Mechanisms of Rhizosphere Microbial Regulation on Ecosystem Multifunctionality Driven by Altitudinal Gradients in Hylodesmum podocarpum
Simple Summary
Abstract
1. Introduction
2. Study Areas and Methods
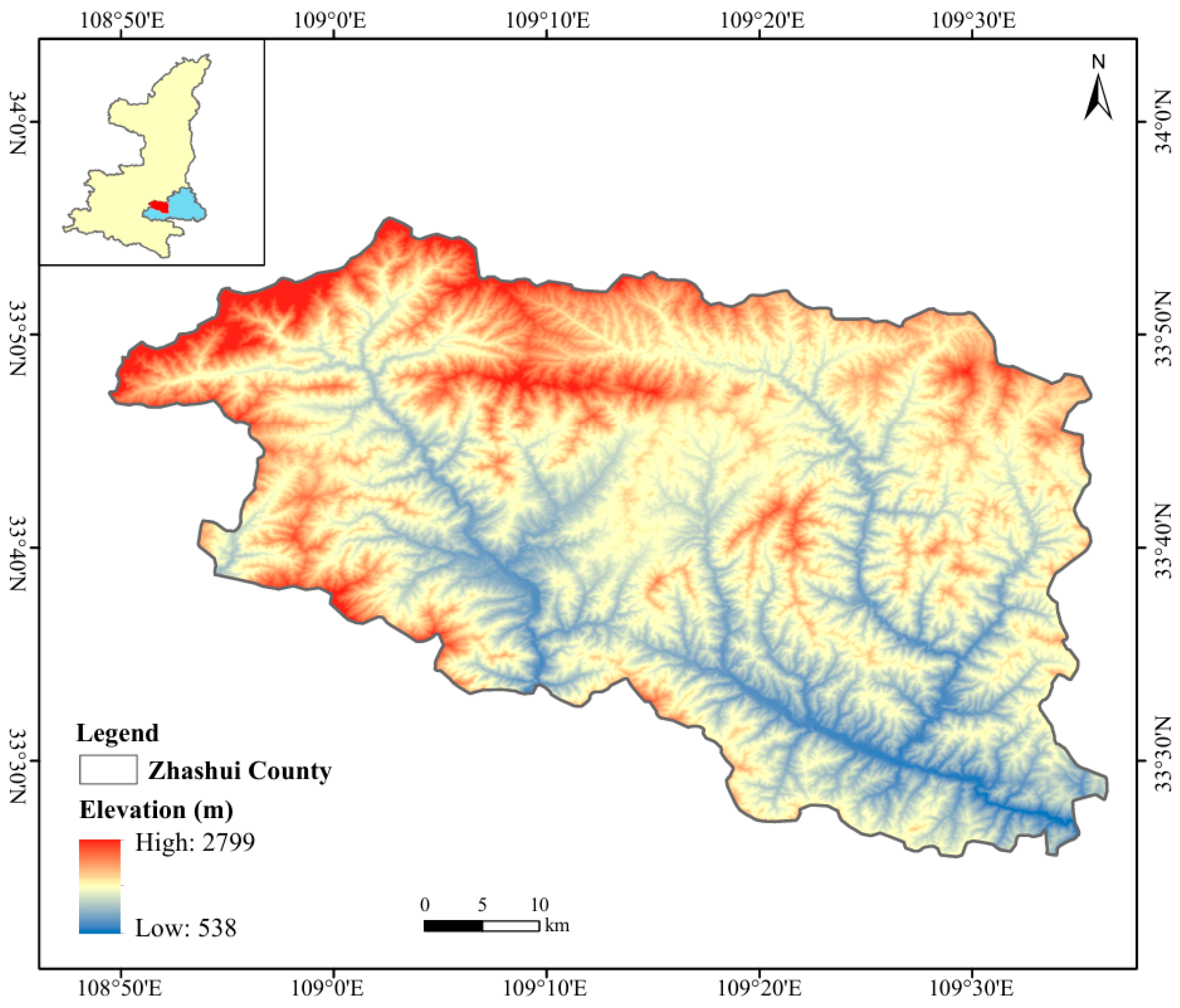
2.1. Study Area
2.2. Sample Collection
2.3. Soil Physicochemical Analysis
2.4. Soil DNA Extraction, High-Throughput Sequencing, and Bioinformatics Analysis
2.5. Determination of Ecosystem Multifunctionality
2.6. Calculation of Soil Element and Enzyme Metering Ratios
2.7. Data Processing
3. Results and Analysis
3.1. Soil and Plant Physical and Chemical Properties
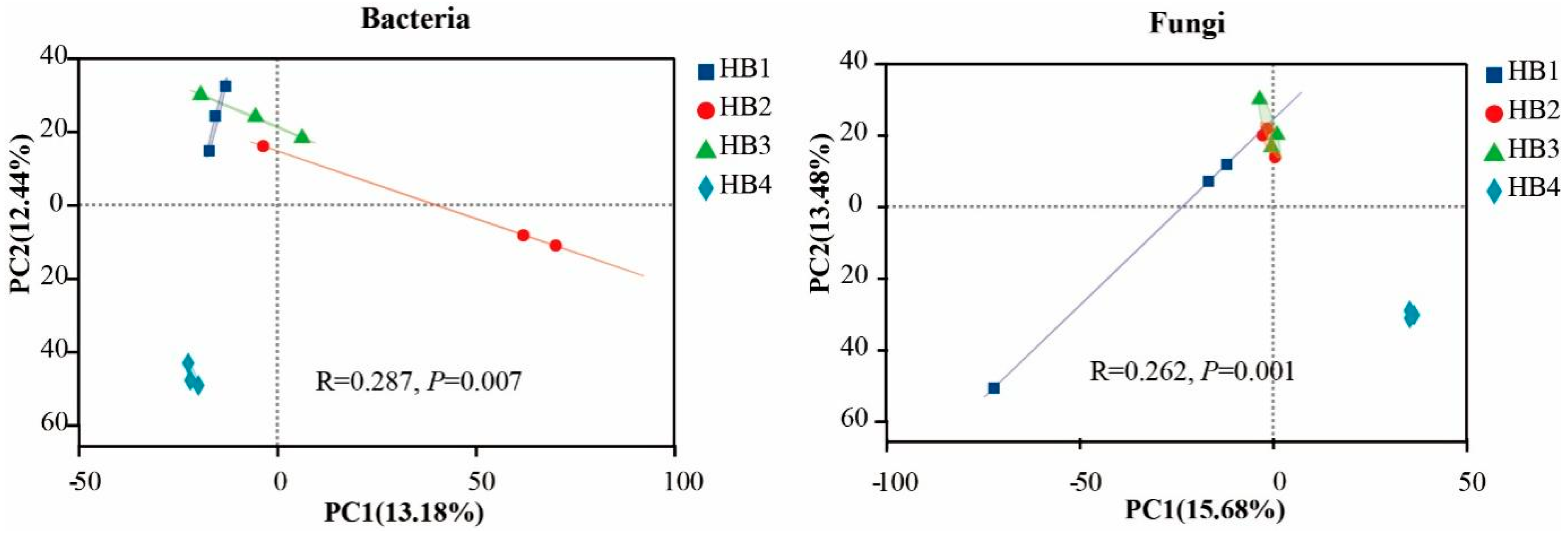
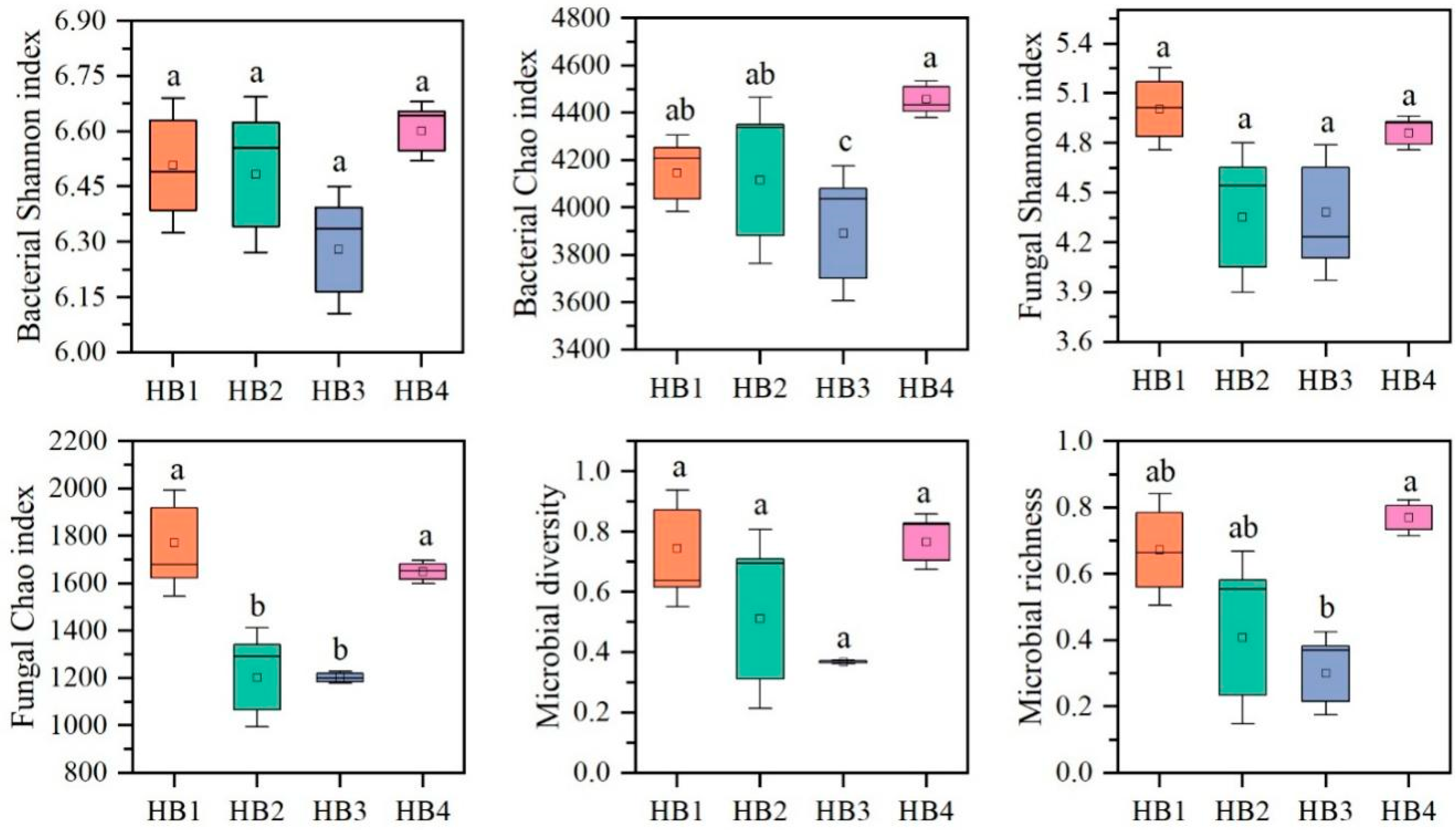
3.2. Soil Microbial Community Structure Characteristics
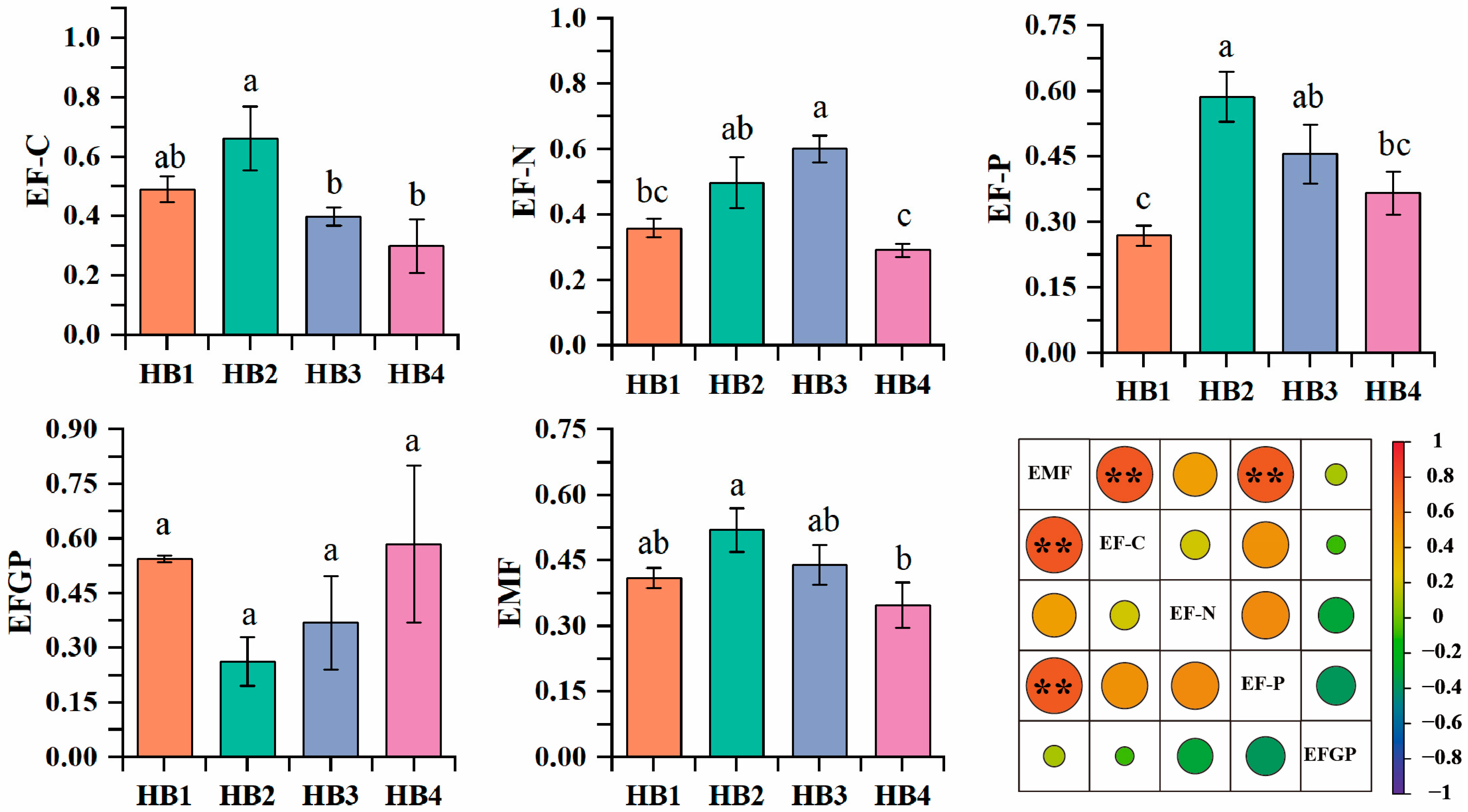
3.3. Soil Ecosystem Multifunctionality
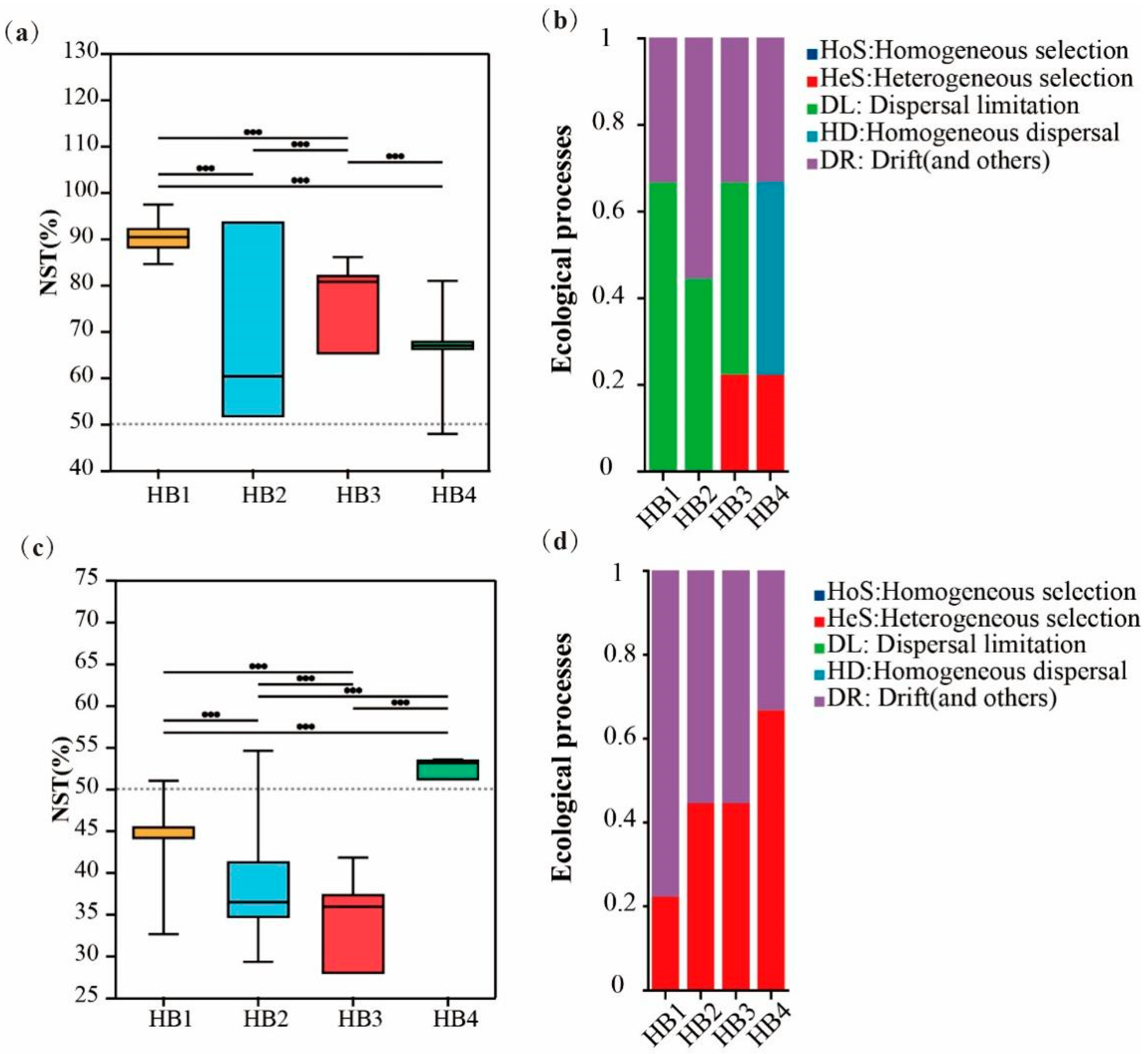
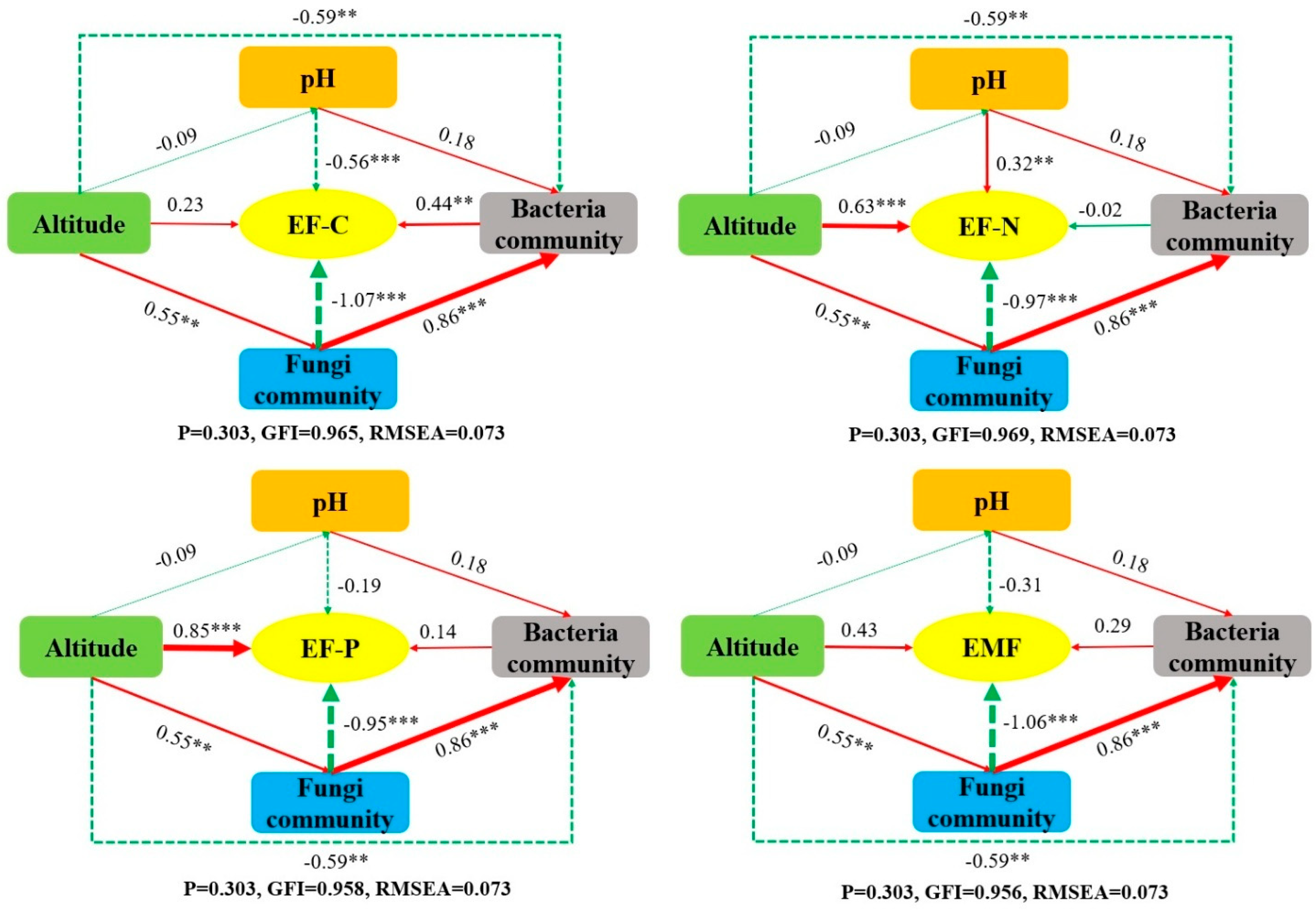
3.4. Relationship Between Soil Microbial Communities and Ecological Functions
4. Discussion
4.1. Effects of Altitude Gradient on Soil Microbial Community Characteristics of Hylodesmum podocarpum
4.2. The Effect of Altitude Gradient on the Multifunctionality of the Soil Ecosystem of Hylodesmum podocarpum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiroyoshi, O.; Kazuaki, O. Desmodium (Leguminosae tribe Desmodieae) of africa, madagascar and the mascarene islands. J. Jpn. Bot. 2019, 94, 135–148. [Google Scholar]
