Gonadal Development and Its Influencing Factors in the Red Swamp Crayfish (Procambarus clarkii): A Review
Simple Summary
Abstract
1. Introduction
2. Gonadal Development in Procambarus clarkii
2.1. Testis Development in Procambarus clarkii
2.1.1. Histological Study of the Testis
2.1.2. Morphology of Procambarus clarkii Sperm
2.1.3. Sperm and Testes Development Stages
2.2. Ovarian Development in Procambarus clarkii
2.2.1. Ovarian Histology
2.2.2. Ovarian Development Patterns
2.2.3. Stages of Oocyte and Ovarian Development
2.3. Genes Associated with Gonadal Development in Procambarus clarkii
3. Factors Influencing Gonadal Development in Procambarus clarkii
3.1. Environmental Factors
3.1.1. Temperature
3.1.2. Salinity
3.1.3. Light
3.1.4. Nutritional Conditions
3.1.5. Toxic Substances
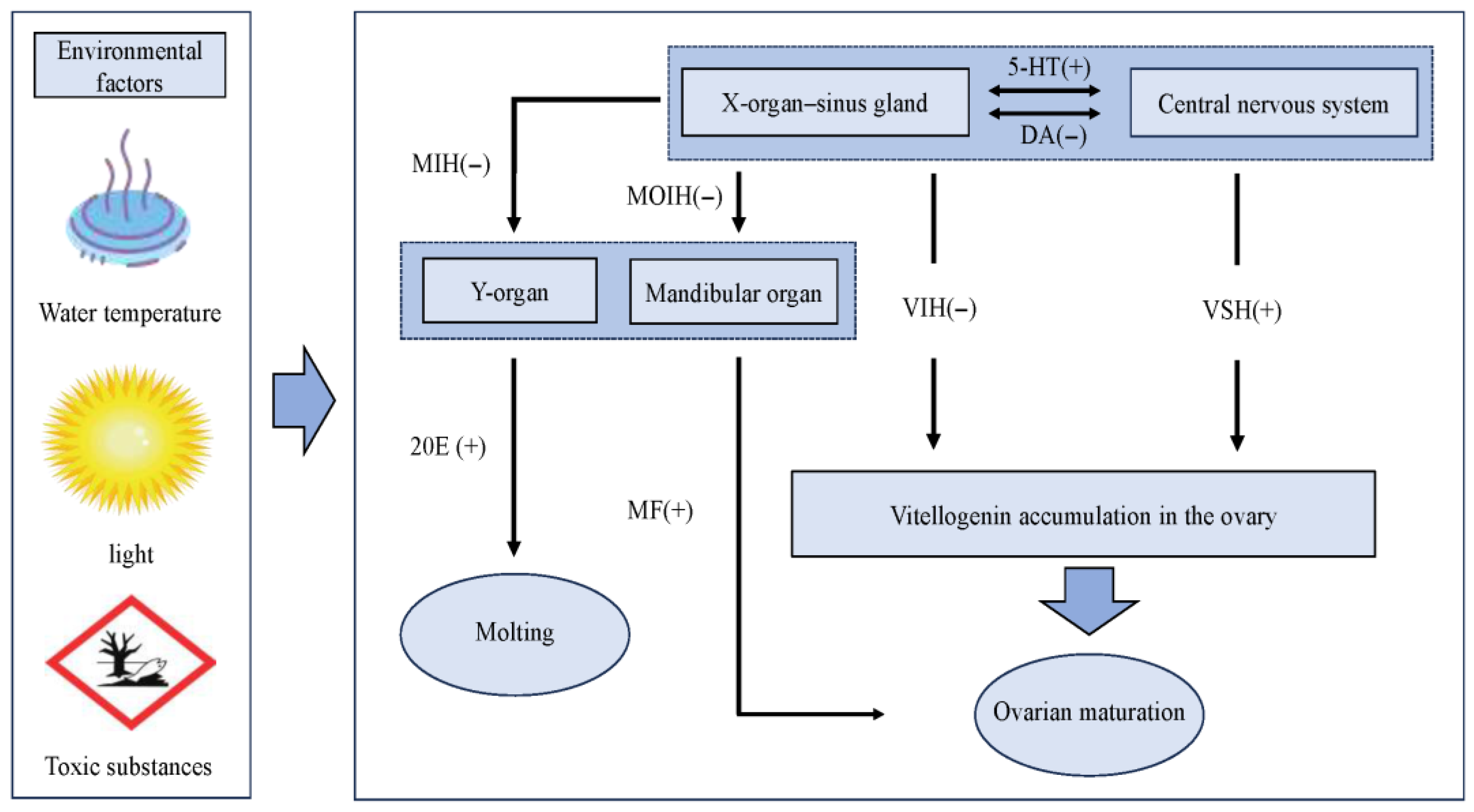
3.2. Eyestalk Ablation
3.3. Hormone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, Y.; Zhao, W.; Tang, Z.; Huang, L.; Zhu, X.; Liang, X.; Yan, A.; Lu, Z.; Yu, Y.; Tang, D.; et al. Comparative transcriptomic analysis of the different developmental stages of ovary in red swamp crayfish Procambarus clarkii. BMC Genom. 2021, 22, 199. [Google Scholar] [CrossRef] [PubMed]
- National Fisheries Technology Extension Center (NFTEC); China Society of Fisheries (CSF). “China Crayfish Industry Development Report (2024)” Was Released in Xuyi, Jiangsu. Available online: http://www.nftec.agri.cn/cyfz/202406/t20240613_8642276.htm (accessed on 26 July 2025).
