1. Introduction
Micropterus salmoides (
M. salmoides), commonly known as the largemouth bass, is a freshwater species indigenous to North America, classified within the family Percoidei, order Perciformes, and genus
Micropterus. Recognized as a significant commercial freshwater species,
M. salmoides is renowned for its vigorous feeding behavior, exceptional culinary qualities, and popularity in competitive angling, establishing it as a prominent freshwater fish in North America and various global regions [
1]. The optimal thermal range for the growth of
M. salmoides is approximately 25 °C, while temperatures exceeding 30 °C can adversely affect its growth performance. This species was introduced to Guangdong Province, China, in 1983, where it has subsequently emerged as a key species in Chinese aquaculture [
2].
M. salmoides exhibits stringent environmental requirements in aquaculture, which can significantly influence its growth, development, and overall health. Inadequate farming conditions may compromise the immune function of
M. salmoides, rendering it vulnerable to a range of diseases and potentially leading to mass mortality events. Recent studies indicate that various viral pathogens have inflicted substantial economic damage on the
M. salmoides sector in China, with notable viral infections such as largemouth bass virus (LMBV), largemouth bass birnavirus (LBBV), and viral hemorrhagic septicemia virus (VHSV). Concurrently, bacterial infections remain a primary challenge hindering the sustainable advancement of
M. salmoides aquaculture. The transmission of bacterial pathogens occurs through contaminated water, feed, equipment, and decaying organisms, facilitating rapid spread within aquaculture environments and complicating prevention and control efforts. Notable bacterial pathogens affecting
M. salmoides farming include
Nocardia seriolae (
N. seriolae),
Aeromonas hydrophila,
Aeromonas wiedemannii,
Vibrio parahaemolyticus,
Edwardsiella piscicida, and
Francisella orientalis [
3,
4,
5,
6,
7]. Due to declining antibiotic use in aquaculture, disease prevention now relies on strategies like lower stocking density, water quality management, and probiotics. While probiotics are gaining attention as an alternative to antibiotics in fish farming, their widespread adoption is limited by the lack of efficient and sustainable aquaculture-specific strains.
In recent years, aquaculture of
M. salmoides has faced significant challenges due to
Nocardia infections, which pose a serious threat to their survival rates. The Nocardia species responsible for nocardiosis infections in farmed fish include
N. asteroides,
N. salmonicida,
N. seriolae, and
N. brasiliensis [
8,
9,
10].
Nocardia typically induces visceral white spot disease, evidenced by external hemorrhaging, subcutaneous abscess formation, and the presence of white nodules in the kidneys and spleen [
11,
12]. The bacterium spreads hematogenously (via the bloodstream), leading to the formation of hyphae and granulomatous nodules in multiple organs. Due to its destructive effects on immune organs and the lack of targeted therapeutic interventions, treatment options remain complex. Standard therapeutic approaches include continuous disinfection using iodine or chlorine, administration of oral antibiotics (such as enrofloxacin and amoxicillin), and the use of traditional Chinese medicinal practices combined with vitamin K. The limited efficacy of these treatments is attributable to the poor penetration of therapeutic agents into the nodules. While vaccination could represent a viable and environmentally sustainable strategy, there are currently no approved vaccines for
Nocardia in fish within China. Therefore, there is an urgent need for innovative strategies to mitigate
Nocardia infections in aquaculture.
For aquaculture, the interaction between gut microbiota and fish diseases is crucial; a balanced gut microbiome boosts host immunity, while dysbiosis can lead to infections and illnesses. As reported, gut microbiota could contribute to disease resistance in fish through several mechanisms: (1) serving as a barrier against pathogens by competing for resources and producing antimicrobial substances [
13]; (2) improving fish health via probiotics, prebiotics, or synbiotics [
14,
15]; and (3) influencing the immune response of the host through the gut–immune axis [
16]. As a key indicator of fish health, gut microbiota present innovative approaches for sustainable aquaculture. Recent studies have identified effective methods for isolating pathogenic and antagonistic bacteria from fish intestines. For example, 65
Bacillus subtilis strains were isolated from the intestines of
Pelteobagrus fulvidraco, with strain F14 demonstrating significant antibacterial activity against
A. hydrophila through crude extract metabolism [
17].
In this study, the intestinal microbiota community of M. salmoides artificially infected with Nocardia was analyzed, and a strain of Bacillus amyloliquefaciens MS05 (BaMS05) with potential resistance to N. seriolae was screened. Its biological characteristics were also explored in this study, providing a potential strategy for the biological control of Nocardia diseases in aquaculture.
2. Materials and Methods
2.1. Experimental Fish and N. seriolae
The M. salmoides used in the experiment were purchased from a farm in Foshan, Guangdong Province, weighing 20 ± 1 g. The experimental fish were confirmed to be free from parasitic and bacterial diseases such as Nocardia through PCR testing. Before the experiment began, the breeding system was thoroughly disinfected with potassium permanganate solution. During the temporary rearing period, the water temperature was strictly controlled at 25 ± 1 °C. The fish were fed twice a day, with the feeding amount being 3% of the fish’s body weight. The temporary rearing period lasted for two weeks, and the experimental fish were deprived of food for 24 h before the challenge experiment.
The strain of N. seriolae (NK0609) from the mandarin fish in our lab, stored at −80 °C, was inoculated onto brain–heart infusion (BHI) agar using streaking and inverted in a 28 °C incubator until single colonies formed. Subsequently, single colonies were picked from the agar and inoculated into BHI liquid medium, which was then cultured under conditions of 28 °C temperature and 150 rpm/min shaking for 4 d. After cultivation, the bacterial suspension was centrifuged at 4000 r/min for 15 min to collect the bacterial precipitate. The bacteria were resuspended in PBS buffer solution and washed twice. Another 4000 rpm/min centrifugation at 15 min collected the bacteria, after which an appropriate amount of PBS was added, and the bacteria were homogenized using a glass homogenizer to prepare a bacterial suspension. The concentration of the bacterial suspension was determined by plate colony count to be 4.6 × 108 CFU/mL. Subsequently, 100 μL of the bacterial suspension was serially diluted with PBS at dilution factors of 10−1 or 10−2 for use in challenge experiments.
