Epigenetic Mechanisms Associated with Livestock Adaptation to Heat Stress
Simple Summary
Abstract
1. Introduction
2. Heat Stress as the Major Factor Influencing Livestock Production
3. Significance of Cellular and Molecular Changes Associated with Livestock Adaptation
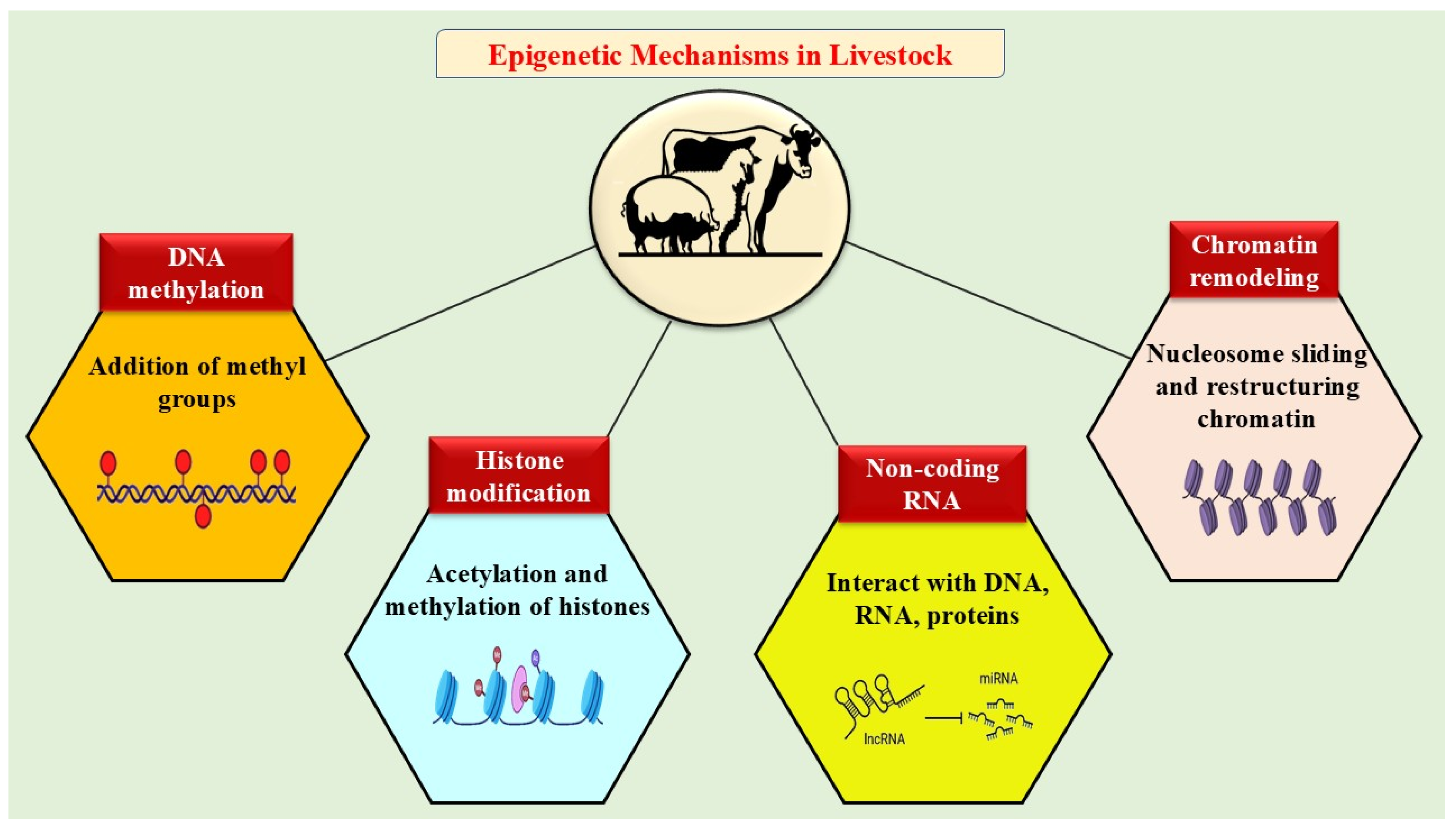
4. Different Epigenetic Mechanisms in Livestock in Response to Environmental Stressors
4.1. DNA Methylation
4.2. Histone Modifications
4.3. Chromatin Remodeling
4.4. Non-Coding RNAs
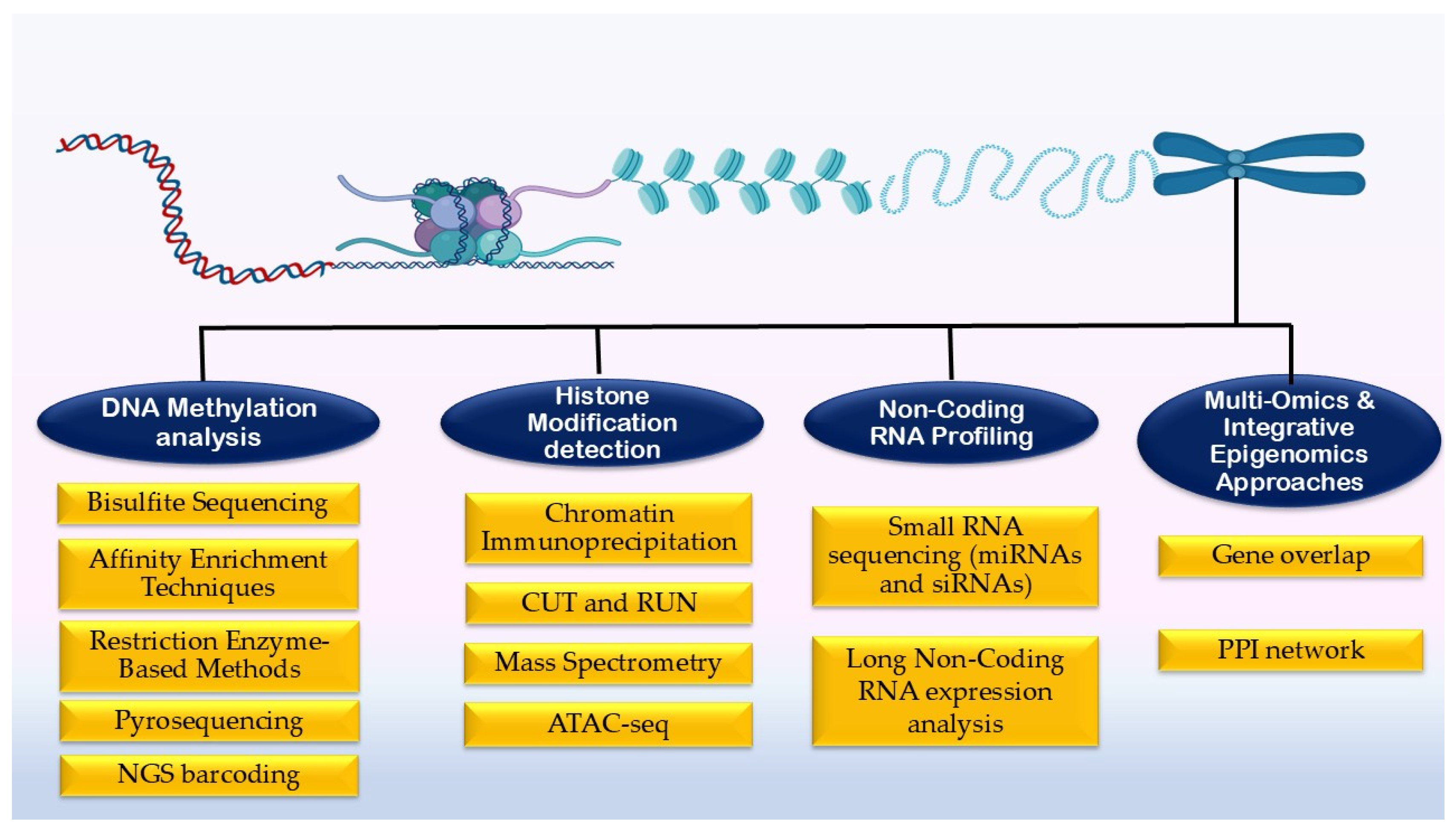
5. Different Methodologies to Quantify Heat Stress-Associated Epigenetic Changes in Livestock
5.1. DNA Methylation Analysis
5.2. Histone Modification Detection Methods
5.3. Non-Coding RNA Profiling
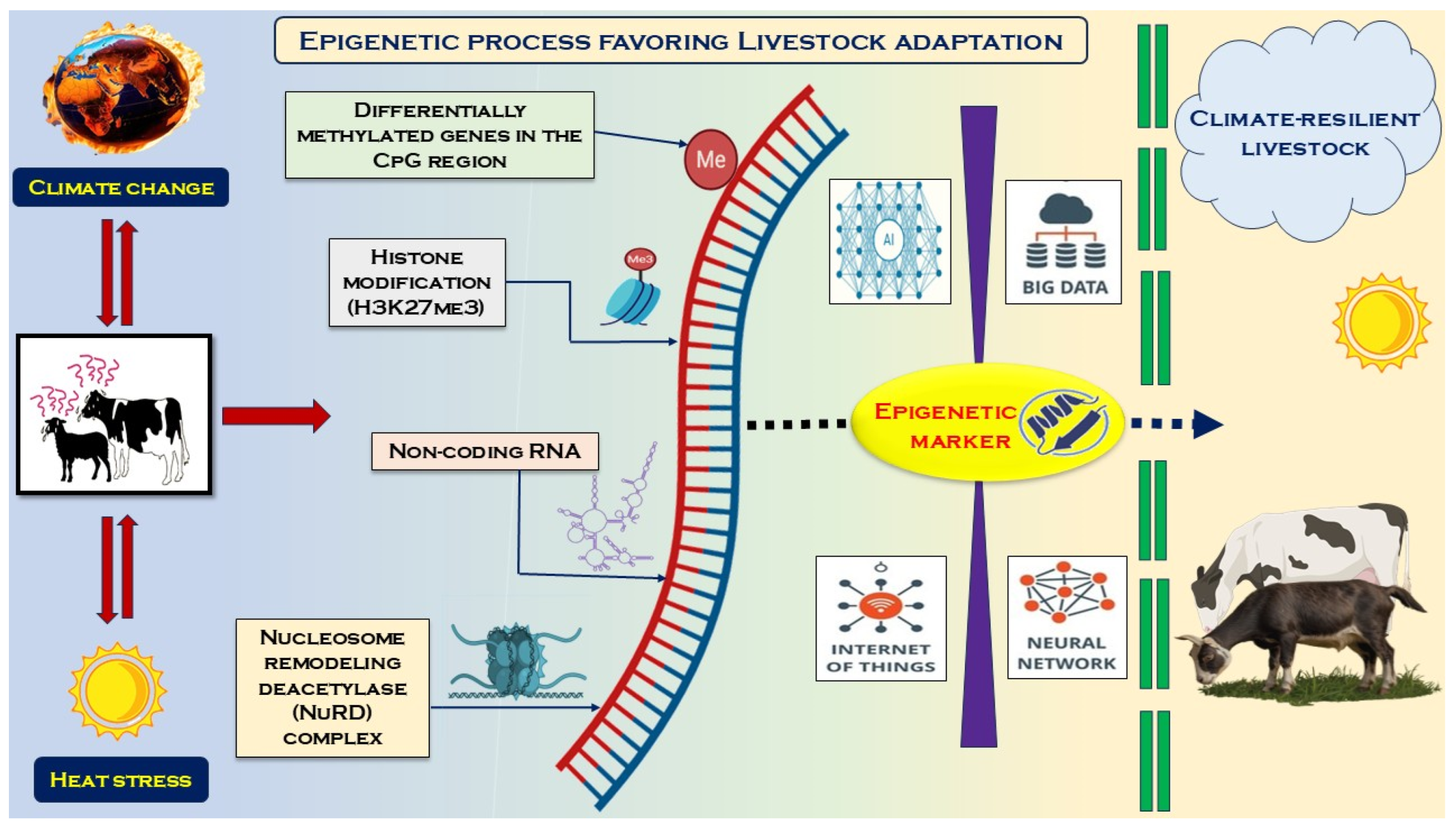
6. Different Methylome Patterns in Livestock Adaptation to Heat Stress
7. Epigenetic Regulation as Response to Heat Stress in Livestock
7.1. Large Ruminants
7.2. Small Ruminants
7.3. Swine
7.4. Chickens
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef]
- IPCC. 2023: Summary for Policymakers. In Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Adaptation of animals to heat stress. Animal 2018, 12, 431–444. [Google Scholar] [CrossRef]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Increases in extreme heat stress in domesticated livestock species during the twenty-first century. Glob. Change Biol. 2021, 27, 5762–5772. [Google Scholar] [CrossRef]
- Niyas, P.A.A.; Chaidanya, K.; Shaji, S.; Sejian, V.; Bhatta, R. Adaptation of livestock to environmental challenges. J. Vet. Sci. Med. Diagn. 2015, 4, 2. [Google Scholar] [CrossRef]
- Del Corvo, M.; Lazzari, B.; Capra, E.; Zavarez, L.; Milanesi, M.; Utsunomiya, Y.T.; Ajmone-Marsan, P. Methylome patterns of cattle adaptation to heat stress. Front. Genet. 2021, 12, 633132. [Google Scholar] [CrossRef] [PubMed]
- Sajjanar, B.; Aalam, M.T.; Khan, O.; Dhara, S.K.; Ghosh, J.; Gandham, R.K.; Gupta, P.K.; Chaudhuri, P.; Dutt, T.; Singh, G.; et al. Genome-wide DNA methylation profiles regulate distinct heat stress response in zebu (Bos indicus) and crossbred (Bos indicus × Bos taurus) cattle. Cell Stress Chaperones 2024, 29, 603–614. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. 2025. Available online: https://www.fao.org/climate-change/en (accessed on 20 July 2025).
- Cheng, M.; McCarl, B.; Fei, C. Climate change and livestock production: A literature review. Atmosphere 2022, 13, 140. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. 2013. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. Available online: https://www.fao.org/3/i3437e/i3437e.pdf (accessed on 20 July 2025).
- Daramola, J.O.; Abioja, M.O.; Onagbesan, O.M. Heat stress impact on livestock production. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 53–73. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef]
- Archana, P.R.; Aleena, J.; Pragna, P.; Vidya, M.K.; Niyas, A.P.A.; Bagath, M.; Krishnan, G.; Manimaran, A.; Beena, V.; Kurien, E.K. Role of Heat Shock Proteins in Livestock Adaptation to Heat Stress. J. Dairy Vet. Anim. Res. 2017, 5, 00127. [Google Scholar] [CrossRef]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 379–397. [Google Scholar] [CrossRef]
- Velichko, A.K.; Markova, E.N.; Petrova, N.V.; Razin, S.V.; Kantidze, O.L. Mechanisms of heat shock response in mammals. Cell. Mol. Life Sci. 2013, 70, 4229–4241. [Google Scholar] [CrossRef]
- AL-Jaryan, I.L.; AL-Thuwaini, T.M.; AL-Jebory, H.H. Heat Shock Protein 70 and Its Role in Alleviating Heat Stress and Improving Livestock Performance. Rev. Agric. Sci. 2023, 11, 234–242. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M.J.O.A.P. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.; Macko, A.R.; Roy, K.S. Genes involved in the thermal tolerance of livestock. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 379–410. [Google Scholar] [CrossRef]
- Grada, A.; Weinbrecht, K. Next-generation sequencing: Methodology and application. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef]