- Hou, Y.; Xu, Q.; Yang, Y.; Jia, R.; Huang, X.; Zhou, L.; Li, B.; Zhu, J. Dynamic impact of one-year integrated rice-crayfish farming on bacterioplankton communities in paddy water. Biology 2024, 13, 1059. [Google Scholar] [CrossRef]
- Qin, L.; Li, W.; Rong, K.; Zhang, T.; Liu, J. Effects evaluation of in vitro incubation and maternal incubation of Procambarus clarkii embryos. J. Fish. China 2024, 48, 130–138. [Google Scholar] [CrossRef]
- Xu, J. Study on Physical and Nutritional Factors Involved in the Growth and Reproduction of the Crayfish Procambarus clarkii. Master’s Thesis, Central China Normal University, Wuhan, China, 2008. [Google Scholar]
- Jin, S.; Jacquin, L.; Xiong, M.; Li, R.; Lek, S.; Li, W.; Zhang, T. Reproductive pattern and population dynamics of commercial red swamp crayfish (Procambarus clarkii) from China: Implications for sustainable aquaculture management. Peer J. 2019, 7, e6214. [Google Scholar] [CrossRef]
- Qin, L.; He, J.; Rong, K.; Guo, C.; Liu, J.; Zhang, T.; Li, W. Changes in secondary sexual characteristics of female red swamp crayfish (Procambarus clarkii) and relationship to ovarian development: Implications for intensive breeding of seedlings. Aquaculture 2024, 592, 741156. [Google Scholar] [CrossRef]
- Huang, W.; Gong, S. Histological studies on the male reproductive system of Procambarus clarkii. J. Fish. China 2012, 36, 514–521. [Google Scholar] [CrossRef]
- Huang, W. Studies on the Testis, Vas Deferens, Spermatophore Histology and Testis’annual Variation of Procambarus clarkii. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2012. [Google Scholar]
- Hu, R.; Shi, W.; Wang, P.; Wan, X.; Shen, H.; Li, H.; Wang, L.; Yang, Z.; Wu, X. Research progress on reproductive biology of important economic shrimps of Palaemonoidea. Mar. Fish. 2021, 43, 485–502. [Google Scholar] [CrossRef]
- Fan, Y. Studies on Development of Reproductive Systems in Cherax quadricarinatus. Master’s Thesis, East China Normal University, Shanghai, China, 2005. [Google Scholar]
- Moses, M.J. Spermiogenesis in the crayfish (Procambarus clarkii) II. Description of stages. J. Biophys. Biochem. Cytol. 1961, 10, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Yazicioglu, B.; Hamr, P.; Kozák, P.; Kouba, A.; Niksirat, H. Fine structure of the spermatozoon in three species of Cambaridae (Arthropoda: Crustacea: Decapoda) Cambarus robustus, Orconectes propinquus and Orconectes rusticus: A comparative biometrical study. Peer J. 2016, 4, e2363. [Google Scholar] [CrossRef]
- Niksirat, H.; Kouba, A.; Pšenička, M.; Kuklina, I.; Kozák, P. Ultrastructure of spermatozoa from three genera of crayfish Orconectes, Procambarus and Astacus (Decapoda: Astacoidea): New findings and comparisons. Zool. Anz. 2013, 252, 226–233. [Google Scholar] [CrossRef]
- Dai, Y.; Gong, X.; Li, B.; Wang, Y.; Huang, W. Reproductive period of Procambarus clarkii in Wuhan area. Chin. J. Zool. 2008, 43, 21–27. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, M. Morphological and histological studies on the ovary development in Parapenaeopsis hardwickii. J. Fish. China 2002, 26, 105–110. [Google Scholar]
- Li, Z.; Zhang, C.; Li, F.; Xiang, J. Histological study on the gonadal development of Exopalaemon carinicauda (Holthuis, 1950). J. Fish. China 2014, 38, 362–370. [Google Scholar]
- Ando, H.; Makioka, T. Structure of the ovary and mode of oogenesis in a freshwater crayfish, Procambarus clarkii (Girard). Zool. Sci. 1998, 15, 893–901. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, Y. Histology of the ovarian structure and development of Penaeus vannamei. Trans. Ocean. Limnol. 2004, 2, 52–58. [Google Scholar] [CrossRef]
- Song, G. Study on the Developmental Process of Female Reproductive System of Procambarus clarkii and the Factor That Affect its Reproductive Performance. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2017. [Google Scholar] [CrossRef]
- King, J.E. A study of the reproductive organs of the common marine shrimp, Penaeus setiferus (Linnaeus). Biol. Bull. 1948, 94, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Jacquin, L.; Huang, F.; Xiong, M.; Li, R.; Lek, S.; Li, W.; Liu, J.; Zhang, T. Optimizing reproductive performance and embryonic development of red swamp crayfish Procambarus clarkii by manipulating water temperature. Aquaculture 2019, 510, 32–42. [Google Scholar] [CrossRef]
- Oluoch, A.O. Breeding biology of the Louisiana red swamp crayfish Procambarus clarkii Girard in Lake Naivasha, Kenya. Hydrobiologia 1990, 208, 85–92. [Google Scholar] [CrossRef]
- Long, X.; Wu, X.; Zhao, L.; Liu, J.; Cheng, Y. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture 2017, 473, 545–553. [Google Scholar] [CrossRef]
- Souza, T.L.; Braga, A.A.; López-Greco, L.S.; Nunes, E.T. Dynamics of oogenesis in ghost shrimp Callichirus major (Crustacea: Axiidea): A morphofunctional and histochemical study. Acta Histochem. 2017, 119, 769–777. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, S.S.; El-Khodary, G.M.; Ghonim, A.Z. Ovarian cycle and scanning electron micrographs of the spawned egg of female mantis shrimp Oratosquilla massavensis (Alexandria, Egypt). J. Basic. Appl. Zool. 2012, 65, 116–124. [Google Scholar] [CrossRef][Green Version]
