Evaluation of Irisin and Interleukin-6 Levels in Saliva Samples of Periodontally Healthy and Stage 3 Grade C Periodontitis Individuals
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Study Design
2.2. Sample Size Calculation
2.3. Participant Grouping
- Periodontally healthy individuals (n = 33);
- Individuals diagnosed with Stage 3 Grade C periodontitis (n = 33).
2.4. Inclusion and Exclusion Criteria
2.4.1. Inclusion Criteria
- Aged between 18 and 60 years;
- No history of systemic disease;
- Classified as either periodontally healthy or having Stage 3 Grade C periodontitis.
2.4.2. Exclusion Criteria
- Use of immunosuppressive medications;
- Pregnancy or lactation;
- Menopause or andropause;
- Periodontal treatment within the past six months;
- Antibiotic use within the past three months;
- Smoking;
- History of systemic disease;
- History of radiotherapy to the head or neck;
- Fewer than 15 remaining teeth.
2.5. Participant Enrollment and Data Collection
2.6. Periodontal Examination
- Plaque Index (PI);
- Probing Depth (PD, mm);
- Clinical Attachment Loss (CAL, mm);
- Bleeding on Probing (BOP, % of sites).
2.7. Saliva Collection and Analysis
- Human Interleukin-6 (IL-6) ELISA Kit (Catalog No: 201-12-1308, SunRed Biotechnology, Shanghai, China);
- Human Irisin ELISA Kit (Catalog No: 201-12-5328, SunRed Biotechnology, Shanghai, China).
2.8. KEGG and Reactome Pathway Enrichment Analysis
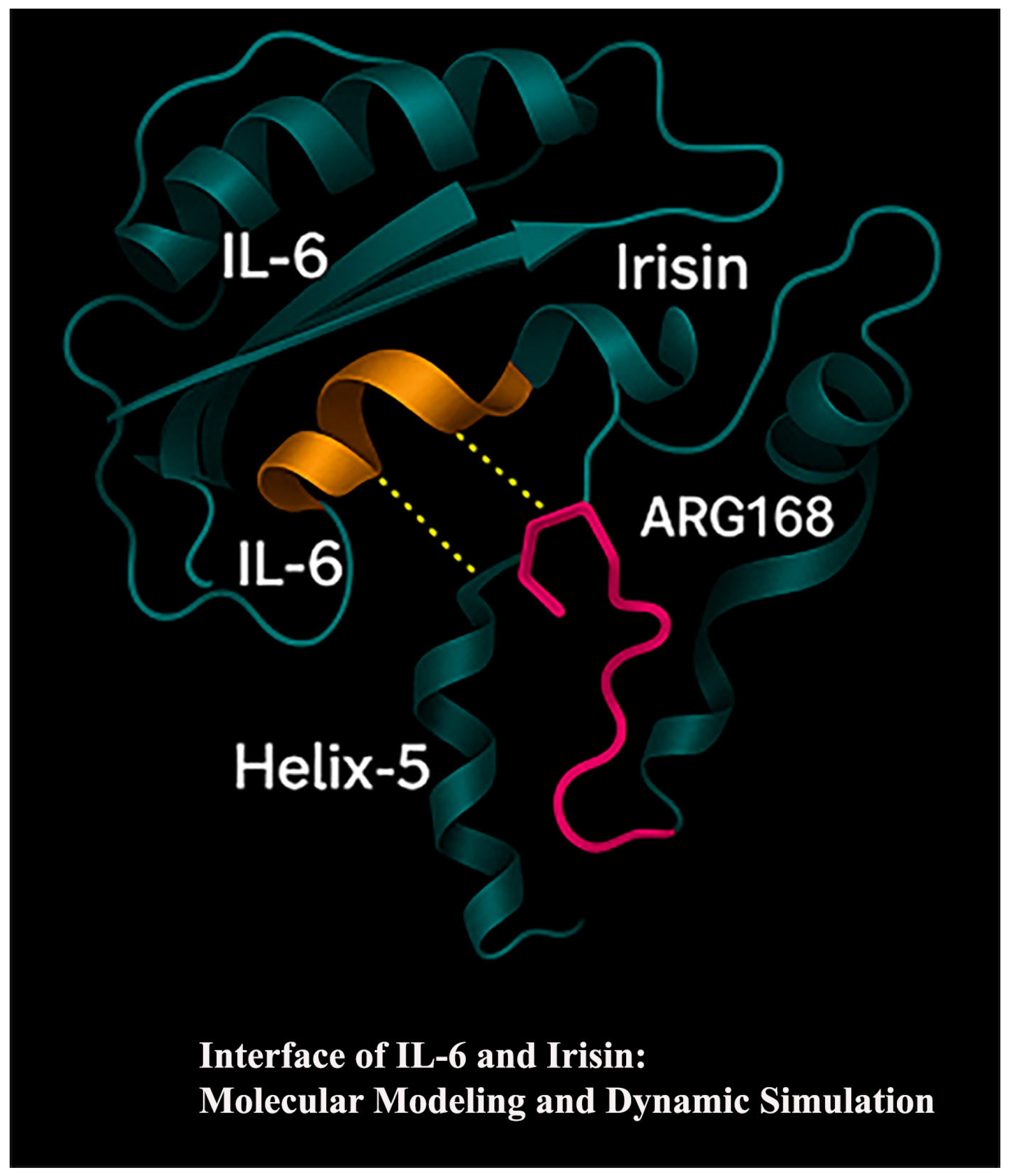
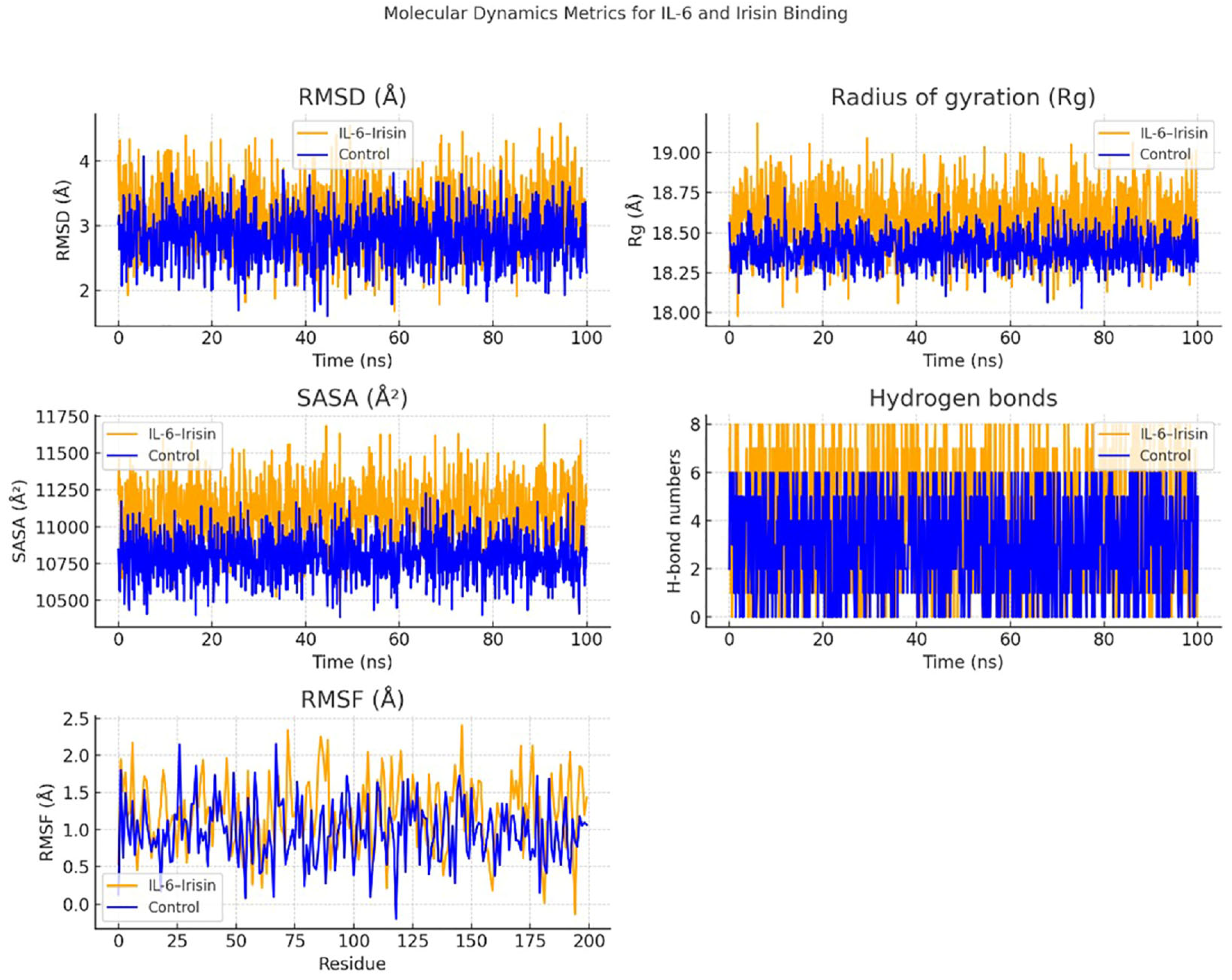
