Mathematical Modeling of Population Dynamics of Pollinators: A Survey
Simple Summary
Abstract
1. Introduction
2. Methodological Framework
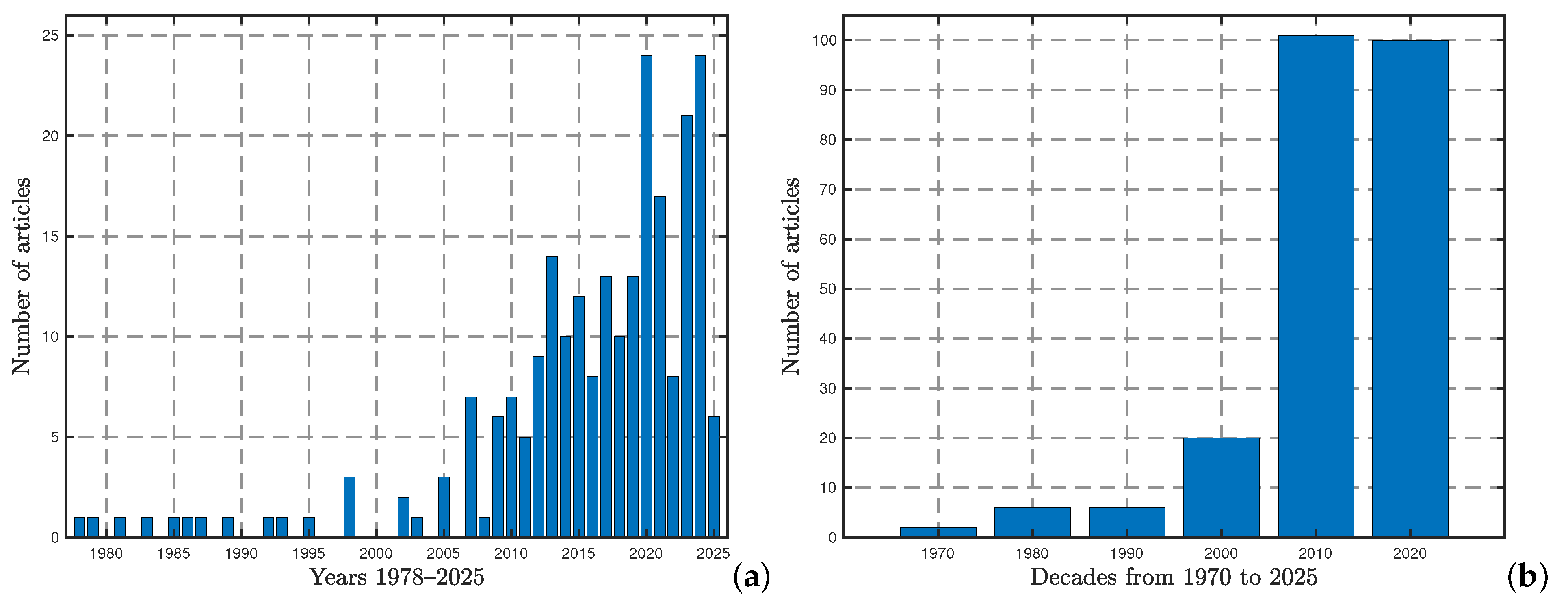
2.1. Framing Questions for a Review and Identifying Relevant Work
- Question 1: What are the studies developed for mathematical modeling of the pollinator population’s dynamics?
- Question 2: What types of modeling approaches were used in those studies?
- Meanwhile, related to the step of identification of the relevant work, we selected two databases, MathSciNet and the Web of Science (WoS), with the following details:
- -
- MathSciNet (https://mathscinet.ams.org/mathscinet/, accessed on 14 April 2025): We searched for the word “pollinator” using the option “search term: anywhere” and found that the response reported a total of 71 items: 69 journal articles and 2 PhD theses.
- -
- WoS (https://www.webofscience.com/, accessed on 14 April 2025). We used the option “all fields” for the platform’s search engine to search for the word “pollinator”, obtaining 26,938 items. Then, by using the keyword “mathematical model” in the option “refine results”, we found 199 items: 198 journal articles and 1 book chapter.
2.2. Assessing the Quality of the Studies
- -
- Brazil (45) and Australia (35) with 4% each;
- -
- India (29), Japan (29), Canada (28), France (28), Mexico (28), the Czech Republic (26), and Spain (25) with 3% each;
- -
- Brazil (22), Hungary (21), and Serbia (16) with 2% each;
- -
- Italy (14), South Africa (13), Denmark (11), Israel (10), Sweden (10), Taiwan (10), the Netherlands (9), New Zealand (9), Chile (8), Russia (8), Norway (7), Argentina (6), and Belgium (5) with 1% each;
- -
- Bulgaria (4), Finland (4), Poland (4), the Republic of Korea (4), Switzerland (4), the Philippines (3), Slovakia (3), Greece (2), Kenya (2), Estonia (2), Ecuador (1), Indonesia (1), Ireland (1), Pakistan (1), Portugal (1), Saudi Arabia (1), Slovenia (1), and Thailand (1) with 0% each.
3. Summarizing the Evidence and Interpreting the Findings
- (1)
- Ordinary differential equations group (see Section 3.1): Here, we distinguish between three types of models based on the methodology used for modeling. First, we considered the Lotka–Volterra models for two populations (see Section 3.1.3). The works of this type are [10,11,15,22,28,30,33,47,55,68,73,76,86,88,91,96,104,106]. The second type was the Lotka–Volterra models for more than two populations (see Section 3.1.2), with the articles being [15,16,19,24,28,30,33,34,37,42,44,45,46,48,51,62,63,64,67,70,71,74,75,78,79,81,87,100,107,114,115]. The third class of ordinary differential equations systems was obtained via application of the compartmental methodology (see Section 3.1.3), and the works of this type are [14,36,38,39,41,61,83,85,89,90,93,98,105,110].
- (2)
- (3)
- (4)
3.1. Mathematical Models Based on Ordinary Differential Equations
3.1.1. Two Population Mathematical Models Using Lotka–Volterra-Like Methodologies
3.1.2. More Than Two Population Mathematical Models Using Lotka–Volterra-Like Methodologies
3.1.3. Mathematical Models Based on Compartmental Methodology
3.2. Mathematical Models Based on Partial Differential Equations
3.3. Network and Patch Mathematical Models
3.4. Other Methodologies
3.5. A Summary of the Topics Studied in the Retained List
- (1)
- Positive bounded solutions: The variables of the mathematical models are the population or the density of the population. Then, the first question of the consistence of the mathematical model with the biological system is for analyzing if the mathematical model’s solutions are positive and bounded. In this sense, the following works [15,19,20,26,28,30,41,50,54,59,65,66,71,75,78,93,102] have explicit results proving that the dynamics of the mathematical systems have positive bounded solutions.
- (2)
- Equilibrium and stability analysis: In the mathematical analysis of dynamical systems, the study of linearization and asymptotic behavior is strongly related to the analysis of stability analysis. In particular, mathematical models are an important tool for characterizing the large time behavior of the system and answering other important questions, like the prevalence or extinction a species of pollinator. The works focused on the development of equilibrium and stability analysis are the following [10,11,12,14,15,19,20,22,26,33,34,36,39,43,44,45,46,47,48,54,55,57,60,61,63,67,68,70,71,73,74,75,76,78,79,81,84,86,90,91,92,93,95,96,98,100,102,103,104,107,111,113,114].
- (3)
- (4)
- Mutualistic interactions: In the case of mathematical models based on networks and patch concepts, there are several topics which have been researched, including coexistence [13,16,18,21,24,25,28,30,33,35,37,39,40,43,44,45,46,51,52,53,54,62,64,65,68,69,70,71,72,80,81,82,92,96,97,99,100,101,103,108], dissipation [28,33,34,48,74,78,94], and eco-evolution [59,67,68,94].
- (5)
- Periodicity of the solution: An interesting question for pollinators strongly related with seasonality is what the periodicity behavior of the populations of the different variables involved in pollination models is. Indeed, the following topics have been researched: periodic orbits [30,47,65,93], non-periodic orbits [28,45,48,65,74], and oscillation [30,53,63].
- (6)
- Numerical solutions and comparison with empirical data: The mathematical models are strongly nonlinear, and the analytical solution cannot be construed. Consequently, numerical solutions of the mathematical models are introduced in order to simulate and calibrate the mathematical models. In the retained list, the authors of [19,25,26,31,32,35,36,37,42,53,56,57,58,60,61,66,67,68,69,75,78,84,85,87,88,89,90,92,94,96,98,99,100,101,103,104,105,106,108,110,111,113,115,116] developed numerical simulations.
- (7)
4. Biological and Applied Problem Typologies in the Retained Literature
- (1)
- Biological consistency and population viability: In this group, we consider the works addressing biological realism in population dynamics and focus on the research of positive bounded solutions. Models in this category ensure that population variables remain biologically meaningful, i.e., non-negative and bounded over time. This foundational consistency is critical for validating ecological interpretations and avoiding spurious predictions. Parametrization typically involves biologically constrained initial conditions and growth functions (e.g., logistic or saturating terms). These models support policy decisions related to conservation thresholds and population viability. Representative works include [15,19,20,26,28,30,41,50,54,59,65,66,71,75,78,93,102].
- (2)
- Long-term dynamics and species persistence: In this group, the problems to study are prevalence, extinction, and asymptotic behavior. These studies examine the conditions under which pollinator populations persist or collapse, often through linearization techniques and Lyapunov-based stability criteria. Parametrization emphasizes sensitivity to reproductive rates, mortality, and interaction coefficients. The results inform long-term sustainability planning and resilience forecasting. Representative works include [10,11,12,14,15,19,20,22,26,33,34,36,39,43,44,45,46,47,48,54,55,57,60,61,63,67,68,70,71,73,74,75,76,78,79,81,84,86,90,91,92,93,95,96,98,100,102,103,104,107,111,113,114].
- (3)
- Regime shifts and critical transitions: In this group of works, the authors focus on the bifurcation analysis and address the study of threshold phenomena and qualitative change. Bifurcation studies identify parameter regimes where small changes induce qualitative shifts in system behavior, such as transitions from coexistence to extinction. These models often employ continuation methods and bifurcation diagrams to explore critical thresholds, with implications for adaptive management and early warning indicators. Representative works include [16,30,53,67,74,94,103,106,107,114].
- (4)
- Mutualism and network structure: In this group, the focus is mutualistic interactions and the study of phenomena like coexistence, dissipation, and eco-evolutionary dynamics. These models incorporate spatial structure, network topology, and evolutionary feedback to explore how mutualistic systems maintain biodiversity. Parametrization includes patch-based connectivity, trait evolution, and interaction matrices. The findings support the design of pollinator corridors, agroecological zoning, and biodiversity incentives. Representative works include [13,16,18,21,24,25,28,30,33,35,37,39,40,43,44,45,46,51,52,53,54,62,64,65,68,69,70,71,72,80,81,82,92,96,97,99,100,101,103,108], as well as dissipation-focused studies [28,33,34,48,74,78,94], and eco-evolutionary dynamics studies [59,67,68,94].
- (5)
- Seasonal and oscillatory behavior: The addressed problem is the temporal variability and seasonality, along with the study of periodicity and oscillations in model solutions. Models in this group address how seasonal forcing and intrinsic dynamics lead to periodic or chaotic population fluctuations. Parametrization incorporates time-dependent coefficients and delay terms. These insights guide seasonal pollination services, crop planning, and phenological synchronization. Representative works include [28,30,45,47,48,53,63,65,74,93].
- (6)
- Simulation and empirical calibration: In this group, we consider works focused on numerical solutions and data comparison and developed for model validation and empirical integration. Due to nonlinear complexity, many models rely on numerical simulations to explore parameter spaces and fit empirical data. Parametrization strategies include optimization techniques, sensitivity analysis, and empirical calibration. These models enhance the credibility of model-based recommendations and support data-driven decision making. Representative works include [19,25,26,31,32,35,36,37,42,53,56,57,58,60,61,66,67,68,69,75,78,84,85,87,88,89,90,92,94,96,98,99,100,101,103,104,105,106,108,110,111,113,115,116].
- (7)
- Intervention and optimization: There are some works on solving the problem of applied control and resource allocation, which are focused on mathematical control. These studies introduce control variables—such as habitat enhancement or pesticide reduction—to optimize ecological outcomes. Parametrization uses Pontryagin’s maximum principle or dynamic programming to derive optimal strategies. The results directly inform cost-effective conservation and adaptive management protocols (see [88,97]).
5. Other Aspects of the Literature Review
5.1. Research Gaps and Future Directions for Control, Stochastic Modeling, and Network-Based PDEs
- (1)
- Optimal Control under Uncertainty: While mathematical control was explored in [88,97], current models rely on deterministic frameworks and assume full observability of system states and parameters. These assumptions limit applicability in real-world settings, where ecological responses to interventions (e.g., pesticide reduction or habitat restoration) are uncertain and data are sparse. Neither study incorporated stochastic perturbations or feedback mechanisms, nor did they address parameter uncertainty or adaptive control strategies. This restricts the robustness and generalizability of the proposed solutions.
- (2)
- Stochastic Modeling: Across the retained list, stochastic formulations are conspicuously absent. Although several studies addressed oscillatory behavior and bifurcation phenomena (e.g., [30,53,63]), they did so within deterministic systems. The lack of stochastic differential equations or probabilistic transitions limits the capacity to model demographic noise, environmental variability, and uncertainty propagation, especially in fragmented landscapes or under climate stress. This gap is critical given the increasing emphasis on resilience and risk-aware ecological planning.
- (3)
- Network-Based PDEs and Patch Dynamics: Numerous studies incorporated network or patch structures in mutualistic systems (e.g., [13,21,28,33,40,65,71,72,96,100]), yet most relied on discrete or compartmental models. Continuous-space PDEs on networks or graph-based domains are rare, and when present, they often lack empirical calibration or realistic topologies. For example, the authors of [28,48,74] explored dissipation and spatial dynamics but did not integrate high-resolution landscape data or adaptive dispersal mechanisms. This limits the ecological realism and policy relevance of spatial predictions.
