From Panels to Pathogen Networks: The Expanding Role of Targeted Sequencing in Veterinary Medicine
Simple Summary
Abstract
1. Introduction
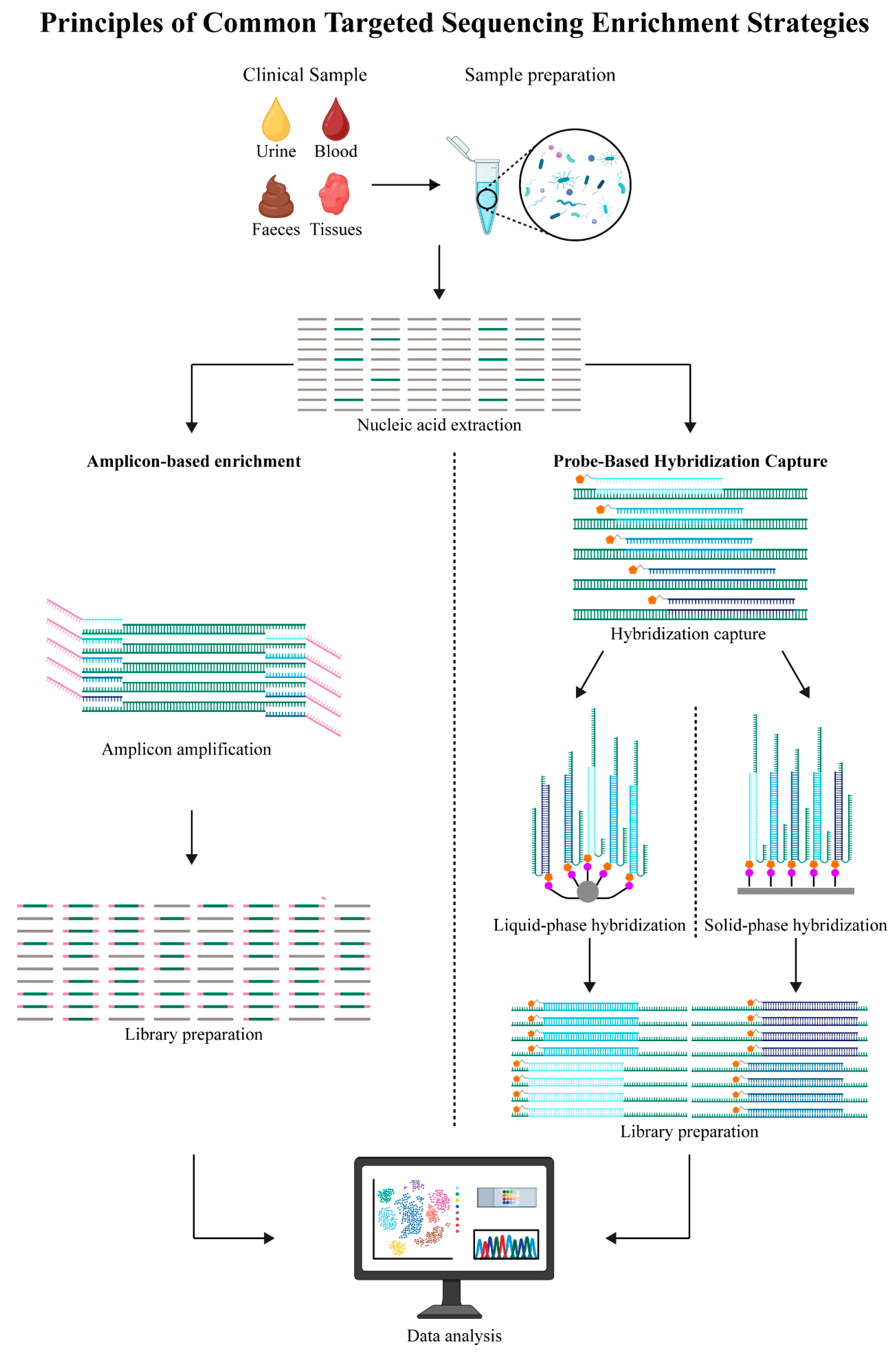
2. Principles of Targeted Sequencing and Target Enrichment Strategies
2.1. Technological Development and Core Characteristics of Targeted Sequencing
2.2. Target Enrichment Strategies for Targeted Sequencing
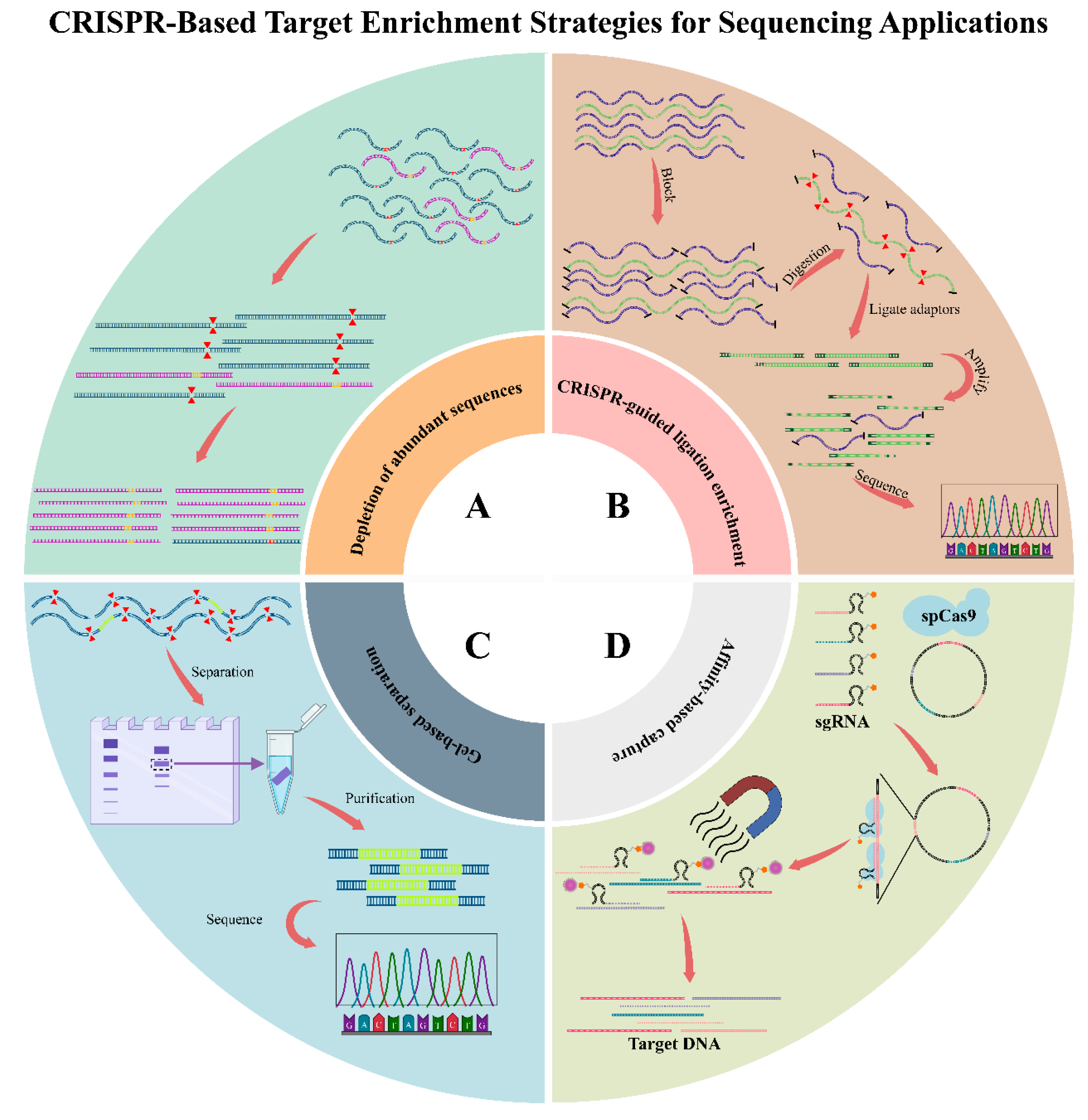
- Abundant sequence depletion: in the CRISPR–Cas9 system, its protospacer adjacent motif (PAM) sequence is specific, which is utilized for differentiating non-target DNA sequences; as a result, Cas protein is capable of the selective degradation of such sequences and the enrichment of target sequence. The strategy is utilized in DASH (Depletion of Abundant Sequences by Hybridization) [51] and CUT-PCR (CRISPR-mediated Ultrasensitive Detection of Target DNA via PCR) [52], efficiently removing high-abundance background sequences for improving the sensitive and specific downstream analyses (Figure 3A) [53,54].
- CRISPR-guided ligation enrichment: Different from depletion-based technologies, the strategy cleaves target sequences under the mediation of Cas9 and the guidance of sgRNAs. After cleavage, the selective ligation of DNA fragments with adaptors is completed, which enables preferential amplification and later sequencing, whereas the uncleaved non-target DNA is not ligated and thereby eliminated from analysis. Typical technologies utilizing the as-mentioned mechanism are FLASH (Finding Low Abundance Sequences by Hybridization) [55] and FUDGE (FUsion Detection from Gene Enrichment) [56]. These two technologies utilize CRISPR–Cas-mediated enrichment for selectively capturing certain genomic regions to conduct deep sequencing analyses (Figure 3B) [54,57,58].