2.2. Detection of Pathogenic Ability of N. seriolae
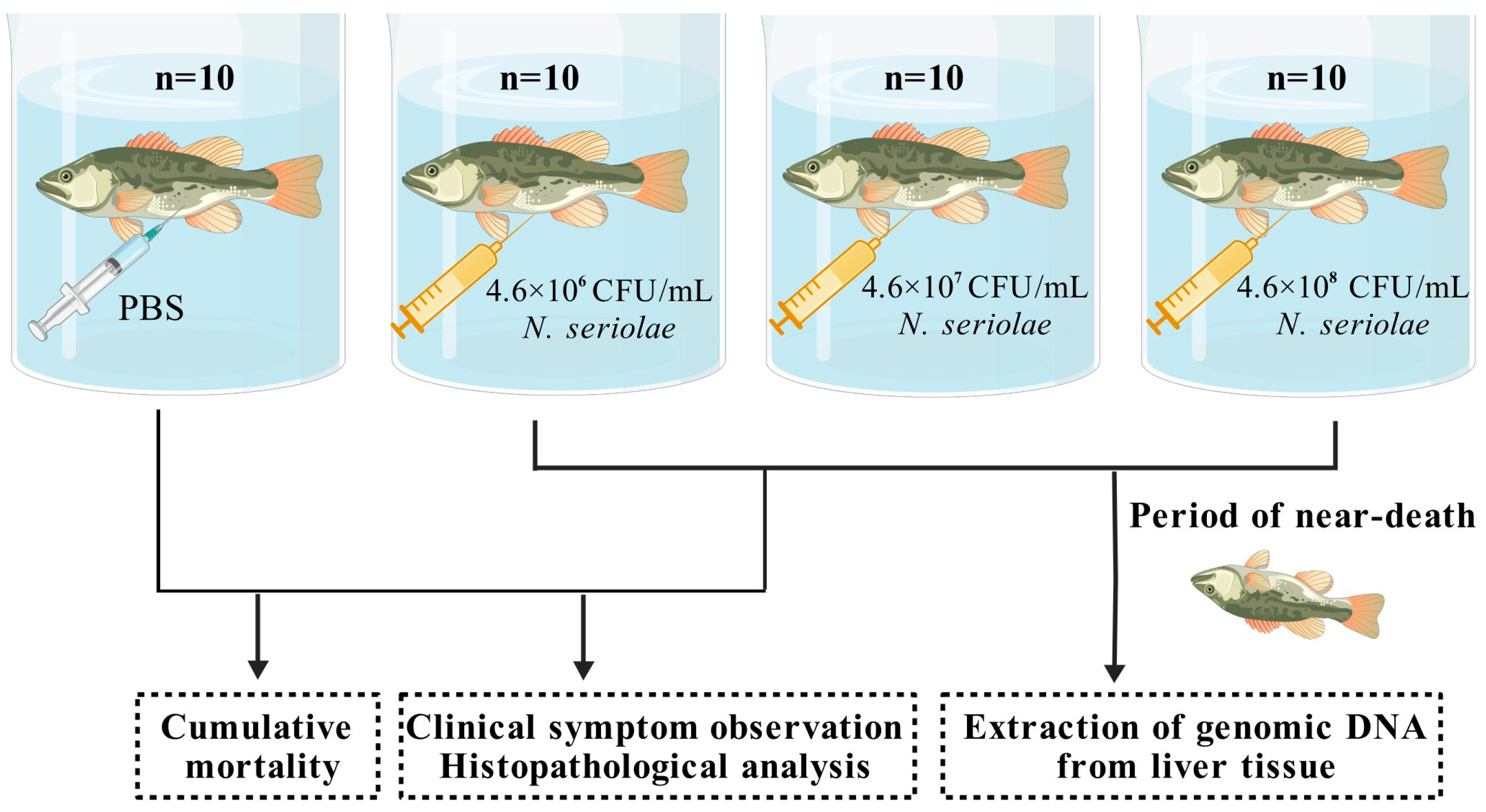
To evaluate the pathogenicity of the
Nocardia strain in
M. salmoides, a challenge test was conducted. Healthy, size-matched fish were randomly divided into control and experimental groups and placed in independent recirculating water aquaculture systems. The control group (
n = 10) was injected with 100 μL of PBS. Three experimental groups (
n = 10 in each group) were injected with
Nocardia suspensions at concentrations of 4.6 × 10
6, 4.6 × 10
7, and 4.6 × 10
8 CFU/mL. Following challenge, fish were monitored continuously. Moribund individuals from experimental groups were randomly selected, anesthetized and dissected for clinical symptom observation and histopathological analysis. Additionally, from the experimental group and the control group, moribund and healthy fish were randomly selected and dissections were performed to collect liver samples (
Figure 1). Genomic DNA was extracted from the liver using the MiniBEST Universal Genomic DNA Extraction Kit Ver.5.0 (Takara, Kyoto, Japan). Detection of
Nocardia in
M. salmoides was performed using specific primers and PCR (
Table 1), following the method outlined by Chen et al. [
18]. The PCR reaction system was set to a final volume of 50 μL: primers (10 μM) 1 μL each, 2 × Taq Plus Master Mix II (Vazyme, Nanjing, China) 25 μL, DNA template 2 μL, with sterile distilled water for volume completion. The optimized thermal cycling parameters were 95 °C for 3 min to activate the polymerase, followed by 32 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 36 s, and the final extension step at 72 °C for 5 min.
2.3. LD50 Determination of N. seriolae Infection in M. salmoides
After different doses of N. seriolae injection in M. salmoides, continuous observation was carried out for 14 d and mortality was calculated by Kaplan–Meier survival curve analysis. The 14 d LD50 of M. salmoides was calculated by the Reed–Muench method, and its calculation formula is as follows: lgLD50 = r (50 − b) + n, r = (m − n)/(a − b), where r is the increase in dose logarithm for every 1 percentage point increase in mortality; a is the cumulative mortality rate greater than 50%; m is the logarithmic dose corresponding to a cumulative mortality greater than 50%; b is the cumulative mortality rate less than 50%; and n is the logarithm of the dose corresponding to the cumulative mortality rate less than 50%.
