3.1. Morphological Parameters
The following morphological parameters were measured: the 50% flowering days, plant height and number of branches. As can be seen from
Table 3, the 50% flowering day of different fertilizer treatments was found to be statistically significant at the level of 0.05% (
p < 0.05). Among different treatments, the mean 50% flowering day varied between 32.33 and 36.39 days. Considering the mean cuts, the latest 50% flowering was observed in the unfertilized treatment and the earliest 50% flowering was observed in the biotreatment. In addition, the inorganic fertilizer application showed the earliest 50% flowering (18.33 days) in the third cutting, and 50% SM application showed the longest 50% flowering day (59.33 day) in the first cutting. Among the fertilizers applied in this study, the earliest mean 50% flowering (32.33 days) occurred with biofertilizer alone, indicating its positive effect. It is thought that the earlier 50% flowering observed in the savory plants inoculated with biofertilizer may be due to the synthesizing of some plant growth-promoting substances. As a key nitrogen-fixing bacterium in the rhizosphere, azotobacter helps roots absorb nutrients by fixing atmospheric nitrogen into ammonium ions, increasing phosphorus availability during the flowering process of plants and reducing soil pH [
33]. So, biotreatment has an indirect effect on flowering day, affecting hormone synthesis and photosynthetic availability.
Moreover, the reduction in days to flowering likely resulted from enhanced soil health, improved water retention, and increased beneficial microorganisms under combined sheep manure and biofertilizer treatment [
34]. When our study was compared with the literature, Hadian et al. [
35] reported that the 50% flowering days of different
S. hortensis L. genotypes varied between 43 and 65 days. It is thought that the differences in the values obtained result from fertilizer type and ecological conditions. On the other hand, in agreement with our results, Pank et al. [
36] reported that the 50% flowering days of
S. hortensis genotypes varied between 29 and 51 days.
Plant height was determined three times before each sampling by measuring the height of 10 randomly selected plants per plot from the soil to the top of the plant, obtaining a mean value for each plot. As shown in
Table 4, mean plant height values were found to be statistically insignificant in biofertilizer and sheep manure treatments but statistically significant in terms of second cutting time (
p < 0.05). According to the treatments, the mean values varied between 26.80 and 30.10 cm. When evaluated according to the cutting time, the highest value was seen in the first cutting of 50% SM (40.93 cm) treatment, followed by unfertilized (39.17 cm) treatment in the first cutting and full SM (39.13 cm) treatment in the first cutting according to the observed tendencies. The lowest plant height was observed in the third cutting of unfertilized (14.97 cm) treatment, followed by the third cutting of 50% SM + Bio (16.20 cm) treatment and third cutting of 50% SM (17.23 cm) treatment according to the observed tendencies. The plant height values showed differences among the treatments depending on the cuttings. Plant height values at later harvests showed a regular decrease among all treatments, with the highest values in the first cutting. Sheep manure treatments (50% SM and full SM) showed higher values compared to biofertilizer alone, unfertilized and inorganic treatments. Further, 50% SM had the highest plant height in the first and second cuts, while full SM had the maximum height in the third cut. So, the treatment of full and 50% SM manure was superior to inoculation with azotobacter treatment.
In this study, the mean values of the plant branch number varied between 12.13 and 14.80. It is obvious from the data in
Table 4 that the highest rate of branching was obtained in savory plants inoculated with the Bio and in the presence of full SM. However, the lowest rate of branching was obtained in unfertilized treatment. When evaluated in terms of cutting time, the highest branch number value was found in the first cutting of Bio + full SM (25.93 number), followed by the first cutting of sole azotobacter (24.10 number), and the first cutting of 50% SM (23.77 cm) treatment. The lowest branch number was determined in the third cutting of unfertilized (6.48 number), followed by third cutting of 50% SM + Bio (7.40 number) and third cutting of unfertilized (7.40 number) treatments according to the observed tendencies. Comparing the treatments, full SM + Bio and sole Bio treatment had positive effects on the branch number of savory in the first and second cuttings. Significant differences were found in the first and second cuttings depending on the treatments. Full SM + Bio and Bio treatments increased the branch number values by 19.33% and 10.91% compared to unfertilized treatment, respectively. In the second harvest, the Bio and full SM + Bio treatments increased the branch number values with 26.07% and 18.73%, respectively.
