S.; Schloissnig, S.; Fei, J.-F.; Dahl, A.; Pang, A.W.C.; Pippel, M.; Winkler, S.; Hastie, A.R.; Young, G.; Roscito, J.G.; et al. The Axolotl Genome and the Evolution of Key Tissue Formation Regulators. Nature 2018, 554, 50–55. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Soto, J.; Hoffman, T.; Lin, X.; Zhang, A.; Chen, S.; Massad, R.N.; Han, X.; Qi, D.; et al. Viscoelastic Extracellular Matrix Enhances Epigenetic Remodeling and Cellular Plasticity. Nat. Commun. 2025, 16, 4054. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Rosenthal, N. Scar-Free Wound Healing and Regeneration in Amphibians: Immunological Influences on Regenerative Success. Differentiation 2014, 87, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages Are Required for Adult Salamander Limb Regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef]
- Tazaki, A.; Tanaka, E.M.; Fei, J.-F. Salamander Spinal Cord Regeneration: The Ultimate Positive Control in Vertebrate Spinal Cord Regeneration. Dev. Biol. 2017, 432, 63–71. [Google Scholar] [CrossRef]
- Altyar, A.E.; El-Sayed, A.; Abdeen, A.; Piscopo, M.; Mousa, S.A.; Najda, A.; Abdel-Daim, M.M. Future Regenerative Medicine Developments and Their Therapeutic Applications. Biomed. Pharmacother. 2023, 158, 114131. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart Regeneration in the Salamander Relies on Macrophage-Mediated Control of Fibroblast Activation and the Extracellular Landscape. npj Regen. Med. 2017, 2, 22. [Google Scholar] [CrossRef]
- Barros, A.L.A.N.; Hamed, A.; Marani, M.; Moreira, D.C.; Eaton, P.; Plácido, A.; Kato, M.J.; Leite, J.R.S.A. The Arsenal of Bioactive Molecules in the Skin Secretion of Urodele Amphibians. Front. Pharmacol. 2022, 12, 810821. [Google Scholar] [CrossRef]
- Xi, X.; Li, B.; Chen, T.; Kwok, H. A Review on Bradykinin-Related Peptides Isolated from Amphibian Skin Secretion. Toxins 2015, 7, 951–970. [Google Scholar] [CrossRef]
- Conceição, K.; Konno, K.; De Melo, R.L.; Antoniazzi, M.M.; Jared, C.; Sciani, J.M.; Conceição, I.M.; Prezoto, B.C.; De Camargo, A.C.M.; Pimenta, D.C. Isolation and Characterization of a Novel Bradykinin Potentiating Peptide (BPP) from the Skin Secretion of Phyllomedusa hypochondrialis. Peptides 2007, 28, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Gross, J.A.; Karasov, W.H. Sublethal Effects of Lead on Northern Leopard Frog (Rana pipiens) Tadpoles. Environ. Toxicol. Chem. 2006, 25, 1383–1389. [Google Scholar] [CrossRef]
- Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Mechkarska, M.; Conlon, J.M. Tigerinin-1R: A Potent, Non-Toxic Insulin-Releasing Peptide Isolated from the Skin of the Asian Frog, Hoplobatrachus rugulosus. Diabetes, Obes. Metab. 2011, 13, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Zaman, S.U.; Asif, F.; Hasan Arnab, M.K.; Rahman, M.M.; Hasan, M. Isolation of Antimicrobial Peptides (AMPs) From Different Sources: A Review. Bangladesh Pharm. J. 2023, 26, 99–111. [Google Scholar] [CrossRef]
- Erspamer, V.; Melchiorri, P.; Falconieri-Erspamer, G.; Negri, L.; Corsi, R.; Severini, C.; Barra, D.; Simmaco, M.; Kreil, G. Deltorphins: A Family of Naturally Occurring Peptides with High Affinity and Selectivity for Delta Opioid Binding Sites. Proc. Natl. Acad. Sci. USA 1989, 86, 5188–5192. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Casciaro, B. Development of Antimicrobial Peptides from Amphibians. Antibiotics 2020, 9, 772. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial Peptides: Opportunities and Challenges in Overcoming Resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Santos, R.; Soares, R.S.; Reis, S.; Carvalho, S.; Rego, P.; Peleteiro, M.C.; Tavares, L.; Oliveira, M. Pexiganan in Combination with Nisin to Control Polymicrobial Diabetic Foot Infections. Antibiotics 2020, 9, 128. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, J.; Trivedi, R.; Ranade, P. Shaping the Future of Antimicrobial Therapy: Harnessing the Power of Antimicrobial Peptides in Biomedical Applications. J. Funct. Biomater. 2023, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Md Fadilah, N.I.; Shahabudin, N.A.; Mohd Razif, R.A.; Sanyal, A.; Ghosh, A.; Baharin, K.I.; Ahmad, H.; Maarof, M.; Motta, A.; Fauzi, M.B. Discovery of Bioactive Peptides as Therapeutic Agents for Skin Wound Repair. J. Tissue Eng. 2024, 15, 20417314241280360. [Google Scholar] [CrossRef]