- Fernandes, M.A.S.; Mendonça, M.I.R.; Marques, J.C.; Madeira, V.M.C. Seasonal changes in the biochemical composition and energy content of the red swamp crayfish Procambarus Clarkii (Girard) in the Lower Mondego River Valley, Portugal. J. Crustac. Biol. 1994, 14, 736–743. [Google Scholar] [CrossRef]
- Guimarães, M.P.; Calado, T.C.d.S.; Barros, M.S.F.d. Gonad development in mature females of tidal spray crab Plagusia depressa (Brachyura: Plagusiidae). Acta Zool. 2021, 102, 227–236. [Google Scholar] [CrossRef]
- Zhao, W. Study on Ovary Development Law and Transcriptome of Different Development Stages of Procambarus clarkii in Guangxi. Master’s Thesis, GuangXi University, Nanning, China, 2020. [Google Scholar] [CrossRef]
- Wang, C. Effects of 17α-hydroxyprogesterone and 5-hydroxytryptamine on the Ovarian Development of Procambarus clarkii. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar]
- Chen, D.; Liu, F.; Zhu, Z.; Lin, Q.; Zeng, C.; Ye, H. Ontogenetic development of gonads and external sexual characters of the protandric simultaneous hermaphrodite peppermint shrimp, Lysmata vittata (Caridea: Hippolytidae). PLoS ONE 2019, 14, e0215406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, J.; Cao, J.; Dai, P.; Chen, B.; Tan, J.; Meng, X.; Luo, K.; Fu, Q.; Wei, P.; et al. Reproductive ability disparity in the Pacific whiteleg shrimp (Penaeus vannamei): Insights from ovarian cellular and molecular levels. Biology 2024, 13, 218. [Google Scholar] [CrossRef]
- Guo, F.; Zhou, X.; Zhao, C.; Xu, Z.; Wang, T. A study on the ovarian development and growth traits of two color populations of Procambarus clarkii. Trans. Ocean. Limnol. 2012, 2, 89–96. [Google Scholar] [CrossRef]
- Du, L.; Hu, Y.; Lu, S.; Li, X.; Li, J.; Lin, H.; Yang, J.; Xu, Y.; Xu, Z. Single-cell RNA sequencing reveals the heterogeneity of hepatopancreas cells and their association with gonadal development in the red swamp crayfish Procambarus clarkii. Aquaculture 2025, 601, 742311. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; Bi, X.; Wu, T.; Liang, Z.; Gao, X.; Chen, T.; Wang, H.; Li, M.; Chen, X. Transcriptomic analysis of Procambarus clarkii ovary at two development stages. J. South. Agric. 2023, 54, 784–796. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, K.; Li, H.; Zhan, M.; Wang, S.; Song, E.; Zhang, Q.; He, J.; He, X.; Xu, M.; et al. Vitellogenin receptor mediates heat adaptability of oocyte development in mud crabs and zebrafish. Nat. Commun. 2025, 16, 3722. [Google Scholar] [CrossRef]
- Yao, H.; Gao, W.; Zhang, J.; Cao, M.; Xiao, W.; Dong, L.; Xie, S.; Tian, J. Effects of dietary histidine on growth, non-specific immune and ovarian development of the red swamp crayfish (Procambarus clarkii). Aquacult Rep. 2024, 36, 102157. [Google Scholar] [CrossRef]
- Mardhiyyah, M.P.; Zakaria, M.F.; Amin-Safwan, A.; Nur-Syahirah, M.; Sung, Y.Y.; Ma, H.; Ikhwanuddin, M. Transcriptome profile and gene expression during different ovarian maturation stages of Macrobrachium rosenbergii (De Man, 1879). Trop. Life Sci. Res. 2024, 35, 77–108. [Google Scholar] [CrossRef]
- Li, J.; Qian, C.; Zhou, W.; Xi, Y.; Cheng, Y.; Li, J. Cement glands as a second sexual characteristic can help to evaluate the ovarian development and reproductive spawning period of the female crayfish Procambarus clarkii. Aquacult Rep. 2024, 35, 101977. [Google Scholar] [CrossRef]
- Crickard, J.B.; Kaniecki, K.; Kwon, Y.; Sung, P.; Greene, E.C. Meiosis-specific recombinase Dmc1 is a potent inhibitor of the Srs2 antirecombinase. Proc. Natl. Acad. Sci. USA 2018, 115, E10041–E10048. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Xue, C.; Zhu, C.; Wu, N.; Chang, G.; Li, J. Molecular cloning and expression analysis of Dmc gene related to gonadal development in Procambarus clarkii. Aquacult Res. 2020, 52, 1321–1326. [Google Scholar] [CrossRef]
- Okutsu, T.; Kang, B.J.; Miwa, M.; Yoshizaki, G.; Maeno, Y.; Wilder, M.N. Molecular cloning and characterization of Dmc1, a gene involved in gametogenesis, from the whiteleg shrimp Litopenaeus vannamei. Fish. Sci. 2010, 76, 961–969. [Google Scholar] [CrossRef]
- Lu, Z.; Ji, Y.; Dai, L.; Wang, D.; Guo, Z.; Xiao, J.; Liang, Z.; Huang, B.; Yang, D.; Wei, Y. Gene cloning and expression characteristics of Dmrt1 gene in Procambarus clarkii. J. South. Agric. 2024, 55, 1207–1215. [Google Scholar] [CrossRef]
- Wan, H.; Liao, J.; Zhang, Z.; Zeng, X.; Liang, K.; Wang, Y. Molecular cloning, characterization, and expression analysis of a sex-biased transcriptional factor sox9 gene of mud crab Scylla paramamosain. Gene 2021, 774, 145423. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhong, J.; Zhang, Z.; Xie, Y.; Wang, Y. Characterization of the foxl2 gene involved in the vtg expression in mud crab (Scylla paramamosain). Gene 2021, 798, 145807. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, W.; Peng, M.; Yang, C.; Chen, X.; Wu, T.; Zeng, D.; Zhao, Y.; Chen, X. Cloning, identification, and functional analysis of the Foxl2 gene in Procambarus clarkii. Genes 2023, 14, 2190. [Google Scholar] [CrossRef]