2.4. Preparation of Samples for Intestinal Flora Analysis
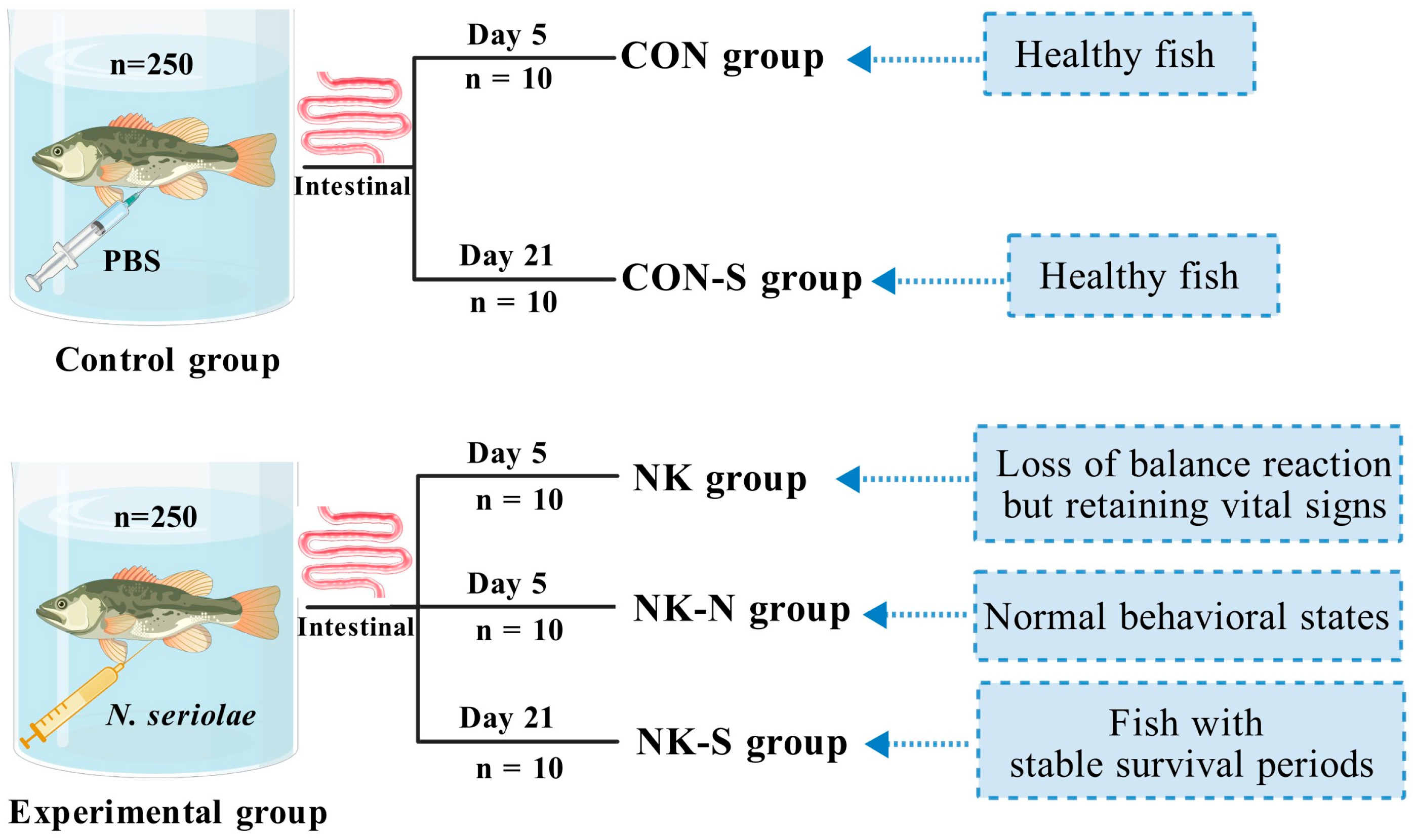
In order to obtain samples for gut microbiota analysis, another challenge experiment was conducted. A total of 500
M. salmoides with consistent body length (8 ± 1 cm) and weight (20 ± 1 g) were selected randomly and divided into two groups (250 fish per group). They were then placed in independent recirculating water aquaculture systems (
Figure 2). The experimental group was intraperitoneally injected with 100 μL of a 5 × 10
7 CFU/mL
N. seriolae suspension, while the control group was injected with the same volume of PBS. At the peak of infection and death (on the 5th day after injection), 10 fish exhibiting near-death states (loss of balance reaction but retaining vital signs) were randomly selected from the experimental group (NK group), 10 fish were selected from the experimental group with normal behavioral states (NK-N group), and 10 healthy fish were randomly selected from the control group (CON group). The external fish surface was disinfected by wiping with 75% ethanol. Using sterile dissecting scissors, an arc-shaped incision was made anterior to the anal vent. The abdominal cavity was opened, the intestinal tract was extracted, and associated adipose tissue was meticulously removed using forceps. The intestinal surface was cleaned by wiping with a 75% ethanol-saturated sterile swab and the sample was placed in a 1.5 mL EP tube. All samples were immediately subjected to DNA extraction.
During the stable infection period (on the 21st day after injection, no more deaths occurred in the experimental fish), 10 fish from the experimental group (NK-S group) and 10 fish from the control group (CON-S group) were randomly selected. The external fish surface was disinfected by wiping with 75% ethanol. Using sterile dissecting scissors, an arc-shaped incision was made anterior to the anal vent. The abdominal cavity was opened, the intestinal tract was extracted, and associated adipose tissue was meticulously removed using forceps. The intestinal surface was cleaned by wiping with a 75% ethanol-saturated sterile swab and the samples were placed in a 1.5 mL EP tube. All samples were immediately subjected to DNA extraction (
Figure 2).