- Gel-based separation: Target DNA fragments are physically separated through gel electrophoresis after CRISPR-mediated cleavage based on size differences. It is used in applications like CRISPR-mediated isolation of specific megabase-sized regions (CISMR) [59] and Cas9-assisted targeting of chromosome segments (Cas9-assisted targeting of chromosome segments, CATCH) [60], which enable the enrichment and downstream analysis of large, specific genomic regions (Figure 3C) [61].
- Affinity-based capture: sgRNAs and modified Cas proteins can be utilized for the specific binding of target DNA sequences, and the latter undergo purification with magnetic beads. It is used in CRISPR–Cap [62] that can efficiently and specifically enrich target regions with no need for ligation or cleavage (Figure 3D) [53,63,64,65].
3. Applications of Targeted Sequencing in the Detection of Animal Pathogens
4. Advantages and Limitations of Targeted Sequencing in Detecting Animal Pathogens
5. Future Development and Outlook
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NGS | Next-Generation Sequencing |
| AMR | Antimicrobial Resistance |
| TGS | Third-Generation Single-Molecule Sequencing |
| WGS | Whole-Genome Sequencing |
| WES | Whole-Exome Sequencing |
| cfDNA | Cell-Free DNA |
| FFPE | Formalin-Fixed, Paraffin-Embedded |
| sgRNAs | Single Guide RNAs |
| SNP | Single-Nucleotide Polymorphism |
| mNGS | Metagenomic Next-Generation Sequencing |
| LAMP | Loop Mediated Isothermal Amplification |
References
- Albert, T.J.; Molla, M.N.; Muzny, D.M.; Nazareth, L.; Wheeler, D.; Song, X.; Richmond, T.A.; Middle, C.M.; Rodesch, M.J.; Packard, C.J.; et al. Direct selection of human genomic loci by microarray hybridization. Nat. Methods 2007, 4, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Mamanova, L.; Coffey, A.J.; Scott, C.E.; Kozarewa, I.; Turner, E.H.; Kumar, A.; Howard, E.; Shendure, J.; Turner, D.J. Target-enrichment strategies for next-generation sequencing. Nat. Methods 2010, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tewhey, R.; Warner, J.B.; Nakano, M.; Libby, B.; Medkova, M.; David, P.H.; Kotsopoulos, S.K.; Samuels, M.L.; Hutchison, J.B.; Larson, J.W.; et al. Microdroplet-based PCR enrichment for large-scale targeted sequencing. Nat. Biotechnol. 2009, 27, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Köser, C.U.; Salfinger, M.; Sougakoff, W.; Heysell, S.K. Targeted next-generation sequencing: A swiss army knife for mycobacterial diagnostics? Eur. Respir. J. 2021, 57, 2004077. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Zhang, Y.; Hu, X.; Tang, M.; Gao, Q. Targeted next-generation sequencing technology showed great potential in identifying spinal tuberculosis and predicting the drug resistance. J. Infect. 2023, 87, e110–e112. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, X.; Cheng, J.; Zhou, H.; Zhang, Y.; Dai, Y. Advances in the application of molecular diagnostic techniques for the detection of infectious disease pathogens (Review). Mol. Med. Rep. 2023, 27, 104. [Google Scholar] [CrossRef]
- Moura, A.; Silva Filho, E.; Barbosa, E.M.; Pereira, W. Comparative analysis of PCR, real-time PCR and LAMP techniques in the diagnosis of Trypanosoma vivax infection in naturally infected buffaloes and cattle in the Brazilian amazon. Pak. Vet. J. 2024, 44, 123–128. [Google Scholar] [CrossRef]
- Mowafy, R.M.; Zeineldin, M.; Okene, I.A.; Nassif, M.; Hafez, A.; Gomaa, N.; El-Habashi, N.; Abdelmegeid, M. Exploring the diagnostic potential of miR-216a and miR-375 for detecting acute pancreatitis in canine model. Pak. Vet. J. 2024, 44, 79–86. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Naguib, D.; Mazeed, A.M.; Ahmed, A.E.; El-tarabili, R.M. Comparative diagnostic efficacy of commonly used serological assays for brucellosis. Pak. Vet. J. 2023, 43, 665–670. [Google Scholar] [CrossRef]
