Mulberroside A: A Multi-Target Neuroprotective Agent in Alzheimer’s Disease via Cholinergic Restoration and PI3K/AKT Pathway Activation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Scopolamine-Induced Mouse Model
2.2. New Object Recognition Test
2.3. Morris Water Maze Test
2.4. Nissl Staining
2.5. Biochemical Analysis
2.6. Immunohistochemistry
2.7. Cell Culture and Treatment Procedures
2.8. Cell Viability Assay
2.9. ROS Levels Assessment
2.10. Aβ1–42 ELISA Assay
2.11. Quantitative Real-Time PCR
2.12. Western Blotting Analysis
2.13. Statistics
3. Results
3.1. The Beneficial Effect of MSA on Scopolamine-Induced Mice
3.1.1. MsA Alleviates Cognitive Impairments in Mice Induced with Scopolamine
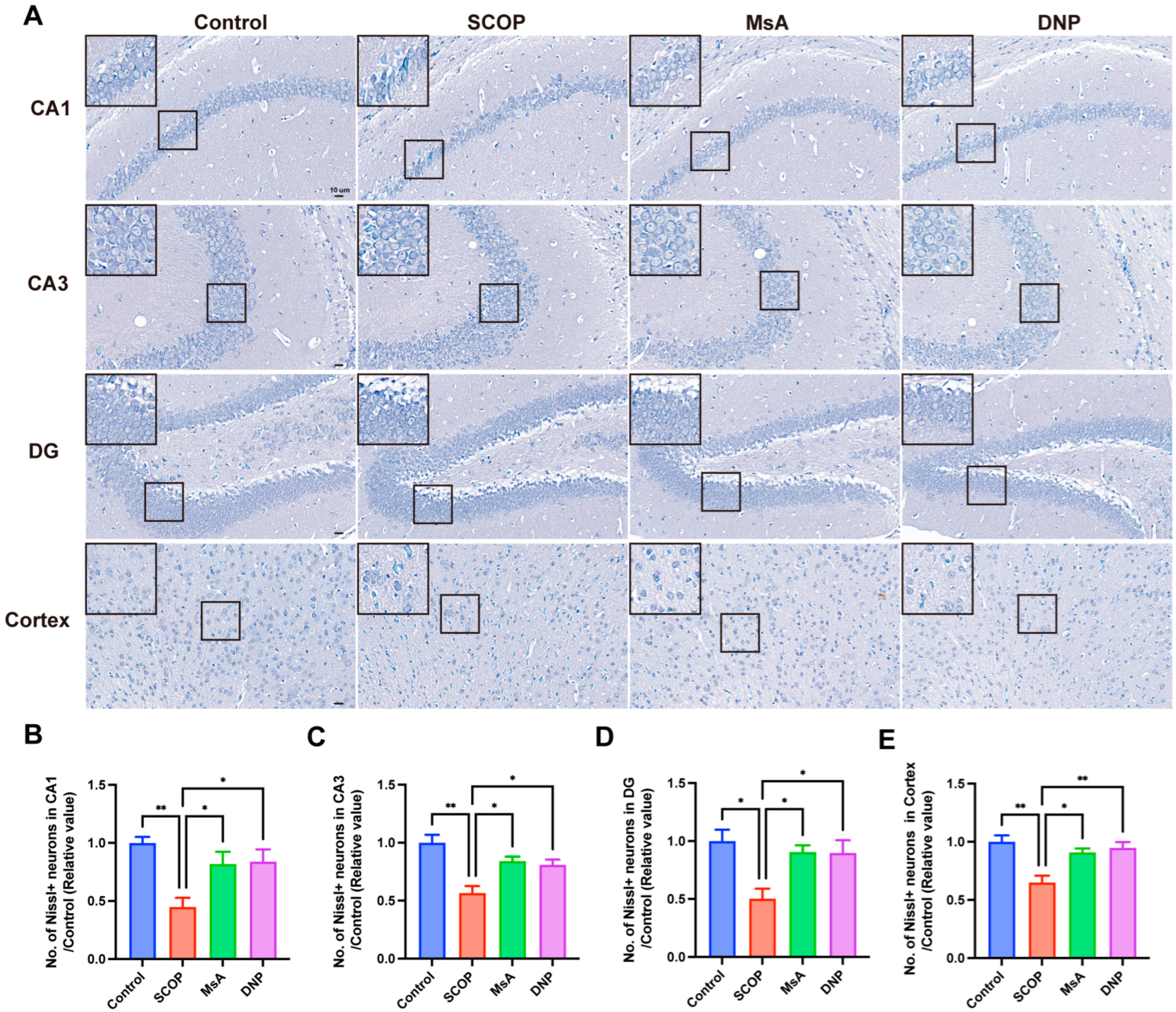
3.1.2. MsA Reduces Neuronal Loss in the Hippocampus and Cortex in Mice Induced with Scopolamine
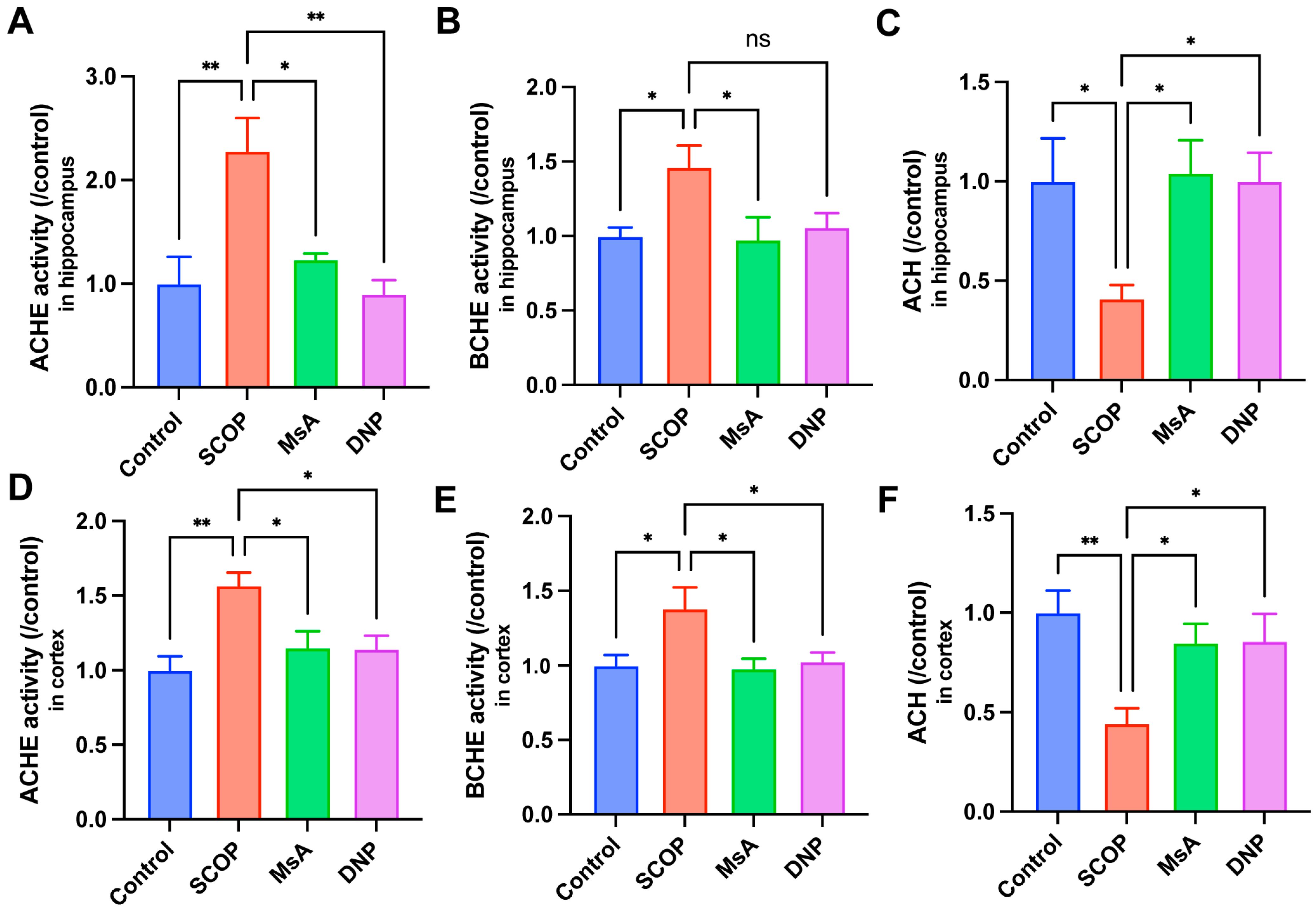
3.1.3. MsA Regulates Cholinergic Metabolism in the Hippocampus and Cortex of Scopolamine-Treated Mice
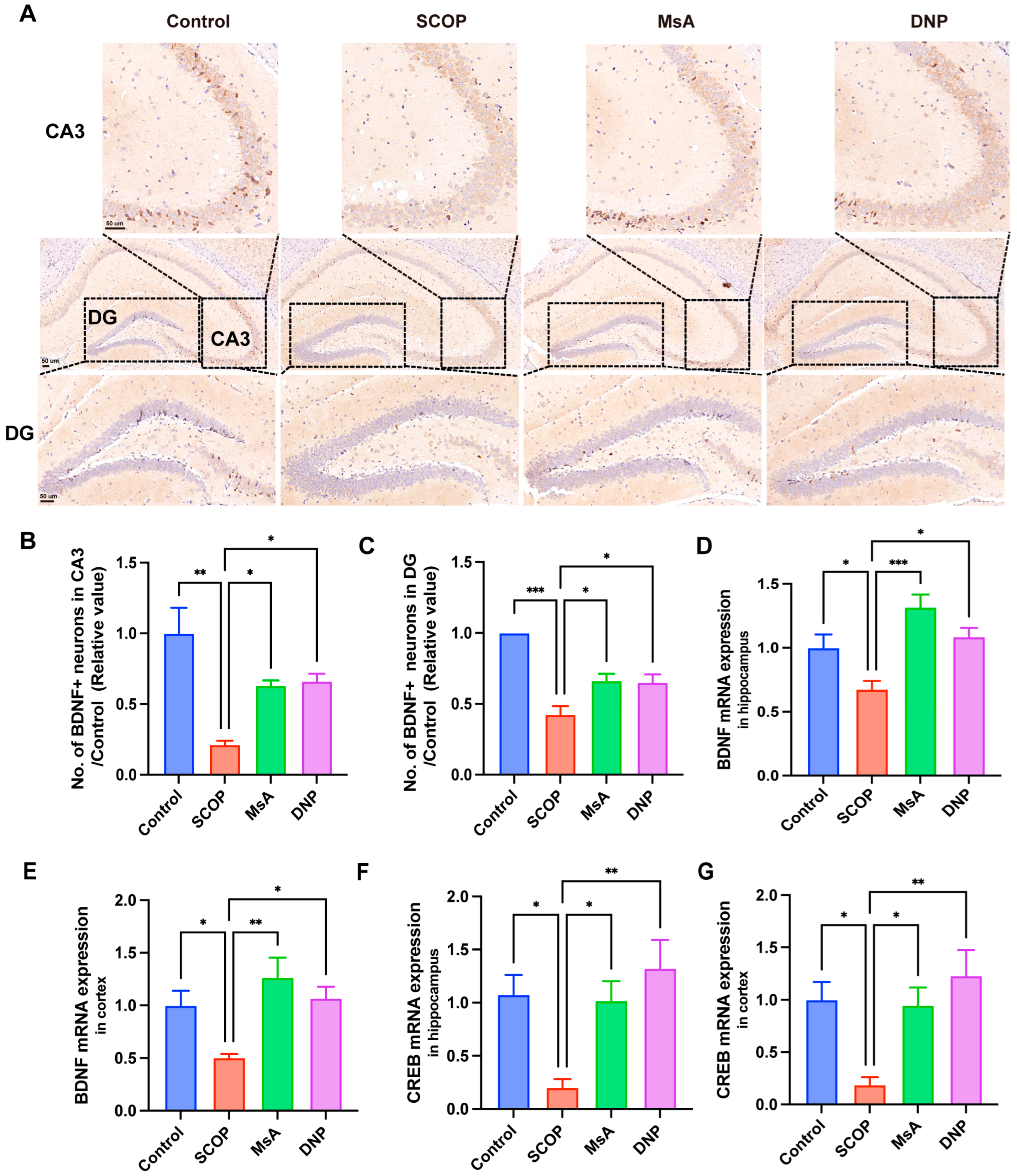
3.1.4. MsA Modulates BDNF and CREB Levels in the Hippocampus and Cortex of Mice Treated with Scopolamine
3.2. The Impact of MSA on N2a/APP695swe Cells
3.2.1. MsA Enhances Cholinergic System Function and Reduces Oxidative Damage in N2a/APP695swe Cells
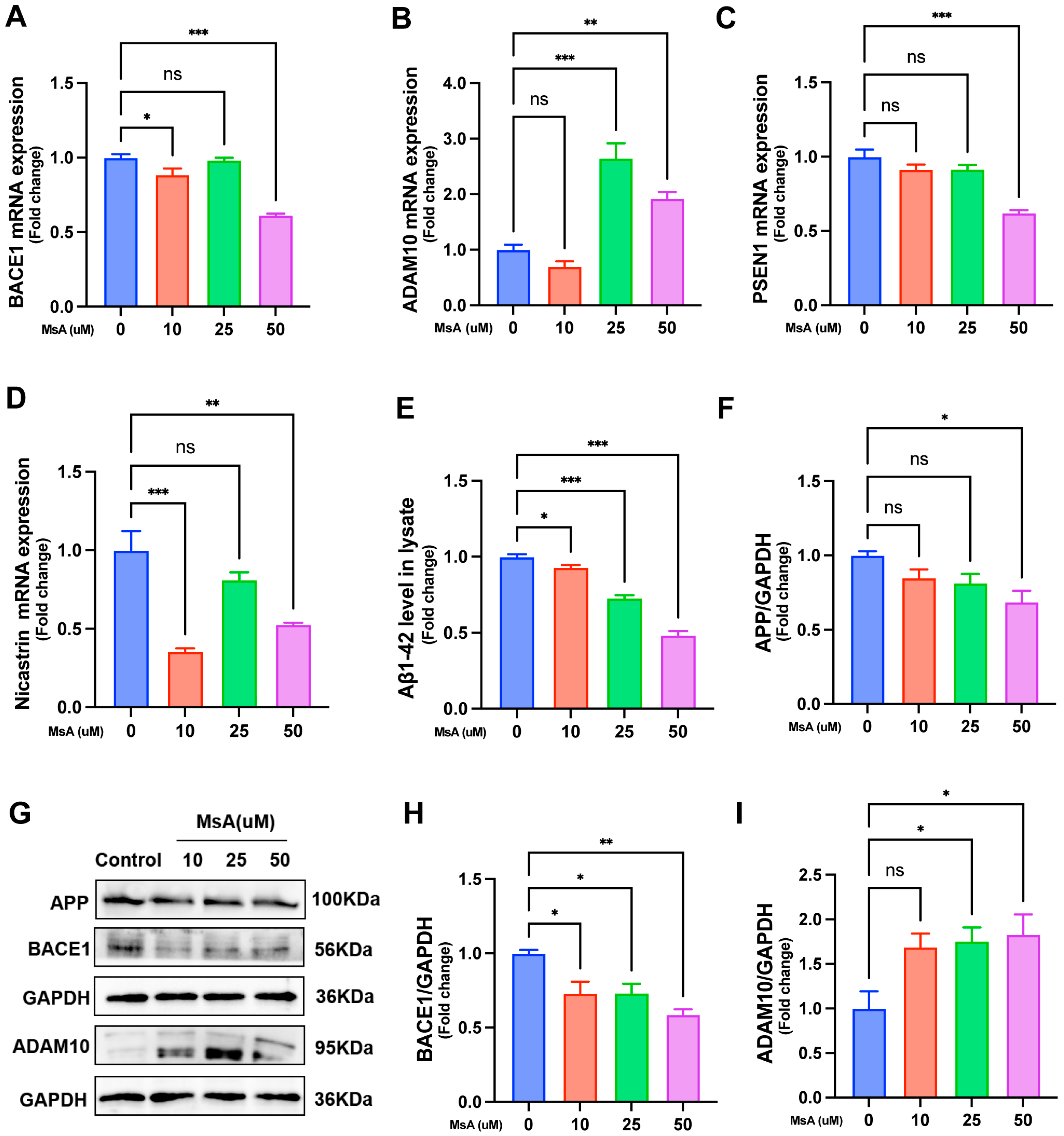
3.2.2. MsA Modulates APP Processing-Related Gene and Protein Expression to Reduce Aβ1–42 Levels in N2a/APP695swe Cells
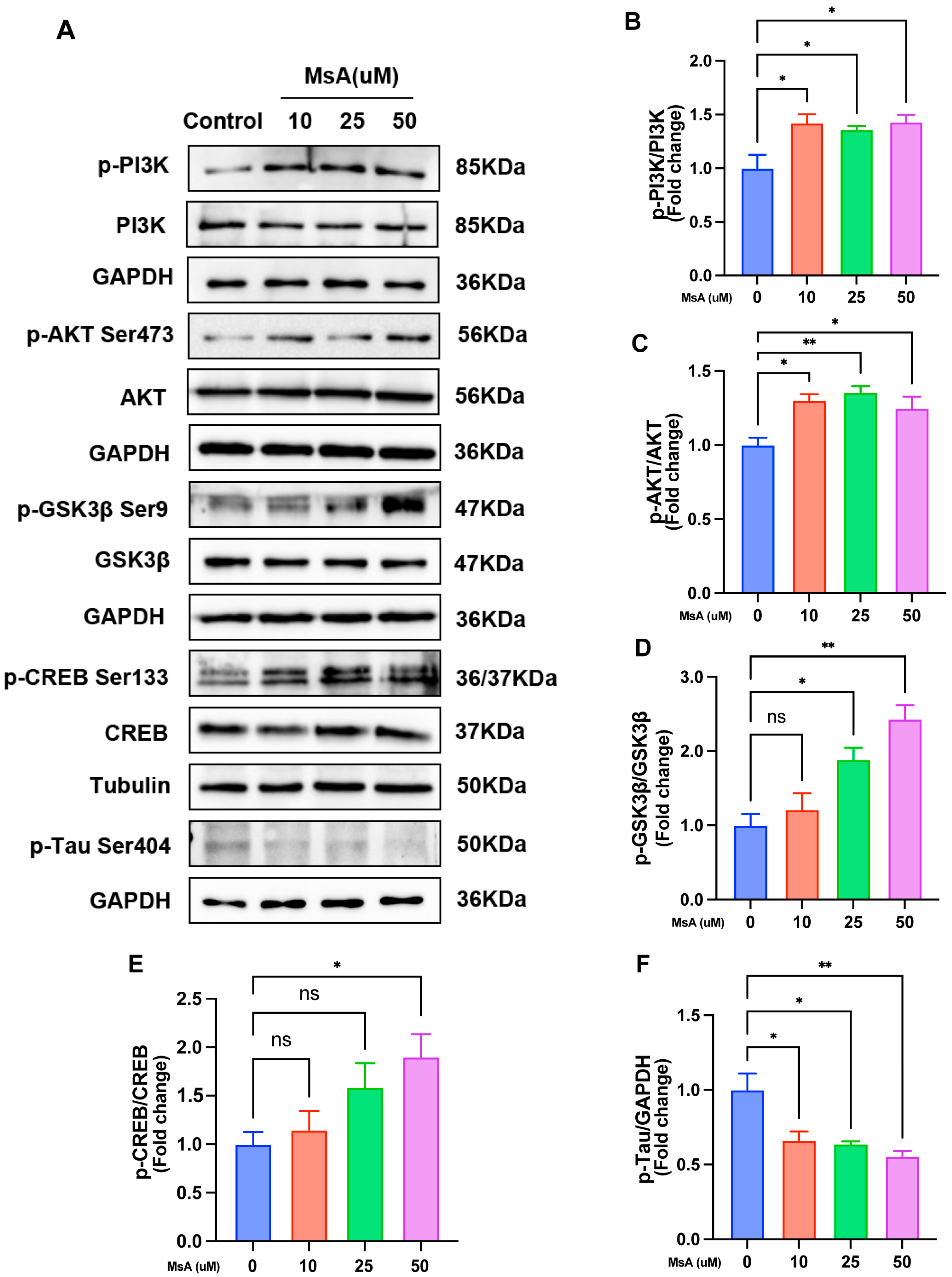
3.2.3. MsA Regulates the PI3K/AKT/GSK3β/CREB Signaling Pathway and Reduces Tau Phosphorylation in N2a/APP695swe Cells
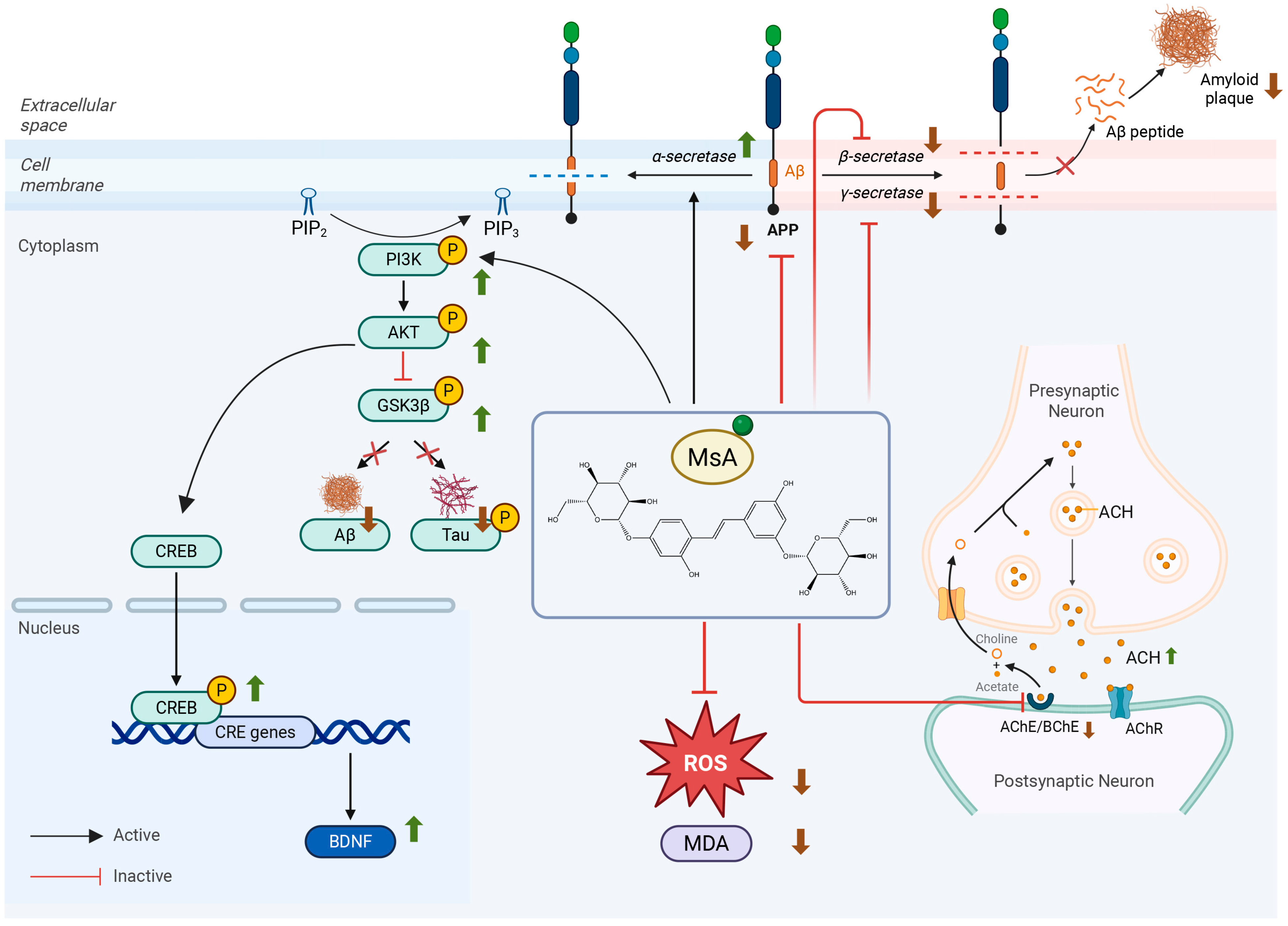
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| MsA | Mulberroside A |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| Aβ | Amyloid-beta |
| BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP response element-binding protein |
| APP | Amyloid precursor protein |
| SCOP | Scopolamine |
| DNP | Donepezil |
| NOR | Novel object recognition |
| MWW | Morris Water Maze |
| MDA | Malondialdehyde |
| CCK-8 | Cell Counting Kit-8 |
| ROS | Reactive oxygen species |
| qRT-PCR | Quantitative real-time PCR |
References