- Phukhatmuen, P.; Meesakul, P.; Suthiphasilp, V.; Charoensup, R.; Maneerat, T.; Cheenpracha, S.; Limtharakul, T.; Pyne, S.G.; Laphookhieo, S. Antidiabetic and antimicrobial flavonoids from the twigs and roots of Erythrina subumbrans (Hassk.) Merr. Heliyon 2021, 7, e06904. [Google Scholar] [CrossRef]
- Huang, H.; Tian, D.; Zhou, L.; Su, H.; Ma, S.; Feng, Y.; Tang, Z.; Zhu, J.; Ji, C.; Fang, J. Effects of afforestation on soil microbial diversity and enzyme activity: A meta-analysis. Geoderma 2022, 423, 115961. [Google Scholar] [CrossRef]
- Peralta, R.M.; Ahn, C.; Gillevet, P.M. Characterization of soil bacterial community structure and physicochemical properties in created and natural wetlands. Sci. Total Environ. 2013, 443, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Baskan, O.; Dengiz, O.; Demirag, İ.T. The land productivity dynamics trend as a tool for land degradation assessment in a dryland ecosystem. Environ. Monit. Assess. 2017, 189, 212. [Google Scholar] [CrossRef]
- Harris, J. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Jiao, S.; Qi, J.; Jin, C.; Liu, Y.; Wang, Y.; Pan, H.; Chen, S.; Liang, C.; Peng, Z.; Chen, B. Core phylotypes enhance the resistance of soil microbiome to environmental changes to maintain multifunctionality in agricultural ecosystems. Glob. Change Biol. 2022, 28, 6653–6664. [Google Scholar] [CrossRef]