- Qiu, G.-F.; Liu, P. On the role of Cdc2 kinase during meiotic maturation of oocyte in the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xing, Z.; Lu, W.; Qian, Z.; Yu, H.; Li, J. Transcriptome analysis of red swamp crawfish Procambarus clarkii reveals genes involved in gonadal development. PLoS ONE 2014, 9, e105122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, F.; Su, N.; Jiang, S.; Yang, Q.; Huang, J.; Yang, L.; Jiang, S. Identification of gonadal associated genes in black tiger shrimp (Penaeus monodon) using transcriptome analysis and high-throughput sequencing. Aquacult Res. 2022, 53, 6595–6605. [Google Scholar] [CrossRef]
- Lu, X.; Peng, D.; Chen, X.; Wu, F.; Jiang, M.; Tian, J.; Liu, W.; Yu, L.; Wen, H.; Wei, K. Effects of dietary protein levels on growth, muscle composition, digestive enzymes activities, hemolymph biochemical indices and ovary development of pre-adult red swamp crayfish (Procambarus clarkii). Aquacult Rep. 2020, 18, 100542. [Google Scholar] [CrossRef]
- Si, J.; He, S.; Cao, X.; Lan, J. High lipolytic capacity improves cold tolerance in red swamp crayfish (Procambarus clarkii). Aquaculture 2025, 595, 741683. [Google Scholar] [CrossRef]
- Wang, L.; Hu, M.; Cai, L.; Wang, Y.; Guan, T.; Zhu, C.; Wang, H.; Wang, G.; Li, J. Effects of light intensity and light duration on growth performance and ovarian development in red swamp crayfish Procambarus clarkii (Girard, 1852). Aquacult Int. 2025, 33, 54. [Google Scholar] [CrossRef]
- Zeng, Q.; Luo, M.; Qin, L.; Guo, C.; Liu, J.; Zhang, T.; Feng, G.; Li, W. Effects of hypoxia stress on survival, antioxidant and anaerobic metabolic enzymes, and related gene expression of red swamp crayfish Procambarus clarkii. Biology 2024, 13, 33. [Google Scholar] [CrossRef]
- Wang, J.; Ye, J.; Zhang, Z.; An, Z.; Wang, T.; Dong, X. Comparison of the nutrient value, nonspecific immunity, and intestinal microflora of red swamp crayfish (Procambarus clarkii) in different culture modes. Aquacult Rep. 2023, 31, 101683. [Google Scholar] [CrossRef]
- Shao, G.-M.; Tan, H.-Y.; Wang, Y.-F. Effect of combined water temperature, stocking density and fish meal: Soybean meal ratio in diet on precocity of the crayfish Procambarus clarkii (Girard). Aquacult Res. 2018, 49, 2081–2083. [Google Scholar] [CrossRef]
- Wu, W.; Xu, Q.; Wan, T.; Jia, L.; Cheng, X.; Li, D.; Bai, Z. Comparison of high temperature tolerance in three population diallel hybrid population of red swamp crayfish (Procambarus clarkii). J. Dalian Oce Univ. 2024, 39, 606–612. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Kuang, T.; Zhu, H.; Jiang, Q.; Zhou, G.; Zhou, G.; Zhu, Y. Effect of warming up to stimulate Procambarus clarkii to breed in winter. Freshw. Fish. 2012, 42, 93–96. [Google Scholar]
- Xu, J.; Yue, C.; Dai, Y.; Wang, Y. Effects of water temperature, photoperiod and diet on survival rate and ovarian development of the crawfish, Procambarus clarkii. J. Cent. China Norm. Univ. (Nat. Sci. Ed.) 2008, 42, 97–101. [Google Scholar] [CrossRef]
- Carmona-Osalde, C.; Rodriguez-Serna, M.; Olvera-Novoa, M.A.; Gutierrez-Yurrita, P.J. Gonadal development, spawning, growth and survival of the crayfish Procambarus llamasi at three different water temperatures. Aquaculture 2004, 232, 305–316. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Xu, W.; Chen, D.; Li, B.; Cheng, Y.; Guo, X.; Dong, W.; Shu, M. The effects of salinities stress on histopathological changes, serum biochemical index, non-specific immune and transcriptome analysis in red swamp crayfish Procambarus clarkii. Sci. Total Environ. 2022, 840, 156502. [Google Scholar] [CrossRef]
- Luo, L.; Yang, L.-S.; Huang, J.-H.; Jiang, S.-G.; Zhou, F.-L.; Li, Y.-D.; Jiang, S.; Yang, Q.-B. Effects of different salinity stress on the transcriptomic responses of freshwater crayfish (Procambarus clarkii, Girard, 1852). Biology 2024, 13, 530. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, L.; Liu, D.; Huang, J.; Jiang, S.; Zhou, F.; Yang, Q.; Jiang, S.; Li, Y.; Tan, L.; et al. Effect of acute salinity stress on metabolism, antioxidant status, and histological structure of Procambarus clarkii. Aquacult Res. 2023, 2023, 2748257. [Google Scholar] [CrossRef]
- Meineri, E.; Rodriguez-Perez, H.; Hilaire, S.; Mesleard, F. Distribution and reproduction of Procambarus clarkii in relation to water management, salinity and habitat type in the Camargue. Aquat. Conserv. 2014, 24, 312–323. [Google Scholar] [CrossRef]
- Nota, A.; Santovito, A.; Gattelli, R.; Tiralongo, F. From fresh to salt waters: First reports of the red swamp crayfish Procambarus clarkii (Girard, 1852) in Mediterranean marine waters. Hydrobiology 2023, 3, 1–10. [Google Scholar] [CrossRef]