2.5. 16S rRNA Amplification and Sequencing and Data Processing and Analysis
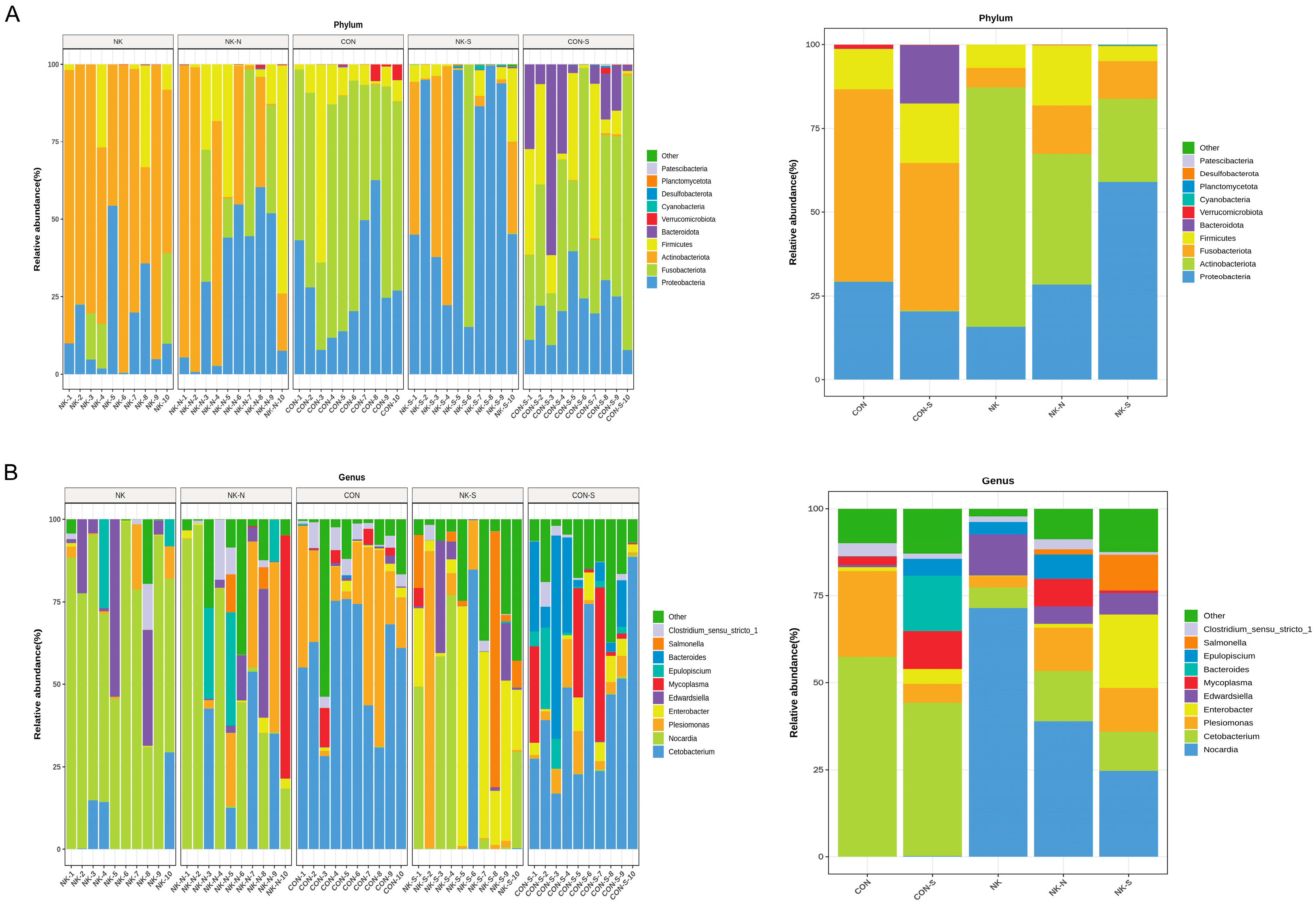
Genomic DNA was extracted from M. salmoides intestinal samples using the MiniBEST Universal Genomic DNA Extraction Kit Ver.5.0 (Takara, Kyoto, Japan). DNA integrity and concentration were verified through agarose gel electrophoresis and NanoDrop spectrophotometry to ensure suitability for subsequent amplification procedures. The DNA amplification and sequencing were carried out by Guangzhou Aiji Biotechnology Co., Ltd. (Guangzhou, China). The process is as follows: Specific primers were designed and synthesized for the 16S rDNA V3-V4 region for targeted amplification. The specific primers were 341F: 5′-CCTACGGGNGGCWGCAG-3′; 805R: 5′-GACTACHVGGGTATCTAATCC-3′. PCR products were purified using AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA). The purified products were quantified using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The amplification success and specificity were verified by 2% agarose gel electrophoresis, and the electrophoresis bands were recovered using the AMPure XT beads recovery kit. The quality of the recovered products was evaluated using library quantification kits for Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) and Illumina (Kapa Biosciences, Woburn, MA, USA) to ensure that the library concentration was no less than 2 nM. Qualified libraries were diluted according to the required sequencing volume, mixed proportionally, and subjected to NaOH denaturation into single strands for sequencing; dual-end sequencing was performed using an NovaSeq 6000 sequencer. For the paired-end data obtained from sequencing, it is first necessary to split the sample data based on barcode information and remove adapters and barcode sequences. Following the procedures of data splicing and filtering, ASV (feature) sequences and abundance tables were acquired while excluding singleton ASVs. Alpha and beta diversity analyses were performed utilizing the resulting ASV (feature) sequences and abundance tables. Using the ASV (feature) sequence file, species were annotated with the SILVA database referencing the NT-16S database, and a statistical analysis of the species abundance across different samples was conducted based on the ASV (feature) abundance table. Finally, differential analysis was carried out between comparison groups based on the statistical data of species abundance.