- Nicoglou, A.; Merlin, F. Epigenetics: A way to bridge the gap between biological fields. Stud. Hist. Philos. Sci. C 2017, 66, 73–82. [Google Scholar] [CrossRef]
- Monk, D.; Mackay, D.J.; Eggermann, T.; Maher, E.R.; Riccio, A. Genomic imprinting disorders: Lessons on how genome, epigenome and environment interact. Nat. Rev. Genet. 2019, 20, 235–248. [Google Scholar] [CrossRef]
- Wang, M.; Ibeagha-Awemu, E.M. Impacts of epigenetic processes on the health and productivity of livestock. Front. Genet. 2021, 11, 613636. [Google Scholar] [CrossRef] [PubMed]
- Ziller, M.J.; Müller, F.; Liao, J.; Zhang, Y.; Gu, H.; Bock, C.; Boyle, P.; Epstein, C.B.; Bernstein, B.E.; Lengauer, T.; et al. Genomic distribution and inter-sample variation of non-CpG methylation across human cell types. PLoS Genet. 2011, 7, 1002389. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.; Farrelly, C.O.; Meade, K.G. Comparative epigenetics: Relevance to the regulation of production and health traits in cattle. Anim. Genet. 2014, 45, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Halušková, J.; Holečková, B.; Staničová, J. DNA methylation studies in cattle. J. Appl. Genet. 2021, 62, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Kalaignazhal, G.; Sejian, V.; Velayudhan, S.M.; Mishra, C.; Rebez, E.B.; Chauhan, S.S.; DiGiacomo, K.; Lacetera, N.; Dunshea, F.R. Applications of Next-Generation Sequencing Technologies and Statistical Tools in Identifying Pathways and Biomarkers for Heat Tolerance in Livestock. Vet. Sci. 2024, 11, 616. [Google Scholar] [CrossRef]
- Kumar, G.; Gurao, A.; Vasisth, R.; Chitkara, M.; Singh, R.; Sriranga, K.R.; Dige, M.S.; Mukesh, M.; Singh, P.; Kataria, R.S. Genome-wide 5′-C-phosphate-G-3′methylation patterns reveal the effect of heat stress on the altered semen quality in Bubalus bubalis. Gene 2024, 906, 148233. [Google Scholar] [CrossRef]
- Reith, R.R.; Gibbs, R.; White, M.R.; Parrish, B.L.; Fuller, A.M.; Schmidt, T.B.; Yates, D.T.; Petersen, J.L. 204 Changes in DNA methylation 5 days after exposure to acute heat stress in beef cattle skeletal muscle. J. Anim. Sci. 2024, 102, 40–41. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Ikonomopoulos, I.; Bannister, A.J. Epigenetics and inheritance of phenotype variation in livestock. Epigenetics Chromatin 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.T.; Zhuang, Z.X.; Chen, C.J.; Liao, H.Y.; Chen, H.L.; Hsueh, H.C.; Chen, C.F.; Chen, S.E.; Huang, S.Y. Effects of acute heat stress on protein expression and histone modification in the adrenal gland of male layer-type country chickens. Sci. Rep. 2021, 11, 6499. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Zhang, H.; Zhang, P.; Shahzad, M.; Du, W.; Zhao, X. Heat-stress impacts on developing bovine oocytes: Unraveling epigenetic changes, oxidative stress, and developmental resilience. Int. J. Mol. Sci. 2024, 25, 4808. [Google Scholar] [CrossRef]
- Ho, L.; Crabtree, G.R. Chromatin remodelling during development. Nature 2010, 463, 474–484. [Google Scholar] [CrossRef]
- Rahman, M.B.; Kamal, M.M.; Rijsselaere, T.; Vandaele, L.; Shamsuddin, M.; Van Soom, A. Altered chromatin condensation of heat-stressed spermatozoa perturbs the dynamics of DNA methylation reprogramming in the paternal genome after in vitro fertilisation in cattle. Reprod. Fertil. Dev. 2014, 26, 1107–1116. [Google Scholar] [CrossRef]
- Nie, L.; Wu, H.J.; Hsu, J.M.; Chang, S.S.; LaBaff, A.M.; Li, C.W.; Wang, Y.; Hsu, J.L.; Hung, M.C. Long Non-Coding RNAs: Versatile Master Regulators of Gene Expression and Crucial Players in Cancer. Am. J. Transl. Res. 2012, 4, 127–150. [Google Scholar]
