Hyperglycemia Modulates the Expression of MAPK13, TSP1, and CXCR2 During Wound Healing in Sprague Dawley Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Processing
2.2. Hematoxylin and Eosin Staining
2.3. Trichrome Staining
2.4. Quantitative Real-Time Polymerase Chain Reaction
2.5. Immunohistochemistry
2.6. Cell Culture and In Vitro Studies
2.7. Statistical Analysis
3. Results
3.1. Histology Revealed Scar Tissue Formation and Decreased Collagen After Healing
3.2. RT-qPCR
3.3. Hyperglycemia Increases the Expression of MAPK13, TSP1, and CXCR2
3.4. Hyperglycemia Regulates Protein Expression of MAPK13, TSP1, and CXCR2
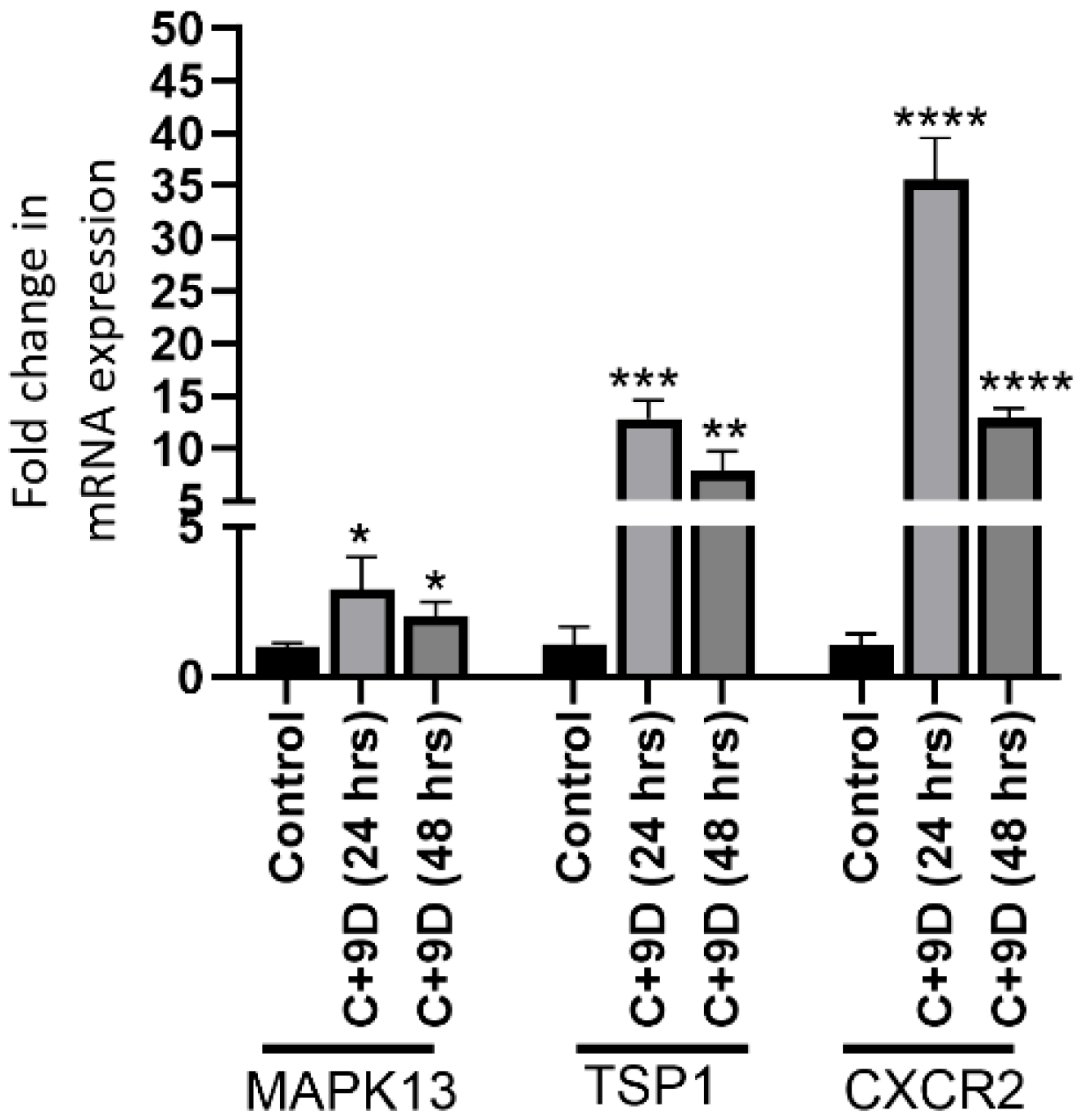
3.5. Hyperglycemia Significantly Increases Gene Expression of MAPK13, TSP1, and CXCR2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-1 | Activating protein 1 |
| CXCR2 | C-X-C chemokine receptor 2 |
| DFUs | Diabetic foot ulcers |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ECM | Extracellular matrix |
| ERKs | Extracellular signal-regulated kinases |
| IL | Interleukin |
| JNKs | c-Jun N-terminal kinases |
| MAPK | Mitogen-activated protein kinase |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| TSP1 | Thrombospondin 1 |
| TGF-β1 | Transforming growth factor beta 1 |
References
- Okorigba, E.M.; Akinade, O.; Emore, E.; Adebayo, A.A.; Aghale, O.S.; Ekarika, E.; Olawale, O.O.; Siaw, T.K.; Azipu, R.U.; Okobi, O.E. Prevalence and Hospitalization Trends of Cardiovascular Disease in Diabetic Populations: An Analysis of the United States Diabetes Surveillance System (USDSS) Data From 2000 to 2022. Cureus 2025, 17, e87886. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Elafros, M.A.; Andersen, H.; Bennett, D.L.; Savelieff, M.G.; Viswanathan, V.; Callaghan, B.C.; Feldman, E.L. Towards prevention of diabetic peripheral neuropathy: Clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022, 21, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Yen, Y.H.; Hung, Y.M.; Wei, J.C.; Tsai, F.J.; Hung, Y.T.; Lin, H.J.; Hwu, C.M.; Hsu, C.C. Diabetic Microvascular Disease and Risk of Peripheral Artery Disease, Foot Ulcer, Leg Infection, and Amputation. Thromb. Haemost. 2025. [Google Scholar] [CrossRef]
- Bowling, F.L.; Rashid, S.T.; Boulton, A.J. Preventing and treating foot complications associated with diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 606–616. [Google Scholar] [CrossRef]