2.9. Molecular Docking Analysis
2.10. Statistical Analysis
3. Results
3.1. Comparison of Clinical and Biochemical Parameters Between Groups
3.2. Correlation Analysis Between Clinical Parameters and Salivary Biomarkers
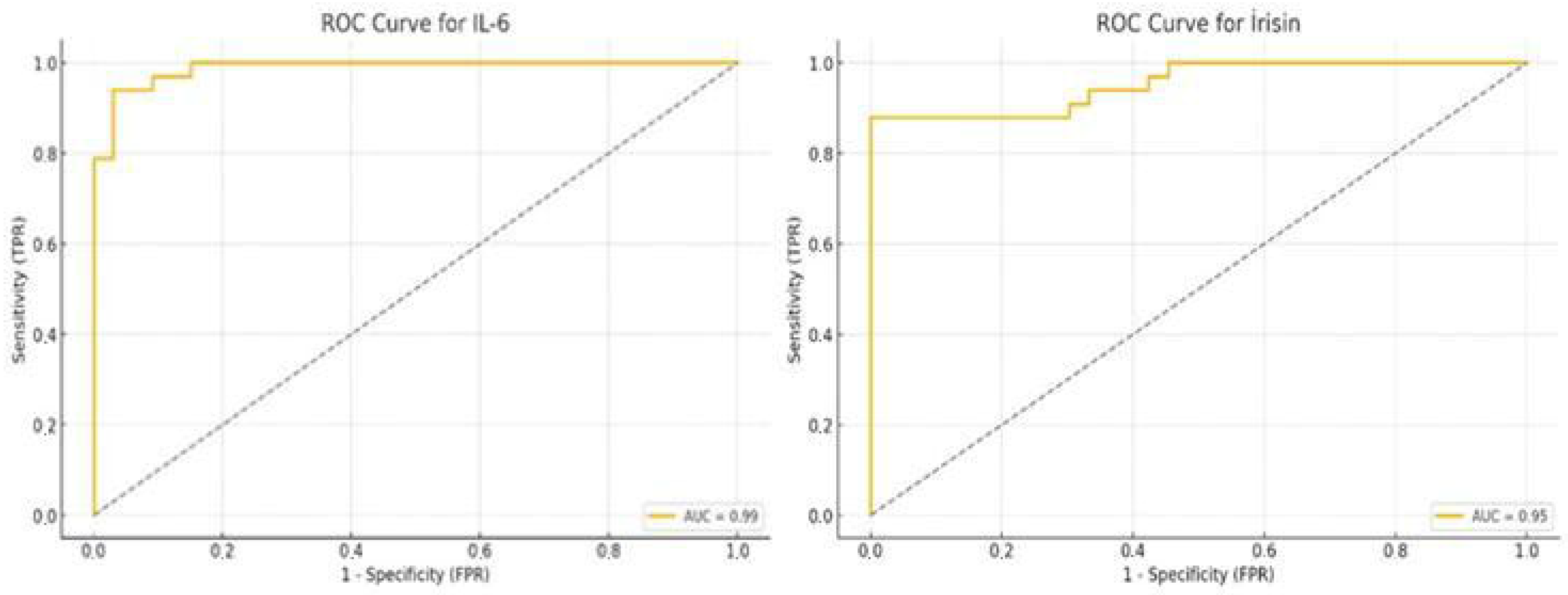
3.3. ROC Curve Analysis of Salivary IL-6 and Irisin
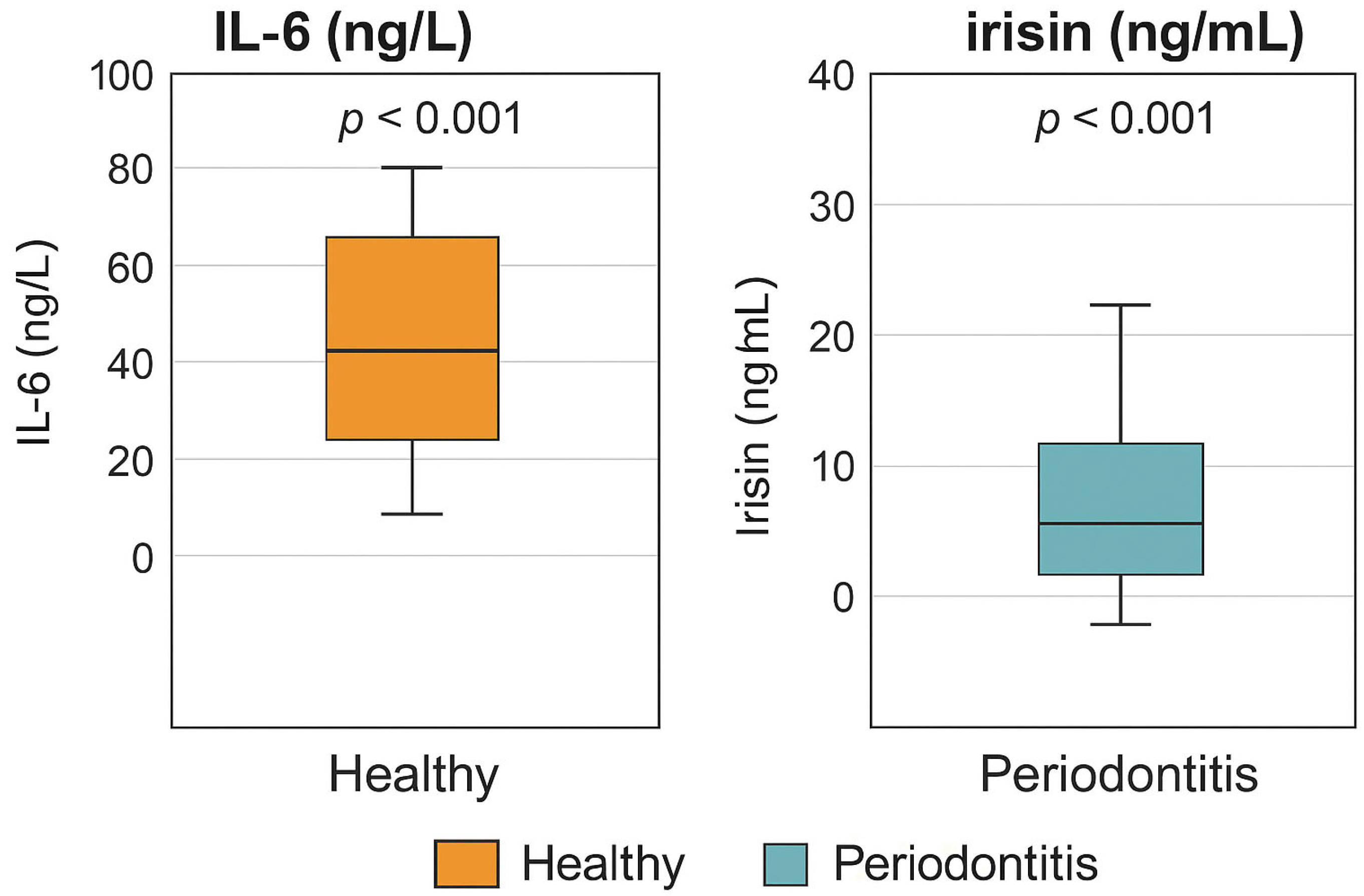
3.4. Visual Comparison of Biomarker Levels
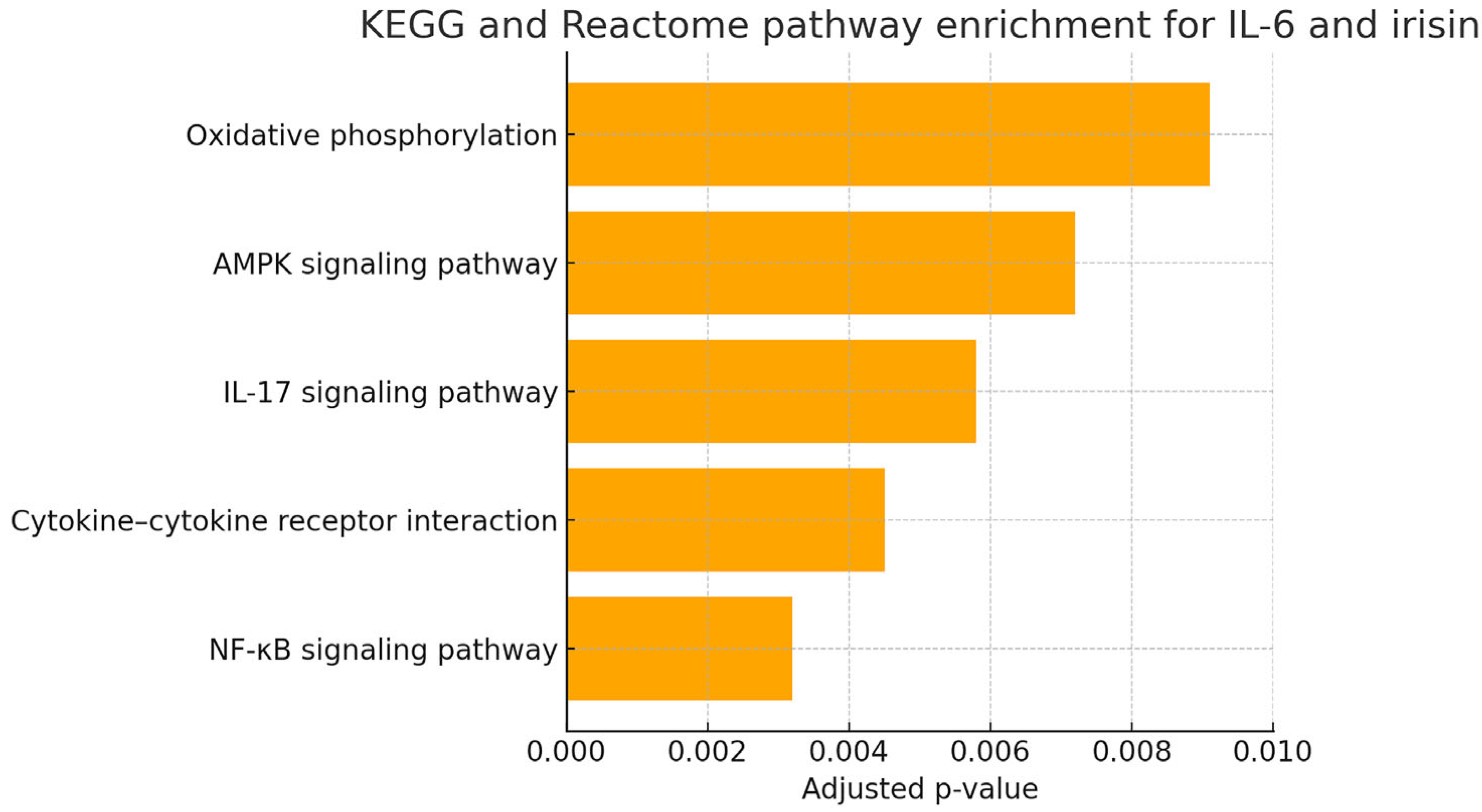
3.5. In Silico Functional Enrichment Analysis of IL-6 and Irisin-Associated Pathways
3.6. IL-6 and Irisin in the Molecular Pathogenesis of Periodontitis: An In Silico Approach
- NF-κB signaling pathway: central to the regulation of inflammation and cytokine production.
- Cytokine–cytokine receptor interaction: mediating immune cell recruitment and activation in periodontal tissues.
- IL-17 signaling pathway: known to exacerbate neutrophilic inflammation and bone resorption.
- AMPK signaling pathway: linking metabolic stress to inflammatory signaling.