- Korczyn, A.D.; Grinberg, L.T. Is Alzheimer Disease a Disease? Nat. Rev. Neurol. 2024, 20, 245–251. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New Insights into Atypical Alzheimer’s Disease in the Era of Biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef]
- Lau, V.; Ramer, L.; Tremblay, M.-È. An Aging, Pathology Burden, and Glial Senescence Build-up Hypothesis for Late Onset Alzheimer’s Disease. Nat. Commun. 2023, 14, 1670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, M.; Villageois, A.; Bonnet, A.M.; Pillon, B.; Rieger, F.; Agid, Y. Acetylcholinesterase and Butyrylcholinesterase Activity in the Cerebrospinal Fluid of Patients with Neurodegenerative Diseases Involving Cholinergic Systems. J. Neurol. Neurosurg. Psychiatry 1987, 50, 538–543. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An Important New Target in Alzheimer’s Disease Therapy. Int. Psychogeriatr. 2002, 14 (Suppl. S1), 77–91. [Google Scholar] [CrossRef]
- Antonino, M.; Marmo, P.; Freites, C.L.; Quassollo, G.E.; Sánchez, M.F.; Lorenzo, A.; Bignante, E.A. Aβ Assemblies Promote Amyloidogenic Processing of APP and Intracellular Accumulation of Aβ42 Through Go/Gβγ Signaling. Front. Cell Dev. Biol. 2022, 10, 852738. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Xia, W.; Zhang, Y.; Wang, C. Targeting Amyloidogenic Processing of APP in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 137. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Daigle, J.G.; Kapinos, L.E.; Coyne, A.; Schiantarelli, J.; Carlomagno, Y.; Cook, C.; Miller, S.J.; Dujardin, S.; Amaral, A.S.; et al. Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer’s Disease. Neuron 2018, 99, 925–940.e7. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Y.; Ni, M.; He, Y.; Li, L.; Hu, Y. Safflower Yellow Alleviates Cognitive Impairment in Mice by Modulating Cholinergic System Function, Oxidative Stress, and CREB/BDNF/TrkB Signaling Pathway. J. Ethnopharmacol. 2025, 340, 118986. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Ardianto, C.; Celia, C.; Sidharta, V.M.; Sasmita, P.K.; Satriotomo, I.; Turana, Y. Brain-Derived Neurotrophic Factor Interplay with Oxidative Stress: Neuropathology Approach in Potential Biomarker of Alzheimer’s Disease. Dement. Neuropsychol. 