- Löbmann, M.T.; Maring, L.; Prokop, G.; Brils, J.; Bender, J.; Bispo, A.; Helming, K. Systems knowledge for sustainable soil and land management. Sci. Total Environ. 2022, 822, 153389. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Jin, H.; Yang, X.; Liu, R.; Yan, Z.; Li, X.; Li, X.; Su, A.; Zhao, Y.; Qin, B. Bacterial community structure associated with the rhizosphere soils and roots of Stellera chamaejasme L. along a Tibetan elevation gradient. Ann. Microbiol. 2018, 68, 273–286. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, K.; Xie, Y.; Li, X.; Zhang, S.; Liu, W.; Huang, Y.; Cui, L.; Wang, S.; Bao, P. Geographical, climatic, and soil factors control the altitudinal pattern of rhizosphere microbial diversity and its driving effect on root zone soil multifunctionality in mountain ecosystems. Sci. Total Environ. 2023, 904, 166932. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1982, 9, 539–579. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C. Nitrogen—Total. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1982; Volume 9, pp. 595–624. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Schinner, F.; Von Mersi, W. Xylanase-, CM-cellulase-and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.Y.; Wang, Y.; Liu, X.; Cheng, J.; Zhang, J.; Baoyin, T.; Bardgett, R.D. High ecosystem multifunctionality under moderate grazing is associated with high plant but low bacterial diversity in a semi-arid steppe grassland. Plant Soil 2020, 448, 265–276. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Niu, S.; Tian, D.; Wu, Q.; Gao, X.; Schellenberg, M.P.; Han, G. Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 2021, 776, 145730. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Ye, S.; Liu, S.; Stirling, E.; Gilbert, J.A.; Faust, K.; Knight, R.; Jansson, J.K.; Cardona, C. Earth microbial co-occurrence network reveals interconnection pattern across microbiomes. Microbiome 2020, 8, 82. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, P.; Xu, L.; Qu, L.; Han, Y.; Zheng, L.; Gong, X. Disproportionate responses between free-living and particle-attached bacteria during the transition to oxygen-deficient zones in the Bohai Seawater. Sci. Total Environ. 2021, 791, 148097. [Google Scholar] [CrossRef]
- Zhang, Y.; Ai, J.; Sun, Q.; Li, Z.; Hou, L.; Song, L.; Tang, G.; Li, L.; Shao, G. Soil organic carbon and total nitrogen stocks as affected by vegetation types and altitude across the mountainous regions in the Yunnan Province, south-western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Jain, V.; Arya, P.; Yagnik, S.M.; Raval, V.H.; Singh, N.A. Microbial diversity of cold-water reservoirs and their prospective applications. In Current Status of Fresh Water Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 49–75. [Google Scholar] [CrossRef]
- Kumar, S.; Suyal, D.C.; Yadav, A.; Shouche, Y.; Goel, R. Microbial diversity and soil physiochemical characteristic of higher altitude. PLoS ONE 2019, 14, e0213844. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, C.; Li, Y.; Deng, Y. Microbial specialists in high-altitude forest soils: Environmental sensitivity and ecological significance. Front. Environ. Sci. Eng. 2025, 19, 30. [Google Scholar] [CrossRef]