- Dinh, T.; Tecilazich, F.; Kafanas, A.; Doupis, J.; Gnardellis, C.; Leal, E.; Tellechea, A.; Pradhan, L.; Lyons, T.E.; Giurini, J.M.; et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012, 61, 2937–2947. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. The role of CXCL8 in chronic nonhealing diabetic foot ulcers and phenotypic changes in fibroblasts: A molecular perspective. Mol. Biol. Rep. 2022, 49, 1565–1572. [Google Scholar] [CrossRef]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Role of fibroblast plasticity and heterogeneity in modulating angiogenesis and healing in the diabetic foot ulcer. Mol. Biol. Rep. 2023, 50, 1913–1929. [Google Scholar] [CrossRef]
- Rai, V.; Le, H.; Agrawal, D.K. Novel mediators regulating angiogenesis in diabetic foot ulcer healing. Can. J. Physiol. Pharmacol. 2023, 101, 488–501. [Google Scholar] [CrossRef]
- Shofler, D.; Rai, V.; Mansager, S.; Cramer, K.; Agrawal, D.K. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Expert Rev. Clin. Immunol. 2021, 17, 681–690. [Google Scholar] [CrossRef]
- Fernandez-Guarino, M.; Hernandez-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Vasalou, V.; Kotidis, E.; Tatsis, D.; Boulogeorgou, K.; Grivas, I.; Koliakos, G.; Cheva, A.; Ioannidis, O.; Tsingotjidou, A.; Angelopoulos, S. The Effects of Tissue Healing Factors in Wound Repair Involving Absorbable Meshes: A Narrative Review. J. Clin. Med. 2023, 12, 5683. [Google Scholar] [CrossRef]
- Strukel, S.; Rai, V. Malignant Transformation of Diabetic Foot Ulcer: Pathophysiology, Molecular Mechanisms, and Clinical Implications. Biocell 2025, 49, 1887. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Keshet, Y.; Seger, R. The MAP kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol. Biol. 2010, 661, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Raza, A.; Crothers, J.W.; McGill, M.M.; Mawe, G.M.; Teuscher, C.; Krementsov, D.N. Anti-inflammatory roles of p38alpha MAPK in macrophages are context dependent and require IL-10. J. Leukoc. Biol. 2017, 102, 1219–1227. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Xing, B.; Van Eldik, L.J. The p38alpha mitogen-activated protein kinase limits the CNS proinflammatory cytokine response to systemic lipopolysaccharide, potentially through an IL-10 dependent mechanism. J. Neuroinflamm. 2014, 11, 175. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Fanning, L.J.; Barry, O.P. p38delta MAPK: Emerging Roles of a Neglected Isoform. Int. J. Cell Biol. 2014, 2014, 272689. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Anshu, T.; Maharana, K.C.; Sinha, S. Molecular insights into diabetic wound healing: Focus on Wnt/beta-catenin and MAPK/ERK signaling pathways. Cytokine 2025, 191, 156957. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Abdul Hamid, Z.; Mohamad Anuar, N.N.; Jalil, J.; Mohd Nor, N.A.; Budin, S.B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582. [Google Scholar] [CrossRef] [PubMed]