Sheep manure and biofertilizer treatments increased the plant height and number of branches in savory plants compared to the unfertilized control. This improvement can be attributed to the role of sheep manure and biofertilizers in promoting plant growth by supplying essential nutrients, enhancing soil properties and microbial activity [
37,
38]. Therefore, the use of sheep manure and Bio treatment in savory will have positive effects on plant height and branch number. Many investigations reported that treatment of azotobacter fertilization alone or with other fertilizers increased vegetative growth (plant height, number of branches and fresh and dry herb weights), nitrogen, phosphorus, and potassium levels in the tissues of medicinal plants [
39].In earlier studies on
S. hortensis L., it was reported that plant height changed between 23.5–39.9 cm [
40], 23.73–30.02 cm [
41] and 32.0–44.70 cm [
42] under different ecological conditions. The present results comply with the mentioned results with respect to plant height. In this study, the number of plant branches’ data corroborate those reported by Aşcı [
43] (20.4–25 number) and Çeri [
42] (16.20–22.73) unit/plant; however, these data are much higher than those of Tansı and Tonçer [
41] (5.36–7.98 number). The variation in branch number among cultivated plants is influenced by genotype, environmental conditions, and applied treatments.
3.2. Fresh and Dry Herb Weights
The fresh herb weight values of the savory were not significantly affected by the different fertilizers, according to statistical analysis of the data, except second cut (
Table 5). In this study, total fresh herb values varied between 10.77 and 16.34 t/ha. The treatment of 50% SM recorded the highest total fresh plant weight, but the lowest total fresh herb weight was obtained in Bio treatment according to the observed tendencies. In terms of cutting time, the highest fresh herb weight was determined in the first cutting of 50% SM (10.19 t/ha) treatment, followed by the first cutting of the IO (7.31 t/ha) treatments according to the observed tendencies. The lowest fresh herb weight was found in the third cutting of 50% SM + Bio (1.49 t/ha), followed by the third cutting of full SM + Bio (1.55 t/ha) and the third cutting of Bio (1.56 t/ha) treatments according to the observed tendencies.
As can be seen from
Table 5, the total dry herb weight values of savory fertilizer treatments were found to be statistically significant at the level of 0.05% (
p < 0.05). The highest weight of dry herbs in terms of cutting time was determined in the first cutting of 50% SM (2.01 t/ha) treatment, followed by the first cutting of full SM (1.58 t/ha) and the first cutting of inorganic (1.40 t/ha) treatments according to the observed tendencies. The lowest dry herb weight value was found in the third cutting of 50% SM + Bio (0.32 t/ha) treatment, followed by the third cutting of Bio (0.35 t/ha) treatments according to the observed tendencies. Total dry herb values were highest in 50% SM (3.18 t/ha) treatment, followed by full SM (2.61 t/ha) and inorganic (2.33 t/ha) treatment. The lowest total dry weight value was obtained from unfertilized (1.99 t/ha) treatment, followed by Bio (2.02 t/ha) and full SM + Bio (2.05 t/ha) treatments. However, plants that were applied with full and 50% SM gave higher fresh and dry yields than those inoculated with biofertilizer, which may be attributed to the high organic matter and macro-element content of the soil. In addition, sheep manure improved the growth, and the yield of growth and development of plants is due to the humic acids and micro- and macronutrients presented in SM (
Table 2). Furthermore, the findings suggest that to achieve high savory yield, the treatment of biological fertilizer alone is insufficient. Although biofertilizers can enhance nutrient availability and stimulate root growth through microbial activity, their sole treatment may not meet the immediate nutrient demands of fast-growing medicinal plants. This is primarily because biofertilizers release nutrients gradually, which may not fulfill peak nutrient requirements, especially under intensive cultivation systems. Thus, for optimal biomass accumulation, biofertilizers perform better when combined with other soil amendments [
44,
45].
