Mitochondrial Reverse Electron Transport: Mechanisms, Pathophysiological Roles, and Therapeutic Potential
Simple Summary
Abstract
1. Introduction
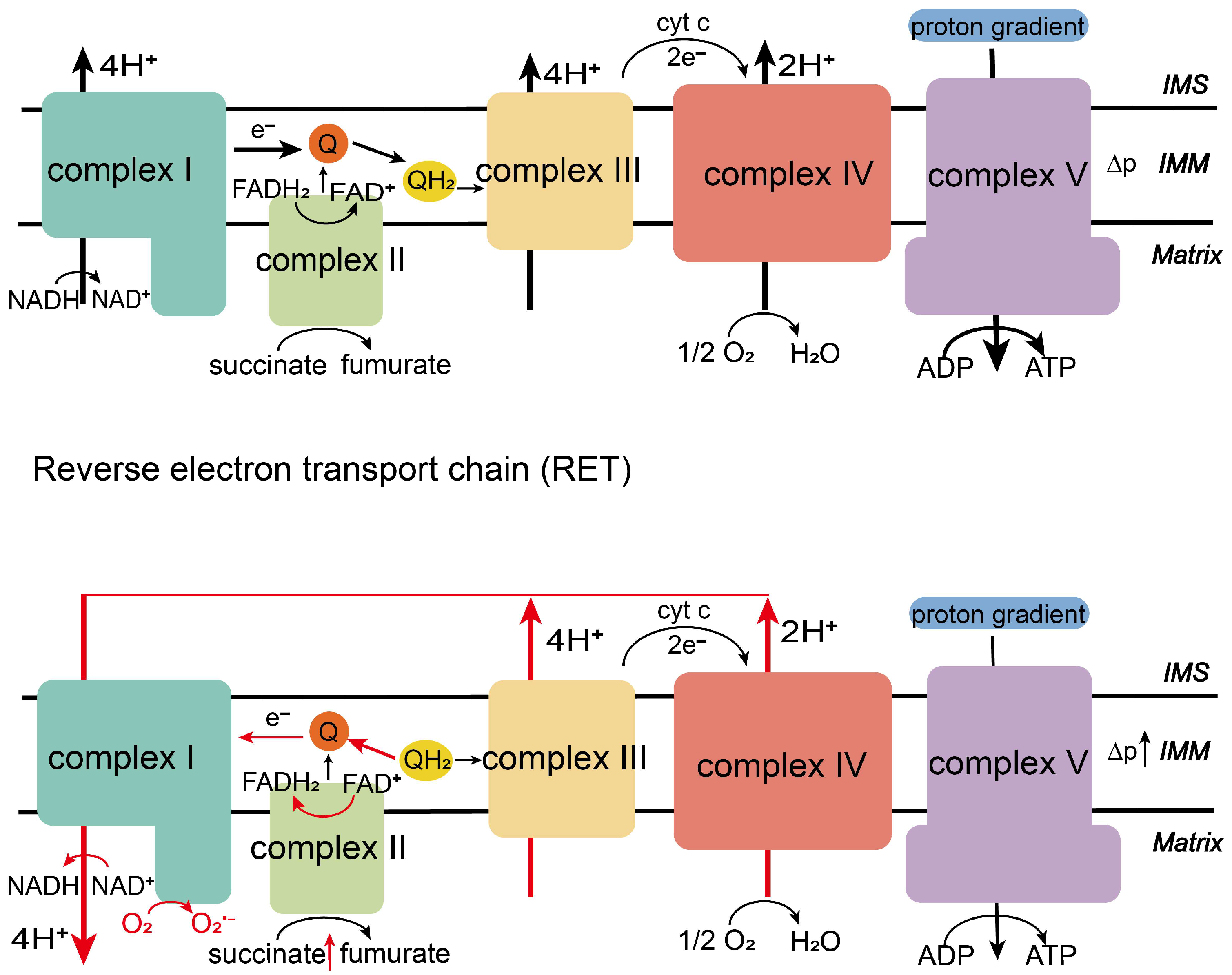
2. Mitochondrial Electron Transport Chain
2.1. Physiological Function of the Electron Transport Chain
2.2. The Production Process of the Electron Transport Chain
3. Mitochondrial Reverse Electron Transport
3.1. Occurrence of RET
3.2. Biological Functions of RET
3.2.1. The Double-Edged Role of RET-ROS
3.2.2. Effects of RET on Energy Metabolism and Physiological Functions
3.2.3. Effects of RET on Unnatural Organismal Death
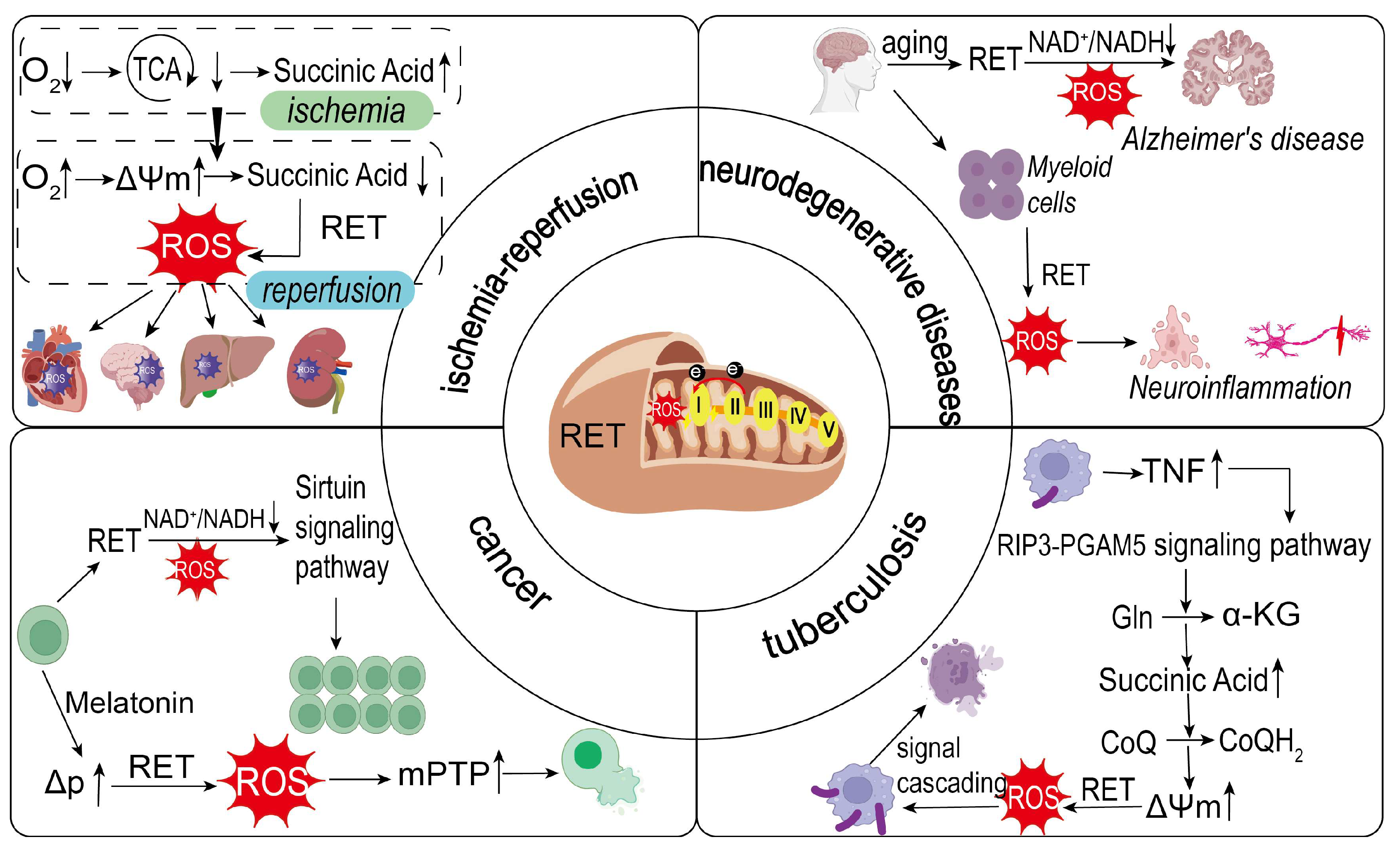
3.3. Relationship of RET to Disease Occurrence and Treatment
3.3.1. RET and Ischemia–Reperfusion (I/R)
3.3.2. RET and Neurodegenerative Diseases
3.3.3. RET and Cancer
3.3.4. RET and Tuberculosis
3.3.5. Treatment of RET-Related Diseases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frey, T.G.; Mannella, C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000, 25, 319–324. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, S.; Buelna-Chontal, M.; Correa, F.; Zazueta, C. Targeting mitochondria for cardiac protection. Curr. Drug Targets 2013, 14, 586–600. [Google Scholar] [CrossRef]
- Enríquez, J.A. Supramolecular Organization of Respiratory Complexes. Annu. Rev. Physiol. 2016, 78, 533–561. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Sanz, A. Mitochondrial reactive oxygen species: Do they extend or shorten animal lifespan? Biochim. Biophys. Acta 2016, 1857, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.W.; Weerapana, E. Cysteine-mediated redox signalling in the mitochondria. Mol. Biosyst. 2015, 11, 678–697. [Google Scholar] [CrossRef]
- Chance, B.; Hollunger, G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J. Biol. Chem. 1961, 236, 1534–1543. [Google Scholar] [CrossRef]
- Chavda, V.; Lu, B. Reverse electron transport at mitochondrial complex I in ischemic stroke, aging, and age-related diseases. Antioxidants 2023, 12, 895. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Zotta, A.; O’Neill, L.A.J.; Yin, M. Unlocking potential: The role of the electron transport chain in immunometabolism. Trends Immunol. 2024, 45, 259–273. [Google Scholar] [CrossRef]
- Li, J.L.; Lin, T.Y.; Chen, P.L.; Guo, T.N.; Huang, S.Y.; Chen, C.H.; Lin, C.H.; Chan, C.C. Mitochondrial function and Parkinson’s disease: From the perspective of the electron transport chain. Front. Mol. Neurosci. 2021, 14, 797833. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.J.; Eren, E.; Goetzl, E.J.; Kapogiannis, D. Mitochondrial electron transport chain protein abnormalities detected in plasma extracellular vesicles in Alzheimer’s disease. Biomedicines 2021, 9, 1587. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Wan, J.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704. [Google Scholar] [CrossRef] [PubMed]