- Al-khlifeh, E.; Alshammari, A.; Alnasarat, H. High incidence of G1 genotype found in the levant revealed by sequence-based association analysis of Echinococcus granulosus (sensu stricto). Pak. Vet. J. 2024, 44, 405–413. [Google Scholar] [CrossRef]
- Xue, M.; Li, Z.; Zhang, P.; Lei, W. Genomic characteristics of ETT2 gene clusters in avian pathogenic escherichia coli identified by whole-genome sequencing. Pak. Vet. J. 2024, 44, 833–839. [Google Scholar] [CrossRef]
- Hilt, E.E.; Ferrieri, P. Next generation and other sequencing technologies in diagnostic microbiology and infectious diseases. Genes 2022, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Chiu, C. The role of metagenomics and next-generation sequencing in infectious disease diagnosis. Clin. Chem. 2021, 68, 115–124. [Google Scholar] [CrossRef]
- Chang, Z.; Deng, J.; Zhang, J.; Wu, H.; Wu, Y.; Bin, L.; Li, D.; Liu, J.; Yu, R.; Lin, H.; et al. Rapid and accurate diagnosis of urinary tract infections using targeted next-generation sequencing: A multicenter comparative study with metagenomic sequencing and traditional culture methods. J. Infect. 2025, 90, 106459. [Google Scholar] [CrossRef]
- Gu, D.; Liu, J.; Wang, J.; Yi, Y.; Chu, Y.; Gao, R.; Liu, H.; She, J.; Lu, B. Integrating DNA and RNA sequencing for enhanced pathogen detection in respiratory infections. J. Transl. Med. 2025, 23, 325. [Google Scholar] [CrossRef]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The oxford nanopore minion: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016, 17, 239. [Google Scholar] [CrossRef]
- Rhoads, A.; Au, K.F. PacBio sequencing and its applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef]
- Jain, M.; Koren, S.; Miga, K.H.; Quick, J.; Rand, A.C.; Sasani, T.A.; Tyson, J.R.; Beggs, A.D.; Dilthey, A.T.; Fiddes, I.T.; et al. Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat. Biotechnol. 2018, 36, 338–345. [Google Scholar] [CrossRef]
- Sereika, M.; Kirkegaard, R.H.; Karst, S.M.; Michaelsen, T.Y.; Sørensen, E.A.; Wollenberg, R.D.; Albertsen, M. Oxford nanopore R10.4 long-read sequencing enables the generation of near-finished bacterial genomes from pure cultures and metagenomes without short-read or reference polishing. Nat. Methods 2022, 19, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R. Target enrichment approaches for next-generation sequencing applications in oncology. Diagnostics 2022, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.M.; Yeung, M.H.Y.; Wong, A.N.N.; Tsang, H.F.; Yu, A.C.S.; Yim, A.K.Y.; Wong, S.C.C. Targeted sequencing approach and its clinical applications for the molecular diagnosis of human diseases. Cells 2023, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Bewicke Copley, F.; Arjun Kumar, E.; Palladino, G.; Korfi, K.; Wang, J. Applications and analysis of targeted genomic sequencing in cancer studies. Comput. Struct. Biotechnol. J. 2019, 17, 1348–1359. [Google Scholar] [CrossRef]
- Paskey, A.C.; Frey, K.G.; Schroth, G.; Gross, S.; Hamilton, T.; Bishop-Lilly, K.A. Enrichment post-library preparation enhances the sensitivity of high-throughput sequencing-based detection and characterization of viruses from complex samples. BMC Genom. 2019, 20, 155. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Soo, J.; Co Ting Keh, L.; Alig, S.; Chabon, J.J.; Sworder, B.J.; Schultz, A.; Jin, M.C.; Scherer, F.; Garofalo, A.; et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat. Biotechnol. 2021, 39, 1537–1547. [Google Scholar] [CrossRef]
- Hitti Malin, R.J.; Dhaenens, C.M.; Panneman, D.M.; Corradi, Z.; Khan, M.; den Hollander, A.I.; Farrar, G.J.; Gilissen, C.; Hoischen, A.; van de Vorst, M.; et al. Using single molecule molecular inversion probes as a cost-effective, high-throughput sequencing approach to target all genes and loci associated with macular diseases. Hum. Mutat. 2022, 43, 2234–2250. [Google Scholar] [CrossRef]