Abd-Allah [
46] reported that the combination of full dose of NPK + biofertilizer increased plant height, fresh and dry weights of
S. hortensis L. In a study by Bakhtiari et al. [
47], which evaluated the effects of combined inorganic (NPK), organic (vermicompost-VC) and biofertilizers (Thiobacillus,
Glomus mosseae) on medicinal savory, optimum growth was observed with VC + NPK treatment. In a study investigating the effects of chitosan and different doses of biofertilizer EM treatment on the yield and quality characteristics of savory plants, it was stated that the plants inoculated with biofertilizer and chitosan spray increased growth and herb yield [
17]. Mosapour and Feizian [
48] reported that the main effect of the treatment of sheep manure biochar (0, 1 and 2% by weight) from soil and humic acid from leaves (0, 200 and 400 mg/L in three stages) on fresh and dry weight of savory shoot, fresh and dry weight of root, and stem diameter was positive in greenhouse conditions, and the highest fresh weight, shoot dry weight, root fresh weight values were obtained in 400 mg/L humic acid + 2% biochar treatment.
In addition, when the studies were examined, our fresh herb yield results (10.76–16.34 t/ha) were found to be higher than the reported values such as Kızıl and Tonçer [
49] (3.90–5.96 t/ha) and Aşcı [
43] (7.91 t/ha) in first year and (10.85 t/ha) in the second year. However, in line with our result, Çeri [
42] reported that the green herb yield varied from 10.46 to 20.36 t/ha.
The dry herb yield (1.99–3.18 t/ha) obtained in the current study was comparable to that of the one reported in a study by Aşcı [
43], who found that the dry herb yield of savory ranged from 3.45 to 4.56 t/ha in different years. In a study conducted by Çeri [
42], the dry herb yield of the savory plant was reported as between 1.26 and 4.24 t/ha in different growth periods. The co-application of sheep manure and biofertilizer exerts multiple beneficial effects on soil and plant performance. Specifically, it contributes to the improvement of soil physical, chemical, and biological properties. Moreover, it enhances the availability and uptake of both macro- and micronutrients in plants. These amendments also influence several physical and biochemical processes within the savory plant system, ultimately resulting in significantly increased dry weight yield [
50]. Thus, these differences may be responsible for the effects of sheep manure and biofertilizers on soil properties, as well as genetic and ecological factors. In the third cutting of summer savory, a noticeable decline in fresh herb weight was detected, commonly attributed to environmental changes such as lower temperatures and shorter photoperiods toward the end of the growing season. This reduction in biomass corresponds with a natural senescence and reduced vegetative growth potential. Consistent with these findings, Khalid [
51] reported that cutting time has significant impacts on the fresh herb yield in medicinal plants. In general, the first cutting yields the highest fresh weight due to optimal vegetative growth under favorable environmental conditions, such as more extended daylight hours and moderate temperatures. Moreover, Kızıl et al. [
52] noted that the second cutting often exhibits a moderate decline in fresh biomass, as the plant regrowth capacity may be affected by prior cutting stress and changing climatic conditions. A substantial reduction in fresh herb weight is typically observed by the third cutting, mainly attributed to the cumulative cutting stress, reduced photosynthetic efficiency, shorter photoperiods, and lower late-season temperatures, which collectively limit vegetative regrowth.
3.3. Essential Oil Contents
The mean values of essential oil (EO) obtained in this study varied between 1.04 and 1.43%
v/
w. Although no statistically significant differences were found among the different cuttings except for the third cutting, the highest essential oil content was obtained in the second cutting with inorganic (2.01%
v/
w) treatment, followed by the second cutting with 50% SM (1.80%
v/
w) treatment and the first cutting with inorganic (1.78%
v/
w) treatment according to the observed tendencies (
Table 6). The lowest essential oil content was obtained in the third cutting with 50% SM (0.26%
v/
w) treatment, followed by the third cutting with the inorganic (0.51%
v/
w) treatment and the third cutting with the unfertilized (0.52%
v/
w) treatment.