- Sengar, G.S.; Deb, R.; Singh, U.; Junghare, V.; Hazra, S.; Raja, T.V.; Alex, R.; Kumar, A.; Alyethodi, R.R.; Kant, R.; et al. Identification of differentially expressed microRNAs in Sahiwal (Bos indicus) breed of cattle during thermal stress. Cell Stress Chaperones 2018, 23, 1019–1032. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, B.; Swain, D.K.; Anand, M.; Yadav, S.; Madan, A.K. Differential expression of miRNAs and related mRNAs during heat stress in buffalo heifers. J. Therm. Biol. 2021, 97, 102904. [Google Scholar] [CrossRef]
- Li, H.; Huang, K.; Wang, P.; Feng, T.; Shi, D.; Cui, K.; Luo, C.; Shafique, L.; Qian, Q.; Ruan, J.; et al. Comparison of Long Non-Coding RNA Expression Profiles of Cattle and Buffalo Differing in Muscle Characteristics. Front. Genet. 2020, 11, 98. [Google Scholar] [CrossRef]
- Zeng, H.; Xia, H.; Wang, X.; Wang, Y.; Fang, J.; Li, S.; Zhai, Y.; Han, Z. Comprehensive Profiling of ceRNA (circRNA-miRNA-mRNA) Networks in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress. Int. J. Mol. Sci. 2023, 24, 888. [Google Scholar] [CrossRef] [PubMed]
- Laporta, J.; Khatib, H.; Zachut, M. Phenotypic and molecular evidence of inter-and trans-generational effects of heat stress in livestock mammals and humans. Animal 2024, 18, 101121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Hu, L.; Zhang, C.; Chen, G.; Hou, L.; Xu, Q.; Wang, Y.; Li, M. Molecular regulation of whole genome DNA methylation in heat stress response of dairy cows. BMC Genom. 2025, 26, 464. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, L.; Zhang, K.; Nie, H.; Gou, X.; Kong, X.; Deng, W. Genetic and Epigenetic Adaptation Mechanisms of Sheep Under Multi-Environmental Stress Environment. Int. J. Mol. Sci. 2025, 26, 3261. [Google Scholar] [CrossRef]
- Cokus, S.J.; Feng, S.; Zhang, X.; Chen, Z.; Merriman, B.; Haudenschild, C.D.; Pradhan, S.; Nelson, S.F.; Pellegrini, M.; Jacobsen, S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 2008, 452, 215–219. [Google Scholar] [CrossRef]
- Parrish, R.R.; Day, J.J.; Lubin, F.D. Direct bisulfite sequencing for examination of DNA methylation with gene and nucleotide resolution from brain tissues. Curr. Protoc. Neurosci. 2012, 60, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; Gnirke, A.; Bell, G.W.; Ramsahoye, B.; Lander, E.S.; Jaenisch, R. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res. 2005, 33, 5868–5877. [Google Scholar] [CrossRef]
- Suzuki, M.; Liao, W.; Wos, F.; Johnston, A.D.; DeGrazia, J.; Ishii, J.; Bloom, T.; Zody, M.C.; Germer, S.; Greally, J.M. Whole-genome bisulfite sequencing with improved accuracy and cost. Genome Res. 2018, 28, 1364–1371. [Google Scholar] [CrossRef]
- Cao, B.; Luo, H.; Luo, T.; Li, N.; Shao, K.; Wu, K.; Sahu, S.K.; Li, F.; Lin, C. The performance of whole genome bisulfite sequencing on DNBSEQ-Tx platform examined by different library preparation strategies. Heliyon 2023, 9, 16571. [Google Scholar] [CrossRef]
- Wojdacz, T.K.; Dobrovic, A. Methylation-sensitive high resolution melting (MS-HRM): A new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007, 35, 41. [Google Scholar] [CrossRef]
- Hernández, H.G.; Tse, M.Y.; Pang, S.C.; Arboleda, H.; Forero, D.A. Optimizing methodologies for PCR-based DNA methylation analysis. BioTechniques 2013, 55, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Coolen, M.W.; Stirzaker, C.; Song, J.Z.; Statham, A.L.; Strbenac, D.; Robinson, M.D.; Clark, S.J. Comparison of methyl-DNA immunoprecipitation (MeDIP) and methyl-CpG binding domain (MBD) protein capture for genome-wide DNA methylation analysis reveal CpG sequence coverage bias. Epigenetics 2011, 6, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Stirzaker, C.; Statham, A.L.; Coolen, M.W.; Song, J.Z.; Nair, S.S.; Strbenac, D.; Speed, T.P.; Clark, S.J. Evaluation of affinity-based genome-wide DNA methylation data: Effects of CpG density, amplification bias, and copy number variation. Genome Res. 2010, 20, 1719–1729. [Google Scholar] [CrossRef]