- Bian, W.; Meng, B.; Li, X.; Wang, S.; Cao, X.; Liu, N.; Yang, M.; Tang, J.; Wang, Y.; Yang, X. OA-GL21, a Novel Bioactive Peptide from Odorrana andersonii, Accelerated the Healing of Skin Wounds. Biosci. Rep. 2018, 38, BSR20180215. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, Y.; Yang, X. Amphibian-Derived Wound Healing Peptides: Chemical Molecular Treasure Trove for Skin Wound Treatment. Front. Pharmacol. 2023, 14, 1120228. [Google Scholar] [CrossRef]
- Silva Ortíz, Y.L.; De Sousa, T.C.; Kruklis, N.E.; Galeano García, P.; Brango-Vanegas, J.; Soller Ramada, M.H.; Franco, O.L. The Role of Amphibian AMPs Against Oxidative Stress and Related Diseases. Antibiotics 2025, 14, 126. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. The Future of Amphibian Immunology: Opportunities and Challenges. Dev. Comp. Immunol. 2024, 160, 105237. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A.; Le Sage, E.H. Heat Stress and Amphibian Immunity in a Time of Climate Change. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220132. [Google Scholar] [CrossRef]
- Hopkins, W.A. Amphibians as Models for Studying Environmental Change. ILAR J. 2007, 48, 270–277. [Google Scholar] [CrossRef]
- Jung, R.; Mercurio, G.; Chellman, I.; Southerland, M.; Baxter, D.; Vølstad, J. Stream Salamanders as Indicators of Stream Quality in Maryland, USA. Appl. Herpetol. 2004, 2, 23–46. [Google Scholar] [CrossRef]
- Welsh, H.H.; Ollivier, L.M. Stream Amphibians as Indicators of Ecosystem Stress: A Case Study from California’s Redwoods. Ecol. Appl. 1998, 8, 1118–1132. [Google Scholar] [CrossRef]
- McIntyre, A.P.; Ojala-Barbour, R.; Jones, J.E.; Kroll, A.J.; Lund, E.M.; Quinn, T.; Ehinger, W.J.; Estrella, S.M.; Schuett-Hames, D.E.; Hayes, M.P. Negative Effects of Forest Harvest on Coastal Tailed Frog (Ascaphus truei) Emerge after a Decade-long Experiment. Ecosphere 2025, 16, e70281. [Google Scholar] [CrossRef]
- Castro-Bastidas, H.A.; Bucio-Pacheco, M.; Serrano, J.M.; Aguillón-Gutiérrez, D.R. Morphological Anomalies in Amphibians of the Sierra Madre Occidental and a Review of Cases Reported in Mexico. Environ. Monit. Assess. 2025, 197, 734. [Google Scholar] [CrossRef]
- Nkwonta, E.; Vanderwolf, K.J.; Ambeau, T.; Davison, S.; Kowalchuk-Reid, A.; Paterson, J.E.; Davy, C.M. Skin PH and Buffering Ability Vary between Two Co-Occurring Semi-Aquatic Frog Species. Conserv. Physiol. 2025, 13, coaf037. [Google Scholar] [CrossRef]
- Ochs, A.E.; Swihart, R.K.; Saunders, M.R. A Comprehensive Review of the Effects of Roads on Salamanders. Landsc. Ecol. 2024, 39, 77. [Google Scholar] [CrossRef]
- Otero, M.A.; Pollo, F.E.; Baraquet, M.; Martino, A.L.; Grenat, P.R. Influence of Fluorite Mining on Ecological Traits of Anuran Amphibian Assemblages from Central Argentina. Environ. Sci. Pollut. Res. 2025, 32, 18516–18524. [Google Scholar] [CrossRef]
- Steigerwald, E.; Chen, J.; Oshiro, J.; Vredenburg, V.T.; Catenazzi, A.; Koo, M.S. Microreserves Are an Important Tool for Amphibian Conservation. Commun. Biol. 2024, 7, 1177. [Google Scholar] [CrossRef] [PubMed]
- Isa, H.R.; Tapley, B.; Michaels, C.J. How Many Data Deficient Amphibians Are Threatened? IUCN Red List Assessments for Amphibian Species Previously Classed as Data Deficient. Herpetol. Bull. 2024, 169, 12–17. [Google Scholar] [CrossRef]
- Welsh, H.H.; Droege, S. A Case for Using Plethodontid Salamanders for Monitoring Biodiversity and Ecosystem Integrity of North American Forests. Conserv. Biol. 2001, 15, 558–569. [Google Scholar] [CrossRef]
- Álvarez-Grzybowska, E.; Urbina-Cardona, N.; Córdova-Tapia, F.; García, A. Amphibian Communities in Two Contrasting Ecosystems: Functional Diversity and Environmental Filters. Biodivers. Conserv. 2020, 29, 2457–2485. [Google Scholar] [CrossRef]
- Zhou, J.; Nelson, T.M.; Rodriguez Lopez, C.; Sarma, R.R.; Zhou, S.J.; Rollins, L.A. A Comparison of Nonlethal Sampling Methods for Amphibian Gut Microbiome Analyses. Mol. Ecol. Resour. 2020, 20, 844–855. [Google Scholar] [CrossRef]
- Bishop, P.J.; Angulo, A.; Lewis, J.P.; Moore, R.D.; Rabb, G.B.; Moreno, J.G. The Amphibian Extinction Crisis—What will it take to put the action into the Amphibian Conservation Action Plan? SAPI EN. S. Surv. Perspect. Integr. Environ. Soc. 2012, 5, 97–111. Available online: https://journals.openedition.org/sapiens/1406 (accessed on 30 December 2025).