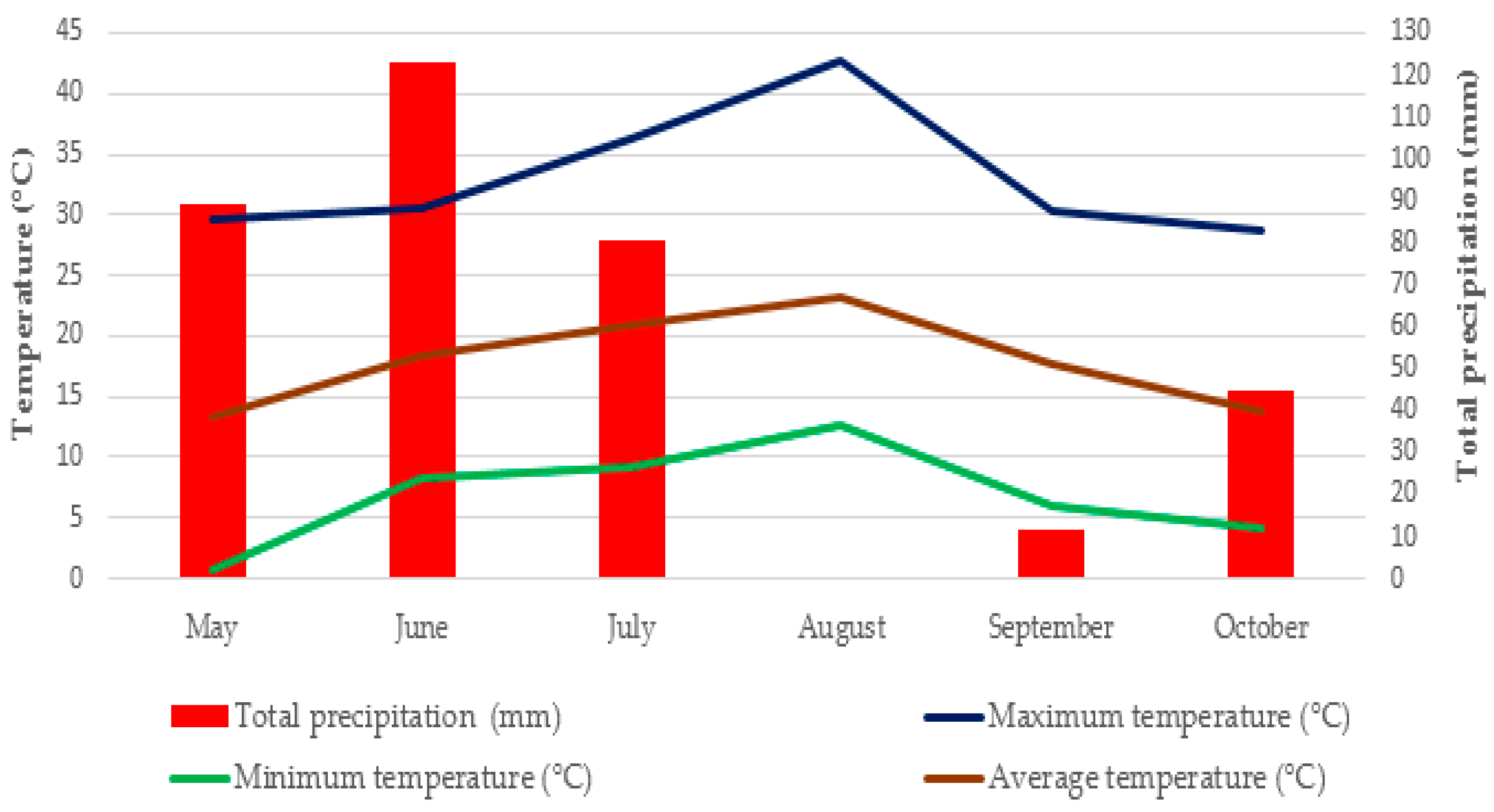
In general, higher essential oil content values were achieved in the second cutting. This pattern is likely due to increasing temperatures during the second cutting (
Figure 1). This observation aligns with previous findings, suggesting that drought stress induces a physiological response characterized by enhanced synthesis of secondary metabolites, which, in turn, contributes to increased essential oil content [
53]. In addition, the levels of secondary metabolites in plants vary in response to seasonal changes, diurnal cycles, and climate conditions [
54]. The effect of azotobacter on the essential oil content of savory herb was minimal. The unfertilized treatment exhibited the highest EO content, suggesting that nutrient limitations may enhance the biosynthesis of secondary metabolites as a plant stress response mechanism. Many studies have shown that nutrient limitation in plants leads to increased accumulation of secondary metabolites, particularly terpenoids and phenolic compounds. In line with the Carbon–Nutrient Balance Hypothesis (CNBH), a reduction in nitrogen availability results in excess carbon, which is subsequently allocated to the biosynthesis of carbon-based secondary metabolites rather than primary growth processes, thereby promoting essential oil production [
55,
56]. Furthermore, EO content was higher in inorganic fertilizer-treated plots compared to those treated with sheep manure. Hence, SM + Bio treatments reduced the essential oil content due to enhancing yield and alleviating stress conditions [
57,
58].