2023, 17, e20230012. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.-Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining Cholinergic Therapy for Alzheimer’s Disease. Brain J. Neurol. 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Choudhury, A.; Islam, A.; Thakur, S.C.; Hassan, M.I. Understanding of Alzheimer’s Disease Pathophysiology for Therapeutic Implications of Natural Products as Neuroprotective Agents. Ageing Res. Rev. 2025, 105, 102680. [Google Scholar] [CrossRef]
- Bhat, B.A.; Almilaibary, A.; Mir, R.A.; Aljarallah, B.M.; Mir, W.R.; Ahmad, F.; Mir, M.A. Natural Therapeutics in Aid of Treating Alzheimer’s Disease: A Green Gateway Toward Ending Quest for Treating Neurological Disorders. Front. Neurosci. 2022, 16, 884345. [Google Scholar] [CrossRef]
- Lu, R.; Wei, Z.; Wang, Z.; Xu, S.; Sun, K.; Cheng, P.; Huang, X.; You, H.; Guo, F.; Liang, S.; et al. Mulberroside a Alleviates Osteoarthritis via Restoring Impaired Autophagy and Suppressing MAPK/NF-κB/PI3K-AKT-mTOR Signaling Pathways. iScience 2023, 26, 105936. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Qian, J.; Miao, H.; Zhang, S.; Hu, Y.; Liu, P.; Xu, L. Mulberroside A Ameliorates CCl4-Induced Liver Fibrosis in Mice via Inhibiting pro-Inflammatory Response. Food Sci. Nutr. 2023, 11, 3433–3441. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, H.; Zhao, T.; Fu, J.; Xu, Y. Mulberroside a from Cortex Mori Enhanced Gut Integrity in Diabetes. Evid.-Based Complement. Altern. Med. 2021, 2021, 6655555. [Google Scholar] [CrossRef]
- Chen, J.; Gou, Z.; Huang, Y.; Yu, Q.; Kim, A.N.; Shi, W.; Zhou, Y. Research Progress on Phytochemicals from Mulberry with Neuroprotective Effects: A Review. Pharmaceuticals 2025, 18, 695. [Google Scholar] [CrossRef]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s Disease Activity of Compounds from the Root Bark of Morus alba L. Arch. Pharm. Res. 2017, 40, 338–349. [Google Scholar] [CrossRef]
- You, S.; Jang, M.; Kim, G.-H. Mori Cortex Radicis Attenuates High Fat Diet-Induced Cognitive Impairment via an IRS/Akt Signaling Pathway. Nutrients 2020, 12, 1851. [Google Scholar] [CrossRef]