- Oxidative phosphorylation: indicating possible involvement of mitochondrial function and redox imbalance.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González Gómez, L.A.; Guedea Preciado, N.E.; Martínez Nieto, M.; Rodríguez Montaño, R.; Alarcón-Sánchez, M.A.; Tor-res-Sánchez, E.D.; Gómez Mireles, J.C.; Acevedo Ambriz, E.; Lomelí Martínez, S.M. Multidisciplinary treatment of epulis fissuratum: A case report. World J. Clin. Cases 2025, 13, 106413. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Foley, J.D.; Bailey, A.L.; Campell, C.L.; Humphries, R.L.; Christodoulides, N.; Floriano, P.N.; Simmons, G.; Bhagwandin, B.; Jacobson, J.W.; et al. Current developments in salivary diagnostics. Biomark. Med. 2010, 4, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Relvas, M.; Mendes-Frias, A.; Gonçalves, M.; Salazar, F.; López-Jarana, P.; Silvestre, R.; Viana da Costa, A. Salivary IL-1β, IL-6, and IL-10 Are Key Biomarkers of Periodontitis Severity. Int. J. Mol. Sci. 2024, 25, 8401. [Google Scholar] [CrossRef]
- Irwin, C.R.; Myrillas, T.T. The role of IL-6 in periodontal disease. Oral Dis. 1998, 4, 43–47. [Google Scholar] [CrossRef]
- Lehrskov, L.L.; Christensen, R.H. The role of interleukin-6 in glucose homeostasis and lipid metabolism. Semin Immunopathol. 2019, 41, 491–499. [Google Scholar] [CrossRef]
- Teles, F.; Wang, Y.; Hajishengallis, G.; Hasturk, H.; Marchesan, J.T. Impact of systemic factors in shaping the periodontal microbiome. Periodontol. 2000 2021, 85, 126–160. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Kornel, A.; Den Hartogh, D.J.; Klentrou, P.; Tsiani, E. Role of the myokine irisin on bone homeostasis. Int. J. Mol. Sci. 2021, 22, 9136. [Google Scholar] [CrossRef]
- Turkmen, E.; Uzun, E.V.; Bozaba, F.; Balci, N.; Toygar, H. Salivary irisin level is higher and related with interleukin-6 in generalized periodontitis. Clin. Oral. Investig. 2023, 27, 3001–3008. [Google Scholar] [CrossRef]
- Khan, S.U.; Ghafoor, S.; Khaliq, S.; Syed, A.R. Salivary Irisin and periodontal clinical parameters in patients of chronic periodontitis and healthy individuals: A novel salivary myokine for periodontal disease. J. Pak. Med. Assoc. 2022, 72, 27–33. [Google Scholar] [PubMed]
- Skelton, M.; Callahan, C.; Levit, M.; Finn, T.R.; Kister, K.; Matsumura, S.; Cantos, A.; Shah, J.; Wadhwa, S.; Yin, M.T. Men with HIV have increased alveolar bone loss. BMC Oral Health 2024, 24, 1248. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Mochizuki, Y.; Miyata, Y.; Obata, Y.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Mukae, H.; Yoshimura, A.; Nishino, T.; et al. Pathological Characteristics of Periodontal Disease in Patients with Chronic Kidney Disease and Kidney Transplantation. Int. J. Mol. Sci. 2019, 20, 3413. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Balci, N.; Kurgan, Ş.; Çekici, A.; Çakır, T.; Serdar, M.A. Free amino acid composition of saliva in patients with healthy periodontium and periodontitis. Clin. Oral Investig. 2021, 25, 4175–4183. [Google Scholar] [CrossRef]
- Hashim, N.T.; Fathima, S.; Hisham, N.M.; Shivappa, P.; Magaogao, M.V.; Islam, M.S.; Ahmed, S.F.; Babiker, R.; Rahman, M.M. Exploring Salivary Alpha-Amylase as a Biomarker in Periodontitis: A Comparative Analysis of Disease Stages and Clinical Correlations. Curr. Issues Mol. Biol. 2024, 46, 12230–12243. [Google Scholar] [CrossRef]
- Padalkar, P.; Yadadi, S.S.; Vivekanandan, G.; Shetty, S.R.; Andhare, M.; Pashine, A.; Vinay, V.; Desai, V.; Shetty, R.M. Salivary periostin levels as a non-invasive biomarker and their clinical correlates among healthy and periodontitis patients-a cross-sectional analytical study. Front. Dent. Med. 2025, 6, 1512252. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontology 2000 1997, 14, 33–53. [Google Scholar] [CrossRef]