Our results are compatible with the essential oil content of savory plants grown under biofertilizer treatment by Amani et al. [
59] and different levels of inorganic fertilizer by Najafian and Zahedifar [
60]. Abd-Allah [
46] reported that the highest oil yield resulted from the plants fertilized with the full dose of NPK + biofertilizer, but the lowest content was produced from treated plants with biofertilizer alone. In another study, it was reported that chitosan and biofertilizer EM treatment increased the amount of essential oil in savory plants [
17]. In addition, Fathi et al. [
61] reported that the Uzbekistan accession exhibited the highest essential oil content (0.75%
v/
w) among the different savory accessions. These differences can be attributed to the biofertilizer treatments, genetic differences, soil and ecological factors. Ecological conditions, such as light quality and intensity, photoperiodic effects, temperature, water, soil, altitude, and wind, may significantly affect the essential oil content and secondary plant metabolites [
62]. In addition, chemical stress (salinity, pH, fertilization, chemical composition and toxins) affects the quality and quantity of essential oil of medicinal and aromatic plants as environmental stress sources [
63].
3.4. Essential Oil Compositions (% v/v)
As shown in
Table 7 and
Table 8, seven major components (carvacrol, thymol, γ-terpinene, α-terpinene, cymol, α-bisabolene, and α-phellandrene) were dominant, representing 29.28–54.58%
v/
v of the total essential oil compounds. The main components, except for carvacrol, were significantly enhanced due to sheep manure + Bio treatments relative to the unfertilized plots.
Carvacrol value means among the treatments varied between 7.56 and 20.95%
v/
v. In the comparison of the cuttings, the highest carvacrol content was obtained in the unfertilized (42.54%
v/
v) treatment in the third cutting, followed by inorganic (26.74%
v/
v) treatment in the third cutting and Bio + full SM (22.98%
v/
v) treatment in the third cutting (
Table 7). Considering the total cutting data, the increase in the carvacrol in the first and second cuttings was less than the third cutting. The lowest carvacrol content was observed in the full SM treatment (0.18%
v/
v), followed by the 50% SM treatment (2.51%
v/
v) and the Bio + 50% SM treatment (4.42%
v/
v) in the second cuttings. Moreover, the lowest carvacrol content was found in the presence of full-dose sheep manure.
The mean thymol values among different fertilizer treatments varied between 0.36 and 5.68%
v/
v. In the comparison of cuttings, the highest thymol content was obtained in the full SM (16.09%
v/
v) treatment in the second cutting, followed by the 50% SM (8.60%
v/
v) treatment in the second cutting and the 50% SM (4.39%
v/
v) treatment in the third cutting (
Table 7). The lowest thymol content was obtained in the third cutting with 50% SM + Bio (0.05%
v/
v) treatment, followed by the third cutting with full SM (0.11%
v/
v) and the second cutting with Bio (0.12%
v/
v), and the first cutting with 50% SM (0.12%
v/
v) treatments with sheep manure.
It was also found that full and 50% doses of sheep manure resulted in a significantly higher thymol content in savory plants compared to both the Bio and the unfertilized treatments. In addition, the combined treatment of Bio and sheep manures showed the lowest thymol content.
The mean γ-terpinene values obtained in this study varied between 6.52 and 10.38%
v/
v. In the comparison of the cuttings, the highest γ-terpinene content was obtained in the inorganic (16.05%
v/
v) treatment of the third cutting, followed by the treatment of full SM + Bio (15.38%
v/
v) in the third cutting and the treatment of inorganic (9.00%
v/
v) in the first cutting (
Table 7). The lowest γ-terpinene content was obtained in the treatment of 50% SM + Bio (3.18%
v/
v) in the second cutting, followed by the treatment of 50% SM (4.83%
v/
v) in the first cutting and the treatment of 50% SM (5.79%
v/
v) in the second cutting.