- Ciccazzo, S.; Esposito, A.; Borruso, L.; Brusetti, L. Microbial communities and primary succession in high altitude mountain environments. Ann. Microbiol. 2016, 66, 43–60. [Google Scholar] [CrossRef]
- Carr, A.; Diener, C.; Baliga, N.S.; Gibbons, S.M. Use and abuse of correlation analyses in microbial ecology. ISME J. 2019, 13, 2647–2655. [Google Scholar] [CrossRef]
- Eheneden, I.; Wang, R.; Zhao, J. Antibiotic removal by microalgae-bacteria consortium: Metabolic pathways and microbial responses. Sci. Total Environ. 2023, 891, 164489. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Hereira-Pacheco, S.; Arias-Del Razo, I.; Miranda-Carrazco, A.; Dendooven, L.; Estrada-Torres, A.; Navarro-Noya, Y.E. Metagenomic analysis of fungal assemblages at a regional scale in high-altitude temperate forest soils: Alternative methods to determine diversity, composition and environmental drivers. PeerJ 2025, 13, e18323. [Google Scholar] [CrossRef]
- Zhao, W.; Soininen, J.; Hu, A.; Liu, J.; Li, M.; Wang, J. The structure of bacteria–fungi bipartite networks along elevational gradients in contrasting climates. Mol. Ecol. 2024, 33, e17442. [Google Scholar] [CrossRef] [PubMed]