- Risco, A.; del Fresno, C.; Mambol, A.; Alsina-Beauchamp, D.; MacKenzie, K.F.; Yang, H.T.; Barber, D.F.; Morcelle, C.; Arthur, J.S.; Ley, S.C.; et al. p38gamma and p38delta kinases regulate the Toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc. Natl. Acad. Sci. USA 2012, 109, 11200–11205. [Google Scholar] [CrossRef]
- Gutierrez, L.S.; Gutierrez, J. Thrombospondin 1 in Metabolic Diseases. Front. Endocrinol. 2021, 12, 638536. [Google Scholar] [CrossRef]
- Kim, C.W.; Pokutta-Paskaleva, A.; Kumar, S.; Timmins, L.H.; Morris, A.D.; Kang, D.W.; Dalal, S.; Chadid, T.; Kuo, K.M.; Raykin, J.; et al. Disturbed Flow Promotes Arterial Stiffening Through Thrombospondin-1. Circulation 2017, 136, 1217–1232. [Google Scholar] [CrossRef]
- Bitar, M.S. Diabetes Impairs Angiogenesis and Induces Endothelial Cell Senescence by Up-Regulating Thrombospondin-CD47-Dependent Signaling. Int. J. Mol. Sci. 2019, 20, 673. [Google Scholar] [CrossRef]
- Maier, K.G.; Han, X.; Sadowitz, B.; Gentile, K.L.; Middleton, F.A.; Gahtan, V. Thrombospondin-1: A proatherosclerotic protein augmented by hyperglycemia. J. Vasc. Surg. 2010, 51, 1238–1247. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Chen, J.; Xu, Y. Thrombospondin-1: A Key Protein That Induces Fibrosis in Diabetic Complications. J. Diabetes Res. 2020, 2020, 8043135. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Lawler, J. The interaction of Thrombospondins with extracellular matrix proteins. J. Cell Commun. Signal. 2009, 3, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Delobel, P.; Ginter, B.; Rubio, E.; Balabanian, K.; Lazennec, G. CXCR2 intrinsically drives the maturation and function of neutrophils in mice. Front. Immunol. 2022, 13, 1005551. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Natsi, A.M.; Gavriilidis, E.; Antoniadou, C.; Eleftheriadou, I.; Anastasiou, I.A.; Tentolouris, A.; Papadimitriou, E.; Eftalitsidis, E.; Kolovos, P.; et al. Interleukin-8/Matrix Metalloproteinase-9 Axis Impairs Wound Healing in Type 2 Diabetes through Neutrophil Extracellular Traps-Fibroblast Crosstalk. Eur. J. Immunol. 2025, 55, e202451664. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Malicevic, U.; Smith, J.; Agrawal, D.K.; Rai, V. Sex-based differences in streptozotocin-induced type 2 diabetes rat models. J. Clin. Transl. Res. 2025, 11, 20–28. [Google Scholar] [CrossRef]