5.2. Limitations of Current Findings
5.3. Methodological Pathways
- (1)
- Graph Laplacians and metapopulation partial differential equations for dispersal modeling;
- (2)
- Bayesian inference and ensemble simulations for uncertainty quantification;
- (3)
- Data assimilation methods for real-time calibration.
5.4. Potential Data Sources
- (1)
- Remote sensing data for habitat fragmentation and land use change;
- (2)
- Citizen science platforms (e.g., iNaturalist or eBird) for species occurrence;
- (3)
- Long-term ecological monitoring networks (e.g., Global Biodiversity Information Facility—GBIF, or Long Term Ecological Research—LTER) for population dynamics.
5.5. Roadmap for Future Work
- (1)
- Development of modular, interoperable modeling platforms that integrate control, stochasticity, and spatial structure;
- (2)
- Co-design of models with stakeholders to ensure contextual relevance and usability;
- (3)
- Formal incorporation of sensitivity analysis and uncertainty quantification;
- (4)
- Establishment of typological benchmarks to compare model performance across ecological and socio-political scenarios.
5.6. Implications of the Retained Modeling Topics for Agricultural Planning, Habitat Management, and Pesticide Regulation
- (1)
- Positive Bounded Solutions: Ensuring that model solutions remain positive and bounded is essential for biological realism, particularly when variables represent population densities. This property supports the development of ecologically valid simulations that can guide agricultural interventions and pesticide thresholds, preventing unintended population collapses.
- (2)
- Equilibrium and Stability Analysis: Stability analysis enables the characterization of long-term system behavior, including species persistence or extinction. In agricultural contexts, it informs crop-pollinator compatibility and resilience, while in habitat management, it supports the design of restoration strategies and ecological corridors.
- (3)
- Bifurcation Analysis: Bifurcation theory reveals how small parameter changes can induce qualitative shifts in system dynamics. This is critical for anticipating nonlinear responses to environmental stressors, such as pesticide application or habitat fragmentation, and for designing adaptive management strategies that avoid tipping points.
- (4)
- Mutualistic Interactions: Modeling mutualistic networks elucidates mechanisms of coexistence, dissipation, and eco-evolutionary dynamics. These insights inform the diversification of cropping systems, the conservation of keystone mutualists, and the regulation of agrochemicals that may disrupt ecological interactions.
- (5)
- Periodicity of Solutions: Seasonal and periodic behaviors in pollinator populations are central to synchronizing agricultural calendars with ecological cycles. Understanding periodicity aids in optimizing planting schedules, flowering periods, and pesticide applications to align with pollinator activity.
- (6)
- Numerical Simulations and Empirical Validation: Given the nonlinear nature of most models, numerical simulations are indispensable for calibration and scenario testing. These simulations support evidence-based agricultural planning and policy evaluation, enabling cost-benefit analyses of proposed interventions.
- (7)
- Mathematical Control: Optimal control frameworks allow for the strategic modulation of system variables to achieve desired ecological or economic outcomes. In agriculture, this translates to resource-efficient practices that sustain pollinator populations, while in regulatory contexts, it supports dynamic policy design responsive to ecological feedback.
5.7. A Particular Comparative Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Details on Counting the Regions
| RR | Affiliations | RR | Affiliations | RR | Affiliations | RR | Affiliations |
|---|---|---|---|---|---|---|---|
| [10] | Mexico (2) | [37] | USA (2), China (2) | [64] | China (1) | [91] | USA (4) |
| [11] | USA (1) | [38] | Australia (3) | [65] | USA (4), China (2), UK (1) | [92] | China (3) |
| [12] | USA (1) | [39] | Netherlands (2), France (1) | [66] | Spain (4) | [93] | USA (6) |
| [13] | USA (2) | [40] | Ecuador (1), Spain (2) | [67] | Mexico (2) | [94] | China (4) |
| [14] | Sweden (2) | [41] | USA (5), Brazil (1) | [68] | Australia (1), UK (1) | [95] | China (1), Brazil (1), Netherlands (1), France (1), Spain (4) |
| [15] | USA (1) | [42] | Mexico (3) | [69] | R. of Korea (3) | [96] | Germany (4) |
| [16] | Italy (1) | [43] | Mexico (1) | [70] | China (1) | [97] | India (3) |
| [17] | UK (3) | [44] | China (2) | [71] | China (1) | [98] | USA (2) |
| [18] | USA (2) | [45] | China (2) | [72] | USA (1) | [99] | China (2) |
| [19] | Mexico (2) | [46] | China (1), USA (2) | [73] | Czech Republic (2) | [100] | Japan (2) |
| [20] | Argentina (2) | [47] | Mexico (2) | [74] | China (3) | [101] | India (3) |
| [21] | Chile (5) | [48] | China (3) | [75] | India (2) | [102] | USA (4) |
| [22] | Israel (2) | [49] | USA (4) | [76] | China (2), USA (1) | [103] | Czech Republic (3) |
| [23] | UK (3), Canada (1), USA (1) | [50] | China (3) | [77] | China (3) | [104] | Bulgaria (3) |
| [24] | Germany (2) | [51] | France (2) | [78] | China (4) | [105] | South African (2), Netherlands (1) |
| [25] | Argentina (2), Germany (1) | [52] | South African (2) | [79] | India (2) | [106] | India (2) |
| [26] | Mexico (2) | [53] | China (1), France (1), USA (1) | [80] | China (3), USA (2) | [107] | Mexico (3) |
| [27] | Canada (3) | [54] | Ecuador (1), Spain (2) | [81] | USA (2), France (1), Switzerland (1) | [108] | South Africa (4) |
| [28] | China (1), USA (3) | [55] | Mexico (2) | [82] | USA (6), UK (1) | [109] | Netherlands (2), Canada (1) |
| [29] | USA (4) | [56] | USA (5), Sweden (2) | [83] | USA (6), New Zeland (6) | [110] | Japan (3) |
| [30] | China (3) | [57] | Canada (2), Australia (2) | [84] | Saudi Arabia (2), Mexico (1), Pakistan (1) | [111] | China (4) |
| [31] | USA (1) | [58] | Belgium (2), Canada (2), Netherlands (1) | [85] | USA (6) | [112] | China (2) |
| [32] | USA (3) | [59] | China (2), USA (1) | [86] | Czech Republic (3) | [113] | Czech Republic (1) |
| [33] | China (2) | [60] | Japan (1) | [87] | USA (3) | [115] | China (1), Canada (1) |
| [34] | China (1) | [61] | South Africa (1), France (2) | [88] | Spain (2), Hungary (6) | [114] | India (3) |
| [35] | Chile (3), USA (1) | [62] | Czech Republic (2) | [89] | Italy (4), Germany (7) | [116] | Czech Republic (1) |
| [36] | UK (5) | [63] | Mexico (2) | [90] | USA (4) |
Appendix B. Details on Journals for Retained List
| Journal | H Index | SJR | Quartile |
|---|---|---|---|
| Agronomy-Basel | 114 | 3.7 | Q1 |
| Alexandria Engineering Journal | 112 | 5.6 | Q1 |
| American Naturalist | 236 | 3 | Q2 |
| Annals of Botany | 215 | 4.1 | Q1 |
| Applied Ecology and Environmental Research | 48 | 0.9 | Q4 |
| Applied Mathematical Modelling. Simulation and Computation for Engineering and Environmental Systems | 150 | 4.2 | Q1 |
| Applied Mathematics and Computation | 182 | 3.1 | Q1 |
| Applied Sciences-Basel | 162 | 2.7 | Q2 |
| Biosystems | 85 | 0.392 | Q2 |
| Boletín de la Sociedad Matemática Mexicana. Third Series | 20 | 0.414 | Q2 |
| Bulletin of Mathematical Biology | 101 | 0.702 | Q1 |
| Chaos, Solitons & Fractals | 175 | 1.184 | Q1 |
| Chaos. An Interdisciplinary Journal of Nonlinear Science | – | – | – |
| Communications in Nonlinear Science and Numerical Simulation | 143 | 0.956 | Q1 |
| Discrete and Continuous Dynamical Systems. Series A | 80 | 1.065 | Q1 |
| Discrete and Continuous Dynamical Systems. Series B. A Journal Bridging Mathematics and Sciences | 65 | 0.735 | Q1 |
| Discrete and Continuous Dynamical Systems. Series S | 43 | 0.514 | Q2 |
| Ecological Modelling | 189 | 0.896 | Q1 |
| Ecological Research | 87 | 0.616 | Q2 |
| Ecology | 345 | 5.5 | Q1 |
| Ecology and Evolution | 109 | 0.858 | Q1 |
| Ecology Letters | 330 | 9.8 | Q1 |
| European Journal of Applied Mathematics | 53 | 0.750 | Q2 |
| Evolution | 227 | 3.4 | Q2 |
| Evolutionary Applications | 95 | 1.362 | q1 |
| Evolutionary Ecology | 96 | 0.645 | Q2 |
| Evolutionary Ecology Research | 82 | – | – |
| International Journal for Parasitology-Parasites and Wildlife | 44 | 0.618 | Q1 |
| International Journal of Bifurcation and Chaos in Applied Sciences and Engineering | 120 | 0.596 | Q1 |
| International Journal of Biomathematics | 38 | 0.527 | Q2 |
| Journal of Applied Ecology | 216 | 6.2 | Q1 |
| Journal of Biological Dynamics | 46 | 0.597 | Q2 |
| Journal of Biological Systems | 39 | 0.487 | Q2 |
| Journal of Ecology | 219 | 6.1 | Q1 |
| Journal of Evolutionary Biology | 148 | 0.921 | Q1 |
| Journal of Mathematical Biology | 111 | 0.921 | Q1 |
| Journal of Mathematics | 30 | 0.322 | Q3 |
| Journal of Statistical Mechanics: Theory and Experiment | 95 | 0.373 | Q3 |
| Journal of the European Mathematical Society | 68 | 3.043 | Q1 |
| Journal of the Royal Society Interface | 177 | 1.025 | Q1 |
| Journal of Theoretical Biology | 178 | 0.532 | Q2 |
| Lobachevskii Journal of Mathematics | 31 | 0.435 | Q2 |
| Mathematical Biosciences | 114 | 0.555 | Q2 |
| Mathematical Methods in the Applied Sciences | 87 | 1.991 | Q1 |
| Modeling Earth Systems and Environment | 66 | 0.654 | Q1 |
| Natural Resource Modeling | 38 | 0.521 | Q2 |
| Nonlinear Analysis. Real World Applications. An International Multidisciplinary Journal | 106 | 1.168 | Q1 |
| Nonlinear Studies. The International Journal | 22 | 0.229 | Q4 |
| Oikos | 210 | 1.438 | Q1 |
| Physica A. Statistical Mechanics and its Applications | 195 | 0.669 | Q2 |
| Physica D: Nonlinear Phenomena | 154 | 0.940 | Q1 |
| PLoS ONE | 467 | 3.3 | Q1 |
| PLoS Pathogens | 260 | 5.5 | Q1 |
| Proceedings of the National Academy of Sciences of the United States of America | 896 | 10.8 | Q1 |
| Royal Society Open Science | 92 | 0.795 | Q1 |
| Scientific Reports | 347 | 4.3 | Q1 |
| Theoretical Ecology | 45 | 0.524 | Q2 |
| Theoretical Population Biology | 99 | 0.563 | Q2 |
References
- Potts, S.G.; Imperatriz-Fonseca, V.L.; Ngo, H.T. IPBES the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2017; p. 552. [Google Scholar]
- Deeksha, M.G.; Khan, M.S.; Kumaranag, K.M. Cuphea hyssopifolia Kunth: A Potential Plant for Conserving Insect Pollinators in Shivalik Foot Hills of Himalaya. Nat. Acad. Sci. Lett. 2023, 46, 137–142. [Google Scholar] [CrossRef]
- Devillers, J. In Silico Bees; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Karlin, S.; Farkash, S. Some Multiallele Partial Assortative Mating Systems for a Polygamous Species. Theoret. Popul. Biol. 1978, 14, 446–470. [Google Scholar] [CrossRef] [PubMed]
- Belsare, P.V.; Sriram, B.; Watve, M.G. The Co-optimization of Floral Display and Nectar Reward. J. Biosci. 2009, 34, 963–967. [Google Scholar] [CrossRef]
- Chen, J.; DeGrandi-Hoffman, G.; Ratti, V.; Kang, Y. Review on mathematical modeling of honeybee population dynamics. Math. Biosci. Eng. 2021, 18, 9606–9650. [Google Scholar] [CrossRef]
- Lozada, E.; Guerrero-Ortiz, C.; Coronel, A.; Medina, R. Classroom Methodologies for Teaching and Learning Ordinary Differential Equations: A Systemic Literature Review and Bibliometric Analysis. Mathematics 2021, 9, 745. [Google Scholar] [CrossRef]
- Khan, K.S.; Kunz, R.; Kleijnen, J.; Antes, G. Five Steps to Conducting a Systematic Review. J. R. Soc. Med. 2003, 96, 118–121. [Google Scholar] [CrossRef]
- Soberon, J.; Rio, C. The Dynamics of a Plant-Pollinator Interaction. J. Theoret. Biol. 1981, 91, 363–378. [Google Scholar] [CrossRef]
- Wells, H. Population Equilibria and Stability in Plant-Animal Pollination Systems. J. Theoret. Biol. 1983, 100, 685–699. [Google Scholar] [CrossRef]
- Armstrong, R. A Patch Model of Mutualism. J. Theoret. Biol. 1987, 125, 243–246. [Google Scholar] [CrossRef]
- Hastings, A.; Wolin, C.L. Within-Patch Dynamics in a Metapopulation. Ecology 1989, 70, 1261–1266. [Google Scholar] [CrossRef]
- Ingvarsson, P.K.; Lundberg, S. The Effect of a Vector-Borne Disease on the Dynamics of Natural Plant-Populations—A Model for Ustilago-Violacea Infection of Lychnis-Viscaria. J. Ecol. 1993, 81, 263–270. [Google Scholar] [CrossRef]
- Jang, S. Dynamics of Herbivore-Plant-Pollinator Models. J. Math. Biol. 2002, 44, 129–149. [Google Scholar] [CrossRef]
- Dercole, F. Border Collision Bifurcations in the Evolution of Mutualistic Interactions. Int. J. Bifurc. Chaos 2005, 15, 2179–2190. [Google Scholar] [CrossRef]
- Stewart-Cox, J.; Britton, N.; Mogie, M. Pollen Limitation or Mate Search Need not Induce an Allee Effect. Bull. Math. Biol. 2005, 67, 1049–1079. [Google Scholar] [CrossRef]
- Smith, C.A.; Wilson, W.G. Evolutionarily Stable Dispersal with Pattern Formation in a Mutualist-Antagonist System. Evol. Ecol. Res. 2007, 9, 987–1004. [Google Scholar]
- Sánchez-Garduño, F.; Breña-Medina, V.E. Spatio-Temporal Dynamics of a Three Interacting Species Mathematical Model Inspired in Physics. In Proceedings of the AIP Conference Proceedings, Ponta Delgada, Portugal, 18–23 May 2008; Dagdug, L., Scherer, L.G.C., Eds.; Universidad Autonoma Metropolitana: Mexico City, Mexico, 2008; Volume 978, pp. 115–134. [Google Scholar]
- Momo, F.R.; Ure, J.E. Stability and Fluctuations in a Three Species System: A Plant with Two Very Different Pollinators. Appl. Ecol. Environ. Res. 2009, 7, 287–296. [Google Scholar] [CrossRef]
- Valdovinos, F.S.; Ramos-Jiliberto, R.; Flores, J.D.; Espinoza, C.; Lópen, G. Structure and Dynamics of Pollination Networks: The Role of Alien Plants. Oikos 2009, 118, 1190–1200. [Google Scholar] [CrossRef]
- Fishman, M.A.; Hadany, L. Plant-Pollinator Population Dynamics. Theor. Popul. Biol. 2010, 78, 270–277. [Google Scholar] [CrossRef]
- Little, T.J.; Shuker, D.M.; Colegrave, N.; Day, T.; Graham, A.L. The Coevolution of Virulence: Tolerance in Perspective. PLoS Pathog. 2010, 6, e1001006. [Google Scholar] [CrossRef]
- Oña, L.; Lachmann, M. Ant Aggression and Evolutionary Stability in Plant-Ant and Plant-Pollinator Mutualistic Interactions. J. Evol. Biol. 2011, 24, 617–629. [Google Scholar] [CrossRef]
- Abramson, G.; Soto, C.A.T.; Oña, L. The Role of Asymmetric Interactions on the Effect of Habitat Destruction in Mutualistic Networks. PLoS ONE 2011, 6, e0021028. [Google Scholar] [CrossRef]
- Sánchez-Garduño, F.; Breña-Medina, V. Searching for Spatial Patterns in a Pollinator-Plant-Herbivore Mathematical Model. Bull. Math. Biol. 2011, 73, 1118–1153. [Google Scholar] [CrossRef] [PubMed]
- Tyson, R.C.; Wilson, J.B.; Lane, W.D. Beyond Diffusion: Modelling Local and Long-Distance Dispersal for Organisms Exhibiting Intensive and Extensive Search Modes. Theor. Popul. Biol. 2012, 79, 70–81. [Google Scholar] [CrossRef]
- Wang, Y.; DeAngelis, D.; Holland, J. Uni-Directional Interaction and Plant-Pollinator-Robber Coexistence. Bull. Math. Biol. 2012, 74, 2142–2164. [Google Scholar] [CrossRef] [PubMed]