- Scalici, M.; Chiesa, S.; Scuderi, S.; Celauro, D.; Gibertini, G. Population structure and dynamics of Procambarus clarkii (Girard, 1852) in a Mediterranean brackish wetland (Central Italy). Biol. Invasions 2010, 12, 1415–1425. [Google Scholar] [CrossRef]
- Farhadi, A.; Huang, Z.; Qiu, B.; Ikhwanuddin, M.; Ma, H. Effect of light condition on the growth performance and biochemical compositions of post-mating female mud crab (Scylla paramamosain). Aquacult Rep. 2021, 21, 100807. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Hu, M.; Cai, L.; Wang, Y.; Zhou, X.; Zhang, L.; Zhu, C.; Wang, H.; Wang, G.; et al. Comparative transcriptome analysis reveals molecular mechanisms of the effects of light intensity and photoperiod on ovarian development in Procambarus clarkii (Girard, 1852). Comp. Biochem. Phys. D 2024, 52, 101329. [Google Scholar] [CrossRef]
- Su, Y.; Xian, J.; Zheng, P.; Wang, L.; Lu, Y.; Zhang, Z.; Zhang, X.; Ma, Y.; Li, J.; Liu, C.; et al. Effect of drought stress on the growth, ovary development, antioxidant capacity, sex hormone content, and reproductive-related gene expression of red swamp crayfish (Procambarus clarkii). Aquacult Rep. 2024, 38, 102352. [Google Scholar] [CrossRef]
- Wang, F.; Dong, S.; Huang, G.; Wu, L.; Tian, X.; Ma, S. The effect of light color on the growth of Chinese shrimp Fenneropenaeus chinensis. Aquaculture 2003, 228, 351–360. [Google Scholar] [CrossRef]
- Toyota, K.; Usami, K.; Mizusawa, K.; Ohira, T. Effect of blue light on the growth of the red swamp crayfish Procambarus clarkii Larvae-Seasonal and sexual differences. Zool. Stud. 2022, 60, e3. [Google Scholar] [CrossRef]
- Hoang, T.; Lee, S.Y.; Keenan, C.P.; Marsden, G.E. Ovarian maturation of the banana prawn, Penaeus merguiensis de Man under different light intensities. Aquaculture 2002, 208, 159–168. [Google Scholar] [CrossRef]
- Luo, J.; Zhuo, H.; Chen, J.; Liang, H.; Wen, C. Cloning, expression and photoperiodic response of gonad-inhibiting hormone (GIH) gene in scalloped spiny lobster Panulirus homarus. J. Dalian Oce Univ. 2021, 36, 727–735. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, J.; Zhou, C.; Lin, Q. Effects of light on the reproductive performance of Lysmata vittata and influences of diets on the growth and development of its larvae. J. Fish. Sci. China 2020, 27, 260–267. [Google Scholar]
- Matsuda, H.; Abe, F.; Tanaka, S. Effect of photoperiod on metamorphosis from phyllosoma larvae to puerulus postlarvae in the Japanese spiny lobster Panulirus japonicus. Aquaculture 2012, 326, 136–140. [Google Scholar] [CrossRef]
- Pang, Z.; Liu, J.; Zhang, Y.; Zhao, Z.; Liu, S.; Lai, X.; Yu, F.; Gao, H. Effects of different photoperiods on growth, gonadal development, and biochemical components of hemolymph in Exopalaemon carinicauda. Prog. Fish. Sci. 2022, 43, 189–196. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, Y.; Wang, Q.; Gu, X.; Xu, G.; Liu, Q. Effects of photoperiod on reproduction performance and egg quality of Cherax quadricarinatus. J. Fish. China 2004, 28, 675–681. [Google Scholar]
- Yuan, C.; Li, W.; Huang, Y.; He, P.; Zheng, Y.; Li, X.; Lu, Z.; Zhang, S.; Li, W.; Peng, J.; et al. Effects of environmental stress of sunning field on ovarian tissue, vitellogenin and nutrient metabolism of Procambarus clarkii. Southwest. China J. Agric. Sci. 2024, 37, 436–445. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, S.; Li, J.; Huang, J.; Li, J.; Cheng, Y. Effects of dietary phospholipid and cholesterol levels on growth, molting performance, and ovary development in female juvenile crayfish (Procambarus clarkii). Aquacult Nutr. 2022, 2022, 4033033. [Google Scholar] [CrossRef]
- Carmona-Osalde, C.; Olvera-Novoa, M.A.; Rodríguez-Serna, M. Effect of the protein-lipids ratio on growth and maturation of the crayfish Procambarus (Austrocambarus) llamasi. Aquaculture 2005, 250, 692–699. [Google Scholar] [CrossRef]
- Peng, D.; Chen, X.; Wen, H.; Wu, F.; Lu, X.; Tian, J.; Liu, W.; Jiang, M.; Yu, L.; Zhang, L.; et al. Effects of dietary lipid levels on growth performance, muscle composition, reproductive performance and hemolymph biochemical indices of Procambarus clarkii broodstock. J. Fish. China 2019, 43, 2175–2185. [Google Scholar] [CrossRef]
- Jin, S.; Jacquin, L.; Li, W.; Li, X.; Liu, J.; Zhang, T. Reduced dietary protein levels do not impair growth and muscle composition in juvenile red swamp crayfish, Procambarus clarkii (Girard, 1852): Implications for pond culture in China. Aquacult Res. 2022, 53, 1435–1445. [Google Scholar] [CrossRef]
- Guo, H.; Wang, M.; Wang, X.; Xiao, K.; Huang, Y.; Hua, H.; Xiong, W.; Liu, W.; Abasubong, K.P.; Qiang, W.; et al. Effect of dietary cholesterol on ovarian development of Chinese mitten crabs (Eriocheir sinensis). Front. Mar. Sci. 2022, 9, 1070829. [Google Scholar] [CrossRef]
- Hou, S.; Li, J.; Huang, J.; Cheng, Y. Effects of dietary phospholipid and cholesterol levels on antioxidant capacity, nonspecial immune response and intestinal microflora of juvenile female crayfish, Procambarus clarkii. Aquacult Rep. 2022, 25, 101245. [Google Scholar] [CrossRef]