- Wang, D.; Ma, S.; Yan, M.; Dong, M.; Zhang, M.; Zhang, T.; Zhang, T.; Zhang, X.; Xu, L.; Huang, X. DNA methylation patterns in the peripheral blood of Xinjiang brown cattle with variable somatic cell counts. Front. Genet. 2024, 15, 1405478. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bissonnette, N.; Laterrière, M.; Gagné, D.; Dudemaine, P.; Roy, J.; Sirard, M.; Ibeagha-Awemu, E.M. Genome-wide DNA methylation and transcriptome integration associates DNA methylation changes with bovine subclinical mastitis caused by Staphylococcus chromogenes. Int. J. Mol. Sci. 2023, 24, 10369. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, 292. [Google Scholar] [CrossRef]
- Milne, T.A.; Zhao, K.; Hess, J.L. Chromatin immunoprecipitation (ChIP) for analysis of histone modifications and chromatin-associated proteins. Methods Mol. Biol. 2009, 538, 409–423. [Google Scholar] [CrossRef] [PubMed]
- O’Geen, H.; Echipare, L.; Farnham, P.J. Using ChIP-seq technology to generate high-resolution profiles of histone modifications. Methods Mol. Biol. 2011, 791, 265–286. [Google Scholar] [CrossRef]
- Haring, M.; Offermann, S.; Danker, T.; Horst, I.; Peterhansel, C.; Stam, M. Chromatin immunoprecipitation: Optimization, quantitative analysis and data normalization. Plant Methods 2007, 3, 11. [Google Scholar] [CrossRef]
- O’Geen, H.; Henry, I.M.; Bhakta, M.S.; Meckler, J.F.; Segal, D.J. A genome-wide analysis of Cas9 binding specificity using ChIP-seq and targeted sequence capture. Nucleic Acids Res. 2015, 43, 3389–3404. [Google Scholar] [CrossRef]
- David, S.; Vitorino Carvalho, A.; Gimonnet, C.; Brionne, A.; Hennequet-Antier, C.; Piégu, B.; Crochet, S.; Couroussé, N.; Bordeau, T.; Bigot, Y. Thermal manipulation during embryogenesis impacts H3K4me3 and H3K27me3 histone marks in chicken hypothalamus. Front. Genet. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Skene, P.J.; Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 2017, 6, e21856. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Okur, H.S.; Wu, S.J.; Codomo, C.A.; Pledger, E.S.; Bryson, T.D.; Henikoff, J.G.; Ahmad, K.; Henikoff, S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019, 10, 1930–1935. [Google Scholar] [CrossRef]
- Thomas, S.P.; Haws, S.A.; Borth, L.E.; Denu, J.M. A practical guide for analysis of histone post-translational modifications by mass spectrometry: Best practices and pitfalls. Methods 2020, 184, 53–60. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Wu, B.; Chang, H.Y.; Greenleaf, W.J. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr. Protoc. Mol. Biol. 2015, 109, 21.29.1–21.29.9. [Google Scholar] [CrossRef] [PubMed]
- Cecere, G. Small RNAs in epigenetic inheritance: From mechanisms to trait transmission. FEBS Lett. 2021, 595, 2953–2977. [Google Scholar] [CrossRef]
- Jo, J.; Ghassemi Nejad, J.; Peng, D.; Kim, H.; Kim, S.; Lee, H. Characterization of Short-Term Heat Stress in Holstein Dairy Cows Using Altered Indicators of Metabolomics, Blood Parameters, Milk MicroRNA-216 and Characteristics. Animals 2021, 11, 722. [Google Scholar] [CrossRef]
- Sengar, G.S.; Deb, R.; Singh, U.; Raja, T.V.; Kant, R.; Sajjanar, B.; Alex, R.; Alyethodi, R.R.; Kumar, A.; Kumar, S.; et al. Differential expression of microRNAs associated with thermal stress in Frieswal (Bos taurus x Bos indicus) crossbred dairy cattle. Cell Stress Chaperones 2018, 23, 155–170. [Google Scholar] [CrossRef]
- Cendron, F.; Rosani, U.; Franzoi, M.; Boselli, C.; Maggi, F.; De Marchi, M.; Penasa, M. Analysis of miRNAs in milk of four livestock species. BMC Genomics 2024, 25, 859. [Google Scholar] [CrossRef]
- Zeng, H.; Li, S.; Zhai, Y.; Chang, H.; Han, Z. Preliminary Transcriptome Analysis of Long Noncoding RNA in Hypothalamic-Pituitary-Mammary Gland Axis of Dairy Cows under Heat Stress. Biomolecules 2023, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Xing, T.; Li, J.; Zhang, L.; Zhao, L.; Jiang, Y.; Gao, F. Unraveling the role of long non-coding RNAs in chronic heat stress-induced muscle injury in broilers. J. Anim. Sci. Biotechnol. 2024, 15, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J. Multi-omics integration strategies for animal epigenetic studies—A review. Anim. Biosci. 2021, 34, 1271–1282. [Google Scholar] [CrossRef]