- Gilman, R.T.; Fabina, N.S.; Abbott, K.C.; Rafferty, N.E. Evolution of Plant-Pollinator Mutualisms in Response to Climate Change. Evol. Appl. 2012, 5, 2–16. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wu, H.; Sun, S. Persistence of Pollination Mutualisms in Plant-Pollinator-Robber Systems. Theor. Popul. Biol. 2012, 81, 243–250. [Google Scholar] [CrossRef]
- Yong, K.E. A Mathematical Model of the Interactions Between Pollinators and Their Effects on Pollination of Almonds. Ph.D. Thesis, The University of Iowa, Iowa City, IA, USA, 2012. [Google Scholar]
- Yong, K.E.; Li, Y.; Hendrix, S. Habitat Choice of Multiple Pollinators in Almond Trees and its Potential Effect on Pollen Movement and Productivity: A Theoretical Approach Using the Shigesada-Kawasaki-Teramoto Model. J. Theoret. Biol. 2012, 305, 103–109. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H. Invasibility of nectarless flowers in plant-pollinator systems. Bull. Math. Biol. 2013, 75, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Dynamics of plant-pollinator-robber systems. J. Math. Biol. 2013, 66, 1155–1177. [Google Scholar] [CrossRef]
- Valdovinos, F.S.; de Espanes, P.M.; Flores, J.D.; Ramos-Jiliberto, R. Adaptive foraging allows the maintenance of biodiversity of pollination networks. Oikos 2013, 122, 907–917. [Google Scholar] [CrossRef]
- Bryden, J.; Gill, R.J.; Mitton, R.A.A.; Raine, N.E.; Jansen, V.A.A. Chronic Sublethal Stress Causes Bee Colony Failure. Ecol. Lett. 2013, 16, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.N.; Wang, Y.S.; Sun, S.; DeAngelis, D.L. Consumer-Resource Dynamics of Indirect Interactions in a Mutualism-Parasitism Food Web Module. Theor. Ecol. 2013, 6, 475–493. [Google Scholar] [CrossRef]
- Khoury, D.S.; Barron, A.B.; Myerscough, M.R. Modelling Food and Population Dynamics in Honey Bee Colonies. PLoS ONE 2013, 8, e59084. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Viso, F.; Revilla, T.; Etienne, R. Shifts in Pollinator Population Structure may JeopardizePollination Service. J. Theoret. Biol. 2014, 352, 24–30. [Google Scholar] [CrossRef]
- Guerrero, G.; Langa, J.; Suárez, A. Biodiversity and Vulnerability in a 3D Mutualistic System. Discret. Contin. Dyn. Syst. 2014, 34, 4107–4126. [Google Scholar] [CrossRef]
- Fagan, W.F.; Bewick, S.; Cantrell, S.; Cosner, C.; Varassin, I.G.; Inouye, D.W. Phenologically Explicit Models for Studying Plant-Pollinator Interactions under Climate Change. Theor. Ecol. 2014, 7, 289–297. [Google Scholar] [CrossRef]
- Sánchez-Garduño, F.; Castellanos, V.; Quilantán, I. Dynamics of a Nonlinear Mathematical Model for Three Interacting Populations. Bol. Soc. Mat. Mex. 2014, 20, 147–170. [Google Scholar] [CrossRef]
- Vargas-De-León, C. Global Stability for Multi-Species Lotka-Volterra Cooperative Systems: One Hyper-Connected Mutualistic-Species. Int. J. Biomath. 2015, 8, 1550039. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H. Stability of Plant-Pollinator-Ant Co-Mutualism. Appl. Math. Comput. 2015, 261, 231–241. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S. Persistence of Pollination Mutualisms in the Presence of Ants. Bull. Math. Biol. 2015, 77, 202–229. [Google Scholar] [CrossRef]
- Wang, Y.; DeAngelis, D.; Holland, J. Dynamics of an Ant-Plant-Pollinator Model. Commun. Nonlinear Sci. Numer. Simul. 2015, 20, 950–964. [Google Scholar] [CrossRef]
- Barradas, I.; Vázquez, V. Improving Pollination Through Learning. J. Biol. Syst. 2015, 23, S77–S90. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wu, H.; Wang, S.K. Invasibility of Nectar Robbers in Pollination-Mutualisms. Appl. Math. Comput. 2015, 250, 908–919. [Google Scholar] [CrossRef]
- Jover, L.F.; Flores, C.O.; Cortez, M.H.; Weitz, J.S. Multiple Regimes of Robust Patterns between Network Structure and Biodiversity. Sci. Rep. 2015, 5, 17856. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, H.; Li, Y. Positive Steady State Solutions of a Pant-Pollinator Model with Diffusion. Discret. Contin. Dyn. Syst. Ser. B 2015, 20, 1805–1819. [Google Scholar] [CrossRef]
- Georgelin, E.; Loeuille, N. Evolutionary Response of Plant Interaction Traits to Nutrient Enrichment Modifies the Assembly and Structure of Antagonistic-Mutualistic Communities. J. Ecol. 2016, 104, 193–205. [Google Scholar] [CrossRef]
- Minoarivelo, H.O.; Hui, C. Invading a Mutualistic Network: To be or not to be Similar. Ecol. Evol. 2016, 6, 4981–4996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Magal, P.; Ruan, S. Oscillations in Age-Structured Models of Consumer-Resource Mutualisms. Discret. Contin. Dyn. Syst. Ser. B 2016, 21, 537–555. [Google Scholar] [CrossRef]
- Guerrero, G.; Langa, J.; Suárez, A. Architecture of attractor determines dynamics on mutualistic complex networks. Nonlinear Anal. Real World Appl. 2017, 34, 17–40. [Google Scholar] [CrossRef]
- Vázquez, V.; Barradas, I. Deceptive Pollination and Insects’ Learning: A Delicate Balance. J. Biol. Dyn. 2017, 11, 299–322. [Google Scholar] [CrossRef]
- Banks, H.T.; Banks, J.E.; Bommarco, R.; Laubmeier, A.N.; Myers, N.J.; Rundlöf, M.; Tillman, K. Modeling Bumble Bee Population Dynamics with Delay Differential Equations. Ecol. Model. 2017, 351, 14–23. [Google Scholar] [CrossRef]
- Bouhours, J.; Mesgaran, M.B.; Cousens, R.D.; Lewis, M.A. Neutral Hybridization Can Overcome a Strong Allee Effect by Improving Pollination Quality. Theor. Ecol. 2017, 10, 319–339. [Google Scholar] [CrossRef]
- Vallaeys, V.; Tyson, R.C.; Lane, W.D.; Deleersnijder, E.; Hanert, E. A Levy-Flight Diffusion Model to Predict Transgenic Pollen Dispersal. J. R. Soc. Interface 2017, 15, 20160889. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Ruan, S. Bifurcation and Temporal Periodic Patterns in a Plant-Pollinator Model with Diffusion and Time Delay Effects. J. Biol. Dyn. 2017, 11, 138–159. [Google Scholar] [CrossRef]
- Yamauchi, A. Joint evolution of interspecific mutualism and regulation of variation of interaction under directional selection in trait space. Theor. Ecol. 2017, 10, 477–491. [Google Scholar] [CrossRef]
- Dumont, Y.; Soulie, J.; Michel, F. Modeling oil Palm Pollinator Dynamics using Deterministic and Agent-Based Approaches. Applications on Fruit Set Estimates. Some Preliminary Results. Math. Methods Appl. Sci. 2018, 41, 8545–8564. [Google Scholar] [CrossRef]
- Revilla, T.; Křivan, V. Competition, Trait-Mediated Facilitation, and the Structure of Plant-Pollinator Communities. J. Theoret. Biol. 2018, 440, 42–57. [Google Scholar] [CrossRef]
- Vázquez, V.; Barradas, I. A Plant-Pollinator System: How Learning Versus Cost-Benefit can Induce Periodic Oscillations. Int. J. Biomath. 2018, 11, 1850024. [Google Scholar] [CrossRef]
- Wang, Y. Global Dynamics of a Competition-Parasitism-Mutualism Model Characterizing Plant-Pollinator-Robber Interactions. Phys. A Stat. Mech. Its Appl. 2018, 510, 26–41. [Google Scholar] [CrossRef]
- Jiang, J.J.; Huang, Z.G.; Seager, T.P.; Lin, W.; Grebogi, C.; Hastings, A.; Lai, Y.C. Predicting Tipping Points in Mutualistic Networks through Dimension Reduction. Proc. Natl. Acad. Sci. USA 2018, 115, E639–E647. [Google Scholar] [CrossRef]
- Usó-Doménech, J.L.; Nescolarde-Selva, J.A.; Lloret-Climent, M.; González-Franco, L. Behavior of Pyrophite Shrubs in Mediterranean Terrestrial Ecosystems (i): Population and Reproductive Model. Math. Biosci. 2018, 297, 58–77. [Google Scholar] [CrossRef]
- Castellanos, V.; Sánchez-Garduño, F. The Existence of a Limit Cycle in a Pollinator-Plant-Herbivore Mathematical Model. Nonlinear Anal. Real World Appl. 2019, 48, 212–231. [Google Scholar] [CrossRef]
- Cropp, R.; Norbury, J. Simulating Eco-Evolutionary Processes in an Obligate Pollination Model with a Genetic Algorithm. Bull. Math. Biol. 2019, 81, 4803–4820. [Google Scholar] [CrossRef]
- Maeng, S.; Lee, J.; Lee, D. Competition-Induced Increase of Species Abundance in Mutualistic Networks. J. Stat. Mech. Theory Exp. 2019, 19, 033502. [Google Scholar] [CrossRef]
- Wang, Y. Pollination-Mutualisms in a Two-Patch System with Dispersal. J. Theoret. Biol. 2019, 476, 51–61. [Google Scholar] [CrossRef]
- Wang, Y. Dynamics of a Plant-Nectar-Pollinator Model and its Approximate Equations. Math. Biosci. 2019, 307, 42–52. [Google Scholar] [CrossRef]
- Valdovinos, F.S. Mutualistic Networks: Moving Closer to a Predictive Theory. Ecology Lett. 2019, 22, 1517–1534. [Google Scholar] [CrossRef]
- Krivan, V.; Revilla, T.A. Plant Coexistence Mediated by Adaptive Foraging Preferences of Exploiters or Mutualists. J. Theor. Biol. 2019, 480, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, H.; Wang, Y. Persistence and Oscillations of Plant-Pollinator-Herbivore Systems. Bull. Math. Biol. 2020, 82, 57. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Mishra, R. A plant-pollinator-pesticide model. Nonlinear Stud. 2020, 27, 1003–1019. [Google Scholar]
- Wang, Y.; Wu, H.; Wang, S. Persistence of Pollination Mutualisms under Pesticides. Appl. Math. Model. 2020, 77, 861–880. [Google Scholar] [CrossRef]
- Wu, H.; Sun, S.; Wang, Y. Bifurcations in a Pollination-Mutualism System with Nectarless Flowers. Discret. Contin. Dyn. Syst.-S 2020, 13, 3213–3229. [Google Scholar]
- Yuan, H.; Wang, Y.; Wu, H.; Wu, S. Persistence of Pollination-Mutualisms under the Effect of Intermediary Resource. Nat. Resour. Model. 2020, 33, e12259. [Google Scholar] [CrossRef]
- Devi, S.; Mishra, R.P. A Mathematical Model to See the Effects of Increasing Environmental Temperature on Plant-Pollinator Interactions. Model. Earth Syst. Environ. 2020, 6, 1315–1329. [Google Scholar] [CrossRef]
- Zhang, H.X.; Liu, X.M.; Wang, Q.; Zhang, W.D.; Gao, J.X. Co-Adaptation Enhances the Resilience of Mutualistic Networks. J. R. Soc. Interface 2020, 17, 20200236. [Google Scholar] [CrossRef]
- Yule, K.M.; Johnson, C.A.; Bronstein, J.L.; Ferrière, R. Interactions among Interactions: The Dynamical Consequences of Antagonism between Mutualists. J. Theor. Biol. 2020, 501, 110334. [Google Scholar] [CrossRef]
- Figueroa, L.L.; Grab, H.; Ng, W.H.; Myers, C.R.; Graystock, P.; McFrederick, Q.S.; McArt, S.H. Landscape Simplification Shapes Pathogen Prevalence in Plant-Pollinator Networks. Ecol. Lett. 2020, 23, 1212–1222. [Google Scholar] [CrossRef]
- Peace, A.; Pattemore, D.; Broussard, M.; Fonseka, D.; Tomer, N.; Bosque-Pérez, N.A.; Crowder, D.; Shaw, A.K.; Jesson, L.; Howlett, B.G.; et al. Orchard Layout and Plant Traits Influence Fruit Yield More Strongly than Pollinator Behaviour and Density in a Dioecious Crop. PLoS ONE 2020, 15, e0231120. [Google Scholar] [CrossRef]
- Khan, A.; Gómez-Aguilar, J.F.; Abdeljawad, T.; Khan, H. Stability and Numerical Simulation of a Fractional Order Plant-Nectar-Pollinator Model. Alex. Eng. J. 2020, 59, 49–59. [Google Scholar] [CrossRef]
- Burnham, P.A.; Alger, S.A.; Case, B.; Boncristiani, H.; Hébert-Dufresne, L.; Brody, A.K. Flowers as Dirty Doorknobs: Deformed Wing Virus Transmitted between Apis Mellifera and Bombus Impatiens through Shared Flowers. J. Appl. Ecol. 2021, 58, 2065–2074. [Google Scholar] [CrossRef]
- Revilla, T.A.; Marcou, T.; Krivan, V. Plant Competition under Simultaneous Adaptation by Herbivores and Pollinators. Ecol. Model. 2021, 455, 109634. [Google Scholar] [CrossRef]
- McPeek, S.J.; Bronstein, J.L.; McPeek, M.A. The Evolution of Resource Provisioning in Pollination Mutualisms. Am. Nat. 2020, 198, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.J.; Garay, J.; Móri, T.F.; Csiszár, V.; Varga, Z.; López, I.; Gámez, M.; Cabello, T. Theoretical Foundation of the Control of Pollination by Hoverflies in a Greenhouse. Agronomy 2021, 11, 167. [Google Scholar] [CrossRef]
- Paxton, R.J.; Schäfer, M.O.; Nazzi, F.; Zanni, V.; Annoscia, D.; Marroni, F.; Bigot, D.; Laws-Quinn, E.R.; Panziera, D.; Jenkins, C.; et al. Epidemiology of a Major Honey Bee Pathogen, Deformed Wing Virus: Potential Worldwide Replacement of Genotype A by Genotype B. Int. J. Parasitol. Parasites Wildl. 2022, 18, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Myers, C.R.; McArt, S.H.; Ellner, S.P. Pathogen Transport Amplifies or Dilutes Disease Transmission Depending on the Host Dose-Response Relationship. Ecol. Lett. 2022, 25, 453–465. [Google Scholar] [CrossRef]
- Hale, K.R.S.; Maes, D.P.; Valdovinos, F.S. Simple Mechanisms of Plant Reproductive Benefits Yield Different Dynamics in Pollination and Seed Dispersal Mutualisms. Am. Nat. 2022, 2022, 202–216. [Google Scholar] [CrossRef]
- Wang, G.; Chen, G.; Zhang, H.-T. Resilience of Hybrid Herbivore-Plant-Pollinator Networks. Chaos 2023, 33, 093129. [Google Scholar] [CrossRef]
- Chen, J.; Rodriguez, J.O.; DeGrandi-Hoffman, G.; Fewell, J.; Harrison, J.; Kang, Y. Impacts of Seasonality and Parasitism on Honey Bee Population Dynamics. J. Math. Biol. 2023, 87, 1–49. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Yao, J.; Wu, X. Bifurcations in a Plant-Pollinator Model with Multiple Delays. J. Math. 2023, 2023, 9950187. [Google Scholar] [CrossRef]
- Wang, X.; Peron, T.; Dubbeldam, J.L.A.; Kéfi, S.; Moreno, Y. Interspecific Competition Shapes the Structural Stability of Mutualistic Networks. Chaos Solitons Fractals 2023, 172, 113507. [Google Scholar] [CrossRef]
- Weyerer, F.; Weinbach, A.; Zarfl, C.; Allhoff, K.T. Eco-Evolutionary Dynamics in Two-Species Mutualistic Systems: One-Sided Population Decline Triggers Joint Interaction Disinvestment. Evol. Ecol. 2023, 37, 981–999. [Google Scholar] [CrossRef]
- Deb, S.; Bhandary, S.; Dutta, P.S. Evading Tipping Points in Socio-Mutualistic Networks via Structure Mediated Optimal Strategy. J. Theor. Biol. 2023, 567, 111494. [Google Scholar] [CrossRef]
- Elzinga, D.C.; Strickland, W.C. Generalized Stressors on Hive and Forager Bee Colonies. Bull. Math. Biol. 2023, 85, 112. [Google Scholar] [CrossRef]
- Ma, Q.; Su, M. Herbivore-Induced Pollinator Limitation Increases Community Stability of Mutualism-Antagonism Continuum. Biosystems 2023, 229, 104929. [Google Scholar] [CrossRef]
- Kawata, S.; Takimoto, G. Pollinator Predation Stabilizes Plant-Pollinator Mutualisms through the Modification of Pollinator Behavior. Ecol. Res. 2023, 38, 360–366. [Google Scholar] [CrossRef]
- Bhandary, S.; Deb, S.; Dutta, P.S. Rising Temperature Drives Tipping Points in Mutualistic Networks. R. Soc. Open Sci. 2023, 10, 221363. [Google Scholar] [CrossRef]
- Kiselev, A.; Nazarov, F.; Ryzhik, L.; Yao, Y. Chemotaxis and Reactions in Biology. J. Eur. Math. Soc. 2023, 25, 2641–2696. [Google Scholar] [CrossRef]
- Marcou, T.; Revilla, T.A.; Křivan, V. Evolutionary Emergence of Plant and Pollinator Polymorphisms in Consumer-Resource Mutualisms. J. Theoret. Biol. 2024, 594, 111911. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.Z.; Georgiev, S.G.; Vulkov, L.G. Analysis of the Influence of Brood Deaths on Honeybee Population. Appl. Sci. 2024, 14, 11412. [Google Scholar] [CrossRef]