- Kluber, L.A.; Smith, J.E.; Myrold, D.D. Distinctive fungal and bacterial communities are associated with mats formed by ectomycorrhizal fungi. Soil Biol. Biochem. 2011, 43, 1042–1050. [Google Scholar] [CrossRef]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wu, J.H.; Sun, Y.X.; Zhang, Y.Y.; Zhao, Y.F.; Huang, Z.; Duan, W.H. Climate and geochemistry at different altitudes influence soil fungal community aggregation patterns in alpine grasslands. Sci. Total Environ. 2023, 881, 163375. [Google Scholar] [CrossRef]
- Yang, L.; Lyu, M.; Li, X.; Xiong, X.; Lin, W.; Yang, Y.; Xie, J. Decline in the contribution of microbial residues to soil organic carbon along a subtropical elevation gradient. Sci. Total Environ. 2020, 749, 141583. [Google Scholar] [CrossRef]
- Lux, J.; Xie, Z.; Sun, X.; Wu, D.; Scheu, S. Changes in microbial community structure and functioning with elevation are linked to local soil characteristics as well as climatic variables. Ecol. Evol. 2022, 12, e9632. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.; Yu, D.; Zhou, J. Altitudinal gradient of microbial biomass phosphorus and its relationship with microbial biomass carbon, nitrogen, and rhizosphere soil phosphorus on the eastern slope of Gongga Mountain, SW China. PLoS ONE 2013, 8, e72952. [Google Scholar] [CrossRef]
- Ding, K.; Chen, L.; Zhang, Y.; Ge, S.; Zhang, Y.; Lu, M.; Shen, Z.; Tong, Z.; Zhang, J. Long-term cover crops boost multi-nutrient cycling and subsurface soil carbon sequestration by alleviating microbial carbon limitation in a subtropical forest. Catena 2024, 244, 108252. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, J.; Liu, Y.; Xu, Q.; Zhu, C.; Ling, N.; Guo, J.; Li, R.; Huang, W.; Guo, S. Relative importance of altitude shifts with plant and microbial diversity to soil multifunctionality in grasslands of north-western China. Plant Soil 2024, 504, 545–560. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; He, Y.; Qu, M.; Zhu, W.; Xue, Y.; Li, J. Interkingdom ecological networks between plants and fungi drive soil multifunctionality across arid inland river basin. Mol. Ecol. 2023, 32, 6939–6952. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanksi, M.E.; Grandy, A.S. Managing agroecosystems for soil microbial carbon use efficiency: Ecological unknowns, potential outcomes, and a path forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Jiang, X.; Hou, X.; Zhou, X.; Xin, X.; Wright, A.; Jia, Z. pH regulates key players of nitrification in paddy soils. Soil Biol. Biochem. 2015, 81, 9–16. [Google Scholar] [CrossRef]
- Fisher, K.A.; Yarwood, S.A.; James, B.R. Soil urease activity and bacterial ureC gene copy numbers: Effect of pH. Geoderma 2017, 285, 1–8. [Google Scholar] [CrossRef]





| Topological | Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | HB1 | HB2 | HB3 | HB4 | HB1 | HB2 | HB3 | HB4 |
| Nodes | 272 | 274 | 273 | 272 | 191 | 251 | 259 | 245 |
| Links | 5138 | 5242 | 5465 | 5422 | 2355 | 3851 | 4085 | 4581 |
| Positive links % | 49.77% | 51.77% | 53.36% | 55.91% | 57.54% | 64.37% | 54.96% | 55.65% |