2.6. Bacillus Spores Isolated from the Gut of M. salmoides
From the NK-S group, M. salmoides with no external damage and in good health were randomly selected. They were anesthetized with 20 mg/L MS-222, then cleaned with 75% alcohol. The intestines were taken out in an aseptic environment on an ultra-clean workbench and placed in a disposable petri dish. The surface of the gut was rinsed three times with cool PBS to ensure that the blood and fat were removed, and then placed in 2 mL PE tube with PBS buffer for homogenization treatment. The homogenized tissue was placed in a water bath at 80 °C for 20 min, coated on Luria-Bertani (LB) agar plate, and the culture inverted in a 37 °C incubator for 24~48 h; after colony growth, different morphological single colonies were selected according to the morphology, size and color of the colony with a sterilizing ring cultured again at 37 °C for 24~48 h, then purified three times to obtain multiple pure strains.
The N. seriolae (NK0609) and the isolated pure strains were cultured until the optical density (OD600) reached 1. After centrifugation and washing, the bacteria were resuspended in PBS to obtain the bacterial suspension. The N. seriolae were spread on the surface of a BHI agar medium. After the bacterial suspension was completely absorbed, a sterile puncher was used to make three holes at equal distances on the agar medium, and 50 μL of the pure strain suspension was added to each hole. The plates were incubated at 37 °C for 4 d. After the cultivation, the size of the inhibition zone was observed, and the strains with significant inhibitory effects on Nocardia were selected as candidate strains for further study.
2.7. Morphological, Biochemical and Molecular Biological Identification of Candidate Bacteria
The screened candidate strain was inoculated into LB liquid medium for cultivation. After 24 h, a sample of the bacterial culture was plated onto LB agar and incubated for 12 to 24 h to assess colony morphology. Concurrently, the bacterial solution was appropriately diluted and subjected to Gram staining using the Biosharp Gram Staining Kit, followed by microscopic examination.
The screened candidate strain was sent to Zhongke Testing Technology Service (Guangzhou) Co., Ltd. (Guangzhou, China). for physiological and biochemical profiling, assessing its spore formation capacity, NaCl tolerance, starch hydrolysis capability, fermentation of D-xylose and L-arabinose, volatile fatty acid (VFA) production, utilization of citrate, fermentation of D-mannitol, and gelatin liquefaction ability, among other characteristics.
The screened candidate strain was inoculated into LB liquid medium and cultured in a shaker at 37 °C for 4 to 8 h until turbid growth was achieved. PCR amplification of the bacterial DNA was performed, targeting the 16S rRNA gene using universal bacterial primers: 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC). The amplified products were sent to Guangzhou Aiji Biotechnology Co., Ltd. for sequencing. Subsequent homology analysis was conducted using the NCBI database to classify the species of the screened candidate strain based on the obtained gene sequences.
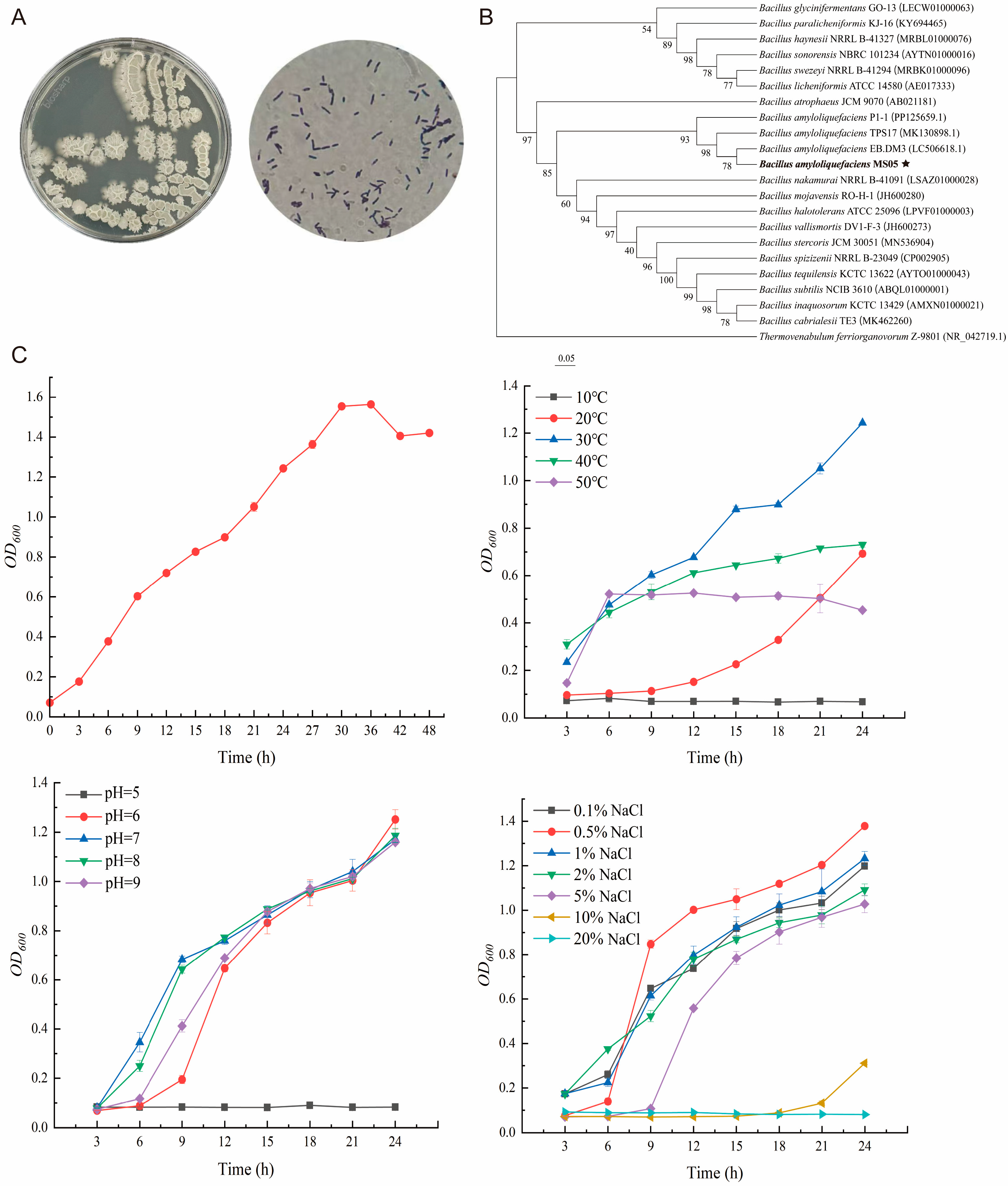
2.8. BaMS05 Growth Curve Determination
BaMS05 strain was inoculated into LB liquid medium and the culture shaken at 37 °C in a constant-temperature shaker (180 rpm) for 24 h to obtain seed solution. Then, the solution was inoculated at a ratio of 1% into 50 mL LB liquid medium and cultivation continued at 37 °C, 180 rpm. Samples were taken every 3 h in the first 30 h of cultivation, and every 6 h thereafter. The optical density of the bacterial solution was determined at 600 nm wavelength using a UV-Vis spectrophotometer, with time as the x-axis and OD600 value as the y-axis, to plot the growth curve of the strain.