- Mugabi, F.; Duffy, K.J.; van Langevelde, F. Behaviours of Honeybees Can Reduce the Probability of Deformed Wing Virus Outbreaks in Varroa Destructor-Infested Colonies. Model. Earth Syst. Environ. 2024, 10, 3745–3761. [Google Scholar] [CrossRef]
- Karmakar, S.; Pal, N. Complex Dynamical Behaviors of a Honeybee-Mite Model in Parameter Plane. Phys. D Nonlinear Phenom. 2024, 468, 134300. [Google Scholar] [CrossRef]
- Castellanos, V.; Dela-Rosa, M.A.; Sánchez-Garduño, F. Dynamics of a Pollinator-Plant-Herbivore Mathematical Model. Lobachevskii J. Math. 2024, 45, 5211–5231. [Google Scholar]
- Yahaya, M.M.; Rodger, J.G.; Landi, P.; Hui, C. Emergence of Structure in Plant-Pollinator Networks: Low Floral Resource Constrains Network Specialisation. Oikos 2024, 2024, e10533. [Google Scholar] [CrossRef]
- Saltini, M.; Barrett, S.C.H.; Deinum, E.E. Evolution from Mixed to Fixed Handedness in Mirror-Image Flowers: Insights from Adaptive Dynamics. Evolution 2024, 79, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Matsumoto, T.K.; Katsuhara, K.R. When Is Lethal Deceptive Pollination Maintained? A Population Dynamics Approach. Ann. Bot. 2024, 134, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, R.; Wang, J.; Cao, J. Threshold Dynamics Scenario of a Plants-Pollinators Cooperative System with Impulsive Effect on a Periodically Evolving Domain. Eur. J. Appl. Math. 2024, 35, 797–822. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Li, Y.K. Permanence and Extinction for the Stochastic Plant-Pollinator Model with Degenerate Diffusion. Int. J. Biomath. 2024, 2024, 2450065. [Google Scholar] [CrossRef]
- Revilla, T. Plant–Pollinator Interaction Model with Separate Pollen and Nectar Dynamics. J. Theor. Biol. 2024, 606, 112096. [Google Scholar] [CrossRef]
- Feng, T.; Wang, H. Complex Dynamics in Plant–Pollinator–Parasite Interactions: Facultative versus Obligate Behaviors and Novel Bifurcations. J. Math. Biol. 2024, 90, 46. [Google Scholar] [CrossRef]
- Biswas, A.; Medda, R.; Pal, S. Dynamics of Predatory Effect on Saturated Plant–Pollinator Mutualistic Relationship. Chaos 2025, 35, 023118. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, S.; Luo, J. Spatial and Temporal Periodic Patterns in a Delayed Diffusive Plant–Pollinator Model with Memory-Based Diffusion. Int. J. Biomath. 2025, 18, 2350093. [Google Scholar] [CrossRef]


| Rank | Journal | Record Count | % of 106 |
|---|---|---|---|
| 1° | Journal of Theoretical Biology | 12 | 11.32 |
| 2° | Bulletin of Mathematical Biology | 8 | 7.55 |
| 3° | Ecology Letters | 4 | 3.7 |
| International Journal of Biomathematics | 4 | 3.77 | |
| Theoretical Ecology | 4 | 3.77 | |
| 4° | Journal of Mathematical Biology | 3 | 2.83 |
| Oikos | 3 | 2.83 | |
| Plos One | 3 | 2.83 | |
| Theoretical Population Biology | 3 | 2.83 |
| Rank | Journal | H Index | SJR 2024 | Quartile SJR | Subject Area and Category |
|---|---|---|---|---|---|
| 1° | Proceedings of the National Academy of Sciences of the United States of America | 896 | 10.8 | Q1 | Multidisciplinary Sciences |
| 2° | Plos One | 467 | 3.3 | Q1 | Multidisciplinary Sciences |
| 3° | Scientific Reports | 347 | 4.3 | Q1 | Multidisciplinary Sciences |
| 4° | Ecology | 343 | 5.5 | Q1 | Ecology |
| 5° | Ecology Letters | 330 | 9.8 | Q1 | Ecology |
| 6° | Plos Pathogens | 260 | 5.5 | Q1 | Microbiology, Parasitology |
| 7° | American Naturalist | 236 | 3.3 | Q2 | Ecology |
| 8° | Evolution | 227 | 3.0 | Q2 | Ecology/Evolutionary Biology |
| 9° | Journal of Ecology | 219 | 6.1 | Q1 | Ecology, Plant Sciences |
| 10° | Journal of Applied Ecology | 216 | 6.2 | Q1 | Biodiversity Conservation, Ecology |
| Author | Institution | Number of Articles |
|---|---|---|
| Yuanshi Wang | Sun Yat-sen University, P. R. China | 16 |
| Hong Wu | Sun Yat-sen University, P. R. China | 7 |
| Faustino Sánchez-Garduño | Universidad Nacional Autónoma de México, Mexico | 6 |
| Fernanda S. Valdovinos | University of California Davis, USA | 4 |
| RR | Model Type | Assumptions | Parameterization | Validation | Key Findings |
|---|---|---|---|---|---|
| [10] | Ordinary differential equations of Lotka–Volterra type | Two-species, mutualistic interaction, closed system, constant environment | Interaction coefficients derived from the ecological literature; intrinsic growth rates assumed to be constant | Analytical exploration of equilibrium points and stability, qualitative phase plane analysis | Demonstrated conditions for mutualistic coexistence and thresholds for collapse due to partner dependency |
| [11] | Ordinary differential Lotka–Volterra equations with ecological feedback | Mutualistic interaction of pollinators and plants, continuous population dynamics, homogeneous environment | Growth rates and interaction, coefficients estimated from empirical observations and the ecological literature | Local stability analysis, numerical simulations exploring population trajectories under varying scenarios | Identification of stable equilibria, limit cycles, and bifurcation points explaining persistence or collapse of mutualistic systems |
| [12] | Discrete patch-based mutualism model | Species occupy spatially distinct patches; mutualistic benefit depends on local density; extinction and colonization are patch-specific | Parameters included colonization rate, extinction probability, and mutualistic benefit per patch; values treated generically for theoretical exploration | Analytical derivation of equilibrium conditions; stability assessed via local perturbation analysis | Mutualism enhances patch occupancy and persistence; spatial structure stabilizes interactions; coexistence possible without obligate dependence |
| [13] | Patch-based metapopulation model | Species occupy discrete habitat patches; extinction risk decreases with population size; colonization depends on local density | Extinction and colonization rates modeled as functions of population size; parameters derived from ecological theory | Analytical derivation of equilibrium distributions; stability analysis of patch ensemble | Demonstrated that spatial structure stabilizes mutualistic systems and intra-patch dynamics critically influence metapopulation persistence |
| [14] | Compartmental disease model with pollination-mediated transmission | Vector-borne fungal disease spreads via pollinators; host recruitment and pollinator visitation drive transmission | Transmission potential and recruitment rates estimated from empirical data; no explicit density dependence | Analytical threshold conditions for disease invasion; bifurcation analysis of host–pathogen dynamics | Identified conditions for disease-induced host extinction; showed that high disease incidence suppresses pollination and drives collapse |
| [15] | Three-species ordinary differential equations model (herbivore, plant, and pollinator) | Non-obligate mutualism; herbivory reduces pollinator visitation; population dynamics are continuous and deterministic | Functional responses and interaction coefficients derived from the ecological literature; visitation rates modeled explicitly | Stability analysis of equilibria; numerical simulations of population trajectories | Showed that herbivory can indirectly promote pollinator persistence; identified conditions for coexistence and oscillatory dynamics |
| [16] | Adaptive dynamics with piecewise smooth bifurcation structure | Evolution of mutualistic traits constrained by physiological costs; trait space bounded; bifurcations occur at viability borders | Trait-dependent fitness functions and bifurcation parameters derived from evolutionary stability conditions | Analytical and numerical bifurcation analysis; detection of border collision bifurcations | Revealed abrupt evolutionary transitions in mutualism; identified critical thresholds for trait viability and coexistence |
| [17] | Cellular automata with pair approximation | Pollination and reproduction modeled as separate processes; spatial clumping affects contact rates; Allee effects are context-dependent | Local and global interaction rules encoded in probabilistic automata; pair approximation used to derive ODE caricatures | Comparison of pair approximation predictions with full simulation outcomes | Demonstrated that spatial structure can eliminate Allee effects; local pollination and colonization enhance reproductive success in small populations |
| [18] | Individual-based spatial model with evolutionary dynamics | Obligate mutualism between plant and pollinator; parasitoid antagonist and dispersal evolves under selection; homogeneous landscape | Dispersal kernels, visitation rates, and mortality probabilities, parameters derived from ecological data and theoretical distributions | Simulation-based pattern formation; comparison with non-spatial ordinary differential equation model; sensitivity to obligacy constraints | Identified evolutionarily stable dispersal distances; showed that obligacy promotes spatial patterning and coexistence and weak obligacy leads to collapse of spatial structure |
| [19] | Reaction, diffusion, advection partial differential equation system | Three-species system: plant, pollinator, herbivore; nonlinear interactions; spatial movement and local reactions; continuous time and space | Diffusion and advection coefficients; interaction terms inspired by physical analogies; parameters selected for dynamical richness | Analytical treatment of reduced subsystems; numerical simulations of full spatiotemporal model | Demonstrated emergence of spatial heterogeneity and traveling waves; showed that mutualism and antagonism interact to produce complex spatial patterns |
| [20] | Three-species ordinary differential equation model with asymmetric mutualism | One plant species with two pollinators differing in efficiency and genetic impact; mutualism and competition coexist; inbreeding affects seed viability | Growth rates, nectar consumption rates, and pollination success calibrated from biological traits; inbreeding modeled as a dynamic feedback | Stability analysis and bifurcation exploration; numerical simulations under varying asymmetry and inbreeding levels | Found that pollinator asymmetry enhances system stability; high inbreeding paradoxically increases population persistence; oscillatory regimes linked to trait divergence |
| [21] | Stochastic metacommunity network model | Pollination network includes native and alien plants; mutualistic interactions structured by network topology; species abundances evolve over time | Degree distribution, nestedness, and modularity used to define network structure; demographic parameters estimated from field data | Simulations of network dynamics under species removal; comparison of full vs. native-only networks | Removal of alien plants destabilizes network structure and reduces species persistence; network topology strongly influences long-term dynamics and resilience |
| [22] | Multi-scale ordinary differential equation model with individual-to-population extrapolation | Pollinator foraging occurs at multiple temporal scales; mutualism affects reproduction and survival; population-level dynamics derived from individual-level interactions | Parameters derived from empirical foraging behavior and floral handling times; interaction terms approximated via Beddington–DeAngelis functional response | Analytical derivation of equilibrium conditions; numerical simulations of long-term dynamics | Demonstrated bi-stability and threshold effects in plant-pollinator systems; highlighted the role of individual-level behavior in shaping population-level outcomes |
| [23] | Conceptual coevolutionary framework with tolerance–virulence trade-offs | Host fitness is reduced by parasite virulence; tolerance evolves independent of resistance; coevolution affects both host and parasite traits | Virulence modeled as a function of parasite density and per-parasite damage; host fitness expressed as a linear function of tolerance and infection burden | Theoretical synthesis of existing models; conceptual validation through comparative analysis of empirical cases | Proposed that tolerance can drive parasite counter-adaptation; emphasized the need for integrated models of host–parasite coevolution, including mutualistic analogs |
| [24] | Game theoretic model of aggression in multi-partner mutualism | Plants interact simultaneously with ants and pollinators; ant aggression affects pollinator survival; fitness interests are misaligned across partners | Aggression modeled as a continuous trait; payoff matrices constructed for plant, ant, and pollinator strategies; parameters derived from ecological scenarios | Analytical derivation of evolutionary stable strategies; threshold analysis of aggression levels | Identified conditions under which pollinators are excluded due to ant aggression; facultative mutualisms more vulnerable than obligate ones; proposed empirical tests for aggression thresholds |
| [25] | Network-based dynamical model with topological asymmetry | Mutualistic networks are asymmetric; specialists interact with generalists, habitat destruction removes nodes and links, and extinction cascades depend on network structure | Network topology defined by degree distribution and nestedness; demographic parameters estimated from empirical pollination networks | Simulations of network collapse under progressive habitat loss; entropy metrics used to assess differential susceptibility | Found that disassortative networks buffer specialist species against extinction; asymmetry promotes resilience under habitat fragmentation; validated predictions with real-world network data |
| [26] | Reaction, diffusion, and advection system | Three-species system (plant, pollinator, herbivore); nonlinear interactions; spatial movement via diffusion and advection; Holling type II functional response | Diffusion and advection coefficients selected for dynamical richness; interaction terms derived from ecological analogies | Analytical proof of existence, positivity, and boundedness; numerical simulations under Dirichlet and Neumann boundary conditions | Showed emergence of spatial patterns and traveling waves; mutualism and antagonism jointly shape spatial heterogeneity and coexistence |
| [27] | Hybrid dispersal model with intensive and extensive search modes | Foraging population divided into two behavioral states—intensive (local) and extensive (long range)—with movement modeled via diffusion and advection, respectively | Search mode parameters calibrated from empirical movement data; dispersal kernels constructed from behavioral observations | Comparison with traditional diffusion models; simulation-based fit to spatial distribution data | Demonstrated superior predictive power of hybrid model; emphasized behavioral heterogeneity as key to dispersal dynamics and ecological forecasting |