- Mullakkalparambil Velayudhan, S.; Sejian, V.; Devaraj, C.; Manjunathareddy, G.B.; Ruban, W.; Kadam, V.; König, S.; Bhatta, R. Novel insights to assess climate resilience in goats using a holistic approach of skin-based advanced NGS technologies. Int. J. Mol. Sci. 2023, 24, 10319. [Google Scholar] [CrossRef]
- Reith, R.R. Heat Stress Changes the Bovine Methylome and Transcriptome and Investigation of Two Novel Genetic Defects in Cattle. Ph.D Thesis, University of Nebraska–Lincoln, Lincoln, NE, USA, 2023. [Google Scholar]
- Stankiewicz, A.M.; Swiergiel, A.H.; Lisowski, P. Epigenetics of stress adaptations in the brain. Brain Res. Bull. 2013, 98, 76–92. [Google Scholar] [CrossRef]
- Flores, K.B.; Wolschin, F.; Amdam, G.V. The role of methylation of DNA in environmental adaptation. Integr. Compar. Biol. 2013, 53, 359–372. [Google Scholar] [CrossRef]
- Sevane, N.; Martínez, R.; Bruford, M.W. Genome-wide differential DNA methylation in tropically adapted Creole cattle and their Iberian ancestors. Anim. Genet. 2019, 50, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Denoyelle, L.; De Villemereuil, P.; Boyer, F.; Khelifi, M.; Gaffet, C.; Alberto, F.; Benjelloun, B.; Pompanon, F. Genetic variations and differential DNA methylation to face contrasted climates in small ruminants: An analysis on traditionally-managed sheep and goats. Front. Genet. 2021, 12, 745284. [Google Scholar] [CrossRef] [PubMed]
- Skibiel, A.L.; Peñagaricano, F.; Amorín, R.; Ahmed, B.M.; Dahl, G.E.; Laporta, J. In utero heat stress alters the offspring epigenome. Sci. Rep. 2018, 8, 14609. [Google Scholar] [CrossRef]
- Li, C.; Sun, D.; Zhang, S.; Wang, S.; Wu, X.; Zhang, Q.; Liu, L.; Li, Y.; Qiao, L. Genome wide association study identifies 20 novel promising genes associated with milk fatty acid traits in Chinese Holstein. PLoS ONE 2014, 9, 96186. [Google Scholar] [CrossRef]
- Fedotova, A.A.; Bonchuk, A.N.; Mogila, V.A.; Georgiev, P.G. C2H2 zinc finger proteins: The largest but poorly explored family of higher eukaryotic transcription factors. Acta Naturae 2017, 9, 47–58. [Google Scholar] [CrossRef]
- Bell, A.W.; McBride, B.W.; Slepetis, R.; Early, R.J.; Currie, W.B. Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J. Anim. Sci. 1989, 67, 3289–3299. [Google Scholar] [CrossRef]
- Livernois, A.M.; Mallard, B.A.; Cartwright, S.L.; Cánovas, A. Heat stress and immune response phenotype affect DNA methylation in blood mononuclear cells from Holstein dairy cows. Sci. Rep. 2021, 11, 11371. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiao, J.; Zhang, Z.; Shang, X.; Chu, Z.; Fu, Y.; Chu, M. Identification and analysis of differentially expressed long non-coding RNAs of Chinese Holstein cattle responses to heat stress. Anim. Biotechnol. 2020, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers, and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, Y.; Li, Q.; Liu, E.; Jin, M.; Zhang, L.; Wei, C. The role of N6-methyladenosine RNA methylation in the heat stress response of sheep (Ovis aries). Cell Stress Chaperones 2019, 24, 333–342. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, J.; Yuan, C.; Jin, M.; Quan, K.; Chu, M.; Wei, C. m6A mRNA methylation analysis provides novel insights into heat stress responses in the liver tissue of sheep. Genomics 2021, 113, 484–492. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, C.; Guo, T.; Liu, J.; Lu, Z. METTL3 and FTO Regulate Heat Stress Response in Hu Sheep Through Lipid Metabolism via m6A Modification. Animals 2025, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Wang, T.; Zhong, X. Modification of N6-methyladenosine RNA methylation on heat shock protein expression. PLoS ONE 2018, 13, 0198604. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, J.; Li, J.; Sun, X.; Chen, C.; Liu, L.; Lv, S.; Qu, M.; Fan, Y.; Zhou, P.; et al. The epigenetic mechanisms of adaption to the hot and humid climate in Hu sheep (Ovis aries). Physiol. Rep. 2024, 12, 16164. [Google Scholar] [CrossRef]