2.9. Effects of Culture Parameters on BaMS05 Growth
Effect of temperature on the growth of BaMS05: the BaMS05 strain was inoculated in LB liquid medium with a 1% inoculum, and placed under 5 temperature gradients of 10 °C, 20 °C, 30 °C, 40 °C, and 50 °C. The culture was shaken at 180 rpm for 24 h. The LB liquid medium without the BaMS05 strain inoculation was set as the control group, with 3 biological replicates for each treatment. Samples were taken every 3 h to measure the OD600 value.
Impact of pH on the growth of BaMS05: A quantity of 50 mL of LB liquid medium was prepared in 200 mL Erlenmeyer flasks. The initial pH of the medium was adjusted using 1 mol/L HCl or 1 mol/L NaOH, with pH gradients set at 4, 5, 6, 7, 8, and 9. The culture medium was sterilized at 121 °C and 0.1 MPa, then cooled to room temperature before use. BaMS05 strain was inoculated with 1% inoculum, with 3 biological replicates for each treatment. All flasks were shaken at 30 °C and 180 rpm for 24 h, with samples taken every 3 h to measure the OD600 values.
The effect of NaCl concentration on the growth of BaMS05: A quantity of 50 mL LB liquid medium was prepared in 200 mL Erlenmeyer flasks, with NaCl concentrations set at 0.1%, 0.5%, 1%, 2%, 5%, 10%, and 20%. The BaMS05 strain was inoculated at 1% inoculum size for each treatment, with 3 biological replicates per treatment. All culture flasks were shaken at 30 °C and 180 rpm for 24 h, with samples taken every 3 h to measure the OD600 value.
2.10. Determination of the Antibacterial Activity of BaMS05
Strain BaMS05 and test pathogens (N. seriolae, A. hydrophila, V. parahaemolyticus, V. alginolyticus, and S. agalactiae) were individually cultured until the optical density (OD600) reached 1. After centrifugation and washing, 100 μL of the bacterial solution was extracted. N. seriolae and S. agalactiae were inoculated on BHI agar, and after complete absorption, a puncher was used to make three equidistant holes on the agar, adding 50 μL of BaMS05 bacterial fermentation liquid to each hole. In parallel, another set of BHI plates was prepared by spreading 100 μL of N. seriolae or S. agalactiae suspensions. After absorption, antibiotic discs containing gentamicin, streptomycin, penicillin, and a blank control disc were placed equidistantly as positive and negative controls. Additionally, after centrifugation and washing, 100 μL of A. hydrophila, V. parahaemolyticus and V. alginolyticus bacterial liquids were inoculated on LB agar, and the same procedures as mentioned above were followed.
2.11. Detection of Colonization Ability of BaMS05 in Gut of M. salmoides
We randomly selected 100 healthy M. salmoides with consistent body length (8 ± 1 cm) and weight (20 ± 1 g), and divided them into a control group (n = 50) and an experimental group (n = 50). All M. salmoides were fasted for 3 d before the experiment. The experimental group was fed feed containing 109 CFU/g of BaMS05 spore bacteria twice, while the control group was fed sterile conventional feed twice, with a one-day interval between each feeding. Subsequently, all M. salmoides were fasted for 7 days. Sampling was done at 0 h of the second feeding, and at 1 h, 6 h, 1 d, 3 d, 5 d, and 7 d thereafter, with 5 M. salmoides being dissected at each time point. During the dissection, the intestines of the experimental M. salmoides were taken out, the intestinal contents were scraped off, the cleaned intestines were washed 3 times with sterile PBS, and the intestines were placed in a 2 mL centrifuge tube. A quantity of 1 mL of sterile PBS was added for homogenization, and the homogenate was heated at 80 °C for 20 min. The slurry was then spread on LB agar culture medium, with 100 μL on each plate, and incubated at 37 °C for 24 h to count the number of bacterial colonies.
2.12. Detection of the Protective Effect of BaMS05 on M. salmoides
BaMS05 and N. seriolae (NK0609) were cultured to 108 CFU/mL. The experimental group was intraperitoneally injected with a mixture of 50 μL of BaMS05 + 50 μL of N. seriolae, while the control group was injected with 50 μL of BaMS05 + 50 μL of PBS, and 50 μL of N. seriolae + 50 μL of PBS buffer, respectively. The death toll of M. salmoides was observed and recorded continuously for 14 d.