| [28] | Three-species ordinary differential equation model with unidirectional consumer–resource interaction | Plant provides nectar to both pollinator and nectar robber; pollinator offers mutualistic service, but robber is exploitative; interaction asymmetry drives coexistence | Functional responses tailored to mutualist and exploiter; parameters derived from empirical studies and theoretical ecology | Stability and persistence analysis; numerical simulations of invasion scenarios and equilibrium dynamics | Identified conditions for stable coexistence despite exploitation; showed that pollinator’s dual role (beneficial and costly) mediates system resilience |
| [29] | Eco-evolutionary model of mutualism under climate change | Plant and pollinator phenologies evolve in response to climate shifts; mutualism persistence depends on synchrony and community composition | Evolutionary rates and phenological traits modeled explicitly; alternative partner availability incorporated as dynamic variables | Simulation of evolutionary trajectories under varying climate scenarios; sensitivity analysis of Allee thresholds | Found that mutualism robustness depends on partner diversity and temporal overlap; identified thresholds beyond which climate-induced asynchrony leads to collapse |
| [30] | Three-species ordinary differential equation model with mutualism and exploitation | Plants interact with pollinators (mutualism) and nectar robbers (parasitism); functional responses differ across interactions and population-level dynamics | Beddington–DeAngelis and Holling type II responses; parameters derived from the ecological literature and theoretical constraints | Analytical conditions for persistence and extinction; bifurcation analysis of coexistence regimes | Identified mechanisms for stable coexistence in presence of robbers; showed that mutualism can persist despite exploitation under specific parameter thresholds |
| [31] | Cross-diffusion partial differential equation model with empirical calibration | Honey bees and solitary bees forage in almond orchards; movement influenced by environmental favorableness and interspecific interactions | Shigesada–Kawasaki–Teramoto framework; parameters calibrated from field data and canopy structure; spectral Galerkin method used for numerical approximation | Numerical simulations of spatial redistribution; empirical validation via bee visitation data and pollen movement patterns | Demonstrated that cross-diffusion leads to increased inter-tree movement and enhanced cross-pollination; model supports use of diverse pollinator assemblages to improve yield |
| [32] | Two-species SKT model with habitat choice and productivity feedback | Pollinators choose habitats based on floral density and competition; movement modeled via cross-diffusion; productivity linked to pollen transfer efficiency | SKT model applied to almond trees; favorableness gradients derived from floral distribution; interspecific effects modeled explicitly | Spectral Galerkin simulations; sensitivity analysis of pollinator redistribution and productivity outcomes | Found that interspecific competition drives honey bees into less favorable zones, increasing cross-pollination; spatial heterogeneity enhances productivity in mixed-variety orchards |
| [33] | Three-species ordinary differential equation model with cheater invasion | Plants classified as nectar secretors or non-secretors; pollinators interact mutualistically with secretors and parasitically with cheaters; fitness depends on nectar availability | Beddington–DeAngelis functional responses for both interactions; efficiency thresholds defined for cheater invasion | Global stability analysis; threshold conditions for persistence and extinction; numerical simulations of invasion dynamics | Showed that nectarless flowers can invade and persist under specific efficiency conditions; identified scenarios where cheaters drive mutualists to extinction, leading to system collapse |
| [34] | Ordinary differential equation model with mutualism and parasitism | Plants and pollinators form a mutualistic pair; nectar robbers exploit plants without providing pollination; pollinators and robbers share a limiting resource without direct interference | Functional responses include Beddington–DeAngelis and Holling type II; parameters varied to explore invasion and persistence scenarios | Analytical derivation of equilibria and stability conditions; numerical simulations of coexistence, extinction, and invasion dynamics | Mutualism can persist despite robber invasion; coexistence possible under intermediate robber efficiency and favorable initial conditions; pollinators not necessarily driven to extinction by robbers |
| [35] | Consumer–resource ordinary differential equation model with adaptive foraging | Pollinators adjust foraging efforts based on floral reward availability; network structure influences interaction strength; mutualism is dynamic and plastic | Empirical pollination networks used to calibrate interaction matrices; adaptive foraging implemented via optimization routines | Simulations of network dynamics under species loss; comparison of static vs. adaptive foraging scenarios | Adaptive foraging enhances biodiversity and network robustness and reduces secondary extinctions by promoting niche partitioning among pollinators and plants |
| [36] | Compartmental model of colony impairment under sublethal stress | Sublethal pesticide exposure impairs individual bees without causing mortality; colony function depends on cumulative impairment; positive density dependence drives collapse | Parameters derived from empirical exposure experiments; impairment modeled as transition between healthy and dysfunctional states | Model fitted to experimental data from bumblebee colonies; comparison with non-impairment models | Sublethal stress leads to colony failure via feedback loops; impairment thresholds generate Allee effects and bistable dynamics; model explains enigmatic patterns of collapse |
| [37] | Three-species consumer–resource model with indirect interactions | Plant provides nectar to mutualist (pollinator) and parasite (robber); indirect interactions emerge via shared resources; feedback loops modulate coexistence | Functional responses calibrated from empirical nectar consumption rates; interaction strengths derived from ecological theory | Analytical derivation of equilibrium and limit cycles; simulations of indirect interaction strength and persistence | Mutualism stabilizes food web module despite parasitism; (+,–) indirect interactions promote coexistence; density-dependent feedback enhances resilience |
| [38] | Compartmental population model of honey bee colony dynamics | Colony composed of brood, hive bees, and foragers; food availability regulates development and foraging onset; mortality and recruitment are stage-specific | Parameters derived from empirical data on bee life stages and foraging behavior; food dynamics modeled via differential equations | Simulations of colony trajectories under varying food and mortality rates; equilibrium and threshold analysis | Identified critical thresholds for forager mortality beyond which colonies collapse; food availability buffers colony resilience; model supports predictive management of hive health |
| [39] | Stage-structured ordinary differential equation model with facultative obligate mutualism | Plant is facultative; pollinator is obligate with life stage structure; external stressors affect larval development and adult survival | Life stage transitions modeled explicitly; demographic rates derived from insect biology and the ecological literature | Analytical stability analysis; bifurcation exploration under varying demographic dominance | Identified hysteresis and collapse thresholds; pollination service vulnerable to shifts in pollinator structure; recovery requires large demographic compensation |
| [40] | Three-dimensional autonomous ordinary differential equation system with cooperative and competitive interactions | Two plant species compete, one pollinator interacts cooperatively with both, and biodiversity emerges from interaction topology | Growth rates, competition coefficients, and mutualistic terms defined via mean-field approximations; handling time neglected | Analytical and numerical exploration of attractors and limit cycles; comparison with reduced competitive system | Demonstrated that cooperative species enhance biodiversity even when driven to extinction; proposed structural vulnerability of key mutualists |
| [41] | Hybrid dynamical model with seasonal and intra-seasonal phenology | Plant and pollinator phenologies respond differently to climate change; demographic outcomes depend on synchrony and lifespan | Zonneveld-type non-autonomous ordinary differential equations for within-season dynamics and discrete-time equations for seasonal transitions | Zonneveld-type non-autonomous ordinary differential equations for within-season dynamics and discrete-time equations for seasonal transitions | Found that short-lived species are highly sensitive to mismatching; hybrid models capture demographic consequences of climate-driven phenological shifts |
| [42] | Reaction, diffusion, and advection system with nonlinear functional responses | Three interacting populations: plant, pollinator, and herbivore; spatial movement via diffusion and advection; non-monotonic response functions | Parameters selected for dynamical richness; functional responses derived from ecological analogies and prior models | Analytical reduction to autonomous ordinary differential equation system; numerical simulations of full spatiotemporal model | Herbivore stabilizes mutualistic dynamics; coexistence enhanced by spatial heterogeneity; limit cycles and attractors depend on interaction strength |
| [43] | Lotka–Volterra cooperative system with delay | One hyper-connected mutualistic species interacts with multiple peripheral species; delays represent interaction lags; no inter-peripheral interactions | Birth rates and interaction coefficients assumed to be positive; delays modeled via distributed kernels | Lyapunov functionals used to prove global stability; analytical derivation of coexistence conditions | Demonstrated global asymptotic stability of coexistence equilibrium; extended classical Lotka-–Volterra framework to nested mutualistic networks |
| [44] | Three-species ordinary differential equation model with dual mutualism and interference | Plant interacts mutually with both pollinator and ant; ant interferes with pollinator access; functional responses differ across interactions | Beddington–DeAngelis for mutualisms and Holling type II for interference; parameters derived from the ecological literature | Stability analysis and threshold exploration; numerical simulations of coexistence and extinction regimes | Identified threshold in ant aggressiveness; coexistence possible under weak interference; strong interference leads to pollinator extinction and mutualism collapse |
| [45] | Extended Beddington–DeAngelis model with asymmetric mutualism | Pollinators and ants interact with plants; ants interfere with pollinators but depend on mutualism for survival, with feedback loops included | Functional responses extended to capture interference; parameters derived from empirical studies and theoretical constraints | Global dynamics analyzed via persistence theory; bifurcation analysis of extinction thresholds | Showed that mutualism can persist under moderate interference; strong ant dependence on pollination stabilizes coexistence; extinction cascades occur under high interference |
| [46] | Three-species dynamical system with extended functional responses | Plant–pollinator and plant-–ant interactions are mutualistic; ant interference modeled explicitly; system includes indirect effects | Extended Beddington-–DeAngelis responses; interaction strengths calibrated from ecological theory and prior models | Analytical derivation of boundary equilibria; numerical simulations of persistence and extinction dynamics | Defined threshold for ant interference; weak interference promotes mutualism synergy; strong interference destabilizes entire system, including ant population |
| [47] | Ordinary differential equation model with pollinator learning and expertise differentiation | Pollinators are divided into novice and expert classes; learning improves pollination efficiency; plant growth benefits from expert visitation | Logistic growth for plants; mutualistic benefit modeled via saturating functional response; learning encoded via efficacy parameter | Analytical derivation of equilibrium and stability; numerical simulations of coexistence dynamics | Showed stable coexistence of plants, novices, and experts; learning enhances mutualistic benefit and system resilience; expertise evolution supports pollination service |
| [48] | Three-species ordinary differential equation model with nectar robbing | Pollinators provide mutualistic service; nectar robbers consume floral resources without pollination; robbers compete indirectly with pollinators | Beddington–DeAngelis functional responses for both mutualism and exploitation; parameters derived from ecological theory | Stability and persistence analysis; bifurcation exploration of coexistence and extinction regimes | Identified conditions for coexistence of robbers, pollinators, and plants; robbers can destabilize mutualism or persist without collapsing the system |
| [49] | Network-based dynamical model with structural variation | Biodiversity depends on network topology (e.g., nestedness, modularity); interaction strength varies across species; life history traits influence persistence | Network structure varied systematically; interaction matrices parameterized across plausible ecological regimes | Simulations across parameter space; biodiversity measured as fraction of surviving species under different topologies | Found multiple regimes linking nestedness to biodiversity; network structure alone can promote or hinder persistence depending on trait configuration |
| [50] | Reaction and diffusion model with positive steady-state analysis | Plant and pollinator populations diffuse spatially; mutualism modeled via Beddington–DeAngelis response; steady states represent ecological coexistence | Diffusion coefficients and growth rates selected for analytical tractability; functional response includes saturation and interference terms | Leray–Schauder degree theory used to prove existence of positive steady states; stability explored via monotone dynamical systems | Demonstrated existence and uniqueness of positive steady states; coexistence depends on growth–mortality balance and spatial diffusion rates |