- Song, J.; Zhao, T.; Wang, C.; Sun, X.; Sun, J.; Zhang, Z. Cell migration in diabetic wound healing: Molecular mechanisms and therapeutic strategies (Review). Int. J. Mol. Med. 2025, 56, 126. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, C.; Bai, J.; Yang, J.; Chen, L.; Yu, Q.; Wen, X.; Yang, Y. Selenomethionine activates MAPK signaling to activate epidermal keratinocyte migration, speeding skin wound healing. Biochem. Biophys. Res. Commun. 2025, 778, 152398. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.; Mao, D.; Iberg, C.A.; Yin-Declue, H.; Sun, K.; Keeler, S.P.; Wikfors, H.A.; Young, D.; Yantis, J.; et al. MAPK13 controls structural remodeling and disease after epithelial injury. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhong, H.; Liu, W.; Wang, Y.; Wang, Y.; Wang, L.; Tang, S.; Zhu, H. Melatonin Alleviates Hyperglycemia-Induced Cardiomyocyte Apoptosis via Regulation of Long Non-Coding RNA H19/miR-29c/MAPK Axis in Diabetic Cardiomyopathy. Pharmaceuticals 2022, 15, 821. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Coderre, L.; Allen, B.G.; Des Rosiers, C. Protecting the heart through MK2 modulation, toward a role in diabetic cardiomyopathy and lipid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yang, X.; Ji, Z.; Li, X.; Tao, Z.; Luo, W.; Yao, Y.; Chen, L.; Ma, G. Update on the Impact of Lipid and Glucose Control on Diabetic Wound Healing. Metab. Open 2025, 28, 100408. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. Cellular senescence controls fibrosis in wound healing. Aging 2010, 2, 627–631. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef]
- Lan, C.C.; Huang, S.M.; Wu, C.S.; Wu, C.H.; Chen, G.S. High-glucose environment increased thrombospondin-1 expression in keratinocytes via DNA hypomethylation. Transl. Res. 2016, 169, 91–101.e3. [Google Scholar] [CrossRef]
- Sweetwyne, M.T.; Murphy-Ullrich, J.E. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012, 31, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Sumi, Y.; Muramatsu, H.; Hata, K.; Muramatsu, T.; Ueda, M. Thrombospondin-1 promotes fibroblast-mediated collagen gel contraction caused by activation of latent transforming growth factor beta-1. J. Dermatol. Sci. 2003, 31, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 CD47 Signalling: From Mechanisms to Medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.S.; Raya-Sandino, A.; Miranda, J.; Fan, S.; Brazil, J.C.; Quiros, M.; Garcia-Hernandez, V.; Liu, Q.; Kim, C.H.; Hankenson, K.D.; et al. Critical role of thrombospondin-1 in promoting intestinal mucosal wound repair. JCI Insight 2024, 9, e180608. [Google Scholar] [CrossRef]
- Streit, M.; Velasco, P.; Riccardi, L.; Spencer, L.; Brown, L.F.; Janes, L.; Lange-Asschenfeldt, B.; Yano, K.; Hawighorst, T.; Iruela-Arispe, L.; et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000, 19, 3272–3282. [Google Scholar] [CrossRef]