- Miller, E.M.; Patterson, N.E.; Marcus Zechmeister, J.; Bejerano Sagie, M.; Delio, M.; Patel, K.; Ravi, N.; Quispe Tintaya, W.; Maslov, A.; Simmons, N.; et al. Development and validation of a targeted next generation DNA sequencing panel outperforming whole exome sequencing for the identification of clinically relevant genetic variants. Oncotarget 2017, 8, 102033. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Uzbas, F.; Opperer, F.; Sönmezer, C.; Shaposhnikov, D.; Sass, S.; Krendl, C.; Angerer, P.; Theis, F.J.; Mueller, N.S.; Drukker, M. BART-Seq: Cost-effective massively parallelized targeted sequencing for genomics, transcriptomics, and single-cell analysis. Genome Biol. 2019, 20, 155. [Google Scholar] [CrossRef]
- Samorodnitsky, E.; Jewell, B.M.; Hagopian, R.; Miya, J.; Wing, M.R.; Lyon, E.; Damodaran, S.; Bhatt, D.; Reeser, J.W.; Datta, J.; et al. Evaluation of hybridization capture versus amplicon-based methods for whole-exome sequencing. Hum. Mutat. 2015, 36, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.; Salvi, S. Cell-Free DNA as Diagnostic Markers: Methods and Protocols, 1st ed.; Springer: New York, NY, USA, 2019; pp. 119–125. [Google Scholar]
- Rosa Rosa, J.M.; Caniego Casas, T.; Leskela, S.; Muñoz, G.; del Castillo, F.; Garrido, P.; Palacios, J. Modified sureselectqxt target enrichment protocol for illumina multiplexed sequencing of FFPE samples. Biol. Proced. Online 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Boers, S.A.; Jansen, R.; Hays, J.P. Understanding and overcoming the pitfalls and biases of next-generation sequencing (ngs) methods for use in the routine clinical microbiological diagnostic laboratory. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Khodakov, D.; Wang, C.; Zhang, D.Y. Diagnostics based on nucleic acid sequence variant profiling: PCR, hybridization, and NGS approaches. Adv. Drug Deliv. Rev. 2016, 105, 3–19. [Google Scholar] [CrossRef]
- Peng, Q.; Xu, C.; Kim, D.; Lewis, M.; DiCarlo, J.; Wang, Y. Targeted single primer enrichment sequencing with single end duplex-UMI. Sci. Rep. 2019, 9, 4810. [Google Scholar] [CrossRef]
- Hess, J.F.; Kotrová, M.; Calabrese, S.; Darzentas, N.; Hutzenlaub, T.; Zengerle, R.; Brüggemann, M.; Paust, N. Automation of amplicon-based library preparation for next-generation sequencing by centrifugal microfluidics. Anal. Chem. 2020, 92, 12833–12841. [Google Scholar] [CrossRef]
- Li, J.; Su, K.; Liu, H.; Zou, Y. Recent advances in magnetically actuated droplet manipulation for biomedical applications. Magnetochemistry 2024, 10, 28. [Google Scholar] [CrossRef]
- Madsen, E.B.; Höijer, I.; Kvist, T.; Ameur, A.; Mikkelsen, M.J. Xdrop: Targeted sequencing of long DNA molecules from low input samples using droplet sorting. Hum. Mutat. 2020, 41, 1671–1679. [Google Scholar] [CrossRef]
- Chen, K.H.; Longley, R.; Bonito, G.; Liao, H.L. A two-step pcr protocol enabling flexible primer choice and high sequencing yield for illumina miseq meta-barcoding. Agronomy 2021, 11, 1274. [Google Scholar] [CrossRef]
- Lee, Y.F.; Consugar, M.; Helzer, K.; Tan, M.H.; Emrick, L.; Bhagat, A.A. Abstract 3953: Highly accurate genetic profiling of circulating tumor cells using a label-free inertial microfluidic approach coupled with droplet pcr-based next-generation sequencing. Cancer Res. 2016, 76, 3953. [Google Scholar] [CrossRef]
- Li, B.; Xu, L.; Guo, Q.; Chen, J.; Zhang, Y.; Huang, W.; Zhang, Z.; Han, L.; Xu, X.; Chu, H. GenSeizer: A multiplex pcr-based targeted gene sequencing platform for rapid and accurate identification of major mycobacterium species. J. Clin. Microbiol. 2021, 59, 10-1128. [Google Scholar] [CrossRef]
- Ribière, C.; Beugnot, R.; Parisot, N.; Gasc, C.; Defois, C.; Denonfoux, J.; Boucher, D.; Peyretaillade, E.; Peyret, P. Targeted Gene Capture by Hybridization to Illuminate Ecosystem Functioning; Humana Press: New York, NY, USA, 2016; pp. 167–182. [Google Scholar]