- You, S.; Jang, M.; Kim, G.-H. Mori Cortex Radicis Extract Protected against Diet-Induced Neuronal Damage by Suppressing the AGE-RAGE/MAPK Signaling Pathway in C. elegans and Mouse Model. J. Funct. Foods 2022, 91, 104996. [Google Scholar] [CrossRef]
- Saha, S.; Pal, D.; Nimse, S.B. Recent Advances in the Discovery of GSK-3 Inhibitors from Synthetic Origin in the Treatment of Neurological Disorders. Curr. Drug Targets 2021, 22, 1437–1462. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Mukherjee, S.; Song, I.-H.; Nimse, S.B. GSK-3 Inhibitors: A New Class of Drugs for Alzheimer’s Disease Treatment. Curr. Drug Targets 2021, 22, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, Y.; Yang, S.; Gong, E.J.; Kim, J.; Ha, N.-C.; Jo, D.-G.; Mattson, M.P.; Lee, J. Akt-Activated GSK3β Inhibitory Peptide Effectively Blocks Tau Hyperphosphorylation. Arch. Pharm. Res. 2024, 47, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.A.; Valero, J. The Role of CREB Signaling in Alzheimer’s Disease and Other Cognitive Disorders. Rev. Neurosci. 2011, 22, 153–169. [Google Scholar] [CrossRef]
- Xia, L.; Zhu, X.; Zhao, Y.; Yang, G.; Zuo, X.; Xie, P.; Chen, C.; Han, Q. Genome-Wide RNA Sequencing Analysis Reveals That IGF-2 Attenuates Memory Decline, Oxidative Stress and Amyloid Plaques in an Alzheimer’s Disease Mouse Model (AD) by Activating the PI3K/AKT/CREB Signaling Pathway. Int. Psychogeriatr. 2019, 31, 947–959. [Google Scholar] [CrossRef]
- Kim, H.G.; Park, G.; Lim, S.; Park, H.; Choi, J.G.; Jeong, H.U.; Kang, M.S.; Lee, M.K.; Oh, M.S. Mori Fructus Improves Cognitive and Neuronal Dysfunction Induced by Beta-Amyloid Toxicity through the GSK-3β Pathway in Vitro and in Vivo. J. Ethnopharmacol. 2015, 171, 196–204. [Google Scholar] [CrossRef]
- Li, J.; Li, H.-X.; Shou, X.-J.; Xu, X.-J.; Song, T.-J.; Han, S.-P.; Zhang, R.; Han, J.-S. Effects of Chronic Restraint Stress on Social Behaviors and the Number of Hypothalamic Oxytocin Neurons in Male Rats. Neuropeptides 2016, 60, 21–28. [Google Scholar] [CrossRef]
- Wang, C.-P.; Zhang, L.-Z.; Li, G.-C.; Shi, Y.; Li, J.-L.; Zhang, X.-C.; Wang, Z.-W.; Ding, F.; Liang, X.-M. Mulberroside a Protects against Ischemic Impairment in Primary Culture of Rat Cortical Neurons after Oxygen-Glucose Deprivation Followed by Reperfusion. J. Neurosci. Res. 2014, 92, 944–954. [Google Scholar] [CrossRef]