- Mo, A.; Wang, J.; Yuan, M.; Zhao, D.; Gu, Z.; Liu, Y.; Huang, H.; Yuan, Y.C. Effect of sub-chronic dietary L-selenomethionine exposure on reproductive performance of red swamp crayfish, (Procambarus clarkii). Environ. Pollut. 2019, 253, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhu, Y.; Yu, H.; Wang, X.; Azm, F.R.A.; Yuan, J.; Tan, Q. Effect of dietary vitamin C on the growth performance, nonspecific immunity and antioxidant ability of red swamp crayfish (Procambarus clarkii). Aquaculture 2021, 541, 736785. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Wen, H.; Wu, F.; Zhang, W.; Gao, W.; Tian, J. Dietary phosphorus requirement of red swamp crayfish (Procambarus clarkia). Aquacult Res. 2022, 53, 1293–1303. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Yin, C.-M.; Niu, R.-G.; Wang, H.; Li, X.-Y.; Zeng, Q.-F.; Lan, J.-F. Symbiotic hemolymph bacteria reduce hexavalent chromium to protect the host from chromium toxicity in Procambarus clarkii. J. Hazard. Mater. 2023, 459, 132257. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Y.; Mo, A.; Yuan, Y. Selenium mitigated cadmium-induced ovarian retardation in female Procambarus clarkii by regulating vitellogenin synthesis and transfer in the hepatopancreas. Ecotoxicol. Environ. Saf. 2024, 288, 117339. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, H.; Qian, Y.; Yang, J. Impairments of cadmium on vitellogenin accumulation in the hepatopancreas of freshwater crab Sinopotamon henanense. Environ. Sci. Pollut. Res. Int. 2017, 24, 18160–18167. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Zhou, F.; Li, J.; Wu, X.; Zhong, X.; Lv, H.; Yi, S.; Gao, Q.; Yang, Z.; et al. Integrated comparative transcriptome and weighted gene co-expression network analysis provide valuable insights into the response mechanisms of crayfish (Procambarus clarkii) to copper stress. J. Hazard. Mater. 2023, 448, 130820. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, N.B.; Avigliano, L.; Loughlin, C.M.; Rodríguez, E.M. The adverse effect of the herbicide atrazine on the reproduction in the intertidal varunid crab Neohelice granulata (Dana, 1851). Reg. Stud. Mar. Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Silveyra, G.R.; Silveyra, P.; Vatnick, I.; Medesani, D.A.; Rodríguez, E.M. Effects of atrazine on vitellogenesis, steroid levels and lipid peroxidation, in female red swamp crayfish Procambarus clarkii. Aquat. Toxicol. 2018, 197, 136–142. [Google Scholar] [CrossRef]
- Saensuwanna, J.K.A.; Jozghorbani, M.; Ho, T.; Szolajska, E.; Sarnowski, T.J.; Udomkit, A. A possible role of the ecdysone receptor in modulating gonad-inhibiting hormone gene expression in the black tiger prawn, Penaeus monodon. Aquaculture 2023, 569, 739393. [Google Scholar] [CrossRef]
- Toyota, K.; Matsushima, H.; Osanai, R.; Okutsu, T.; Yamane, F.; Ohira, T. Dual roles of crustacean female sex hormone during juvenile stage in the kuruma prawn Marsupenaeus japonicus. Gen. Comp. Endocrinol. 2023, 344, 114374. [Google Scholar] [CrossRef]
- Shui, Y.; Tang, X.; Xu, Z.; Zhou, X. Histological study on the ovary development induced by eyestalk ablation in red swamp crayfish Procambarus clarkii. Chin. J. Zool. 2016, 51, 449–454. [Google Scholar] [CrossRef]
- Tang, M.; Lu, Z.; Qin, Z.; Yang, G.; Babu, V.S.; Zhang, M.; Xu, Z.; Zhao, L.; Pan, G.; Lin, L. Examination of the potential role of CHH in regulating the expression of IAGBP gene through the eyestalk-testis pathway. Aquaculture 2022, 547, 737455. [Google Scholar] [CrossRef]
- Wang, M.; Geng, X.; Li, X.; Ye, H. Regulation of CHH family in eyestalk by A-type allatostatin in the mud crab Scylla paramamosain. Aquaculture 2023, 568, 739349. [Google Scholar] [CrossRef]
- Qiu, L.; Zhao, C.; Wang, P.; Fan, S.; Yan, L.; Xie, B.; Jiang, S.; Wang, S.; Lin, H. Genomic structure, expression, and functional characterization of checkpoint kinase 1 from Penaeus monodon. PLoS ONE 2018, 13, e0198036. [Google Scholar] [CrossRef]
- Yin, H. Effects of Eyestalk Ablation on the Molting, Growth and Gonad Development of Procambarus clarkia. J. Anhui Agric. Sci. 2007, 35, 4818–4819. [Google Scholar] [CrossRef]
- Chen, T.; Ren, C.; Jiang, X.; Zhang, L.; Li, H.; Huang, W.; Hu, C. Mechanisms for type-II vitellogenesis-inhibiting hormone suppression of vitellogenin transcription in shrimp hepatopancreas: Crosstalk of GC/cGMP pathway with different MAPK-dependent cascades. PLoS ONE 2018, 13, e0194459. [Google Scholar] [CrossRef] [PubMed]
- Uawisetwathana, U.; Leelatanawit, R.; Klanchui, A.; Prommoon, J.; Klinbunga, S.; Karoonuthaisiri, N. Insights into eyestalk ablation mechanism to induce ovarian maturation in the black tiger shrimp. PLoS ONE 2011, 6, e24427. [Google Scholar] [CrossRef]
- Wang, T.; He, K.; Blaney, L.; Chung, J.S. Testosterone and steroidogenic genes in the male blue crab Callinectes sapidus and their relationship with insulin-like androgenic gland factor (IAG) and crustacean female sex hormone (CFSH). Aquaculture 2023, 568, 739297. [Google Scholar] [CrossRef]