- Meisel, J.D.; Miranda, M.; Skinner, O.S.; Wiesenthal, P.P.; Wellner, S.M.; Jourdain, A.A.; Ruvkun, G.; Mootha, V.K. Hypoxia and intra-complex genetic suppressors rescue complex I mutants by a shared mechanism. Cell 2024, 187, 659–675.e18. [Google Scholar] [CrossRef]
- Mangalhara, K.C.; Varanasi, S.K.; Johnson, M.A.; Burns, M.J.; Rojas, G.R.; Moltó, P.B.E.; Sainz, A.G.; Tadepalle, N.; Abbott, K.L.; Mendiratta, G.; et al. Manipulating mitochondrial electron flow enhances tumor immunogenicity. Science 2023, 381, 1316–1323. [Google Scholar] [CrossRef]
- Conley, K.E. Mitochondria to motion: Optimizing oxidative phosphorylation to improve exercise performance. J. Exp. Biol. 2016, 219, 243–249. [Google Scholar] [CrossRef]
- Bulkeley, E.A.; Foutouhi, A.; Wigney, K.; Santistevan, A.C.; Collins, C.; McNabb, B.; Meyers, S. Effects from disruption of mitochondrial electron transport chain function on bull sperm motility. Theriogenology 2021, 176, 63–72. [Google Scholar] [CrossRef]
- Billingham, L.K.; Stoolman, J.S.; Vasan, K.; Rodriguez, A.E.; Poor, T.A.; Szibor, M.; Jacobs, H.T.; Reczek, C.R.; Rashidi, A.; Zhang, P.; et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 2022, 23, 692–704. [Google Scholar] [CrossRef]
- Shin, Y.C.; Latorre-Muro, P.; Djurabekova, A.; Zdorevskyi, O.; Bennett, C.F.; Burger, N.; Song, K.; Xu, C.; Paulo, J.A.; Gygi, S.P.; et al. Structural basis of respiratory complex adaptation to cold temperatures. Cell 2024, 187, 6584–6598.e17. [Google Scholar] [CrossRef]
- Saraste, M. Oxidative phosphorylation at the fin de siècle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Efremov, R.G.; Baradaran, R.; Sazanov, L.A. The architecture of respiratory complex I. Nature 2010, 465, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Trumpower, B.L. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J. Biol. Chem. 1990, 265, 11409–11412. [Google Scholar] [CrossRef]
- Yankovskaya, V.; Horsefield, R.; Törnroth, S.; Luna-Chavez, C.; Miyoshi, H.; Léger, C.; Byrne, B.; Cecchini, G.; Iwata, S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 2003, 299, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Brandt, U.; Trumpower, B. The protonmotive Q cycle in mitochondria and bacteria. Crit. Rev. Biochem. Mol. Biol. 1994, 29, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Babcock, G.T.; Wikström, M. Oxygen activation and the conservation of energy in cell respiration. Nature 1992, 356, 301–309. [Google Scholar] [CrossRef]
- Rich, P.R. Mitochondrial cytochrome c oxidase: Catalysis, coupling and controversies. Biochem. Soc. Trans. 2017, 45, 813–829. [Google Scholar] [CrossRef]
- Boyer, P.D. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997, 66, 717–749. [Google Scholar] [CrossRef]