- Salces-Ortiz, J.; González, C.; Bolado-Carrancio, A.; Rodríguez-Rey, J.C.; Calvo, J.H.; Muñoz, R.; Serrano, M.M. Ovine HSP90AA1 gene promoter: Functional study and epigenetic modifications. Cell Stress Chaperones 2015, 20, 1001–1012. [Google Scholar] [CrossRef]
- Zou, J.; Wei, L.; Mo, Z.; Liang, Y.; Lu, J.; Zou, J.; Wang, F.; Wu, S.; He, H.E.; Li, W.; et al. Impact of Heat Stress on Ovarian Function and circRNA Expression in Hu Sheep. Animals 2025, 15, 2063. [Google Scholar] [CrossRef]
- Li, Y.; Kong, L.; Deng, M.; Lian, Z.; Han, Y.; Sun, B.; Guo, Y.; Liu, G.; Liu, D. Heat Stress-Responsive Transcriptome Analysis in the Liver Tissue of Hu Sheep. Genes 2019, 10, 395. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Hao, Y.; Cui, Y.; Gu, X. Genome-wide DNA methylation profiles changes associated with constant heat stress in pigs as measured by bisulfite sequencing. Sci. Rep. 2016, 6, 27507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Green, M.P.; Marth, C.D.; Liu, F.; Le, H.H.; Lynch, G.S.; Bell, A.W.; Leury, B.J.; Dunshea, F.R.; Cottrell, J.J. Gestational heat stress alters skeletal muscle gene expression profiles and vascularity in fetal pigs in a sexually dimorphic manner. J. Anim. Sci. Biotechnol. 2022, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, Y.; Zou, H.; Jiang, Q. Analysis of long non-coding RNAs in skeletal muscle of Bama Xiang pigs in response to heat stress. Trop. Anim. Health Prod. 2021, 53, 259. [Google Scholar] [CrossRef]
- Sun, M.H.; Jiang, W.J.; Li, X.H.; Lee, S.H.; Heo, G.; Zhou, D.; Guo, J.; Cui, X.S. High Temperature–Induced m6A Epigenetic Changes Affect Early Porcine Embryonic Developmental Competence in Pigs. Microsc. Microanal. 2023, 29, 2174–2183. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, F.; Chen, Q.; Cai, J.; Hu, J.; Shen, J.; Zhang, J. Long Noncoding RNA and mRNA Profiling of Hypothalamic-Pituitary-Mammary Gland Axis in Lactating Sows under Heat Stress. Genomics 2020, 112, 3668–3676. [Google Scholar] [CrossRef]
- Yu, Z.; Yong, Y.; Liu, X.; Ma, X.; Abd El-Aty, A.M.; Li, L.; Zhong, Z.; Ye, X.; Ju, X. Insights and Implications for Transcriptomic Analysis of Heat Stress-Induced Intestinal Inflammation in Pigs. BMC Genom. 2024, 25, 1110. [Google Scholar] [CrossRef]
- Cramer, T.; Rosenberg, T.; Kisliouk, T.; Meiri, N. Early-life epigenetic changes along the corticotropin-releasing hormone (CRH) gene influence resilience or vulnerability to heat stress later in life. Mol. Psychiatry 2019, 24, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, A.; Thirunalasundari, T.; Shanmugam, M.; Uthrakumar, A.; Suji, S.; Rajkumar, U. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones 2018, 23, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Karami, K.; Sabban, J.; Cerutti, C.; Devailly, G.; Foissac, S.; Gourichon, D.; Hubert, A.; Hubert, J.N.; Leroux, S.; Zerjal, T.; et al. Molecular responses of chicken embryos to maternal heat stress through DNA methylation and gene expression. bioRxiv 2024, 2024-04. [Google Scholar] [CrossRef] [PubMed]
- Kisliouk, T.; Ziv, M.; Meiri, N. Epigenetic control of translation regulation: Alterations in histone H3 lysine 9 post-translation modifications are correlated with the expression of the translation initiation factor 2B (Eif2b5) during thermal control establishment. Dev. Neurobiol. 2010, 70, 100–113. [Google Scholar] [CrossRef]
- Kisliouk, T.; Cramer, T.; Meiri, N. Methyl CpG level at distal part of heat-shock protein promoter HSP 70 exhibits epigenetic memory for heat stress by modulating recruitment of POU 2F1-associated nucleosome-remodeling deacetylase (Nu RD) complex. J. Neurochem. 2017, 141, 358–372. [Google Scholar] [CrossRef]
- Yossifoff, M.; Kisliouk, T.; Meiri, N. Dynamic changes in DNA methylation during thermal control establishment affect CREB binding to the brain-derived neurotrophic factor promoter. Eur. J. Neurosci. 2008, 28, 2267–2277. [Google Scholar] [CrossRef]