- Yu, R.; Wen, S.; Wang, Q.; Wang, C.; Zhang, L.; Wu, X.; Li, J.; Kong, L. Mulberroside a Repairs High Fructose Diet-Induced Damage of Intestinal Epithelial and Blood-Brain Barriers in Mice: A Potential for Preventing Hippocampal Neuroinflammatory Injury. J. Neurochem. 2021, 157, 1979–1991. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Q.; Zhao, F.; Yin, J.; Liu, H.; Wang, L.; Liu, B. Mulberroside A Alleviates Myocardial Infarction by Inhibiting Oxidative Stress and Apoptosis via the PI3K/Akt Signaling Pathway. Bratisl. Med. J. 2025, 2025, 1–9. [Google Scholar] [CrossRef]
- Xue, H.; Feng, Z.; Yuan, P.; Qiao, L.; Lou, Q.; Zhao, X.; Ma, Q.; Wang, S.; Shen, Y.; Ye, H.; et al. Restrained Mitf-Associated Autophagy by Mulberroside A Ameliorates Osteoclastogenesis and Counteracts OVX-Induced Osteoporosis in Mice. Cell Death Discov. 2024, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, J.; Shi, L. Protective Function of Cis-Mulberroside a and Oxyresveratrol from Ramulus mori against Ethanol-Induced Hepatic Damage. Environ. Toxicol. Pharmacol. 2008, 26, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, L.; Zeng, X.; Zhong, G.; Ying, M.; Huang, M.; Bi, H. Down-Regulation of P-Gp Expression and Function after Mulberroside A Treatment: Potential Role of Protein Kinase C and NF-Kappa B. Chem. Biol. Interact. 2014, 213, 44–50. [Google Scholar] [CrossRef]
- Wang, C.-P.; Wang, Y.; Wang, X.; Zhang, X.; Ye, J.-F.; Hu, L.-S.; Kong, L.-D. Mulberroside A Possesses Potent Uricosuric and Nephroprotective Effects in Hyperuricemic Mice. Planta Med. 2011, 77, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, L. Anti-Inflammatory and Analgesic Properties of Cis-Mulberroside A from Ramulus mori. Fitoterapia 2010, 81, 214–218. [Google Scholar] [CrossRef]