- Hinkle, P.C.; Kumar, M.A.; Resetar, A.; Harris, D.L. Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry 1991, 30, 3576–3582. [Google Scholar] [CrossRef]
- Lin, C.S.; Sharpley, M.S.; Fan, W.; Waymire, K.G.; Sadun, A.A.; Carelli, V.; Ross-Cisneros, F.N.; Baciu, P.; Sung, E.; McManus, M.J.; et al. Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc. Natl. Acad. Sci. USA 2012, 109, 20065–20070. [Google Scholar] [CrossRef]
- Szalárdy, L.; Zádori, D.; Klivényi, P.; Toldi, J.; Vécsei, L. Electron transport disturbances and neurodegeneration: From Albert Szent-Györgyi’s concept (Szeged) till novel approaches to boost mitochondrial bioenergetics. Oxidative Med. Cell. Longev. 2015, 2015, 498401. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Dehghani, M.A.; Karami, S.Z. Study of respiratory chain dysfunction in heart disease. J. Cardiovasc. Thorac. Res. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Rosen, P.C.; Sprenger, H.G.; Puszynska, A.M.; Mann, J.L.; Roessler, J.M.; Cangelosi, A.L.; Henne, A.; Condon, K.J.; Zhang, T.; et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science 2021, 374, 1227–1237. [Google Scholar] [CrossRef]
- Kumar, R.; Landry, A.P.; Guha, A.; Vitvitsky, V.; Lee, H.J.; Seike, K.; Reddy, P.; Lyssiotis, C.A.; Banerjee, R. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem. 2022, 298, 101435. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Ojha, R.; Tantray, I.; Rimal, S.; Mitra, S.; Cheshier, S.; Lu, B. Regulation of reverse electron transfer at mitochondrial complex I by unconventional Notch action in cancer stem cells. Dev. Cell 2022, 57, 260–276.e9. [Google Scholar] [CrossRef]
- Chance, B.; Hollunger, G. The interaction of energy and electron transfer reactions in mitochondria. IV. The pathway of electron transfer. J. Biol. Chem. 1961, 236, 1562–1568. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Superoxide production by NADH: Ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 2004, 382, 511–517. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Zhang, M.; Huang, C.; Song, Y.; Xu, F.; Zhang, J.; Li, J.; He, M.; Li, Y.; et al. SQR mediates therapeutic effects of H2S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 2020, 6, eaaz5752. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Scialò, F.; Sriram, A.; Fernández-Ayala, D.; Gubina, N.; Lõhmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enríquez, J.A.; et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Pham, L.; Arroum, T.; Wan, J.; Pavelich, L.; Bell, J.; Morse, P.T.; Lee, I.; Grossman, L.I.; Sanderson, T.H.; Malek, M.H.; et al. Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease—Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer. Redox Biol. 2024, 78, 103426. [Google Scholar] [CrossRef]
- Martins, D.; Kathiresan, M.; English, A.M. Cytochrome c peroxidase is a mitochondrial heme-based H2O2 sensor that modulates antioxidant defense. Free Radic. Biol. Med. 2013, 65, 541–551. [Google Scholar] [CrossRef]