- Gaudin, M.; Desnues, C. Hybrid capture-based next generation sequencing and its application to human infectious diseases. Front. Microbiol. 2018, 9, 2924. [Google Scholar] [CrossRef]
- Gnirke, A.; Melnikov, A.; Maguire, J.; Rogov, P.; LeProust, E.M.; Brockman, W.; Fennell, T.; Giannoukos, G.; Fisher, S.; Russ, C.; et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat. Biotechnol. 2009, 27, 182–189. [Google Scholar] [CrossRef]
- Okou, D.T.; Steinberg, K.M.; Middle, C.; Cutler, D.J.; Albert, T.J.; Zwick, M.E. Microarray-based genomic selection for high-throughput resequencing. Nat. Methods 2007, 4, 907–909. [Google Scholar] [CrossRef]
- Chen, X.; Ni, G.; He, K.; Ding, Z.L.; Li, G.M.; Adeola, A.C.; Murphy, R.W.; Wang, W.Z.; Zhang, Y.P. Capture Hybridization of Long-Range DNA Fragments for High-Throughput Sequencing; Springer: New York, NY, USA, 2018; pp. 29–44. [Google Scholar]
- Wylezich, C.; Calvelage, S.; Schlottau, K.; Ziegler, U.; Pohlmann, A.; Höper, D.; Beer, M. Next-generation diagnostics: Virus capture facilitates a sensitive viral diagnosis for epizootic and zoonotic pathogens including SARS-CoV-2. Microbiome 2021, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.S.; Meissner, B.; Chavez, E.A.; Ben Neriah, S.; Ennishi, D.; Jones, M.R.; Shulha, H.P.; Chan, F.C.; Boyle, M.; Kridel, R.; et al. Assessment of capture and amplicon-based approaches for the development of a targeted next-generation sequencing pipeline to personalize lymphoma management. J. Mol. Diagn. 2018, 20, 203–214. [Google Scholar] [CrossRef] [PubMed]
- García García, G.; Baux, D.; Faugère, V.; Moclyn, M.; Koenig, M.; Claustres, M.; Roux, A.F. Assessment of the latest NGS enrichment capture methods in clinical context. Sci. Rep. 2016, 6, 20948. [Google Scholar] [CrossRef]
- Gu, W.; Crawford, E.D.; O’Donovan, B.D.; Wilson, M.R.; Chow, E.D.; Retallack, H.; DeRisi, J.L. Depletion of abundant sequences by hybridization (DASH): Using Cas9 to remove unwanted high-abundance species in sequencing libraries and molecular counting applications. Genome Biol. 2016, 17, 41. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, J.; Hwang, G.H.; Kim, S.; Kim, H.S.; Ye, S.; Kim, K.; Park, J.; Park, D.Y.; Cho, Y.K.; et al. CUT-PCR: CRISPR-mediated, ultrasensitive detection of target DNA using PCR. Oncogene 2017, 36, 6823–6829. [Google Scholar] [CrossRef]
- Aalipour, A.; Dudley, J.C.; Park, S.-m.; Murty, S.; Chabon, J.J.; Boyle, E.A.; Diehn, M.; Gambhir, S.S. Deactivated crispr associated protein 9 for minor-allele enrichment in cell-free DNA. Clin. Chem. 2018, 64, 307–316. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, L.; Ying, L.; Zhao, Z.; Chu, P.K.; Yu, X.-F. A CRISPR–Cas9-triggered strand displacement amplification method for ultrasensitive DNA detection. Nat. Commun. 2018, 9, 5012. [Google Scholar] [CrossRef]
- Quan, J.; Langelier, C.; Kuchta, A.; Batson, J.; Teyssier, N.; Lyden, A.; Caldera, S.; McGeever, A.; Dimitrov, B.; King, R.; et al. FLASH: A next-generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. Nucleic Acids Res. 2019, 47, e83. [Google Scholar] [CrossRef] [PubMed]
- Stangl, C.; de Blank, S.; Renkens, I.; Westera, L.; Verbeek, T.; Valle-Inclan, J.E.; González, R.C.; Henssen, A.G.; van Roosmalen, M.J.; Stam, R.W.; et al. Partner independent fusion gene detection by multiplexed CRISPR-Cas9 enrichment and long read nanopore sequencing. Nat. Commun. 2020, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.M.; Crinnion, L.A.; Hewitt, S.; Bates, J.; Robinson, R.; Carr, I.M.; Sheridan, E.; Adlard, J.; Bonthron, D.T. Cas9-based enrichment and single-molecule sequencing for precise characterization of genomic duplications. Lab. Investig. 2020, 100, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Greenberg, D.; Powell, J.; Höijer, I.; Ameur, A.; Strahl, M.; Ellis, E.; Jonasson, I.; Mouro Pinto, R.; Wheeler, V.C.; et al. Amplification-free, crispr-cas9 targeted enrichment and smrt sequencing of repeat-expansion disease causative genomic regions. bioRxiv 2017. [Google Scholar] [CrossRef]