- Simões, L.A.R.; Normann, R.S.; Chung, J.S.; Vinagre, A.S. A brief and updated introduction to the neuroendocrine system of crustaceans. Mol. Cell Endocrinol. 2024, 590, 112265. [Google Scholar] [CrossRef]
- Jayasankar, V.; Tomy, S.; Wilder, M.N. Insights on molecular mechanisms of ovarian development in Decapod Crustacea: Focus on vitellogenesis-stimulating factors and pathways. Front. Endocrinol. 2020, 11, 577925. [Google Scholar] [CrossRef]
- Goudeau, M.; Lachaise, F.; Carpentier, G.; Goxe, B. High titers of ecdysteroids are associated with the secretory process of embryonic envelopes in the european lobster. Tissue Cell 1990, 22, 269–281. [Google Scholar] [CrossRef]
- Young, N.J.; Webster, S.G.; Rees, H.H. Ecdysteroid profiles and vitellogenesis in Penaeus monodon (Crustacea: Decapoda). Invertebr. Reprod. Dev. 1993, 24, 107–117. [Google Scholar] [CrossRef]
- Tiu, S.H.K.; Hui, J.H.L.; Mak, A.S.C.; He, J.; Chan, S. Equal contribution of hepatopancreas and ovary to the production of vitellogenin (PmVg1) transcripts in the tiger shrimp, Penaeus monodon. Aquaculture 2006, 254, 666–674. [Google Scholar] [CrossRef]
- Sathapondecha, P.; Thepsuwan, T.; Chotigeat, W. Induction of vitellogenesis in female banana shrimp, Fenneropenaeus merguiensis by leucine-tyrosine-arginine motif-containing protein 5 (LYRM5). Aquaculture 2019, 512, 734292. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, J.; Liu, X.; Sun, C.; Zhou, Q.; Wang, A.; Chen, J.; Liu, B. Effects of different levels of Antarctic krill oil on the ovarian development of Macrobrachium rosenbergii. Animals 2024, 14, 3313. [Google Scholar] [CrossRef]
- Nguyen, A.H.T.; Glendinning, S.; Ventura, T. A refined roadmap to decapod sexual manipulation. Rev. Aquacult 2023, 15, 1654–1663. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, B.; Tan, K.; Yi, S.; Li, Y. Effects of two exogenous proteins on the insulin-like androgenic gland hormone gene expression in Procambarus clarkii. Aquacult Res. 2021, 52, 6602–6611. [Google Scholar] [CrossRef]
- Huang, X.; Ye, H.; Huang, H.; Yang, Y.; Gong, J. An insulin-like androgenic gland hormone gene in the mud crab, Scylla paramamosain, extensively expressed and involved in the processes of growth and female reproduction. Gen. Comp. Endocrinol. 2014, 204, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A. Editorial: Sex determination and developmental mechanism of crustaceans and shellfish, volume II. Front. Endocrinol. 2023, 14, 1155209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, L.; Jiang, W.; Abasubong, K.P.; Zhang, C.; Zhang, D.; Li, X.; Jiang, G.; Chi, C.; Liu, W. Effects of dietary icariin supplementation on the ovary development-related transcriptome of Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Phys. D 2021, 37, 100756. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Gong, F.; Yang, F.; Jiang, Q.; Xu, Y.; Xia, W. A comparison of eating safety and quality of live and dead freshwater crayfish (Procambarus clarkii) at different stages. Food Res. Int. 2022, 159, 111630. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, K.; Yang, B.; Li, B.; Shen, X.; Du, Z. Dopamine receptor (DAR) and dopa decarboxylase (DDC) mediate hepatopancreas antibacterial innate immune reactions in Procambarus clarkii. Int. J. Biol. Macromol. 2022, 214, 140–151. [Google Scholar] [CrossRef]
- Felice, B.D.; Pascalis, F.D.; Manenti, R.; Pavlovic, R.; Cesare, F.d.; Nasti, R.; Beretta, G.; Parolini, M. Differential biochemical and behavioral responses induced by cocaine and benzoylecgonine exposure to the red swamp crayfish Procambarus clarkii. Sci. Total Environ. 2022, 844, 157025. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Yu, G.; Ge, F.; Xu, R.; Jin, Z.; Xie, X.; Zhu, D. Comparative transcriptomic characterization of the ovary in the spawning process of the mud crab Scylla paramamosain. Dev. Growth Differ. 2024, 66, 274–284. [Google Scholar] [CrossRef] [PubMed]

| Structure | Morphological Characteristics | Function |
|---|---|---|
| Seminiferous tubules | Spherical units (10–16 μm) enveloped by connective tissue | Germ cell development |
| Collecting ducts | Three-tiered system: primary (connects single tubule), secondary (5–8 primaries converge), and tertiary (central lumen) | Sperm transport to vas deferens |
| Right vas deferens | Patent duct connection, secretory epithelium (anterior/middle), and thickened muscular wall (posterior) | Sperm transport and spermatophore formation |
| Left vas deferens | Junction sealed by epithelial filaments and no sperm content observed | Non-functional for sperm transfer |
| Stage | Morphological Features of Spermatid Nuclei |
|---|---|
| Stage I | Nuclei appear capsule-shaped or sparsely pancake-shaped; nucleoplasm is sparse. |
| Stage II | Nuclei are nearly rounded; degree of nuclear condensation is higher than in Stage I. |
| Stage III | Nuclei are nearly rounded and slightly irregular; a distinct vesicular structure is apparent on one side of the nucleus. |
| Stage IV | Nuclei are smooth and rounded-quadrate; the vesicular structure is less conspicuous than in Stage III. |