- Wang, P.L.; Oido-Mori, M.; Fujii, T.; Kowashi, Y.; Kikuchi, M.; Suetsugu, Y.; Tanaka, J.; Azuma, Y.; Shinohara, M.; Ohura, K. Effect of anti-CD14 antibody on experimental periodontitis induced by Porphyromonas gingivalis lipopolysaccharide. Jpn. J. Pharmacol. 2002, 89, 176–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boggess, K.A.; Lieff, S.; Murtha, A.P.; Moss, K.; Beck, J.; Offenbacher, S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet. Gynecol. 2003, 101, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Yaghobee, S.; Hasannia, S.; Hamidzadeh, F.; Valadan Tahbaz, S.; Shahmohammadi, R.; Poursafar, F. Evaluation of Pentraxin-3 and Interleukin-6 Levels in Serum and Gingival Crevicular Fluid in Patients with Generalized Periodontitis and Periodontal Health Controls before and after Scaling and Root Planing. Front. Dent. 2025, 22, 1. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Nagarajan, R.; Akers, D.; Miller, C.S. Targeted salivary biomarkers for discrimination of periodontal health and disease(s). Front. Cell Infect. Microbiol. 2015, 5, 62. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 2–18. [Google Scholar] [CrossRef]
- Kerkis, I.; da Silva, Á.P.; Araldi, R.P. The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neu-rological conditions. Front. Immunol. 2024, 15, 1400533. [Google Scholar] [CrossRef]
- Rodríguez-Montaño, R.; Ruiz-Gutiérrez, A.d.C.; Martínez-Rodríguez, V.M.d.C.; Gómez-Sandoval, J.R.; Guzmán-Flores, J.M.; Becerra-Ruiz, J.S.; Zamora-Perez, A.L.; Guerrero-Velázquez, C. Levels of IL-23/IL-17 Axis in Plasma and Gingival Tissue of Periodontitis Patients According to the New Classification. Appl. Sci. 2022, 12, 8051. [Google Scholar] [CrossRef]
- Ando, Y.; Tsukasaki, M.; Huynh, N.C.; Zang, S.; Yan, M.; Muro, R.; Nakamura, K.; Komagamine, M.; Komatsu, N.; Okamoto, K.; et al. The neutrophil-osteogenic cell axis promotes bone destruction in periodontitis. Int. J. Oral Sci. 2024, 16, 18. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Graves, D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Sahin, I.; Kalayci, M.; Sarman, E.; Kaya, N.; Yilmaz, O.F.; Turk, A.; et al. Irisin: A potentially candidate marker for myocardial infarction. Peptides 2014, 55, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Perdonsini, L.S.C.; Roth, J.V.S.; Ribas, P.A.T.; Borges, M.O.; Takarada, H.K.; Reitmeyer, F.B.; Zielak, J.C.; Steffens, J.P. Expression of endogenous Irisin and associated markers during periodontitis progression and repair in male rats. Periodont. Implant. Res. 2025, 9, 15. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating irisin in healthy, young individuals: Day–night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Al-Nimer, M.S. Interaction between inflammatory bowel disease, physical activity, and myokines: Assessment of serum irisin levels. World J. Gastroenterol. 2024, 30, 2923–2926. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Schuster, J.L.; Stevens, J.; Dawson, D., 3rd; Kryscio, R.J.; Lin, Y.; Thomas, M.V.; Miller, C.S. Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. J. Clin. Immunol. 2013, 33, 271–278. [Google Scholar] [CrossRef]
- Toraman, A.; Sağlam, E.; Savran, L.; Köseoğlu, S. Evaluation of Salivary Il-38 Levels in Periodontitis: A Cross-Sectional Study. J. Interferon Cytokine Res. 2025, 45, 76–82. [Google Scholar] [CrossRef]