- Bennett-Baker, P.E.; Mueller, J.L. CRISPR-mediated isolation of specific megabase segments of genomic DNA. Nucleic Acids Res. 2017, 45, e165. [Google Scholar] [CrossRef]
- Gabrieli, T.; Sharim, H.; Fridman, D.; Arbib, N.; Michaeli, Y.; Ebenstein, Y. Selective nanopore sequencing of human BRCA1 by Cas9-assisted targeting of chromosome segments (CATCH). Nucleic Acids Res. 2018, 46, e87. [Google Scholar] [CrossRef]
- Stevens, R.C.; Steele, J.L.; Glover, W.R.; Sanchez-Garcia, J.F.; Simpson, S.D.; O’Rourke, D.; Ramsdell, J.S.; MacManes, M.D.; Thomas, W.K.; Shuber, A.P. A novel CRISPR/Cas9 associated technology for sequence-specific nucleic acid enrichment. PLoS ONE 2019, 14, e0215441. [Google Scholar] [CrossRef]
- Lee, J.; Lim, H.; Jang, H.; Hwang, B.; Lee, J.H.; Cho, J.; Lee, J.H.; Bang, D. CRISPR-Cap: Multiplexed double-stranded DNA enrichment based on the CRISPR system. Nucleic Acids Res. 2018, 47, e1. [Google Scholar] [CrossRef]
- Gilpatrick, T.; Lee, I.; Graham, J.E.; Raimondeau, E.; Bowen, R.; Heron, A.; Downs, B.; Sukumar, S.; Sedlazeck, F.J.; Timp, W. Targeted nanopore sequencing with Cas9-guided adapter ligation. Nat. Biotechnol. 2020, 38, 433–438. [Google Scholar] [CrossRef]
- Slesarev, A.; Viswanathan, L.; Tang, Y.; Borgschulte, T.; Achtien, K.; Razafsky, D.; Onions, D.; Chang, A.; Cote, C. CRISPR/Cas9 targeted CAPTURE of mammalian genomic regions for characterization by NGS. Sci. Rep. 2019, 9, 3587. [Google Scholar] [CrossRef]
- Xu, X.; Xia, Q.; Zhang, S.; Gao, J.; Dai, W.; Wu, J.; Wang, J. CRISPR-assisted targeted enrichment-sequencing (CATE-seq). bioRxiv 2019. [Google Scholar] [CrossRef]
- Lee, J.S.; Mackie, R.S.; Harrison, T.; Shariat, B.; Kind, T.; Kehl, T.; Löchelt, M.; Boucher, C.; VandeWoude, S. Targeted enrichment for pathogen detection and characterization in three felid species. J. Clin. Microbiol. 2017, 55, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.; Hawkins, I.K.; Ilha, M.R.S.; Woldemeskel, M.W.; Saliki, J.T.; Wilkes, R.P. Evaluation of targeted next-generation sequencing for detection of bovine pathogens in clinical samples. J. Clin. Microbiol. 2018, 56, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.; Ilha, M.R.S.; Engiles, J.B.; Wilkes, R.P. Evaluation of targeted next-generation sequencing for detection of equine pathogens in clinical samples. J. Vet. Diagn. Investig. 2021, 33, 227–234. [Google Scholar] [CrossRef]
- Periyasamy, D.; Huang, Y.; Hill, J.E. Targeted syndromic next-generation sequencing panel for simultaneous detection of pathogens associated with bovine reproductive failure. J. Clin. Microbiol. 2025, 63, e01433-24. [Google Scholar] [CrossRef]
- Kattoor, J.J.; Mlalazi-Oyinloye, M.; Nemser, S.M.; Wilkes, R.P. Development of a targeted NGS assay for the detection of respiratory pathogens including SARS-CoV-2 in felines. Pathogens 2024, 13, 335. [Google Scholar] [CrossRef]
- Kattoor, J.J.; Nikolai, E.; Qurollo, B.; Wilkes, R.P. Targeted next-generation sequencing for comprehensive testing for selected vector-borne pathogens in canines. Pathogens 2022, 11, 964. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhouse, M. Achieving global targets for antimicrobial resistance. Science 2016, 353, 874–875. [Google Scholar] [CrossRef]