- Treberg, J.R.; Quinlan, C.L.; Brand, M.D. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J. Biol. Chem. 2011, 286, 27103–27110. [Google Scholar] [CrossRef]
- Wong, H.S.; Monternier, P.A.; Brand, M.D. S1QELs suppress mitochondrial superoxide/hydrogen peroxide production from site IQ without inhibiting reverse electron flow through Complex I. Free Radic. Biol. Med. 2019, 143, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.T.; Lerner, C.A.; Watson, M.A.; Wong, H.S.; Gerencser, A.A.; Brand, M.D. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem. J. 2023, 480, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Scialò, F.; Sriram, A.; Stefanatos, R.; Spriggs, R.V.; Loh, S.H.Y.; Martins, L.M.; Sanz, A. Mitochondrial complex I derived ROS regulate stress adaptation in Drosophila melanogaster. Redox Biol. 2020, 32, 101450. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.M.; Ryan, D.G.; Prag, H.A.; Chowdhury, S.R.; Marques, E.; Turner, K.; Gruszczyk, A.V.; Yang, M.; Wolf, D.M.; Miljkovic, J.L.; et al. Pro-inflammatory macrophages produce mitochondria-derived superoxide by reverse electron transport at complex I that regulates IL-1β release during NLRP3 inflammasome activation. Nat. Metab. 2025, 7, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.C.; Wang, H.F.; Cai, Y.Y.; Yang, D.; Alolga, R.N.; Liu, B.; Li, J.; Huang, F.Q. Ginsenoside Rb1 inhibits astrocyte activation and promotes transfer of astrocytic mitochondria to neurons against ischemic stroke. Redox Biol. 2022, 54, 102363. [Google Scholar] [CrossRef] [PubMed]
- Rimal, S.; Tantray, I.; Li, Y.; Pal Khaket, T.; Li, Y.; Bhurtel, S.; Li, W.; Zeng, C.; Lu, B. Reverse electron transfer is activated during aging and contributes to aging and age-related disease. EMBO Rep. 2023, 24, e55548. [Google Scholar] [CrossRef]
- Roca, F.J.; Whitworth, L.J.; Prag, H.A.; Murphy, M.P.; Ramakrishnan, L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science 2022, 376, eabh2841. [Google Scholar] [CrossRef]
- Yin, Z.; Burger, N.; Kula-Alwar, D.; Aksentijević, D.; Bridges, H.R.; Prag, H.A.; Grba, D.N.; Viscomi, C.; James, A.M.; Mottahedin, A.; et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat. Commun. 2021, 12, 707. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Willis, C.M.; Krzak, G.; Hamel, R.; Pirvan, L.; Ionescu, R.B.; Reisz, J.A.; Prag, H.A.; Garcia-Segura, M.E.; Wu, V.; et al. Mitochondrial complex I activity in microglia sustains neuroinflammation. Nature 2024, 628, 195–203. [Google Scholar] [CrossRef]
- Prag, H.A.; Murphy, M.P.; Krieg, T. Preventing mitochondrial reverse electron transport as a strategy for cardioprotection. Basic. Res. Cardiol. 2023, 118, 34. [Google Scholar] [CrossRef]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879, Erratum in J. Biol. Chem. 2019, 294, 7966. [Google Scholar] [CrossRef]