| [51] | Adaptive dynamics model with trait-mediated trade-offs | Plants evolve interaction traits under nutrient enrichment; traits affect both mutualistic and antagonistic interactions; ecological trade-off is convex | Trait values modulate interaction strength; nutrient enrichment modeled as external forcing; parameters derived from ecological theory | Analytical exploration of evolutionary equilibria; simulations of community assembly under enrichment gradient | Evolution modifies community structure and alleviates priority effects; nutrient enrichment promotes plant diversification into attractive and defensive phenotypes |
| [52] | Eco-evolutionary network model with trait similarity | Invader success depends on trait similarity to resident species; mutualistic interactions are trait-mediated; network structure influences invasibility | Trait distributions and propagule pressure varied systematically; network metrics (nestedness, modularity) used to define recipient community | Simulations of invasion scenarios; robustness and resilience metrics used to assess impact | Trait dissimilarity enhances invasiveness; network stability better predicts invasibility than topology; multiple introductions increase invasion success |
| [53] | Age-structured partial differential equation model for unidirectional mutualism | Consumer species structured by age; resource species provides benefit without reciprocal cost; interaction includes both positive and negative effects | Age-dependent interaction kernels, Michaelis-–Menten saturation for resource uptake; parameters derived from biological life history traits | Hopf bifurcation and stability analysis; numerical simulations of periodic solutions | Identified conditions for oscillatory dynamics; age structure induces complex feedbacks; coexistence depends on balance between benefit and exploitation |
| [54] | Ordinary differential equation system with attractor geometry in mutualistic networks | Bipartite network of plants and pollinators; mutualistic and competitive interactions; attractor architecture governs long-term dynamics | Growth rates and competition and cooperation coefficients defined via network topology; nestedness encoded in interaction matrices | Morse decomposition and Lyapunov function construction; numerical simulations of attractor transitions | Attractor structure determines biodiversity outcomes; nestedness enhances coexistence; topological robustness linked to dynamical stability |
| [55] | Two-species ordinary differential equation model with behavioral learning | Plants attract pollinators deceptively without offering rewards; pollinators learn to avoid deceptive flowers over time; learning affects visitation rates | Learning encoded via a dynamic cost function; interaction terms modeled with saturating responses; parameters derived from behavioral ecology | Stability and bifurcation analysis; numerical simulations of coexistence and extinction regimes | Learning reduces pollinator visitation to deceptive plants; coexistence depends on deception cost and learning rate; oscillatory dynamics emerge under intermediate conditions |
| [56] | Delay differential equation model for bumblebee colonies | Bumblebee population structured by colony stages; time delays represent developmental lags; external pressures affect reproduction and survival | Life history parameters derived from Bombus terrestris data; delays calibrated from empirical colony development timelines | Numerical simulations using spline approximations; sensitivity analysis under resource and pesticide stress | Delay structure captures seasonal dynamics and vulnerability; model predicts colony collapse under combined stressors; useful for evaluating conservation strategies |
| [57] | Genetic hybridization model with Allee effect mitigation | Small populations suffer from pollen limitation and genetic Allee effects; hybridization with co-flowering species improves pollination quality | Single-locus, two-allele genetic model; pollinators modeled as catalytic agents; parameters derived from kinetic reaction theory | Stability analysis of trivial and hybrid equilibria; phase-plane exploration of invasion dynamics | Neutral hybridization removes or reduces Allee thresholds; hybridization facilitates invasion and persistence; implications for conservation and invasion biology |
| [58] | Fractional-order diffusion model with Lévy flights | Bee-mediated pollen dispersal follows truncated Lévy flight patterns; long-distance dispersal events drive transgene spread; Brownian motion underestimates risk | Dispersal kernels fitted to empirical pollen movement data; fractional diffusion operator used to interpolate between Brownian and Lévy regimes | Numerical solution of fractional PDEs; comparison with classical diffusion predictions; threshold analysis for isolation distances | Lévy-based models predict significantly larger dispersal ranges; isolation distances must be revised upward; model improves risk assessment for GM pollen escape |
| [59] | Reaction, diffusion, delay system model with Hopf bifurcation | Plant–pollinator system with spatial diffusion and time delay; unidirectional consumer–resource interaction; periodic patterns emerge from instability | Diffusion coefficients and delay terms derived from ecological reasoning; functional responses include saturation and interference | Hopf bifurcation analysis via center manifold and normal form theory; numerical simulations of spatially homogeneous and inhomogeneous solutions | Identified conditions for temporal and spatial oscillations; delay and diffusion jointly drive pattern formation; bifurcation structure predicts ecological transitions |
| [60] | Trait-based evolutionary model with directional selection | Mutualism evolves via trait matching; regulation of trait variation includes homeostasis, developmental stability, and partner acceptability; selection is directional | Trait space modeled as continuous; mutation bias and environmental noise incorporated; parameters derived from evolutionary theory | Analytical exploration of joint evolution; stability analysis of trait distributions under different regulatory regimes | Developmental stability promotes mutualism evolution; trait regulation affects partner specificity; coevolutionary feedback shapes mutualistic trait architecture |
| [61] | Hybrid deterministic and agent-based model for oil palm pollination | Pollination by Elaeidobius spp. weevils depends on male inflorescence availability; fruit set linked to pollinator dynamics; spatial heterogeneity matters | Deterministic model uses metapopulation dynamics; agent-based model simulates individual weevil behavior; parameters from plantation data | Comparative simulations of both models; fruit set estimates validated against field observations; sensitivity analysis of inflorescence ratios | Agent-based model captures fine-scale dynamics; deterministic model predicts population thresholds; both approaches inform optimal pollinator management for yield improvement |
| [62] | Adaptive consumer–resource model with trait-mediated facilitation | Two plants compete for pollinators; pollinators adapt preferences based on plant abundance; facilitation and competition co-occur via trait mediation | Functional responses include adaptive foraging; trait distributions influence interaction strength; parameters derived from optimal foraging theory | Isoleg analysis and ideal free distribution framework; numerical simulations of coexistence and exclusion regimes | Adaptive preferences reduce niche overlap and promote coexistence; trait-mediated facilitation alters community structure; coexistence depends on balance between facilitation and competition |
| [63] | Ordinary differential equation model with behavioral learning and cost-benefit trade-offs | Pollinators learn to avoid deceptive plants; plant population includes rewarding and non-rewarding individuals; learning affects visitation rates | Learning encoded via dynamic feedback; cost-benefit parameters derived from ecological theory and behavioral studies | Hopf bifurcation analysis; numerical simulations of periodic and damped oscillations | Learning induces sustained or damped oscillations; coexistence depends on deception cost and learning rate; behavioral adaptation stabilizes mutualism |
| [64] | Three-species dynamical system with competition, parasitism, and mutualism | Plants interact with pollinators (mutualism) and nectar robbers (parasitism); pollinators and robbers compete indirectly for floral resources | Functional responses include Beddington-–DeAngelis and Holling type II; parameters derived from the ecological literature and invasion theory | Global stability and persistence analysis; bifurcation diagrams of coexistence regimes | Coexistence possible under intermediate parasitism and competition; mutualism can persist despite robbers; extinction thresholds depend on interaction efficiency |
| [65] | Dimension-reduced model of mutualistic network collapse | High-dimensional mutualistic networks exhibit tipping points; dimension reduction captures essential dynamics; stochastic perturbations affect resilience | Reduction to 2D system using weighted averaging of empirical networks; parameters derived from 59 real-world datasets | Comparison of reduced model predictions with full network simulations; robustness tested under structural perturbations | Reduced model accurately predicts tipping points; resilience depends on network structure and interaction strength; framework generalizable to other complex systems |
| [66] | Compartmental population model for pyrophite shrub dynamics | Ulex parviflorus population structured by age and reproductive status; fire regimes influence biomass and regeneration; Mediterranean ecosystem context | Growth, flowering, and seed dispersal modeled via nonlinear differential equations; parameters calibrated from field data in Castellón, Spain | Numerical simulations of post-fire recovery and reproductive cycles; sensitivity analysis of biomass and seed bank dynamics | Pyrophite shrubs exhibit structured recovery under fire disturbance; reproductive success depends on spatial distribution and ecological thresholds |
| [67] | Three-species nonlinear ordinary differential equation model with limit cycle emergence | Plant–pollinator mutualism coupled with herbivory; herbivores reduce plant biomass and indirectly affect pollinator visitation; functional responses are nonlinear | Type IV functional responses for herbivory and mutualism; parameters selected for dynamical richness and ecological realism | Hopf-–Andronov bifurcation theorem applied; Lyapunov coefficient used to confirm stability of limit cycle; numerical simulations support analytical results | Demonstrated existence of a stable limit cycle; herbivory can destabilize mutualism and induce oscillatory dynamics; coexistence depends on interaction strength and saturation effects |
| [68] | Eco-evolutionary simulation model with genetic algorithm | Obligate pollination mutualism; plant and pollinator traits evolve under fitness trade-offs; genetic algorithm simulates adaptive dynamics | Heuristic Lotka–Volterra-type model; fitness landscapes and trait distributions encoded in algorithm; parameters varied across simulations | Zero-isocline analysis and trait distribution mapping; robustness tested across multiple evolutionary runs | Trade-offs between cost and benefit shape mutualist niches; genetic algorithm reveals multiple stable eco-evolutionary regimes; obligate mutualism can persist under constrained trait evolution |
| [69] | Network-based model with exploitative competition | Pollinators compete for shared plant resources; exploitative competition affects network topology and species abundance; mutualistic links evolve adaptively | Interaction matrix constructed from rewiring rules; competition strength varied systematically; parameters derived from network theory | Analytical inversion of interaction matrix; simulations of network evolution under competition pressure | Exploitative competition increases plant abundance; pollinator hubs emerge asymmetrically; network rewiring enhances mutualistic benefit while minimizing competition cost |
| [70] | Two-patch ordinary differential equation model with dispersal and mutualism | Pollinators and plants interact in two spatial patches; dispersal affects persistence and abundance; mutualism is patch-dependent | Resource-service exchange modeled via nonlinear terms; dispersal rates and survival thresholds calibrated from theoretical ecology | Stability analysis of equilibria; numerical simulations of dispersal scenarios and population trajectories | Dispersal enhances pollinator abundance even under low plant density; patch quality influences mutualistic outcomes; small dispersal can outperform non-dispersal in both persistence and productivity |
| [71] | Three-species ordinary differential equation model with intermediary nectar dynamics | Nectar acts as intermediary resource between plant and pollinator; nectar dynamics influence mutualism persistence; nectar rapidly reaches quasi-steady state in reduced model | Nectar decay, production, and consumption rates derived from the ecological literature; reduced model assumes fast nectar dynamics | Analytical comparison of full and reduced models; bifurcation analysis and numerical simulations | Initial nectar density critically affects pollinator survival; reduced model captures long-term dynamics; nectar-mediated feedback shapes coexistence thresholds |
| [72] | Conceptual synthesis of mutualistic network theory | Network structure and species traits jointly determine mutualism dynamics; adaptive foraging and trait matching improve predictive capacity | Parameters drawn from empirical datasets and theoretical models; emphasis on trait-based and mechanistic approaches | Comparative review of modeling frameworks; integration of empirical validation strategies | Advocates for biologically grounded models; trait-based and adaptive mechanisms enhance prediction of network responses to perturbations |
| [73] | Two-plant, one-animal model with adaptive foraging | Animal mediator (mutualist or exploiter) adapts foraging preferences; plant coexistence mediated by behavioral feedback; animal density fixed | Preferences evolve to maximize fitness; generalized isocline framework used; competition strength varied systematically | Differential inclusion and sliding mode analysis; numerical simulations of coexistence regimes | Exploiter generalism promotes coexistence under strong competition; mutualist specialization yields alternative stable states; adaptive behavior reshapes competitive outcomes |