2.13. Statistical Analysis
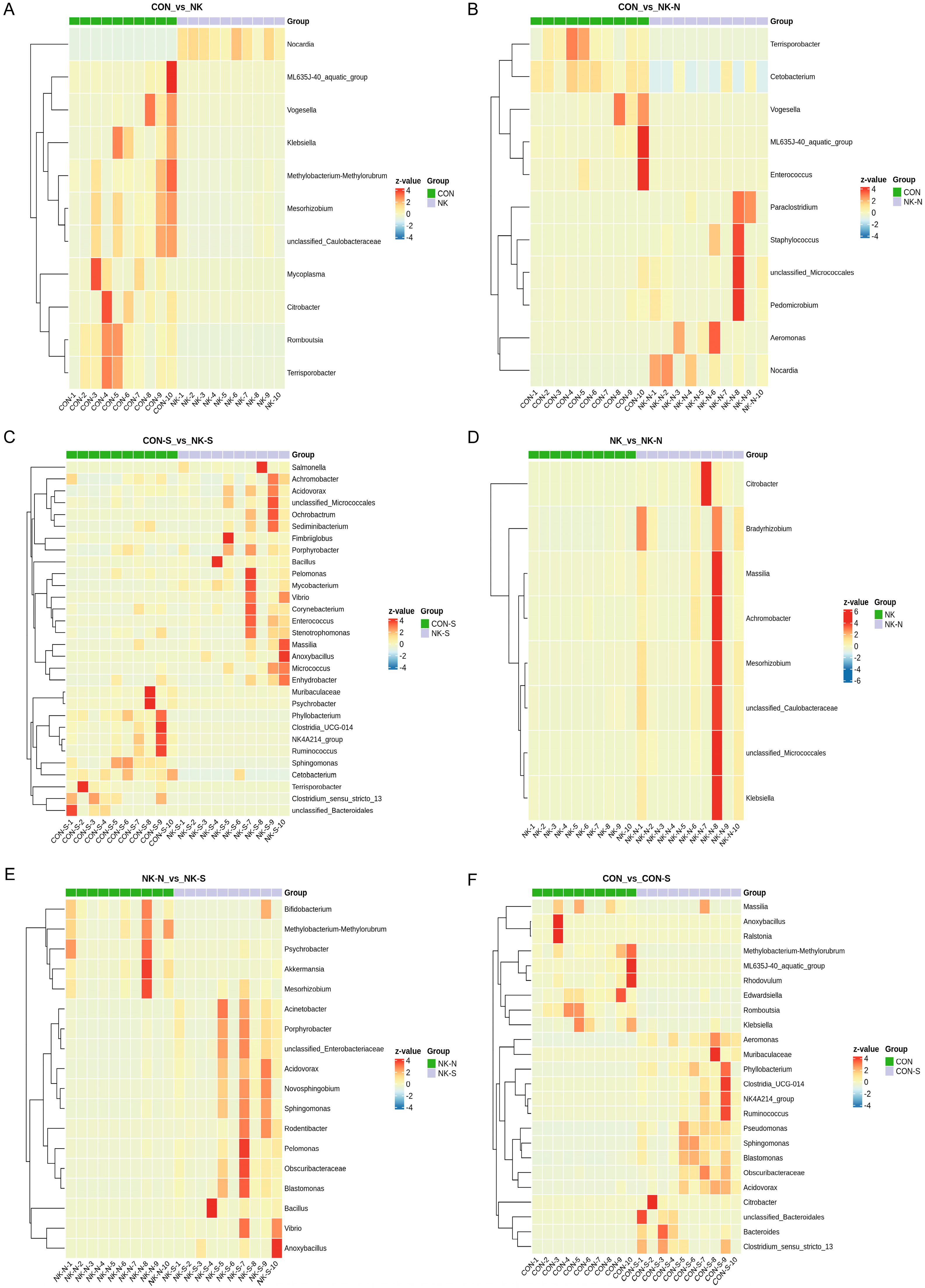
All data were subjected to three independent repeated experiments, and the statistical analysis of the data was conducted using GraphPad Prism 9.5 software. First, Shapiro–Wilk normality test and Levene’s homogeneity of variance test were conducted. Since the measured data met both the normal distribution and homogeneity of variance assumptions, Student’s t-test was used for pairwise comparisons between groups to analyze the species with differences. Additionally, the LEfSe method was used to analyze the characteristics of differentially abundant species between groups; the non-parametric Kruskal–Wallis (KW) sum-rank test was utilized to detect the differences in species abundance among different groups; then, the Wilcoxon rank-sum test was applied to verify the consistency of the differences in differential species among subgroups within different groups; finally, LDA discriminant analysis was employed to estimate the influence of these differential species on the distinction between groups. Statistical analysis results were considered statistically significant at p < 0.05.
4. Discussion
Currently, the
Nocardia infection in aquaculture is quite challenging, often causing significant economic losses. Therefore, exploring methods for preventing and controlling
Nocardia infection in fish is an important subject. Our finding is that
N. seriolae infection reduces gut microbiota diversity in
M. salmoides. This is consistent with previous studies, demonstrating the link between intestinal flora imbalance and susceptibility to disease in fish [
20]. Furthermore, based on the observation of an increase in the proportion of
Bacillus spores in
M. salmoides that remained healthy after
N. seriolae infection, we isolated, screened, and ultimately obtained a strain of starch-degrading BaMS05. This strain can inhibit the growth of
N. seriolae and other common pathogens of
M. salmoides, both in vivo and in vitro, and can colonize the gut of
M. salmoides. This study not only deepens our understanding of the gut microbiota of
M. salmoides and its relationship with
N. seriolae, but also provides a potential new technical approach for the prevention and control of
N. seriolae infection in
M. salmoides.
Nocardia infection does not cause sepsis or acute immune reactions, but progressively invades various cells of fish, including white blood cells, with a long latency period. The infected fish are asymptomatic or have mild symptoms in the early stages of the disease, and by the time obvious symptoms or death are discovered, the infection has often been present for a long time, making treatment more difficult. This study found that
Nocardia infection causes severe damage to important physiological and immune organs in fish, which is consistent with previous research results [
21]. The important physiological and immune organs, such as the liver and kidneys of diseased fish, suffer severe damage, leading to decreased immune function. Moreover,
Nocardia is a Gram-positive bacterium, and there are few drugs approved for use in aquaculture that are effective against Gram-positive bacteria in China. Besides, the susceptibility of
Nocardia to antibacterial drugs is not well understood, and there is a lack of scientific basis for drug use. Due to various reasons, the treatment of
Nocardia infection in fish is difficult, resulting in long duration, low cure rate, and high mortality rate [
22]. Researchers are seeking alternative treatment methods for fish nocardiosis, such as vaccination, and it has been reported that researchers have developed an oral vaccine using probiotics recombinantly expressing the major antigen of
N. seriolae in fish, which has shown good immune protection [
23]. Yet, there are still no commercially available vaccines for
Nocardia in fish. Even if commercialization of vaccines for
Nocardia in fish is achieved in the future, there are still issues related to aquaculture costs as well as bacterial variations.