- Blanco-Pintos, T.; Regueira-Iglesias, A.; Seijo-Porto, I.; Balsa-Castro, C.; Castelo-Baz, P.; Nibali, L.; Tomás, I. Accuracy of pe-riodontitis diagnosis obtained using multiple molecular biomarkers in oral fluids: A systematic review and me-ta-analysis. J. Clin. Periodontol. 2023, 50, 1420–1443. [Google Scholar] [CrossRef]
- Rocha, A.C.S.D.; Orlandini, R.K.; Motta, A.C.F.; Pinheiro, J.B.; Silva, G.A.E.; Oliveira, V.C.; Lourenço, A.G. Variability of salivary analytes under daily conditions and their implications for periodontitis biomarkers. Front. Dent. Med. 2024, 5, 1369186. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]

| Parameter | Healthy (C, n = 33) | Periodontitis (P, n = 33) | Test | p-Value |
|---|---|---|---|---|
| Age (years) | 28.73 ± 5.20 | 45.70 ± 6.77 | Mann–Whitney U | p < 0.001 * |
| Sex (Male/Female) | 15/18 | 16/17 | Chi-square | 0.82 (n.s.) |
| BMI (kg/m2) | 23.34 ± 1.10 | 23.46 ± 1.13 | t-test | 0.685 |
| Educational background | 55% Univ./45% HS | 52% Univ./48% HS | Chi-square | 0.79 (n.s.) |
| Oral hygiene (regular brushing ≥2/day) | 58% | 55% | Chi-square | 0.77 (n.s.) |
| PD (mm) | 1.77 ± 0.36 | 5.02 ± 0.40 | t-test | p < 0.001 * |
| BOP (%) | 7.08 ± 1.27 | 42.32 ± 6.25 | Mann–Whitney U | p < 0.001 * |
| CAL (mm) | 0.00 ± 0.00 | 5.58 ± 0.56 | Mann–Whitney U | p < 0.001 * |
| PI | 0.58 ± 0.13 | 2.08 ± 0.24 | t-test | p < 0.001 * |
| IL-6 (ng/L) | 25.07 ± 5.28 | 71.76 ± 7.54 | Mann–Whitney U | p < 0.001 * |
| Irisin (ng/mL) | 9.94 ± 3.09 | 28.85 ± 7.99 | t-test | p < 0.001 * |
| Group | Variable | Biomarker | Spearman Rho | p-Value |
|---|---|---|---|---|
| Control (C) | BMI | IL-6 (ng/L) | 0.429 | 0.013 * |
| Periodontitis (P) | PI | IL-6 (ng/L) | 0.403 | 0.020 * |
| All groups (C + P) | Age | IL-6 (ng/L) | 0.673 | p < 0.001 * |
| All groups (C + P) | Age | Irisin (ng/mL) | 0.683 | p < 0.001 * |
| All groups (C + P) | PI | IL-6 (ng/L) | 0.821 | p < 0.001 * |
| All groups (C + P) | PI | Irisin (ng/mL) | 0.728 | p < 0.001 * |
| All groups (C + P) | PD | IL-6 (ng/L) | 0.739 | p < 0.001 * |
| All groups (C + P) | PD | Irisin (ng/mL) | 0.726 | p < 0.001 * |
| All groups (C + P) | BOP | IL-6 (ng/L) | 0.772 | p < 0.001 * |
| All groups (C + P) | BOP | Irisin (ng/mL) | 0.722 | p < 0.001 * |
| All groups (C + P) | CAL | IL-6 (ng/L) | 0.803 | p < 0.001 * |
| All groups (C + P) | CAL | Irisin (ng/mL) | 0.693 | p < 0.001 * |
| Marker | AUC | Cut-Off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| IL-6 | 0.99 | 37.87 | 93.9 | 97.0 |
| Irisin | 0.95 | 14.91 | 87.9 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saribas, E.; Kandemir, M.; Erkan, R.E.C.; Tuncer, M.C. Evaluation of Irisin and Interleukin-6 Levels in Saliva Samples of Periodontally Healthy and Stage 3 Grade C Periodontitis Individuals. Biology 2025, 14, 1188. https://doi.org/10.3390/biology14091188
Saribas E, Kandemir M, Erkan REC, Tuncer MC. Evaluation of Irisin and Interleukin-6 Levels in Saliva Samples of Periodontally Healthy and Stage 3 Grade C Periodontitis Individuals. Biology. 2025; 14(9):1188. https://doi.org/10.3390/biology14091188
Chicago/Turabian StyleSaribas, Ebru, Müzeyyen Kandemir, Revsa Evin Canpolat Erkan, and Mehmet Cudi Tuncer. 2025. "Evaluation of Irisin and Interleukin-6 Levels in Saliva Samples of Periodontally Healthy and Stage 3 Grade C Periodontitis Individuals" Biology 14, no. 9: 1188. https://doi.org/10.3390/biology14091188
APA StyleSaribas, E., Kandemir, M., Erkan, R. E. C., & Tuncer, M. C. (2025). Evaluation of Irisin and Interleukin-6 Levels in Saliva Samples of Periodontally Healthy and Stage 3 Grade C Periodontitis Individuals. Biology, 14(9), 1188. https://doi.org/10.3390/biology14091188