- Lenhardt, P.P.; Schäfer, R.B.; Theissinger, K.; Brühl, C.A. An Expert-Based Landscape Permeability Model for Assessing the Impact of Agricultural Management on Amphibian Migration. Basic Appl. Ecol. 2013, 14, 442–451. [Google Scholar] [CrossRef]
- Silva-Alves, V.D.; Neves, M.O.; Seba, M.D.F.R.; Santos Filho, M.D.; Silva, D.J. Da Amphibian Diversity: Where Everything Starts to Flood, Cáceres Municipality, North Pantanal, Central-West Brazil. Pap. Avulsos Zool. 2023, 63, e202363033. [Google Scholar] [CrossRef]
- Hayes, T.B.; Falso, P.; Gallipeau, S.; Stice, M. The Cause of Global Amphibian Declines: A Developmental Endocrinologist’s Perspective. J. Exp. Biol. 2010, 213, 921–933. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Van Meter, R.J.; Glinski, D.A.; Purucker, S.T.; Henderson, W.M. Influence of Exposure to Pesticide Mixtures on the Metabolomic Profile in Post-Metamorphic Green Frogs (Lithobates clamitans). Sci. Total Environ. 2018, 624, 1348–1359. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond Biomarkers and towards Mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Miller, D.; Gray, M.; Storfer, A. Ecopathology of Ranaviruses Infecting Amphibians. Viruses 2011, 3, 2351–2373. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Dalpasso, A.; Seglie, D.; Eusebio Bergò, P.; Ciracì, A.; Compostella, M.; Laddaga, L.; Manica, M.; Marino, G.; Pandolfo, I.; Soldato, G.; et al. Effects of Temperature and Precipitation Changes on Shifts in Breeding Phenology of an Endangered Toad. Sci. Rep. 2023, 13, 14573. [Google Scholar] [CrossRef] [PubMed]
- Benard, M.F.; Greenwald, K.R. Environmental Drivers of Amphibian Breeding Phenology across Multiple Sites. Diversity 2023, 15, 253. [Google Scholar] [CrossRef]
- Todd, B.D.; Scott, D.E.; Pechmann, J.H.K.; Gibbons, J.W. Climate Change Correlates with Rapid Delays and Advancements in Reproductive Timing in an Amphibian Community. Proc. R. Soc. B Biol. Sci. 2011, 278, 2191–2197. [Google Scholar] [CrossRef]
- Ochoa-Ochoa, L.; Whittaker, R.; Ladle, R. The Demise of the Golden Toad and the Creation of a Climate Change Icon Species. Conserv. Soc. 2013, 11, 291. [Google Scholar] [CrossRef]
- Alan Pounds, J.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread Amphibian Extinctions from Epidemic Disease Driven by Global Warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Alton, L.A.; Franklin, C.E. Drivers of Amphibian Declines: Effects of Ultraviolet Radiation and Interactions with Other Environmental Factors. Clim. Chang. Responses 2017, 4, 6. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of Habitat Loss and Fragmentation on Amphibians: A Review and Prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, Y.; Dong, M.; Li, W. Human Activities and Climate Change Are the Main Factors of Amphibian Extinction. Glob. Ecol. Conserv. 2025, 62, e03747. [Google Scholar] [CrossRef]
- Collins, J.P. Amphibian Decline and Extinction: What We Know and What We Need to Learn. Dis. Aquat. Organ. 2010, 92, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, A.; Robertson, H.; Prieler, R.; Dubey, A.; Finlayson, C. Recognizing Diversity in Wetlands and Farming Systems to Support Sustainable Agriculture and Conserve Wetlands. Mar. Freshw. Res. 2025, 76, MF24017. [Google Scholar] [CrossRef]
- Falcón-Espitia, N.; Ríos-Orjuela, J.C.; Perez-Rojas, S.; Plazas-Cardona, D.; Arias-Escobar, A. Impacts of Habitat Transformation on Amphibian and Reptile Communities in a Heterogeneous Andean Landscape. bioRxiv 2025. [Google Scholar] [CrossRef]
- Nowakowski, A.J.; Frishkoff, L.O.; Thompson, M.E.; Smith, T.M.; Todd, B.D. Phylogenetic Homogenization of Amphibian Assemblages in Human-Altered Habitats across the Globe. Proc. Natl. Acad. Sci. USA 2018, 115, E3454–E3462. [Google Scholar] [CrossRef]
- LAURANCE, W.F.; USECHE, D.C. Environmental Synergisms and Extinctions of Tropical Species. Conserv. Biol. 2009, 23, 1427–1437. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans Sp. Nov. Causes Lethal Chytridiomycosis in Amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef]
- Gray, M.J.; Miller, D.L.; Hoverman, J.T. Ecology and Pathology of Amphibian Ranaviruses. Dis. Aquat. Organ. 2009, 87, 243–266. [Google Scholar] [CrossRef]