| Negative links% | 50.23% | 48.23% | 46.64% | 44.09% | 42.46% | 35.63% | 45.04% | 44.35% |
| Average degree | 37.779 | 38.263 | 40.073 | 39.868 | 24.66 | 31.437 | 31.544 | 36.502 |
| Graph density | 0.139 | 0.14 | 0.147 | 0.147 | 0.13 | 0.129 | 0.122 | 0.146 |
| Modularity | 0.811 | 0.823 | 0.768 | 0.776 | 0.831 | 0.837 | 0.849 | 0.751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.; Wang, L.; Nian, L.; Wang, M.; Li, Y.; Mao, Z. Mechanisms of Rhizosphere Microbial Regulation on Ecosystem Multifunctionality Driven by Altitudinal Gradients in Hylodesmum podocarpum. Biology 2025, 14, 1126. https://doi.org/10.3390/biology14091126
Liang K, Wang L, Nian L, Wang M, Li Y, Mao Z. Mechanisms of Rhizosphere Microbial Regulation on Ecosystem Multifunctionality Driven by Altitudinal Gradients in Hylodesmum podocarpum. Biology. 2025; 14(9):1126. https://doi.org/10.3390/biology14091126
Chicago/Turabian StyleLiang, Kunlun, Li Wang, Lili Nian, Mingyan Wang, Yang Li, and Zhuxin Mao. 2025. "Mechanisms of Rhizosphere Microbial Regulation on Ecosystem Multifunctionality Driven by Altitudinal Gradients in Hylodesmum podocarpum" Biology 14, no. 9: 1126. https://doi.org/10.3390/biology14091126
APA StyleLiang, K., Wang, L., Nian, L., Wang, M., Li, Y., & Mao, Z. (2025). Mechanisms of Rhizosphere Microbial Regulation on Ecosystem Multifunctionality Driven by Altitudinal Gradients in Hylodesmum podocarpum. Biology, 14(9), 1126. https://doi.org/10.3390/biology14091126