- Rosenberg, T.; Marco, A.; Kisliouk, T.; Haron, A.; Shinder, D.; Druyan, S.; Meiri, N. Embryonic heat conditioning in chicks induces transgenerational heat/immunological resilience via methylation on regulatory elements. FASEB J. 2022, 36, 22406. [Google Scholar] [CrossRef]
| Species | Breed | Organ/Tissue (Sample Size) | Epigenetic Modifications | Genes/Alleles | Pathways | Reference |
|---|---|---|---|---|---|---|
| Cattle | Holstein | Mammary gland (7) | DNA methylation (hypomethylation) | PRKG1, PI4KA, AGO2, TRIM | Regulation of calcium levels, influencing milk synthesis, gene transcription and silencing | [1] |
| Liver (10) | DNA methylation | AGER, ZMAT5, ZNF608, ZNF395, MED1, H2AY | Mediates cellular stress response, transcriptional regulation and innate immune defense | |||
| Nellore | Peripheral blood mononuclear cells (25) | DNA methylation (hypomethylation) | ELOVL5, FADS1, MAP3K1, NTN1, RASA3, PRDX, PDE5A | Regulating immune and inflammatory pathways | [6] | |
| Angus | Peripheral blood mononuclear cells (25) | DNA methylation (hypermethylation) | ATG16L2, CACNA1C, GADD45A | Modulating cell autophagy, calcium signaling and genomic integrity | ||
| Holstein | Peripheral blood mononuclear cells (12) | DNA methylation (hypomethylation) | IL15, BCL2L12, HSPB9, NDRG1 | Vital role in the immunological landscape and chaperone-like activity | [82] | |
| DNA methylation (hypermethylation) | APC2, BNIP3 | Regulating tumor metastasis and autophagy | ||||
| Hariana and Vrindavani | Peripheral blood mononuclear cells (6) | DNA methylation | DERL3, GCLC and PPP1R15A | Signature genes for adaptative traits and stress response | [7] | |
| bta-miR-107, bta-miR-1284, bta-miR-2326, bmiR-2396, bta-miR-2441, bta-miR-342, bta-miR-411c-5p, bta-miR-6121-3p and bta-miR-885 | Modulation of stress responses through methylation of microRNA genes | |||||
| Holstein | Hypothalamus, pituitary and mammary gland tissue (6) | Non-coding RNA | MSTRG.6147.5, MSTRG.8643.1, MSTRG.6147.5, MSTRG.8643.1, MSTRG.8646.1, MSTRG.12225.1, MSTRG.16080.1, MSTRG.16082.2, MSTRG.16082.1, MSTRG.16081.1 | Cellular response to heat stress and physiological processes related to lactation | [40] | |
| Buffalo | Murrah Heifers | PBMCs (12) | Non-coding RNA | bta-mir-142, bta-mir-1248, bta-mir-2332 and bta-mir-2478 | Regulating thermotolerance | [38] |
| Goat | - | Ear biopsy (21) | DNA methylation | AGPAT4 | Lipid synthesis in milk production | [77] |
| Sheep | - | Ear biopsy (22) | DNA methylation | SLIT3 | Influences muscle development | [77] |
| - | Lymphocytes (16) | DNA methylation | HSP90AA1 promotor | Regulating the transcription of heat shock protein genes | [90] | |
| Hu | Blood (20) | DNA methylation (hypermethylation) | ADCY9, PRKACB, CREB5, TPO | Pathways repressing thyroid hormone secretion and thermogenesis | [89] | |
| POMC, MC2R, ADCY9, PRKACB, CREB5 and SP1 | Modulating ACTH–cortisol signaling loop | |||||
| Hu | Hepatocytes and preadipocytes (3) | m6A RNA methylation | Wnt, TGF-β, AMPK, HSP60, HSP70, HSP110 | Influences lipid deposition and exerts control on heat shock proteins | [87] | |
| Hu | Ovaries (6) | Non-coding RNA | 152 differentially expressed circRNAs | Apoptosis, mitophagy and FoxO signaling pathway | [91] | |
| Hu | Liver | Non-coding RNA | Lnc_001782 | Regulates liver function | [92] | |
| Swine | DLY Pigs (crossbreeds between Landrace × Yorkshire sows and Duroc boars) | Longissimus dorsi (16) | DNA methylation | PFKFB1, PGK1, PDK3, CPTIB, CPTIA, LEPR, CLIC2, RYR, SMPX, MYH11, COL16A1, COL4A3, HSP27, HSP70, HSP90, CRYAB, DNAJC5 | Alterations in DNA methylation patterns within genes involved in energy homeostasis, lipid metabolism, cellular protection mechanisms and calcium signaling pathways | [94] |