| [74] | Three-species ordinary differential equation model with food and toxin production | Plant produces both nectar (mutualism) and toxin (defense); pollinator and herbivore interact with plant via distinct pathways; trade-offs govern coexistence | Functional responses include saturation and inhibition; toxin production modeled as dynamic trait; parameters derived from ecological experiment | Hopf bifurcation and persistence analysis; numerical simulations of oscillatory and steady-state regimes | Toxin production modulates herbivore suppression and pollinator survival; coexistence possible via intermediate defense levels; excessive defense leads to collapse |
| [75] | Three-species ordinary differential equation model with pesticide-induced mortality | Pesticides reduce pollinator survival and indirectly affect plant reproduction; mutualism depends on energetic reward; extinction thresholds exist | Mortality rates and reward thresholds derived from ecological theory; pesticide effects modeled as additive mortality terms | Stability analysis of equilibria; numerical simulations under varying pesticide intensities | High energetic reward can buffer pesticide impact; low reward leads to plant extinction; mutual dependence is sensitive to pesticide pressure |
| [76] | Ordinary differential equation model with nonlinear mutualism and pesticide feedback | Pollinator mortality increases with pesticide exposure; mutualism modeled via Beddington-–DeAngelis response; plant growth depends on pollination service | Pesticide toxicity modeled as a dynamic variable; parameters derived from empirical studies and theoretical ecology | Global stability and persistence analysis; bifurcation diagrams of extinction and coexistence regimes | Mutualism persists under moderate pesticide levels; excessive toxicity leads to collapse; coexistence thresholds depend on pollinator resilience and plant reward rates |
| [77] | Replicator and ordinary differential equation hybrid model with floral deception | Plants produce nectar-rich or nectarless flowers; pollinators adopt selective or non-selective foraging strategies; evolutionary game dynamics shape population structure | Nectar cost and cheater efficiency encoded in replicator equations; interaction terms derived from behavioral ecology | Bifurcation analysis of periodic and steady-state regimes; numerical simulations of invasion and persistence scenarios | Nectarless flowers can persist via cyclic dynamics; pollinator learning affects strategy evolution; coexistence depends on cost-benefit asymmetry and foraging discrimination |
| [78] | Three-species ordinary differential equation model with intermediary nectar dynamics | Nectar acts as intermediary resource between plant and pollinator; nectar dynamics influence persistence; plant cannot survive without pollination | Nectar decay, production, and consumption rates derived from ecological experiments; reduced model assumes fast nectar equilibrium | Analytical comparison of full and reduced models; global dynamics and persistence conditions derived rigorously | Initial nectar density determines persistence; low decay rates favor coexistence; intermediary resource mediates survival thresholds and system resilience |
| [79] | Nonlinear ordinary differential equation model with temperature-dependent mortality | Rising environmental temperature increases pollinator mortality and reduces plant reproduction; mutualism is sensitive to thermal stress | Temperature effects modeled via exponential mortality terms; ecological parameters derived from the climate and pollination literature | Local and global stability analysis; numerical simulations under varying temperature regimes | Elevated temperature reduces pollinator persistence and plant biomass; mutualism collapses under extreme warming; mitigation requires cooling interventions |
| [80] | Network-based co-adaptation model with dynamic link weights | Mutualistic networks adapt both structurally and dynamically; co-adaptation enhances resilience under perturbations; link weights evolve with species abundance | Link weights updated via feedback rules; empirical networks used for calibration; heterogeneity and connectance preserved | Comparative simulations of static, adaptive, and co-adaptive models; robustness tested under species loss scenarios | Co-adaptation increases resilience without altering connectance; dynamic feedback buffers against coextinction; model generalizable to other complex systems |
| [81] | Three-species ordinary differential equation model with antagonism between mutualists | Two mutualists share a partner species but interact antagonistically; antagonism may be consumptive or non-consumptive; life stages explicitly modeled | Interaction strengths and specialization levels varied systematically; antagonism encoded as direct negative feedback | Stability and persistence analysis; bifurcation exploration of oscillatory and extinction regimes | Antagonism reduces mutualist persistence; indirect effects dominate at high antagonism rates; specialization modulates system resilience |
| [82] | Empirical network analysis with epidemiological modeling | Landscape simplification alters plant-pollinator network structure; pathogen prevalence shaped by diet breadth and connectance; dilution effect emerges in complex networks | Eleven empirical networks analyzed; pathogen prevalence measured via molecular assays; network metrics computed from field data | Structural equation modeling and simulation of disease dynamics; robustness tested across landscape gradients | Simplified landscapes increase pathogen prevalence; higher connectance reduces outbreak risk; dominant species’ diet breadth mediates community-level infection patterns |
| [83] | Delay differential equation model with empirical calibration | Fruit yield in dioecious crops depends on orchard layout, flower sex ratio, and pollinator density; pollinator behavior has diminishing returns at high density | Empirical data from kiwifruit orchards in New Zealand; Latin hypercube sampling used for sensitivity analysis | Simulations of fruit set under varying orchard configurations; model validated against field observations | Plant traits and layout more influential than pollinator density; optimal yield achieved with 65–75% female flowers and bees per 1000 flowers |
| [84] | Fractional-order differential model with Atangana-–Baleanu derivative | Nectar acts as intermediary resource; fractional calculus captures memory effects and non-locality in pollination dynamics | Fractional order varied systematically; stability analyzed via Picard-–Lindelöf method | Numerical simulations using Adams–Bashforth scheme; stability tested across fractional orders | Fractional models outperform classical ODEs in capturing system memory; persistence depends on nectar dynamics and fractional order |
| [85] | Mechanistic transmission model with empirical viral assays | Deformed wing virus (DWV) transmits between bee species via shared flowers; transmission is bidirectional and density-dependent | Laboratory experiments with Apis mellifera and Bombus impatiens; viral load quantified via molecular assays | Mathematical simulations of transmission dynamics; dilution effect tested via floral abundance scenarios | DWV spreads via shared floral resources; increasing floral abundance reduces transmission; managing Varroa mites in honeybees mitigates spillover |
| [86] | Ordinary differential equation model with adaptive foraging by herbivores and pollinators | Two plants compete for shared mutualists and exploiters; animal preferences adapt to plant density; indirect interactions shape coexistence | Trait-mediated interactions modeled via isolegs and ideal free distribution; parameters varied across consumer abundance | Stability analysis of alternative states; simulations of coexistence under adaptive behavior | Adaptive preferences promote coexistence at high consumer abundance; low abundance leads to specialization and exclusion; insect decline alters plant community structure |
| [87] | Adaptive evolutionary model of nectar provisioning traits | Plants evolve nectar traits (production rate and reservoir volume) under selective pressures from pollinator interactions; traits co-evolve as a suite | Cost-benefit trade-offs modeled explicitly; ecological constraints include pollinator conversion efficiency and plant productivity | Analytical exploration of evolutionary equilibria; simulations of trait dynamics under varying ecological conditions | Higher nectar provisioning evolves under pollinator limitation, compensatory investment across traits stabilizes mutualism; indirect selection shapes trait architecture |
| [88] | Stochastic–deterministic hybrid model for hoverfly pollination control | Tomato flowers lack nectar; hoverfly density must be supplemented via feeding; pollination success depends on adult density and feeding strategy | Stochastic model estimates required hoverfly density; deterministic optimal control model minimizes feeding cost; parameters derived from greenhouse crop data | Simulation of pollinator dynamics and fruit yield; theoretical validation of control strategy under economic constraints | Supplementary feeding maintains economically viable hoverfly density; optimal control reduces cost; model supports hoverfly-based pollination in nectar-deficient crops |
| [89] | Epidemiological synthesis with genotype replacement modeling | DWV-A and DWV-B genotypes co-circulate in honeybee populations; DWV-B exhibits higher transmission and virulence; genotype interference affects prevalence | Global dataset (2008–2021) analyzed; mathematical model incorporates genotype competition and host co-infection dynamics | Empirical prevalence data from Germany, Italy, and the UK; model predictions compared to observed genotype shifts | DWV-B is replacing DWV-A globally; genotype interference drives replacement; implications for wild pollinators and beekeeping practices |
| [90] | Dose–response transmission model with pathogen transport | Pathogen transport via mechanical vectors (e.g., pollinators) alters exposure distribution; transmission depends on host dose–response curve | Two transmission scenarios modeled: amplification and dilution; dose–response functions derived from empirical infection thresholds | Analytical derivation of infection risk under varying transport regimes; simulations of disease spread in pollinator networks | Transport amplifies or dilutes transmission depending on dose–response shape; oversimplified models misestimate risk; framework improves epidemiological predictions |
| [91] | Consumer–resource ordinary differential equation models with reproductive benefit mechanisms | Plant reproductive benefits arise via pollination or seed dispersal; benefits affect seed set, germination, or recruitment; mutualism may be obligate or facultative | Foraging rate functions and benefit pathways modeled explicitly; parameters varied across ecological scenarios | Stability and bifurcation analysis; simulations of low-density thresholds and Allee effects | Pollination and seed dispersal mutualisms exhibit distinct dynamics; bistability and collapse thresholds depend on benefit mechanism and partner density |
| [92] | Network-based resilience analysis with dimension reduction | Hybrid ecological networks include mutualism, herbivory, and antagonism; resilience assessed via species contribution and extinction vulnerability | Interaction matrices constructed from hybrid network topology; resilience evaluated via reduced-dimensional metrics | Simulation of species removal and perturbation scenarios; resilience mapped across species categories | Strong contributors to resilience are more extinction-prone; plants are most vulnerable; network structure influences robustness under perturbation |
| [93] | Non-autonomous nonlinear ordinary differential equation model with seasonal forcing and parasitism | Honey bee population dynamics influenced by seasonal egg-laying and parasitism (e.g., Varroa mites); seasonality modulates colony resilience | Seasonality encoded via time-dependent birth rates; parasitism modeled as density-dependent mortality; parameters derived from empirical data | Hopf bifurcation analysis; simulations of collapse and recovery under seasonal and parasitic stress | Seasonality can stabilize or destabilize colonies depending on timing; parasitism induces collapse via bifurcation; synergistic effects shape colony survival |
| [94] | Delay differential equation model with dual time lags | Plant–pollinator interactions subject to two distinct delays (e.g., response and maturation); delays influence stability and oscillatory behavior | Characteristic equations derived from delay structure; delays treated as bifurcation parameters; ecological rates assumed to be constant | Center manifold and normal form theory applied; numerical simulations of periodic solutions and stability regions | Multiple delays induce Hopf bifurcations and periodic dynamics; delay asymmetry affects system persistence; explicit conditions derived for oscillation onset |
| [95] | Analytical framework for structural stability in mutualistic–competitive networks | Mutualistic networks include interspecific competition among plants and pollinators; structural stability defined as coexistence feasibility under perturbation | Total of 50 empirical networks used; competition encoded via weighted adjacency matrices; mutualism modeled with saturating responses | Analytical derivation of feasibility domains; numerical simulations of stability under network rewiring | Competitive structure strongly influences coexistence; mutualism alone insufficient for stability; new metric links network architecture to resilience thresholds |
| [96] | Eco-evolutionary model with adaptive disinvestment | One-sided population decline triggers adaptive reduction in mutualistic investment; feedback loops can accelerate collapse or stabilize interaction | Trade-off between independent growth and mutualistic investment modeled as concave function; adaptation rates varied systematically | Simulations of co-evolutionary trajectories; bifurcation analysis of collapse and recovery regimes | Disinvestment by undisturbed partner precedes collapse; slow adaptation or high initial investment delays extinction; co-evolution essential for recovery |
| [97] | Socio-mutualistic network model with optimal conservation strategy | Pollinator dynamics coupled with human conservation norms; tipping points emerge from structural and behavioral feedbacks | Network topology varied across nestedness levels; conservation norms applied selectively to pollinator nodes | Dynamical analysis of reduced model; simulations across empirical and synthetic networks | Optimal conservation strategy prevents collapse with minimal intervention; intermediate nestedness most responsive; social norms amplify resilience |