- Alves-Ferreira, G.; Talora, D.C.; Solé, M.; Cervantes-López, M.J.; Heming, N.M. Unraveling Global Impacts of Climate Change on Amphibians Distributions: A Life-History and Biogeographic-Based Approach. Front. Ecol. Evol. 2022, 10, 987237. [Google Scholar] [CrossRef]
- López-de Sancha, A.; Boix, D.; Benejam, L.; Briggs, L.; Davidson, T.A.; Fahy, J.C.; Frutos-Aragón, V.; Greaves, H.M.; Lemmens, P.; Mehner, T.; et al. Amphibian Conservation in Europe: The Importance of Pond Condition. Biodivers. Conserv. 2025, 34, 1559–1574. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Schmidt, B.R.; Martel, A.; Pasmans, F.; Muths, E.; Cunningham, A.A.; Weldon, C.; Fisher, M.C.; Bosch, J. Mitigating Amphibian Chytridiomycoses in Nature. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160207. [Google Scholar] [CrossRef]
- Moraes, L.J.C.D.L.; Rainha, R.D.N.; Werneck, F.D.P.; Oliveira, A.F.D.S.; Gascon, C.; Carvalho, V.T. De Amphibians and Reptiles from a Protected Area in Western Brazilian Amazonia (Reserva Extrativista Do Baixo Juruá). Pap. Avulsos Zool. 2022, 62, e202262054. [Google Scholar] [CrossRef]
- Belasen, A.M.; Russell, I.D.; Zamudio, K.R.; Bletz, M.C. Endemic Lineages of Batrachochytrium Dendrobatidis Are Associated With Reduced Chytridiomycosis-Induced Mortality in Amphibians: Evidence From a Meta-Analysis of Experimental Infection Studies. Front. Vet. Sci. 2022, 9, 756686. [Google Scholar] [CrossRef]
- Crawford, J.A.; Peterman, W.E.; Kuhns, A.R.; Phillips, C.A. Effectiveness of Rapid Sampling Assessments for Wetland-Breeding Amphibians. Ecol. Indic. 2023, 154, 110736. [Google Scholar] [CrossRef]
- Chuang, M.-F.; Cheng, Y.-J.; Andersen, D.; Borzée, A.; Wu, C.-S.; Chang, Y.-M.; Yang, Y.-J.; Jang, Y.; Kam, Y.-C. Increasing Salinity Stress Decreases the Thermal Tolerance of Amphibian Tadpoles in Coastal Areas of Taiwan. Sci. Rep. 2022, 12, 9014. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Le Sage, E.H. Batrachochytrium Fungi: Stealth Invaders in Amphibian Skin. Curr. Opin. Microbiol. 2021, 61, 124–132. [Google Scholar] [CrossRef]
- Le Sage, E.H.; Reinert, L.K.; Ohmer, M.E.B.; LaBumbard, B.C.; Altman, K.A.; Brannelly, L.A.; Latella, I.; McDonnell, N.B.; Saenz, V.; Walsman, J.C.; et al. Diverse Relationships between Batrachochytrium Infections and Antimicrobial Peptide Defenses Across Leopard Frog Populations. Integr. Comp. Biol. 2024, 64, 921–931. [Google Scholar] [CrossRef]
- Melo-Dias, M.; Souza-Cruz, P.G.D.; Moreira, I.G.; Almeida Curi, N.H.D.; Carvalho, N.S.D.; Freitas, M.A.D.; Rosa, C. Invasive Amphibians and Reptiles Living in Brazil. S. Am. J. Herpetol. 2023, 29, 38–65. [Google Scholar] [CrossRef]
- De Oliveira Ferronato, B.; Hoefer, A.M.; Booksmythe, I.; Ubrihien, R.; Georges, A. City Dwellers: Habitat Connectivity and Demographic Responses of a Semi-Aquatic Turtle in Australia. Urban Ecosyst. 2024, 27, 2201–2212. [Google Scholar] [CrossRef]
- Belasen, A.M.; Amses, K.R.; Clemons, R.A.; Becker, C.G.; Toledo, L.F.; James, T.Y. Habitat Fragmentation in the Brazilian Atlantic Forest Is Associated with Erosion of Frog Immunogenetic Diversity and Increased Fungal Infections. Immunogenetics 2022, 74, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Belasen, A.M.; Bletz, M.C.; Leite, D.D.S.; Toledo, L.F.; James, T.Y. Long-Term Habitat Fragmentation Is Associated With Reduced MHC IIB Diversity and Increased Infections in Amphibian Hosts. Front. Ecol. Evol. 2019, 6, 236. [Google Scholar] [CrossRef]
- Mendelson, J.R.; Lips, K.R.; Gagliardo, R.W.; Rabb, G.B.; Collins, J.P.; Diffendorfer, J.E.; Daszak, P.; Ibáñez, D.R.; Zippel, K.C.; Lawson, D.P.; et al. Confronting Amphibian Declines and Extinctions. Science 2006, 313, 48. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Sillero, N. Climate Change in Action: Local Elevational Shifts on Iberian Amphibians and Reptiles. Reg. Environ. Chang. 2021, 21, 101. [Google Scholar] [CrossRef]
- Čeirāns, A.; Pupins, M.; Skute, A.; Nekrasova, O.; Kirjusina, M.; Combroux, I.; Grac, C.; Kvach, Y.; Van Der Zon, K.A.E.; Theissinger, K.; et al. Identification and Use of Suitable Metrics for Calling Male Count-Based Community Assessments in Amphibian Monitoring in Temperate Europe. Ecol. Indic. 2024, 168, 112771. [Google Scholar] [CrossRef]