| Large White × Landrace | Fetal longissimus dorsi (LD) muscle (8) | DNA methylation | MTA1, NCOR1, DMAP1, CTBP1, EID1, PPARGC1B/PGC-1β, SREBF1/ADD1, COL4A2, LAMA5 | Regulatory pathways involving transcriptional silencing, adipogenesis, fibrogenesis and angiogenesis along with sex-specific differences in gene expression | [95] | |
| - | Oocytes | m6A RNA methylation | - | Mediated by regulators of m6A modification, especially YTHDF2, METTL3 and FTO | [97] | |
| Crossbred pigs | Colon (6) | Non-coding RNA | MSTRG.13202.5, MSTRG.28207.43, MSTRG.30039.11, MSTRG.34871.3, MSTRG.47709.5, MSTRG.50167.1 and MSTRG.8273.18 | Regulation of intestinal inflammation | [99] | |
| - | Hypothalamus, pituitary and mammary gland (6) | Non-coding RNA | MSTRG.17186, MSTRG.5366 | Regulation of lactational performance | [98] | |
| Bama Xiang pigs | Longissimus dorsi muscles (10) | Non-coding RNA | 365 lncRNAs were identified | Muscle development and lipid metabolism | [96] | |
| Chicken | Cobb | Anterior hypothalamus | DNA methylation | BDNF and DNMT3A | Dynamic DNA methylation changes in BDNF gene promoter occurred during thermal adaptation, suggesting epigenetic regulation of neurotrophic factors | [105] |
| Preoptic anterior hypothalamus | Histone modifications (H3K9 acetylation and H3K9 dimethylation) | Eif2b5 | Key regulator of global protein synthesis during thermal adaptation, highlighting a dynamic epigenetic mechanism | [103] | ||
| Anterior hypothalamus | DNA methylation, chromatin modifiers (NURD remodeling complex) | HSP70 | An epigenetic marker of the heat stress response, revealing a molecular basis for thermotolerance variability | [104] | ||
| Naked chicken | Brain tissue (6) | DNA methylation (hypermethylation) | HSP90α, HSP90β and HSP70 | Epigenetic regulation of promotor genes for stress response | [101] | |
| Paraventricular nucleus | DNA methylation, DNA hydroxymethylation, histone modification (H3K27ac) | CRH introns | A dual epigenetic mechanism, comprising histone modifications and DNA methylation, within the CRH gene-regulatory region, influences stress resilience or vulnerability later in life | [100] | ||
| Broiler | Hypothalamus (54) | Histone modification (H3K4me3 and H3K27me3) | - | Molecular memory of environmental exposure, influencing thermal adaptation in chickens during later life | [60] | |
| Layer type (L2 strain) | Adrenal gland (192) | Histone modification (H3K27me3) | - | Modulation of adrenal H3K27me3 correlates with adrenal function and may play a crucial role in regulating thermotolerance in chickens | [32] | |
| Cobb strain broiler | Anterior preoptic hypothalamus (80) | DNA methylation | HSP25, SOCS3 | Conferring transgenerational mechanisms and enhancing both thermal tolerance and immune resilience in offspring | [106] | |
| Broiler | Muscles (12) | Non-coding RNA | 68 lncRNAs | Apoptosis and fibrosis-related pathways | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aravindh, S.; Silpa, M.V.; Voggu, S.P.; Rebez, E.B.; Kalaignazhal, G.; Srinivas, M.V.; Dunshea, F.R.; Sejian, V. Epigenetic Mechanisms Associated with Livestock Adaptation to Heat Stress. Biology 2025, 14, 1154. https://doi.org/10.3390/biology14091154
Aravindh S, Silpa MV, Voggu SP, Rebez EB, Kalaignazhal G, Srinivas MV, Dunshea FR, Sejian V. Epigenetic Mechanisms Associated with Livestock Adaptation to Heat Stress. Biology. 2025; 14(9):1154. https://doi.org/10.3390/biology14091154
Chicago/Turabian StyleAravindh, Sundar, Mullakkalparambil Velayudhan Silpa, Santhi Priya Voggu, Ebenezer Binuni Rebez, Gajendirane Kalaignazhal, Mouttou Vivek Srinivas, Frank Rowland Dunshea, and Veerasamy Sejian. 2025. "Epigenetic Mechanisms Associated with Livestock Adaptation to Heat Stress" Biology 14, no. 9: 1154. https://doi.org/10.3390/biology14091154
APA StyleAravindh, S., Silpa, M. V., Voggu, S. P., Rebez, E. B., Kalaignazhal, G., Srinivas, M. V., Dunshea, F. R., & Sejian, V. (2025). Epigenetic Mechanisms Associated with Livestock Adaptation to Heat Stress. Biology, 14(9), 1154. https://doi.org/10.3390/biology14091154