The mean values of α-terpinene varied between 3.48 and 7.22% v/v. In the comparison of the cuttings, the highest α-terpinene content was obtained in the third cutting of the full SM + Bio (10.02% v/v) treatment, followed by the first cutting of the full SM + Bio (6.14% v/v) treatment and the inorganic (5.71% v/v) treatment of the first cutting. The lowest α-terpinene content was obtained in the unfertilized (1.32% v/v) treatment of the third cutting, followed by the first cutting of the 50% SM (1.42% v/v) treatment and the Bio (2.01% v/v) treatment of the first cutting. The results showed that the combination of Bio and 50% of sheep manure produced highest α-terpinene content compared to other treatments. So, the combination of SM + Bio treatment was significantly higher than other treatments.
In our study, we noticed a decrease in thymol levels when Bio and SM alone were applied, while γ-terpinene showed a notable increase in response to Bio + SM treatments. This observation aligns with the findings of Sarmoum et al. [
64] and Raffo et al. [
65], highlighting that the biosynthesis of terpenoids happens through different pathways. Similarly, Kachur and Suntres [
66] reported that thymol and carvacrol are positional-isomers, sharing a similar chemical structure with a hydroxyl group and an isopropyl group attached to a benzene ring, while terpinenes, which have a similar chemical structure to α-phellandrene, are isomeric hydrocarbons differing in the location of their carbon–carbon double bonds in their chemical skeletons. Although the biosynthesis of secondary metabolites is genetically controlled, it is strongly affected by environmental influences [
67]. In addition, the use of organic and biofertilizers can also increase the EOC by enhancing the uptake of P and N, which are the major prerequisites for the primary and secondary metabolism in most medicinal plants [
68,
69]. Similar to our results, Edris et al. [
70] found that the relative percentage of certain constituents of marjoram essential oil was affected by fertilization type and level. Gharib et al. [
71] reported that the interaction treatment of nitrogen-fixer strains + compost treatment in
Majorana hortensis had the highest percent of γ-terpinene, α-terpinene and α-phellandren. Our results were in harmony with those obtained by Gharib et al. [
71], Amzallag et al. [
68], and Bernstein et al. [
69].
Additionally, it was observed that the contents of α-carvacrol, thymol, and γ-terpinene increased in the third harvests. Likewise, seasonal changes occurring over the cutting period affect the content and component of essential oil [
72]. Climatic changes occurring over the approximately 4-month period difference between the first and last cuttings influenced essential oil component. In the third cutting, decreasing temperatures correlated with increases in key components. Similarly, many studies reported that carvacrol and thymol contents decrease under high-temperature conditions, likely due to the thermal degradation of these phenolic monoterpenes or altered biosynthetic enzyme activity. Additionally, elevated temperatures may inhibit their biosynthesis pathways, resulting in reduced accumulation in aromatic plants, such as thyme and oregano [
73,
74]. Therefore, a comparison of the experimental data with literature values revealed that current results were consistent with those reported by [
73,
74].
In previous studies, the essential oil of summer savory was also found to have good antioxidant activity, owing to the presence of the dominating oxygenated monoterpenes, thymol and carvacrol [
75]. Therefore, it is considered that the antioxidant activities in savory extracts are related to the high content of these components. Other important terpenoids found in the essential oil are γ-terpinene, myrcene, p-cymene, linalool, β-caryophyllene, α-pinene, and some derivatives [
76,
77].
In contrast, Çeri [
42] reported that carvacrol (55.94–65.24%
v/
v), γ-terpinene (21.67–27.00%
v/
v) and p-cymene (9.47–16.21%
v/
v) were prominent as the main components of leaf essential oil of
S. hortensis. The difference in the results from our study may be due to differences in chemotypes, as well as the part of the plant utilized [
72].
In addition, the obtained results were in agreement with findings mentioned by Abd-Allah [
46], who reported that the highest component of savory oil was carvacrol, and the treatment of biofertilizer alone decreased the carvacrol percentage. Similarly, Esmaielpour et al. [
78] obtained the highest carvacrol content (62.90%
v/
v) when 50% washed mushroom compost was applied. Our results are consistent with the results obtained from the study conducted by Hadi et al. [
79] with biological nitrogen fertilizers and worm compost.