The gut microbiota of vertebrates is a complex microbial ecosystem, comprising diverse and abundant bacteria, archaea, and fungi. These gut microbial communities enhance metabolic capacity and provide a range of beneficial effects to their hosts, such as nutrient digestion, immune function, and resistance to invading pathogens. In the gut, there is a large population of symbiotic microorganisms that provide an excellent microecological environment for fish. In terms of nutrient digestion, the gut microbiota can produce vitamins, amino acids, digestive enzymes, various growth factors, and other metabolites. Major enzymes include carbohydrases, phosphatases, esterases, lipases, peptidases, cellulases, and proteases, which enzymes play a crucial role in the digestion of nutrients in the intestines [
24]. Increasing research is focusing on the relationship between gut microbiota and the immune response and disease resistance of fish [
25]. The interaction between the host and the gut microbial community is the basis for immune development. The genes of the microbiota or microbial cell populations exist within the organism and have unforeseen benefits and effects on overall health, from regulating the immune system to promoting growth and reproduction of the organism. The microbiota within the body have the ability to produce a variety of compounds through their genes, which have various benefits. Studies have shown that gut microbial communities can produce vitamins and help with food digestion, nutrient storage, and promote healthy metabolism. The microbiota also help maintain the integrity of the intestinal barrier cells, preventing harmful bacteria and toxins from penetrating the intestinal barrier, thus serving as an immune barrier [
26].
Probiotics are defined as live microorganisms introduced into the gastrointestinal tract with food or water to promote health by enhancing the internal microbial balance. In recent years, probiotics have also been widely recognized as an environmentally friendly disease prevention method in aquaculture, especially in controlling bacterial fish diseases. The roles of probiotics in aquaculture include improving growth performance, disease resistance, immune enhancement, health status, balancing fish functional mechanisms, sustainability of intestinal microbiota, water quality (as bio-remediation to improve water quality), and enrichment of nutrients for zooplankton [
27,
28]. In this study, we focused on analyzing the antibacterial effect of BaMS05 and found that it inhibits the growth of various fish pathogenic bacteria, although its other effects are currently unclear. According to existing research, some
Bacillus bacteria can inhibit the growth of pathogenic bacteria by producing antimicrobial peptides, biosurfactants, or competitive inhibition [
29]. It is speculated that BaMS05 may also have a similar mechanism of action, such as directly inhibiting the growth of
N. seriolae by secreting antimicrobial substances, or indirectly inhibiting its growth by competing for nutrients and attachment sites. However, these hypotheses require further experimental verification. In addition, for probiotics to function in fish, an important prerequisite is that they must be able to colonize the gut of fish. From this perspective, endogenous probiotics in the host may be more suitable in aquaculture, as selecting potential probiotics from the host or local environment can enhance the colonization ability in the intestines or improve the survival and growth of fry. Compared to exogenous probiotics, endogenous probiotics have several advantages, such as higher host safety, easier colonization and effectiveness, wider temperature and salinity adaptability, and stronger environmental adaptability. For example, Clements et al. found that the
Vibrio bacteria mainly exists in the digestive tract of marine fish, especially in the hindgut of many herbivorous fish, providing the host with fatty acids and vitamins [
30,
31].
Clostridium butyricum, successfully used as a probiotic in aquaculture, enhances rainbow trout resistance to
Vibriosis, stimulates immune responses, and improves the survival rate of Japanese flounder [
32].
In the aquaculture industry, common probiotics used include
Bacillus, lactic acid bacteria, butyric acid bacteria, and yeast. Lactic acid bacteria play an important role in maintaining the balance of the fish intestinal microbial community by inhibiting the reproduction of harmful bacteria through acidic metabolites or bacteriocins [
33]. Butyric acid bacteria promote the proliferation of beneficial bacteria, inhibit the growth of pathogens, repair intestinal mucosa, reduce the occurrence of intestinal inflammation, and enhance the host’s immune function [
34].
Bacillus is the most widely used probiotic in aquaculture because it can form spores resistant to environmental stress and adapt to harsh farming conditions. In addition,
Bacillus produces various hydrolytic enzymes, such as proteases, amylases, cellulases, and lipases that help aquatic animals effectively utilize nutrients in feed; some
Bacillus strains can also inhibit the growth of pathogens and enhance the immune response of aquatic animals, making them high-quality probiotic additives [
35]. Although studies show that host-derived probiotics have advantages for host health, research on terrestrial animal gut microbes is more advanced and the characteristics of probiotics derived from terrestrial animals are more stable. On the other hand, the use of fish-derived probiotics in aquaculture is still in the early stages, so currently probiotics used in aquaculture mainly rely on probiotics derived from terrestrial animals, requiring further research and development. In this study, we obtained the starch-degrading
Bacillus BaMS05, which can depress the proliferation of common pathogens in
M. salmoides both in vivo and in vitro and can colonize the intestine of
M. salmoides, showing potential in nocardiosis prevention and treatment in
M. salmoides. Additionally, introducing BaMS05 into aquaculture systems may have an impact on the ecological environment. On the one hand, BaMS05 may improve the aquaculture environment by inhibiting the growth of pathogenic bacteria such as
N. seriolae, thereby promoting the healthy growth of fish [
36]. On the other hand, the introduction of BaMS05 may also alter the existing microbial community structure in the water body, potentially affecting other beneficial microorganisms. Therefore, future research should further explore the mechanism of action of BaMS05, assess its long-term ecological impacts in aquaculture systems, and formulate reasonable application strategies to ensure its safety and efficacy.