- Kaunert, M.D.; Brown, R.K.; Spear, S.; Johantgen, P.B.; Popescu, V.D. Restoring Eastern Hellbender (Cryptobranchus a. alleganiensis) Populations through Translocation of Headstarted Individuals. Popul. Ecol. 2024, 66, 93–107. [Google Scholar] [CrossRef]
- Gururaja, K.V.; Dinesh, K.P.; Priti, H.; Ravikanth, G. Mud-Packing Frog: A Novel Breeding Behaviour and Parental Care in a Stream Dwelling New Species of Nyctibatrachus (Amphibia, Anura, Nyctibatrachidae). Zootaxa 2014, 3796, 33–61. [Google Scholar] [CrossRef]
- Silva, L.A.D.; Carvalho, P.S.; Pereira, E.A.; Fadel, R.M.; Dantas, S.P.; Brandão, R.A.; Santana, D.J. Richness, Diversity Patterns, and Taxonomic Notes of Amphibians from the Tocantins State. Biota Neotrop. 2020, 20, e20190838. [Google Scholar] [CrossRef]
- Arifin, U. Current Knowledge of Amphibian Diversity in Sumatra, and Its Significance for Conservation. Oryx 2024, 58, 462–467. [Google Scholar] [CrossRef]
- Measey, G.J.; Vimercati, G.; De Villiers, F.A.; Mokhatla, M.; Davies, S.J.; Thorp, C.J.; Rebelo, A.D.; Kumschick, S. A Global Assessment of Alien Amphibian Impacts in a Formal Framework. Divers. Distrib. 2016, 22, 970–981. [Google Scholar] [CrossRef]
- Valencia-Aguilar, A.; Cortés-Gómez, A.M.; Ruiz-Agudelo, C.A. Ecosystem Services Provided by Amphibians and Reptiles in Neotropical Ecosystems. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 257–272. [Google Scholar] [CrossRef]
- Hilje, B.; Chaves, G.; Klank, J.; Timmerman, F.; Feltham, J.; Gillingwater, S.; Piraino, T.; Rojas, E. Amphibians and Reptiles of the Tirimbina Biological Reserve: A Baseline for Conservation, Research and Environmental Education in a Lowland Tropical Wet Forest in Costa Rica. Check List 2020, 16, 1633–1655. [Google Scholar] [CrossRef]
- Ortega-Andrade, H.M.; Rodes Blanco, M.; Cisneros-Heredia, D.F.; Guerra Arévalo, N.; López De Vargas-Machuca, K.G.; Sánchez-Nivicela, J.C.; Armijos-Ojeda, D.; Cáceres Andrade, J.F.; Reyes-Puig, C.; Quezada Riera, A.B.; et al. Red List Assessment of Amphibian Species of Ecuador: A Multidimensional Approach for Their Conservation. PLoS ONE 2021, 16, e0251027. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Guerrero, P.A.; Graham, C.H. Evaluating Multiple Causes of Amphibian Declines of Ecuador Using Geographical Quantitative Analyses. Ecography 2013, 36, 756–769. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, H.; Zhang, L. Husbandry, Captive Breeding, and Field Survey of Chinese Giant Salamander (Andrias davidianus). In Salamanders; Seifert, A.W., Currie, J.D., Eds.; Springer US: New York, NY, USA, 2023; Volume 2562, pp. 75–92. ISBN 978-1-0716-2658-0 978-1-0716-2659-7. [Google Scholar]
- Wang, X.; Song, Y.; Li, J.; Liu, H.; Xu, X.; Lai, R.; Zhang, K. A New Family of Antimicrobial Peptides from Skin Secretions of Rana pleuraden. Peptides 2007, 28, 2069–2074. [Google Scholar] [CrossRef]
- Canestrelli, D.; Zampiglia, M.; Nascetti, G. Widespread Occurrence of Batrachochytrium dendrobatidis in Contemporary and Historical Samples of the Endangered Bombina pachypus along the Italian Peninsula. PLoS ONE 2013, 8, e63349. [Google Scholar] [CrossRef]
- Lau, Q.; Igawa, T.; Kosch, T.A.; Dharmayanthi, A.B.; Berger, L.; Skerratt, L.F.; Satta, Y. Conserved Evolution of MHC Supertypes among Japanese Frogs Suggests Selection for Bd Resistance. Animals 2023, 13, 2121. [Google Scholar] [CrossRef]
- Watabe, K.; Nakashima, N.; Koizumi, N. Conservation of Frog Habitats for Land Consolidation Projects in Paddy Field Areas. Ecol. Civ. Eng. 2021, 24, 95–110. [Google Scholar] [CrossRef]
- Mulcahy, D.G.; Lee, J.L.; Miller, A.H.; Chand, M.; Thura, M.K.; Zug, G.R. Filling the BINs of Life: Report of an Amphibian and Reptile Survey of the Tanintharyi (Tenasserim) Region of Myanmar, with DNA Barcode Data. Zookeys 2018, 757, 85–152. [Google Scholar] [CrossRef]
- Stuart, S.N.; Hoffmann, M.; Chanson, J.S.; Cox, N.A.; Berridge, R.J.; Ramani, P.; Young, B.E. (Eds.) Threatened Amphibians of the World; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2008; ISBN 9788496553415. [Google Scholar]