- Szibor, M.; Schreckenberg, R.; Gizatullina, Z.; Dufour, E.; Wiesnet, M.; Dhandapani, P.K.; Debska-Vielhaber, G.; Heidler, J.; Wittig, I.; Nyman, T.A.; et al. Respiratory chain signalling is essential for adaptive remodelling following cardiac ischaemia. J. Cell. Mol. Med. 2020, 24, 3534–3548. [Google Scholar] [CrossRef]
- Shi, F.L.; Yuan, L.S.; Wong, T.S.; Li, Q.; Li, Y.P.; Xu, R.; You, Y.P.; Yuan, T.; Zhang, H.R.; Shi, Z.J.; et al. Dimethyl fumarate inhibits necroptosis and alleviates systemic inflammatory response syndrome by blocking the RIPK1-RIPK3-MLKL axis. Pharmacol. Res. 2023, 189, 106697. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Jiang, C.; Chang, W.Y.; Zhang, H.; An, J.; Ho, F.; Chen, P.; Zhang, H.; Junqueira, C.; Amgalan, D.; et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 2023, 56, 2523–2541.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Wang, F.; Mao, G.; Wu, J.; Han, R.; Sheng, R.; Qin, Z.; Ni, H. TIGAR reduces neuronal ferroptosis by inhibiting succinate dehydrogenase activity in cerebral ischemia. Free Radic. Biol. Med. 2024, 216, 89–105. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Bi, Y.; Li, W.; Piao, J.; Ren, X. PANoptosis: A new era for anti-cancer strategies. Life Sci. 2024, 359, 123241. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.L.; Li, Q.; Xu, R.; Yuan, L.S.; Chen, Y.; Shi, Z.J.; Li, Y.P.; Zhou, Z.Y.; Xu, L.H.; Zha, Q.B.; et al. Blocking reverse electron transfer-mediated mitochondrial DNA oxidation rescues cells from PANoptosis. Acta Pharmacol. Sin. 2024, 45, 594–608. [Google Scholar] [CrossRef]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960, 70, 68–78. [Google Scholar]
- Prag, H.A.; Gruszczyk, A.V.; Huang, M.M.; Beach, T.E.; Young, T.; Tronci, L.; Nikitopoulou, E.; Mulvey, J.F.; Ascione, R.; Hadjihambi, A.; et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res. 2021, 117, 1188–1201. [Google Scholar] [CrossRef]
- Tabata Fukushima, C.; Dancil, I.S.; Clary, H.; Shah, N.; Nadtochiy, S.M.; Brookes, P.S. Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions. Redox Biol. 2024, 70, 103047. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, A.B.; Vinogradov, A.D. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta 1990, 1019, 151–158. [Google Scholar] [CrossRef]
- Gallinat, A.; Vilahur, G.; Padró, T.; Badimon, L. Network-Assisted Systems Biology Analysis of the Mitochondrial Proteome in a Pre-Clinical Model of Ischemia, Revascularization and Post-Conditioning. Int. J. Mol. Sci. 2022, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, N.R.; Hjortbak, M.V.; Lassen, T.R.; Støttrup, N.B.; Johnsen, J.; Tonnesen, P.T.; Larsen, S.; Kimose, H.H.; Bøtker, H.E. Cardioprotective effect of succinate dehydrogenase inhibition in rat hearts and human myocardium with and without diabetes mellitus. Sci. Rep. 2020, 10, 10344. [Google Scholar] [CrossRef]