- Xing, S.; Li, Q.; Xiong, B.; Chen, Y.; Feng, F.; Liu, W.; Sun, H. Structure and Therapeutic Uses of Butyrylcholinesterase: Application in Detoxification, Alzheimer’s Disease, and Fat Metabolism. Med. Res. Rev. 2021, 41, 858–901. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of Butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Reid, G.A.; Darvesh, S. Butyrylcholinesterase-Knockout Reduces Brain Deposition of Fibrillar β-Amyloid in an Alzheimer Mouse Model. Neuroscience 2015, 298, 424–435. [Google Scholar] [CrossRef]
- Maurice, T.; Strehaiano, M.; Siméon, N.; Bertrand, C.; Chatonnet, A. Learning Performances and Vulnerability to Amyloid Toxicity in the Butyrylcholinesterase Knockout Mouse. Behav. Brain Res. 2016, 296, 351–360. [Google Scholar] [CrossRef]
- Gandhi, S.; Refolo, L.M.; Sambamurti, K. Amyloid Precursor Protein Compartmentalization Restricts Beta-Amyloid Production: Therapeutic Targets Based on BACE Compartmentalization. J. Mol. Neurosci. 2004, 24, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Thompson, R.; Zhang, H.; Xu, H. APP Processing in Alzheimer’s Disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2023, 6, fcad356. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Sah, S.P. Insulin Signaling Pathway and Related Molecules: Role in Neurodegeneration and Alzheimer’s Disease. Neurochem. Int. 2020, 135, 104707. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen Synthase Kinase-3 Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef] [PubMed]
- Limantoro, J.; de Liyis, B.G.; Sutedja, J.C. Akt Signaling Pathway: A Potential Therapy for Alzheimer’s Disease through Glycogen Synthase Kinase 3 Beta Inhibition. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 147. [Google Scholar] [CrossRef]
- Hur, E.-M.; Zhou, F.-Q. GSK3 Signalling in Neural Development. Nat. Rev. Neurosci. 2010, 11, 539–551. [Google Scholar] [CrossRef]
- Amidfar, M.; De Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The Role of CREB and BDNF in Neurobiology and Treatment of Alzheimer’s Disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Wang, Q.M.; Roach, P.J.; Fiol, C.J. Use of a Synthetic Peptide as a Selective Substrate for Glycogen Synthase Kinase 3. Anal. Biochem. 1994, 220, 397–402. [Google Scholar] [CrossRef]
- Grimes, C.A.; Jope, R.S. CREB DNA Binding Activity Is Inhibited by Glycogen Synthase Kinase-3 Beta and Facilitated by Lithium. J. Neurochem. 2001, 78, 1219–1232. [Google Scholar] [CrossRef]