- Group, I.S.S.C.A.S.; Wren, S.; Borzée, A.; Marcec-Greaves, R.; Angulo, A. Amphibian Conservation Action Plan: A Status Review and Roadmap for Global Amphibian Conservation; IUCN SSC Occasional Paper No. 57; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2024; ISBN 978-2-8317-2279-5. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Corbera, E.; Reyes-García, V. Traditional Ecological Knowledge and Global Environmental Change: Research Findings and Policy Implications. Ecol. Soc. 2013, 18, art72. [Google Scholar] [CrossRef] [PubMed]
- Werdel, T.J.; Matarrita-Cascante, D.; Lucero, J.E. State of Traditional Ecological Knowledge in the Wildlife Management Profession. J. Wildl. Manage 2024, 88, e22579. [Google Scholar] [CrossRef]
- Sudlovenick, E.; Jenkins, E.; Loseto, L. Comparative Review of One Health and Indigenous Approaches to Wildlife Research in Inuit Nunangat. One Health 2024, 19, 100846. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.C.; Hilchey, K.G. A Review of Citizen Science and Community-Based Environmental Monitoring: Issues and Opportunities. Environ. Monit. Assess. 2011, 176, 273–291. [Google Scholar] [CrossRef]
- Chapman, J.M.; Algera, D.; Dick, M.; Hawkins, E.E.; Lawrence, M.J.; Lennox, R.J.; Rous, A.M.; Souliere, C.M.; Stemberger, H.L.J.; Struthers, D.P.; et al. Being Relevant: Practical Guidance for Early Career Researchers Interested in Solving Conservation Problems. Glob. Ecol. Conserv. 2015, 4, 334–348. [Google Scholar] [CrossRef]
- Pottier, P.; Kearney, M.R.; Wu, N.C.; Gunderson, A.R.; Rej, J.E.; Rivera-Villanueva, A.N.; Pollo, P.; Burke, S.; Drobniak, S.M.; Nakagawa, S. Vulnerability of Amphibians to Global Warming. Nature 2025, 639, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Blachnik, M.; Sołtysiak, M.; Dąbrowska, D. Predicting Presence of Amphibian Species Using Features Obtained from GIS and Satellite Images. ISPRS Int. J. Geo-Inf 2019, 8, 123. [Google Scholar] [CrossRef]
- Sahlean, T.C.; Papeș, M.; Strugariu, A.; Gherghel, I. Ecological Corridors for the Amphibians and Reptiles in the Natura 2000 Sites of Romania. Sci. Rep. 2020, 10, 19464. [Google Scholar] [CrossRef] [PubMed]
| Main Compound | Compound Name | Amphibian Species | Origin | Medical Use | Mechanism/Activity | Reference |
|---|---|---|---|---|---|---|
| Alkaloid | Zetekitoxin-AB | Atelopus zeteki | Panama | Antibacterial | MIC 10 µg/mL against E. coli | [105] |
| Bufotenine | Bufo gargarizans | China | Antitumor | Inhibits cell proliferation and induces apoptosis | [131] | |
| Pyrrolizidine | Melanophryniscus spp. | Argentina, Brazil, Paraguay, Uruguay | Antibacterial | MIC 3.9 µg/mL against S. aureus, and 31.3 µg/mL against E. coli | [132] | |
| Peptide | Bombesin-LS | Bombina orientalis | Korea | Hormonal | Stimulates release of gastrin | [133] |
| RP-Bombesin | Boana raniceps | Argentina | Antibacterial, Hormonal | MIC 25 µg/mL (S. aureus), stimulates gastrin | [106] | |
| Brevinin-1 | Lithobates pipiens | Canada | Antibacterial, Antidiabetic | MIC 26 µg/mL (E. coli), stimulates insulin | [126] | |
| Brevinin-2GU | Hylarana guntheri | China | Immunomodulatory | Reduces TNF-α, suppresses IFN-γ | [111] | |
| Magainin-1 | Xenopus laevis | Africa | Antibacterial | MIC 5 µg/mL (E. coli), 50 µg/mL (S. aureus) | [120] | |
| Magainin A | Bombina maxima | China | Anticancer, Antiviral | Inhibits tumor growth, antiviral properties | [112,113] | |
| Cruzioseptin-16 | Cruziohyla calcarifer | Costa Rica | Antibacterial | Resistant to multiple bacteria | [114] | |
| Esculentin-1a | Lithobates areolatus | USA | Antibacterial, Wound Healing | Stimulates keratinocyte migration | [115] | |
| Ocellatin-4 | Leptodactylus spp. | South America | Antibacterial | MIC 64 µM (E. coli, S. aureus) | [116] | |
| Proline-arginine (RP-27) | Rhinophrynus dorsalis | Mexico | Antidiabetic | Stimulates insulin secretion | [107] | |
| Syphaxin | Leptodactylus syphax | Brazil | Antibacterial | Low MICs against bacteria | [108] | |
| Andersonin-C1 | Odorrana margaretae | China | Antioxidant | Scavenges ROS via amphipathic helix formation | [101] | |
| Andersonin-G1 | Odorrana andersonii | China | Antioxidant | Inhibits lipid peroxidation and chelates metals | [101] | |
| Andersonin-H3 | Odorrana margaretae | China | Antioxidant | Radical scavenging, sequence | [101] | |