- Sorby-Adams, A.; Prime, T.A.; Miljkovic, J.L.; Prag, H.A.; Krieg, T.; Murphy, M.P. A model of mitochondrial superoxide production during ischaemia-reperfusion injury for therapeutic development and mechanistic understanding. Redox Biol. 2024, 72, 103161. [Google Scholar] [CrossRef]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef]
- Oh, C.J.; Kim, M.J.; Lee, J.M.; Kim, D.H.; Kim, I.Y.; Park, S.; Kim, Y.; Lee, K.B.; Lee, S.H.; Lim, C.W.; et al. Inhibition of pyruvate dehydrogenase kinase 4 ameliorates kidney ischemia-reperfusion injury by reducing succinate accumulation during ischemia and preserving mitochondrial function during reperfusion. Kidney Int. 2023, 104, 724–739. [Google Scholar] [CrossRef]
- Yu, B.; Jin, L.; Yao, X.; Zhang, Y.; Zhang, G.; Wang, F.; Su, X.; Fang, Q.; Xiao, L.; Yang, Y.; et al. TRPM2 protects against cisplatin-induced acute kidney injury and mitochondrial dysfunction via modulating autophagy. Theranostics 2023, 13, 4356–4375. [Google Scholar] [CrossRef]
- Yang, K.L.; Li, W.H.; Liu, Y.J.; Wei, Y.J.; Ren, Y.K.; Mai, C.D.; Zhang, S.Y.; Zuo, Y.; Sun, Z.Z.; Li, D.L.; et al. Hydrogen Sulfide Attenuates Neuroinflammation by Inhibiting the NLRP3/Caspase-1/GSDMD Pathway in Retina or Brain Neuron following Rat Ischemia/Reperfusion. Brain Sci. 2022, 12, 1245. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and mitochondrial cascades in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Rimal, S.; Li, W.; Khaket, T.P.; Li, Y.; Tantray, I.; Li, Y.; Bhurtel, S.; Grinberg, L.T.; Spina, S.; Sillero, M.I.C.; et al. Deregulation of mitochondrial reverse electron transport alters the metabolism of reactive oxygen species and NAD+/NADH and presents a therapeutic target in Alzheimer’s disease. Ageing Neur. Dis. 2024, 4, 4. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Willis, C.M.; Hamel, R.; Krzak, G.; Reisz, J.A.; Prag, H.A.; Wu, V.; Xiang, Y.; van den Bosch, A.M.R.; Nicaise, A.M.; et al. Mitochondrial reverse electron transport in myeloid cells perpetuates neuroinflammation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Cardona, L.R.; Kong, H.; Vasan, K.; McElroy, G.S.; Werner, M.; Kihshen, H.; Reczek, C.R.; Weinberg, S.E.; Gao, P.; et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 2020, 585, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Florido, J.; Martinez-Ruiz, L.; Rodriguez-Santana, C.; López-Rodríguez, A.; Hidalgo-Gutiérrez, A.; Cottet-Rousselle, C.; Lamarche, F.; Schlattner, U.; Guerra-Librero, A.; Aranda-Martínez, P.; et al. Melatonin drives apoptosis in head and neck cancer by increasing mitochondrial ROS generated via reverse electron transport. J. Pineal Res. 2022, 73, e12824. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Estaji, F.; Kamali, A.; Keikha, M. Strengthening the global response to tuberculosis: Insights from the 2024 WHO global TB report. J. Clin. Tuberc. Other Mycobact. Dis. 2025, 39, 100522. [Google Scholar] [CrossRef]