- Fahnestock, M. Brain-Derived Neurotrophic Factor: The Link Between Amyloid-β and Memory Loss. Future Neurol. 2011, 6, 627–639. [Google Scholar] [CrossRef]
- Rosa, E.; Fahnestock, M. CREB Expression Mediates Amyloid β-Induced Basal BDNF Downregulation. Neurobiol. Aging 2015, 36, 2406–2413. [Google Scholar] [CrossRef]
- Song, J.-H.; Yu, J.-T.; Tan, L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol. Neurobiol. 2015, 52, 1477–1493. [Google Scholar] [CrossRef]
- Caccamo, A.; Maldonado, M.A.; Bokov, A.F.; Majumder, S.; Oddo, S. CBP Gene Transfer Increases BDNF Levels and Ameliorates Learning and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2010, 107, 22687–22692. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective Effects of Brain-Derived Neurotrophic Factor in Rodent and Primate Models of Alzheimer’s Disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.L.; Lin, C.-J.; Huang, C.-C.; Chang, L.-C. Curcumin-Derived Carbon Quantum Dots: Dual Actions in Mitigating Tau Hyperphosphorylation and Amyloid Beta Aggregation. Colloids Surf. B Biointerfaces 2024, 234, 113676. [Google Scholar] [CrossRef]
- Chen, M.-H.; Liu, X.-Z.; Qu, X.-W.; Guo, R.-B.; Zhang, L.; Kong, L.; Yu, Y.; Liu, Y.; Zang, J.; Li, X.-Y.; et al. ApoE-Modified Liposomes Encapsulating Resveratrol and Salidroside Alleviate Manifestations of Alzheimer’s Disease in APP/PS-1 Mice. Drug Dev. Ind. Pharm. 2023, 49, 559–571. [Google Scholar] [CrossRef]
- She, L.; Sun, J.; Xiong, L.; Li, A.; Li, L.; Wu, H.; Ren, J.; Wang, W.; Liang, G.; Zhao, X. Ginsenoside RK1 Improves Cognitive Impairments and Pathological Changes in Alzheimer’s Disease via Stimulation of the AMPK/Nrf2 Signaling Pathway. Phytomed. Int. J. Phytother. Phytopharm. 2024, 122, 155168. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Wang, C.; Teng, Z.; Li, Y. Curcumin Decreases Hyperphosphorylation of Tau by Down-Regulating Caveolin-1/GSK-3β in N2a/APP695swe Cells and APP/PS1 Double Transgenic Alzheimer’s Disease Mice. Am. J. Chin. Med. 2017, 45, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective Effects of Ginsenoside Rg2 against Memory Impairment and Neuronal Death Induced by Aβ25-35 in Rats. J. Ethnopharmacol. 