| Antioxidin-RP1 | Rana pleuraden | China | Antioxidant | Scavenges ABTS, DPPH radicals | [109] | |
| APBSP | Lithobates catesbeianus | USA | Antioxidant | Quenches DPPH, hydroxyl, superoxide radicals | [130] | |
| Cathelicid-OA1 | Odorrana andersonii | China | Antioxidant, Wound healing | Boosts catalase, GSH; accelerates skin healing | [124] | |
| Nigroain-B-MS1 | Hylarana maosuoensis | China | Antioxidant | High ABTS and DPPH scavenging | [110] | |
| OA-VII2 | Odorrana andersonii | China | Antioxidant | Enhances CAT, SOD in vivo | [101,134] | |
| OA-GL21 | Odorrana andersonii | China | Antioxidant | ABTS scavenging activity | [101] | |
| Pleurain-A1 | Rana pleuraden | China | Antioxidant | ABTS, NO scavenging | [101,109] | |
| Buforin-2 | Bufo bufo gargarizans | Unknown | Antimicrobial | Broad spectrum activity incl. fungi | [135] | |
| Dermaseptin-S1 | Phyllomedusa sauvagii | South America | Antimicrobial | Binds and disrupts membranes | [136] | |
| Esculentin-1 | Rana esculenta | Europe | Antimicrobial, Antidiabetic | Broad MIC, insulin-stimulating | [127] | |
| Hymenochirin-1B | Hymenochirus boettgeri | Africa | Antidiabetic, Anticancer | Insulin release and tumor inhibition | [127,137] | |
| Dermaseptin-PP | Phyllomedusa palliata | South America | Anticancer | Induces apoptosis in tumor cells | [138,139] | |
| XLAsp-P1 | Xenopus laevis | Africa | Anticancer | Membrane disruption of cancer cells | [128] | |
| Phylloseptin-L2 | Hylomantis lemur | Costa Rica | Antidiabetic | Stimulates insulin secretion | [117,127] | |
| Plasticin-L1 | Leptodactylus laticeps | South America | Antidiabetic | Enhances insulin release | [118,127] | |
| Pseudhymenochirin-1Pb | Pseudhymenochirus merlini | Africa | Antidiabetic, Anticancer | Multiple bioactivities | [140,141] | |
| Alyteserin-2a | Alytes obstetricans | Europe | Antidiabetic | Increases insulin release | [142] | |
| Amolopin | Amolops loloensis | Unknown | Antidiabetic | Enhances insulin release in vitro | [127] | |
| Bombesin (protein fractions) | Bombina variegata | Europe | Antidiabetic | Stimulates insulin secretion | [127,129] | |
| Brevinin-1CBb | Lithobates septentrionalis | Canada | Antidiabetic | Increases insulin secretion, low cytotoxicity | [143] | |
| Caerulein-B1 | Xenopus borealis | Unknown | Antidiabetic | In vitro insulinotropic peptide | [127,144] | |
| CPF-AM1 | Xenopus amieti | Africa | Antidiabetic | Stimulates GLP-1 release | [145] | |
| Dermaseptin B4 | Phyllomedusa trinitatis | South America | Antidiabetic | Unknown insulin-stimulating mechanism | [146,147] | |
| Esculentin-2Cha | Lithobates chiricahuensis | USA | Antidiabetic | GLP-1 activity, plasma insulin increase | [148] | |
| GM-14 | Bombina variegata | Europe | Antidiabetic | Stimulates insulin secretion | [127,129] | |
| IN-21 | Bombina variegata | Europe | Antidiabetic | Insulin-releasing bioactive peptide | [127,129] | |
| GPPGPA | Andrias davidianus | China | Antidiabetic | α-glucosidase inhibitor | [149] | |
| Magainin-AM1 | Xenopus amieti | Nigieria, Africa | Antidiabetic | GLP-1 release, antimicrobial | [145,150] | |
| Ocellatin-L2 | Leptodactylus laticeps | South America | Antidiabetic | Boosts insulin output | [118,151] | |
| Palustrin-2CBa | Lithobates catesbeianus | USA | Antidiabetic | Insulin secretion enhancer | [143] | |
| PGLa-AM1 | Xenopus amieti | Africa | Antidiabetic | GLP-1 enhancement | [145,152] | |
| Ranateurin-2CBc | Lithobates catesbeianus | USA | Antidiabetic | Increases insulin release | [143] | |
| Temporin-DRa | Rana draytonii | USA | Antidiabetic | Low toxicity, insulinotropic | [153] | |
| Temporin-Oe | Rana ornativentris | Unknown | Antidiabetic | Moderate insulin stimulation | [153] | |
| Tigerinin-1R | Rana tigerina | Unknown | Antidiabetic | Regulates glucose metabolism | [145,154] | |
| Xenopsin | Xenopus amieti | Africa | Antidiabetic | Insulin-releasing short peptide | [145,152] | |
| AH90 | Odorrana grahami | Unknown | Wound Healing | Stimulates TGF-β1 in healing | [155] | |
| Temporin-1RNb | Rana nigromaculata | East Asia | Antimicrobial | Inhibits multiple pathogens | [135,156] | |
| Nigrocin-1 | Rana nigromaculata | East Asia | Antimicrobial | Effective against Gram-negative bacteria | [135] | |