| Stage V | Nuclei exhibit evident angular spines; a rounded structure is discernible within the nucleus. |
| Stage VI | Mature sperm exhibits a helical quadrangular shape; four distinct helically arranged radial arms extend from the nucleus; a distinct concentric structure is visible within the nucleus. |
| Stage | Month | Histological Features |
|---|---|---|
| Spermatogonia Stage | Jan–Mar | Seminiferous tubules are predominantly populated by spermatogonia and spermatocytes; mature sperm are seldom found in the vas deferens. |
| Sperm Maturation Stage I | Apr–Jun | The proportion of tubules containing spermatids and spermatozoa is markedly increased; sustentacular cells are prominent; the vas deferens is generally filled with mature sperm. |
| Spermatocyte Stage | Jul–Aug | Following sperm release, a new spermatogenic cycle commences; the proportion of tubules containing spermatocytes is significantly increased; spermatids at various metamorphic stages remain observable. |
| Sperm Maturation Stage II | Sep–Oct | The proportion of tubules containing spermatids and spermatozoa is significantly increased again (features analogous to Apr–Jun period); vas deferens is generally filled with mature sperm; empty tubules following sperm release are observable. |
| Spermatogonia Recovery Stage | Nov–Dec | Cells within the majority of seminiferous tubules revert to spermatogonia and spermatocyte stages (features analogous to Jan–Mar period); some tubules containing cells undergoing spermiogenesis are still discernible. |
| Phase | Features |
|---|---|
| Oogonia (proliferation phase) | The oocytes have large nuclei and little cytoplasm. |
| Pre-vitellogenic (small growth phase) | The oocytes increase rapidly in size, with an increase in cytoplasm, and the nucleoplasm is filled with granules. |
| Vitellogenic (large growth phase) | This phase is further divided into early, middle, and late stages. The oocytes continue to grow, and yolk granules start to accumulate and gradually increase in size. |
| Mature oocytes | At this stage, the nucleus disappears, the yolk granules are numerous and large in size, and the oocytes separate from the follicular cells. |
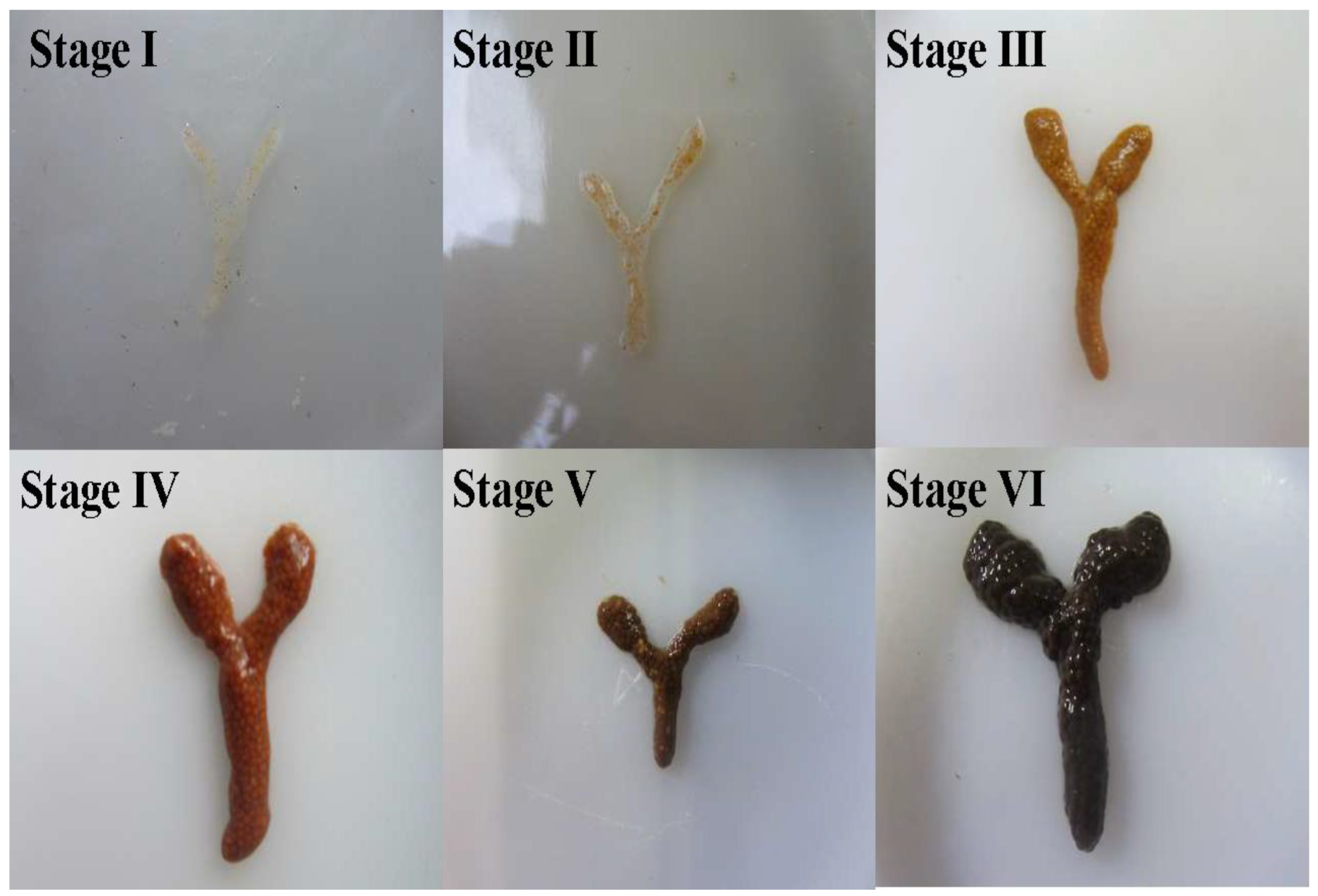
| Ovarian Stage | Color | Ovarian Morphology | References |
|---|---|---|---|
| Stage I | Grayish-white | Translucent thread-like structure, with oocytes indistinct to the naked eye, positioned medially to the hepatopancreas | [33] |
| Stage II | Pale yellow | Filamentous structure containing minute oocytes visible macroscopically; oocytes exhibit blurred boundaries and remain inseparable | [34] |
| Stage III | Yellow | Narrow ribbon-like structure with clearly outlined but incompletely developed oocytes | [35] |
| Stage IV | Light brown | Slender striated structure with discernible oocytes still adherent; follicular membranes become separable upon rupture | [29] |
| Stage V | Brownish-black | Rod-shaped structure containing uniformly plump oocytes that are minimally separable, fully occupying the cephalothoracic cavity | [15] |
| Stage VI | Dark brown | Fragile rod-shaped structure retaining only residual immature oocytes, with ovarian membranes prone to rupture | [30] |
| Decapod Crustaceans | Types of Light Sources | Light Intensity | Photoperiod | Additional Parameters | References |
|---|---|---|---|---|---|
| Penaeus merguiensis | Daylight fluorescent tubes (36 W) | 2 lx | 12 L∶12 D | 27 °C | [71] |
| Panulirus japonicus | Incandescent lamps (40 W) | 70–100 lx | 14 L∶10 D | 25 °C | [74] |
| Procambarus clarkii | Fluorescent lamps (15–25 W) | 50–500 lx | 16 L:8 D | 25–28 °C | [58] |
| Lysmata vittata | Tubular fluorescent lamps (25 W) | 1000 lx | 12 L∶12 D | 25 °C | [73] |
| Exopalaemon carinicauda | LED retrofit tubes (5 W) | 1000 lx | 8 L:16 D | 23–25 °C | [75] |
| Cherax quadricarinatus | Incandescent lamps (200 W) | 3000 lx | 16 L:8 D | 28 °C | [76] |
| Panulirus homarus | Energy-saving lamps (80 W) | 3350 lx | 14 L∶10 D | 29–32 °C | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, D.; Guo, C.; Zhang, T.; Liu, J.; Li, W. Gonadal Development and Its Influencing Factors in the Red Swamp Crayfish (Procambarus clarkii): A Review. Biology 2025, 14, 1138. https://doi.org/10.3390/biology14091138
Wang Y, Xu D, Guo C, Zhang T, Liu J, Li W. Gonadal Development and Its Influencing Factors in the Red Swamp Crayfish (Procambarus clarkii): A Review. Biology. 2025; 14(9):1138. https://doi.org/10.3390/biology14091138
Chicago/Turabian StyleWang, Yuchi, Dengge Xu, Chao Guo, Tanglin Zhang, Jiashou Liu, and Wei Li. 2025. "Gonadal Development and Its Influencing Factors in the Red Swamp Crayfish (Procambarus clarkii): A Review" Biology 14, no. 9: 1138. https://doi.org/10.3390/biology14091138
APA StyleWang, Y., Xu, D., Guo, C., Zhang, T., Liu, J., & Li, W. (2025). Gonadal Development and Its Influencing Factors in the Red Swamp Crayfish (Procambarus clarkii): A Review. Biology, 14(9), 1138. https://doi.org/10.3390/biology14091138