- Roca, F.J.; Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Prag, H.A.; Aksentijevic, D.; Dannhorn, A.; Giles, A.V.; Mulvey, J.F.; Sauchanka, O.; Du, L.; Bates, G.; Reinhold, J.; Kula-Alwar, D.; et al. Ischemia-selective cardioprotection by malonate for ischemia/reperfusion injury. Circ. Res. 2022, 131, 528–541. [Google Scholar] [CrossRef]
- Abe, J.; Vujic, A.; Prag, H.A.; Murphy, M.P.; Krieg, T. Malonate given at reperfusion prevents post-myocardial infarction heart failure by decreasing ischemia/reperfusion injury. Basic Res. Cardiol. 2024, 119, 691–697. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, Y.; Song, X.; Zeng, J.; Wang, Z.; Wang, Z.; Zhang, H.; Xu, J.; Li, W.; Gong, Z.; et al. Baicalin attenuates neuronal damage associated with SDH activation and PDK2-PDH axis dysfunction in early reperfusion. Phytomedicine 2024, 129, 155570. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Parks, J.K.; Parker, W.D., Jr.; Bennett, J.P., Jr. The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim. Biophys. Acta 1999, 1453, 49–62. [Google Scholar] [CrossRef]
- Ajike, R.A.; Afolabi, O.A.; Alabi, B.A.; Ajayi, A.F.; Oyekunle, O.S.; Lawal, S.K.; Olojede, S.O.; Nku-Ekpang, O.A.; Hezekiah, O.S.; Hammed, O.S. Sequential administration of febuxostat and vitamin E protects against testicular ischemia/reperfusion injury via inhibition of sperm DNA damage in Wistar rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–15. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef]
- Capeloa, T.; Krzystyniak, J.; d’Hose, D.; Rodriguez, A.C.; Payen, V.L.; Zampieri, L.X.; Van de Velde, J.A.; Benyahia, Z.; Pranzini, E.; Vazeille, T.; et al. MitoQ inhibits human breast cancer cell migration, invasion and clonogenicity. Cancers 2022, 14, 1516. [Google Scholar] [CrossRef]
- Capeloa, T.; Van de Velde, J.A.; d’Hose, D.; Lipari, S.G.; Derouane, F.; Hamelin, L.; Bedin, M.; Vazeille, T.; Duhoux, F.P.; Murphy, M.P.; et al. Inhibition of mitochondrial redox signaling with MitoQ prevents metastasis of human pancreatic cancer in mice. Cancers 2022, 14, 4918. [Google Scholar] [CrossRef] [PubMed]
- Lebigot, E.; Gaignard, P.; Dorboz, I.; Slama, A.; Rio, M.; de Lonlay, P.; Héron, B.; Sabourdy, F.; Boespflug-Tanguy, O.; Cardoso, A.; et al. Impact of mutations within the [Fe-S] cluster or the lipoic acid biosynthesis pathways on mitochondrial protein expression profiles in fibroblasts from patients. Mol. Genet. Metab. 2017, 122, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Oskuye, Z.Z.; Mehri, K.; Mokhtari, B.; Bafadam, S.; Nemati, S.; Badalzadeh, R. Cardioprotective effect of antioxidant combination therapy: A highlight on MitoQ plus alpha-lipoic acid beneficial impact on myocardial ischemia-reperfusion injury in aged rats. Heliyon 2024, 10, e28158. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.; Ren, M.; Chen, Y.; Zhang, J.; Li, J.; Gao, F.; Bao, Y.; Huang, Y.; Yang, X.; et al. Metformin induces apoptosis and ferroptosis of ovarian cancer cells under energy stress conditions. Cells 2025, 14, 213. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, H.; Liu, Z.; Li, Q.; Guo, Y.; Chen, Y.; Chang, Y.; Cui, H. Carnitine palmitoyltransferase 1 (CPT1) alleviates oxidative stress and apoptosis of hippocampal neuron in response to beta-Amyloid peptide fragment Aβ25-35. Bioengineered 2021, 12, 5440–5449. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Guan, L.; Zhang, H.; Huang, Y.; Johnson, C.H.; Wu, Z.; Gonzalez, F.J.; Yu, A.; Huang, P.; et al. Carnitine palmitoyltransferase 1C regulates cancer cell senescence through mitochondria-associated metabolic reprograming. Cell Death Differ. 2018, 25, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Freyberger, A.; Lawrenz, B.; Schladt, L.; Schmuck, G.; Ellinger-Ziegelbauer, H. Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Sci. Rep. 2017, 7, 45465. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Hou, J.; Zheng, Y.; Jiang, T.; Zhao, X.; Cai, J.; Huang, J.; He, H.; Xu, J.; Qian, S.; et al. NAD+-boosting agent nicotinamide mononucleotide potently improves mitochondria stress response in Alzheimer’s disease via ATF4-dependent mitochondrial UPR. Cell Death Dis. 2024, 15, 744. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, H.; Lan, W.; Yang, J.; Tang, Y. Nicotinamide ribose ameliorates myocardial ischemia/reperfusion injury by regulating autophagy and regulating oxidative stress. Exp. Ther. Med. 2024, 27, 187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Hu, C.; Wang, B.; Liu, X.; Wu, Q.; Xu, D.; Shi, Z.; Sun, C. Mitochondrial Reverse Electron Transport: Mechanisms, Pathophysiological Roles, and Therapeutic Potential. Biology 2025, 14, 1140. https://doi.org/10.3390/biology14091140
Bao Y, Hu C, Wang B, Liu X, Wu Q, Xu D, Shi Z, Sun C. Mitochondrial Reverse Electron Transport: Mechanisms, Pathophysiological Roles, and Therapeutic Potential. Biology. 2025; 14(9):1140. https://doi.org/10.3390/biology14091140
Chicago/Turabian StyleBao, Yanyu, Cuilan Hu, Bing Wang, Xiongxiong Liu, Qingfeng Wu, Dan Xu, Zheng Shi, and Chao Sun. 2025. "Mitochondrial Reverse Electron Transport: Mechanisms, Pathophysiological Roles, and Therapeutic Potential" Biology 14, no. 9: 1140. https://doi.org/10.3390/biology14091140
APA StyleBao, Y., Hu, C., Wang, B., Liu, X., Wu, Q., Xu, D., Shi, Z., & Sun, C. (2025). Mitochondrial Reverse Electron Transport: Mechanisms, Pathophysiological Roles, and Therapeutic Potential. Biology, 14(9), 1140. https://doi.org/10.3390/biology14091140