| Nigrocin-2 | Rana nigromaculata | East Asia | Antimicrobial | Broad antimicrobial spectrum | [135] | |
| Maximin-H5 | Bombina maxima | Asian | Antimicrobial | Inhibits S. aureus growth | [106,157] | |
| Japonicin-2 | Rana chaochiaoensis | China | Antimicrobial | Effective against S. aureus and E. coli | [158] | |
| Dermaseptin-B3 | Phyllomedusa bicolor | South America | Anticancer | Inhibits PC-3 prostate tumor cells | [147,159] | |
| Temporin-1CEa | Rana chensinensis | China | Anticancer | Selective cytotoxicity in cancer cells | [160] | |
| Temporin-Vc | Lithobates virgatipes | USA | Antidiabetic | High insulin-releasing activity | [127,153] | |
| RK-13 | Agalychnis calcarifer | Costa Rica | Antidiabetic | Short insulinotropic peptide | [161] |
| Region/Country | Conservation Strategy/Program | Target Species | Effectiveness/Outcome | Reference |
|---|---|---|---|---|
| Central America | Amphibian Ark/Captive Breeding | Atelopus spp., Craugastor spp. | reintroductions limited by habitat threats | [2,244] |
| Australia | Disease management (chytrid fungus) | Litoria spp., Taudactylus spp. | ex situ programs | [245] |
| Europe | Natura 2000/EU Habitats Directive | Bombina bombina, Alytes spp. | Enhanced protection of critical breeding habitats | [246,247] |
| USA | Headstart and release programs | Cryptobranchus a. alleganiensis | Viable strategy for rebuilding eastern hellbender populations | [248] |
| India | Community education & monitoring programs | Nyctibatrachus spp., Indirana spp. | Improved local awareness; limited enforcement capacity | [1,249] |
| Brazil | Protected area expansion (Amazon reserves) | Leptodactylus spp., Dendrobates spp. | Positive buffer against deforestation-related declines | [67,250] |
| Colombia | Red List and priority site mapping | Andean stream frogs | identify hotspots for focused conservation | [67,251] |
| South Africa | Biodiversity stewardship programs | Breviceps spp., Hyperolius spp. | Enhanced private land protection; requires long-term funding | [252] |
| Costa Rica | Environmental education + long-term monitoring | Craugastor spp., Hyalinobatrachium spp. | Improved data collection; increased local conservation action | [253] |
| France | Reintroduction of Bombina variegata | Bombina variegata | Successful site-level reestablishment; requires continuous monitoring | [254] |
| Ecuador | Yasuní National Park amphibian inventories | Pristimantis spp., Dendrobatidae | High biodiversity documentation; improved policy influence | [255,256] |
| China | Habitat protection and legislation (e.g., CITES enforcement) | Rana chensinensis, Andrias davidianus | Controlled trade and supported captive breeding | [257,258] |
| Italy | Genetic monitoring and relocation programs | Salamandra atraaurorae | Maintained genetic diversity in isolated populations | [259] |
| Japan | Wetland reserves for endemic frogs | Glandirana rugosa, Buergeria spp. | Enhanced breeding site protection | [260,261] |
| Thailand | Integrated conservation and development | Kalophrynus spp., Leptolalax spp. | Community acceptance increased with benefit-sharing | [262] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wickramasingha, B.; West, J.; Bellanthudawa, B.K.A.; Graziano, M.P.; Sursinghe, T.D. The Multifaceted Importance of Amphibians: Ecological, Biomedical, and Socio-Economic Perspectives. Biology 2026, 15, 98. https://doi.org/10.3390/biology15010098
Wickramasingha B, West J, Bellanthudawa BKA, Graziano MP, Sursinghe TD. The Multifaceted Importance of Amphibians: Ecological, Biomedical, and Socio-Economic Perspectives. Biology. 2026; 15(1):98. https://doi.org/10.3390/biology15010098
Chicago/Turabian StyleWickramasingha, Buddhika, Josh West, Bellanthudawage Kushan Aravinda Bellanthudawa, Michael P. Graziano, and Thilina D. Sursinghe. 2026. "The Multifaceted Importance of Amphibians: Ecological, Biomedical, and Socio-Economic Perspectives" Biology 15, no. 1: 98. https://doi.org/10.3390/biology15010098
APA StyleWickramasingha, B., West, J., Bellanthudawa, B. K. A., Graziano, M. P., & Sursinghe, T. D. (2026). The Multifaceted Importance of Amphibians: Ecological, Biomedical, and Socio-Economic Perspectives. Biology, 15(1), 98. https://doi.org/10.3390/biology15010098