In contrast, Ghojavand et al. [
80] reported that the thymol content of plants inoculated with any of the biofertilizers, alone or together, increased statistically compared to the unfertilized treatment. Toaima et al. [
17] determined that the main components of the essential oil of savory plants were γ-terpinene and carvacrol, and the highest levels of γ-terpinene and carvacrol were detected in oils obtained from plants treated with chitosan and biofertilizer. Fathi et al. [
61] noticed that the values of carvacrol, thymol and γ-terpinene in different origins of savory oils were found from 0.004 to 28.07%
v/
v, from 1.95 to 41.13%
v/
v, and from 2.15 to 84.03%
v/
v, respectively. Dardioti et al. [
81] investigated the diversity of
Satureja pilosa essential oil compounds and reported that the thymol,
p-cymene, and carvacrol amounts had the highest variation.
The mean values of cymol varied between 2.12 and 5.90% v/v. In the comparison of the cuttings, the highest cymol content was obtained in the inorganic treatment of the third cutting (9.49% v/v), followed by the inorganic treatment of the first cutting (5.06% v/v) and the 50% SM treatment of the second cutting (5.05% v/v). The lowest cymol content was obtained in the unfertilized treatment of the third cutting (0.04% v/v).
So, inorganic fertilizer gave savory plants a high cymol content, in comparison with inoculation with the sheep manure and unfertilized treatment (
Table 8). In addition, full and 50% doses of sheep manure increased the mean cymol content compared to the sheep manure + Bio and unfertilized treatment. Ruberto and Baratta [
82] reported that α-terpinene and γ-terpinene (isomeric monoterpenes) aromatic compounds have powerful antioxidant properties. Thus, these valuable aromatic compounds observed in the savory plant are very important because they prevent the oxidation of other compounds by preventing or delaying the beginning or proliferation of oxidative chain reactions [
83]
The mean α-bisabolene values among the treatments ranged from 2.97%
v/
v to 5.28%
v/
v. In the comparison of the cuttings, the highest α-bisabolene content was obtained in the inorganic (7.09%
v/
v) treatment in the third cutting, followed by the unfertilized (5.29%
v/
v) treatment in the third cutting and the Bio + full SM (4.82%
v/
v) treatment in the third cutting (
Table 8). The lowest α-bisabolene content was obtained in the full SM (0.79%
v/
v) treatment in the second cutting, followed by the Bio (1.03%
v/
v) treatment in the second cutting and the 50% SM (2.09%
v/
v) treatment in the first cutting. Rioba et al. [
84] reported that high phosphorus applied to the
Chamomilla recutita plant increased the α-bisabolol content. This result is consistent with our study; high α-bisabolol was obtained from the treatment of sheep manure with high phosphorus content. the α-bisabolene compound is an organic compound with a therapeutic effect and acts as an antioxidant; like the most important antioxidant, vitamin E, it can also be used as an alternative for vitamin E. Therefore, since this compound is used as an effective ligand alternative to vitamin E, the savory plant can be used to prevent or treat some different conditions such as cancer, eye disorders and others [
85]. Therefore, savory herbs were found to be suitable for the production of α-bisabolene as an antioxidant vitamin E in food, and it would also have economic value for the grower.
The mean α-phellandrene values obtained in this study ranged from 1.86% v/v to 6.44% v/v. In different cuttings, the highest α-phellandrene content was obtained in 50% SM + Bio (9.76% v/v) treatment in the second cutting, followed by Bio (7.18% v/v) treatment in the first cutting and unfertilized (7.16% v/v) treatment in the second cutting. The lowest α-phellandrene content was obtained with unfertilized (0.03% v/v) treatment in the third cutting, followed by Bio + full SM (0.15% v/v) treatment in the third cutting and inorganic (0.17% v/v) treatment in the third cutting.
Alpha-phellandrene and oils rich in this molecule have been proven to be potential biopesticides, larvicides and insect repellents, while recent studies have reported antimicrobial and antitumoral effects of alpha-phellandrene as a pure compound and in synergy with drugs [
86]. So, α-phellandrene was found in higher concentrations in Bio + 50% SM treatment compared to other treatments. Therefore, Bio + 50% SM treatment may play a significant role in enhancing antimicrobial and antitumoral effects and healthy living.
Totally, thirty-four essential oil compositions were detected, and twenty-seven essential oil compositions were found as minor. The minor essential oil compositions values of the savory grown under different-dose SM + biofertilizer treatments are given in
Tables S2–S6.