- Theocharidis, G.; Thomas, B.E.; Sarkar, D.; Mumme, H.L.; Pilcher, W.J.R.; Dwivedi, B.; Sandoval-Schaefer, T.; Sirbulescu, R.F.; Kafanas, A.; Mezghani, I.; et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat. Commun. 2022, 13, 181. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Takedachi, M.; Mori, K.; Kubota, M.; Yamada, S.; Kitamura, M.; Murakami, S. High glucose-induced oxidative stress increases IL-8 production in human gingival epithelial cells. Oral Dis. 2016, 22, 578–584. [Google Scholar] [CrossRef]
- Kinoshita, K.; Tanjoh, K.; Noda, A.; Sakurai, A.; Yamaguchi, J.; Azuhata, T.; Utagawa, A.; Moriya, T. Interleukin-8 production from human umbilical vein endothelial cells during brief hyperglycemia: The effect of tumor necrotic factor-alpha. J. Surg. Res. 2008, 144, 127–131. [Google Scholar] [CrossRef]
- Escuin-Ordinas, H.; Li, S.; Xie, M.W.; Sun, L.; Hugo, W.; Huang, R.R.; Jiao, J.; de-Faria, F.M.; Realegeno, S.; Krystofinski, P.; et al. Cutaneous wound healing through paradoxical MAPK activation by BRAF inhibitors. Nat. Commun. 2016, 7, 12348. [Google Scholar] [CrossRef]
- Lai, Y.H.; Lee, P.Y.; Lu, C.Y.; Liu, Y.R.; Wang, S.C.; Liu, C.C.; Chang, Y.C.; Chen, Y.H.; Su, C.C.; Li, C.Y.; et al. Thrombospondin 1-induced exosomal proteins attenuate hypoxia-induced paraptosis in corneal epithelial cells and promote wound healing. FASEB J. 2021, 35, e21200. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Shih, H.B.; Maxhimer, J.B.; Cook, K.L.; Ghosh, A.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol. 2014, 37, 25–34. [Google Scholar] [CrossRef]




| Forward Primer (5′—3′) | Reverse Primer (5′—3′) | |
|---|---|---|
| MAPK13 | GAATGACTAGGGTCTGCTTCTG | GCCTGGGCTACATGTGAATA |
| TSP-1 | CCTCGTCACATTGGCTGGAA | CTTGAGTCTGGCCTACGGTTTT |
| CXCR2 | CTGCTGGCTTCCCTACAACA | ATCTCGTTCTGGCGTTCACA |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tran, T.; Patel, J.; Ho, V.; Teshome, B.; Rai, V. Hyperglycemia Modulates the Expression of MAPK13, TSP1, and CXCR2 During Wound Healing in Sprague Dawley Rats. Biology 2026, 15, 26. https://doi.org/10.3390/biology15010026
Tran T, Patel J, Ho V, Teshome B, Rai V. Hyperglycemia Modulates the Expression of MAPK13, TSP1, and CXCR2 During Wound Healing in Sprague Dawley Rats. Biology. 2026; 15(1):26. https://doi.org/10.3390/biology15010026
Chicago/Turabian StyleTran, Tommy, Jaylan Patel, Vy Ho, Betelhem Teshome, and Vikrant Rai. 2026. "Hyperglycemia Modulates the Expression of MAPK13, TSP1, and CXCR2 During Wound Healing in Sprague Dawley Rats" Biology 15, no. 1: 26. https://doi.org/10.3390/biology15010026
APA StyleTran, T., Patel, J., Ho, V., Teshome, B., & Rai, V. (2026). Hyperglycemia Modulates the Expression of MAPK13, TSP1, and CXCR2 During Wound Healing in Sprague Dawley Rats. Biology, 15(1), 26. https://doi.org/10.3390/biology15010026