2021, 266, 113466. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.F.; Colón, D.; Fonseca-Pinto, R. A Memory Failure Computational Model in Alzheimer-like Disease via Continuous Delayed Hopfield Network with Lurie Control System Based Healing. Neurocomputing 2025, 636, 129967. [Google Scholar] [CrossRef]


| Target | Primer Sequence (5′ to 3′) | Primer Length (bp) |
|---|---|---|
| BACE1 | F: CTGCCATCACTGAATCGGACAAG R: TCACCAGGGAGTCAAGAAGGG | 126 |
| ADAM10 | F: TTGATGATGGTGTTC R: GATGCCTGTGTTCAATCACTTCTTC | 119 |
| PSEN1 | F: TGTGGTTGGTGAATATGGCTGAAG R: TCTCCGCTCTTTGTGTGTATACTTG | 87 |
| Nicastrin | F: TCTGCTCTATGGGTTCCTGGTTAG R: GAGACCGCCATGTAGTGTGAAG | 114 |
| BDNF | F: CGACGACATCACTGGCTGACAC R: GAGGCTCCAAAGGCACTTGACTG | 150 |
| CREB | F: CTGAAGAAGCAGCACGGAAGAGAG R: TTCAAGCACTGCCACTCTGTTCTC | 122 |
| GAPDH | F: AACTCCCACTCCTTCCACCTTCCG R: TCCACCACCCTGTTGCCTGTAG | 113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, J.; Li, Y.; Guo, J.; Jin, Z.; Qiao, S.; Zhang, Y.; Li, G.; Liu, H.; Wu, C. Mulberroside A: A Multi-Target Neuroprotective Agent in Alzheimer’s Disease via Cholinergic Restoration and PI3K/AKT Pathway Activation. Biology 2025, 14, 1114. https://doi.org/10.3390/biology14091114
Li J, Wang J, Li Y, Guo J, Jin Z, Qiao S, Zhang Y, Li G, Liu H, Wu C. Mulberroside A: A Multi-Target Neuroprotective Agent in Alzheimer’s Disease via Cholinergic Restoration and PI3K/AKT Pathway Activation. Biology. 2025; 14(9):1114. https://doi.org/10.3390/biology14091114
Chicago/Turabian StyleLi, Jin, Jiawen Wang, Yaodong Li, Jingyi Guo, Ziliang Jin, Shourong Qiao, Yunxia Zhang, Guoyin Li, Huazhen Liu, and Changjing Wu. 2025. "Mulberroside A: A Multi-Target Neuroprotective Agent in Alzheimer’s Disease via Cholinergic Restoration and PI3K/AKT Pathway Activation" Biology 14, no. 9: 1114. https://doi.org/10.3390/biology14091114
APA StyleLi, J., Wang, J., Li, Y., Guo, J., Jin, Z., Qiao, S., Zhang, Y., Li, G., Liu, H., & Wu, C. (2025). Mulberroside A: A Multi-Target Neuroprotective Agent in Alzheimer’s Disease via Cholinergic Restoration and PI3K/AKT Pathway Activation. Biology, 14(9), 1114. https://doi.org/10.3390/biology14091114