| [98] | Generalized stressor model for hive and forager bees | Stressors affect bees via transmissibility, lethality, impairment, and timing; colony collapse emerges from labor destabilization and precocious foraging | Stressor attributes generalized across multiple dimensions; model integrates prior CCD frameworks; parameters derived from empirical studies | Validated against emergent colony behaviors; simulations of collapse thresholds under stressor variation | Sublethal stressors sufficient to trigger collapse; timing and impairment level critical; model supports holistic stressor management in conservation |
| [99] | Ordinary differential equation based community model with antagonism–mutualism continuum | Herbivores reduce pollinator visitation via indirect limitation; mutualistic and antagonistic interactions co-occur; stability assessed across interaction gradients | Interaction strengths varied systematically; network architecture encoded via adjacency matrices; parameters derived from ecological literature | Temporal and compositional stability analyzed; simulations of species persistence and network robustness | Pollinator limitation enhances both temporal and compositional stability; herbivory indirectly promotes persistence; network architecture–stability relationship shifts under limitation |
| [100] | Behaviorally modified predator–prey–mutualism model | Predation on pollinators alters foraging behavior; behavioral avoidance stabilizes mutualism; direct predation alone insufficient for stability | Pollinator behavior modeled via adaptive response functions; predation rates and avoidance thresholds varied across scenarios | Stability analysis of modified mutualism; simulations of predator-induced behavioral shift | Behavioral modification stabilizes mutualism under predation; avoidance behavior critical for persistence; predator pressure reshapes interaction dynamics |
| [101] | Network-based thermal response model with tipping point detection | Climate warming alters species-level physiological traits; mutualistic networks exhibit tipping points under thermal stress; generalists play stabilizing roles | Total of 139 empirical networks analyzed; thermal sensitivity encoded via trait-dependent growth and mortality rates | Reduced two-dimensional model used for bifurcation analysis; simulations across temperature gradients | Rising temperature induces rapid transitions in low-strength networks; generalists delay collapse; network structure and thermal traits jointly determine resilience |
| [102] | Partial differential equation system with chemotaxis-enhanced reaction rates | Chemotaxis enhances biological reaction efficiency; organisms move toward chemical gradients; surface chemotaxis considered for biological realism | Chemotactic flux modeled via Keller–Segel-type terms; reaction rates compared with pure diffusion; parameters derived from biological signaling contexts | Analytical estimates of convergence rates; sharp inequalities derived for Fokker–Planck operators | Chemotaxis significantly enhances reaction success; convergence to equilibrium accelerated; framework applicable to immune signaling, reproduction, and pollination |
| [103] | Trait-based evolutionary model with trophic structure | Plants and pollinators co-evolve under mutualistic and competitive pressures; trait polymorphisms emerge via Darwinian dynamics; niche breadth influences diversification | Trait matching and competition encoded via continuous trait space; ecological rates derived from trophic interactions | Analytical derivation of evolutionary branching conditions; simulations of polymorphism emergence | Broad plant niches promote phenotypic diversification; mutualistic generalism triggers cascading trait divergence; co-diversification driven by bottom-up control |
| [104] | Delay differential equation model with brood mortality | Brood deaths induced by insecticide exposure affect colony dynamics; time delay represents developmental lag; collapse thresholds explored | Mortality and delay parameters derived from empirical bee life cycle data; insecticide effects modeled as external forcing | Stability and sensitivity analysis of equilibria; numerical simulations with real data | Brood mortality significantly reduces colony resilience; no Hopf bifurcation within biologically plausible delay range; model supports policy design for insecticide regulation |
| [105] | Stochastic branching process model with behavioral resistance | Grooming and hygienic behaviors reduce DWV outbreak probability in Varroa-infested colonies; transmission occurs via vector and contact routes | Transmission probabilities and behavioral efficacy derived from empirical studies; branching process theory applied to outbreak dynamics | Analytical computation of outbreak probabilities; simulations across behavioral scenarios | Hygienic behavior most effective at reducing DWV outbreaks; grooming reduces vector transmission to near zero; behavioral selection enhances colony survival |
| [106] | Nonlinear dynamical system with bifurcation analysis in parameter space | Honeybee—mite interactions exhibit multistability, chaos, and bifurcation structures; parameter variation reveals complex attractor landscapes | Interaction rates and mortality parameters varied systematically; bifurcation parameters mapped across biologically relevant ranges | Numerical exploration of phase space; identification of Arnold tongues, jellyfish structures, and saddle regions | System exhibits rich dynamical regimes including chaos and multistability; parameter tuning critical for colony persistence; visual structures aid in ecological interpretation |
| [107] | Three-species nonlinear ordinary differential equation model with limit cycle dynamics | Pollinators and plants form a mutualistic pair; herbivores consume plants and indirectly reduce pollinator visitation; functional responses are of type IV | Saciety and saturation effects encoded via nonlinear terms; ecological rates selected for dynamical richness | Hopf–Andronov bifurcation theorem applied; Lyapunov coefficient used to confirm stability of limit cycle; numerical simulations support analytical results | Herbivory destabilizes mutualism and induces oscillatory dynamics; coexistence depends on interaction strength and saturation thresholds |
| [108] | Consumer–resource network model with adaptive foraging | Floral resource availability constrains pollinator specialization; network structure emerges from adaptive foraging and resource depletion | Lotka–Volterra framework with dynamic floral resource pool; parameters varied across resource and animal density gradients | Generalized linear models used to explain nestedness, modularity, and specialization; simulations across 3812 networks | Low floral resource availability reduces specialization; resource dynamics distinct from plant density; adaptive foraging mediates network structure |
| [109] | Adaptive dynamics model of floral handedness evolution | Mirror-image flowers evolve from mixed to fixed handedness; stylar orientation affects pollination efficiency and mating patterns; inbreeding depression penalizes geitonogamy | Trait evolution modeled via adaptive dynamics; genetic architecture assumed plausible; ecological parameters include pollinator efficiency and plant density | Stability analysis of dimorphic vs. monomorphic states; population genetics simulations validate transitions | Dimorphic enantiostyly evolves under moderate inbreeding and high pollination efficiency; ecological shifts may reverse dimorphism; model supports convergence in floral asymmetry |
| [110] | Population dynamics model of lethal deceptive pollination | Plants trap and kill pollinators without reward; coexistence depends on vegetative reproduction and sex transition rates; disturbances affect demographic stability | Empirical data from Arisaema species used to calibrate model; disturbance scenarios include deer abundance, habitat loss, and plant theft | Stability analysis and extinction thresholds explored; simulations under multiple disturbance regimes | Lethal deception maintained under high vegetative reproduction and slow sex transition; co-extinction risk elevated under strong attraction and anthropogenic disturbance |
| [111] | Impulsive reaction–diffusion model on a periodically evolving domain | Plant–pollinator system subject to periodic habitat changes and impulsive disturbances; domain evolution affects dispersal and persistence | Ecological reproduction index defined; impulsive effects modeled via discontinuous terms; domain evolution rate treated as bifurcation parameter | Upper–lower solution method applied; numerical simulations of extinction and persistence scenarios | High domain evolution rate promotes pollinator survival; impulsive effects can override spatial benefits; coexistence depends on timing and magnitude of impulses |
| [112] | Stochastic partial differential equation model with degenerate diffusion | Plant–pollinator dynamics influenced by environmental noise; degenerate diffusion captures spatial heterogeneity and dispersal limitations | Diffusion coefficients vary with population density; stochastic terms modeled via Brownian motion; permanence conditions derived analytically | Stochastic comparison principle and Lyapunov function used; extinction and persistence regions identified | Degenerate diffusion enhances extinction risk under low density; stochasticity can stabilize or destabilize dynamics; permanence requires bounded noise intensity |
| [113] | Mechanistic ordinary differential equation model with separate pollen and nectar dynamics | Pollinators consume nectar and transfer pollen independently; interspecific pollen transfer reduces pollination efficiency; mutualism may shift to antagonism | Pollen and nectar modeled as distinct resources; pollinator efficiency and interference encoded via saturating functions | Analytical derivation of pollination efficiency; simulations of mutualism-antagonism transitions | Separation of pollen and nectar dynamics reveals hidden antagonism; interspecific pollen transfer reduces plant fitness; ecological context determines net interaction outcome |
| [115] | Nonlinear dynamical system with predation on pollinators | Predators consume pollinators and destabilize mutualism; plant—pollinator interaction saturates at high density; predator–pollinator dynamics coupled | Saturated mutualism modeled via Holling-type functions; predation rate and initial density varied across simulations | Stability and bifurcation analysis; numerical exploration of transcritical and Hopf bifurcations | High predator density leads to pollinator extinction; mutualistic strength buffers predation impact; coexistence possible under low predation and strong mutualism |
| [114] | Tripartite ordinary differential equation model with facultative and obligate mutualism and parasitism | Plant–pollinator–parasite system; pollinators may be facultative or obligate; parasites affect pollinator fitness and system stability | Facultative and obligate behavior encoded via growth and mortality terms; bifurcation parameters include parasite conversion and death rates | Bifurcation analysis of subsystems and full model; numerical simulations of multistability and oscillatory regimes | Facultative pollinators enhance resilience via multistability; obligate systems prone to collapse; novel bifurcation patterns (e.g., wavebow) characterize amplitude transitions |
| [116] | Delayed reaction, diffusion model with memory-based diffusion | Pollinator movement influenced by spatial memory; delay affects diffusion and pattern formation; periodicity emerges from delay–diffusion interplay | Memory delay incorporated via modified Fick’s law; diffusion coefficients and delay terms varied systematically | Hopf bifurcation and Lyapunov–Schmidt reduction applied; simulations of spatial and temporal periodic solution | Memory-based diffusion induces spatial heterogeneity and oscillations; delay triggers stability switch; model captures biologically realistic movement biases |
| Modeling Domain | Assumptions | Parametrization | Validation | Findings | Policy | Works |
|---|---|---|---|---|---|---|
| Biological consistency and population viability | Non-negative, bounded population variables. | Logistic or saturating growth; constrained initial conditions. | Analytical consistency. | Avoids spurious extinction or explosion. | Supports viability thresholds. | [15,19,20] |
| Long-term dynamics and species persistence | Equilibrium-based persistence or extinction. | Reproduction, mortality, and interaction sensitivity. | Stability via Lyapunov function and linearization. | Identifies resilience thresholds. | Informs sustainability planning. | [10,11,12] |
| Regime shifts and critical transitions | Threshold-driven qualitative change. | Bifurcation parameters. | Bifurcation diagrams. | Reveals tipping points. | Enables adaptive management. | [16,30,53] |
| Mutualism and network structure | Mutualistic coexistence and spatial structure. | Patch connectivity and trait evolution. | Network simulations. | Biodiversity maintenance. | Corridor design and zoning. | [13,16,18] |
| Seasonal and oscillatory behavior | Seasonal forcing and delay effects. | Time-dependent coefficients. | Periodicity analysis. | Captures seasonal fluctuations. | Supports crop planning. | [30,47,65] |
| Simulation and empirical calibration | Empirical realism. | Optimization and sensitivity analysis. | Simulation observed data. | Enhances credibility. | Enables data-driven decisions. | [19,25] |
| Intervention and optimization | Ecological outcomes modifiable via control. | Pontryagin’s maximum principle and dynamic programming. | Optimality conditions. | Cost-effective strategies. | Informs adaptive conservation. | [88,97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huancas, F.; Coronel, A.; Lozada, E.; Torres, J. Mathematical Modeling of Population Dynamics of Pollinators: A Survey. Biology 2025, 14, 1308. https://doi.org/10.3390/biology14091308
Huancas F, Coronel A, Lozada E, Torres J. Mathematical Modeling of Population Dynamics of Pollinators: A Survey. Biology. 2025; 14(9):1308. https://doi.org/10.3390/biology14091308
Chicago/Turabian StyleHuancas, Fernando, Anibal Coronel, Esperanza Lozada, and Jorge Torres. 2025. "Mathematical Modeling of Population Dynamics of Pollinators: A Survey" Biology 14, no. 9: 1308. https://doi.org/10.3390/biology14091308
APA StyleHuancas, F., Coronel, A., Lozada, E., & Torres, J. (2025). Mathematical Modeling of Population Dynamics of Pollinators: A Survey. Biology, 14(9), 1308. https://doi.org/10.3390/biology14091308