- Yasein, G.; Zahid, O.; Minter, E.; Ashraf, K.; Rashid, I.; Shabbir, M.Z.; Betson, M.; Sargison, N.D.; Chaudhry, U. A novel metabarcoded deep amplicon sequencing tool for disease surveillance and determining the species composition of trypanosoma in cattle and other farm animals. Acta Trop. 2022, 230, 106416. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, U.; Ali, Q.; Rashid, I.; Shabbir, M.Z.; Ijaz, M.; Abbas, M.; Evans, M.; Ashraf, K.; Morrison, I.; Morrison, L.; et al. Development of a deep amplicon sequencing method to determine the species composition of piroplasm haemoprotozoa. Ticks Tick-Borne Dis. 2019, 10, 101276. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.A.; Haniford, L.S.E.; Cooper, A.; Carrillo, C.D.; Blais, B.W.; Lau, C.H.-F. Exploiting a targeted resistome sequencing approach in assessing antimicrobial resistance in retail foods. Environ. Microbiome 2023, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Arena, F.; Rolfe, P.A.; Doran, G.; Conte, V.; Gruszka, S.; Clarke, T.; Adesokan, Y.; Giani, T.; Rossolini, G.M. Rapid resistome fingerprinting and clonal lineage profiling of carbapenem-resistant klebsiella pneumoniae isolates by targeted next-generation sequencing. J. Clin. Microbiol. 2014, 52, 987–990. [Google Scholar] [CrossRef]
- Leung, K.S.-S.; Tam, K.K.-G.; Ng, T.T.-L.; Lao, H.-Y.; Shek, R.C.-M.; Ma, O.C.K.; Yu, S.-H.; Chen, J.-X.; Han, Q.; Siu, G.K.-H.; et al. Clinical utility of target amplicon sequencing test for rapid diagnosis of drug-resistant Mycobacterium tuberculosis from respiratory specimens. Front. Microbiol. 2022, 13, 974428. [Google Scholar] [CrossRef]
- Schwab, T.C.; Perrig, L.; Göller, P.C.; Guebely De la Hoz, F.F.; Lahousse, A.P.; Minder, B.; Günther, G.; Efthimiou, O.; Omar, S.V.; Egger, M.; et al. Targeted next-generation sequencing to diagnose drug-resistant tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2024, 24, 1162–1176. [Google Scholar] [CrossRef]
- Sodja, E.; Koren, S.; Toplak, N.; Truden, S.; Žolnir-Dovč, M. Next-generation sequencing to characterise pyrazinamide resistance in Mycobacterium tuberculosis isolates from two Balkan countries. J. Glob. Antimicrob. Resist. 2022, 29, 507–512. [Google Scholar] [CrossRef]
- Street, T.L.; Sanderson, N.D.; Barker, L.; Kavanagh, J.; Cole, K.; Group, T.G.I.; Llewelyn, M.; Eyre, D.W. Target enrichment improves culture-independent detection of neisseria gonorrhoeae and antimicrobial resistance determinants direct from clinical samples with nanopore sequencing. Microb. Genom. 2024, 10, 001208. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Zuo, Y.; Li, T.; Liu, L.; Shen, Z.; Shen, J.; Zhang, R.; Wang, S. Multiplexed target enrichment enables efficient and in-depth analysis of antimicrobial resistome in metagenomes. Microbiol. Spectr. 2022, 10, e02297-22. [Google Scholar] [CrossRef]
- Itarte, M.; Calvo, M.; Martínez-Frago, L.; Mejías-Molina, C.; Martínez-Puchol, S.; Girones, R.; Medema, G.; Bofill-Mas, S.; Rusiñol, M. Assessing environmental exposure to viruses in wastewater treatment plant and swine farm scenarios with next-generation sequencing and occupational risk approaches. Int. J. Hyg. Environ. Health 2024, 259, 114360. [Google Scholar] [CrossRef]
- Felice, V.; Ball, C.; Ganapathy, K.; Catelli, E.; Di Francesco, A. Gene targeted sequencing analysis of mycoplasma gallisepticum strains in poultry flocks from middle east and South Asia. Pak. Vet. J. 2020, 40, 397–399. [Google Scholar] [CrossRef]
- Matthews, M.C.; Cooke, D.M.; Kerr, T.J.; Loxton, A.G.; Warren, R.M.; Ghielmetti, G.; Streicher, E.M.; Witte, C.S.; Miller, M.A.; Goosen, W.J. Evidence of Mycobacterium bovis DNA in shared water sources at livestock–wildlife–human interfaces in KwaZulu-Natal, South Africa. Front. Vet. Sci. 2025, 12, 1483162. [Google Scholar] [CrossRef]
- Schuele, L.; Lizarazo-Forero, E.; Strutzberg-Minder, K.; Schütze, S.; Löbert, S.; Lambrecht, C.; Harlizius, J.; Friedrich, A.W.; Peter, S.; Rossen, J.W.A.; et al. Application of shotgun metagenomics sequencing and targeted sequence capture to detect circulating porcine viruses in the Dutch–German border region. Transbound. Emerg. Dis. 2022, 69, 2306–2319. [Google Scholar] [CrossRef] [PubMed]
- Osikowicz, L.M.; Hojgaard, A.; Maes, S.; Eisen, R.J.; Stenglein, M.D. A bioinformatics pipeline for a tick pathogen surveillance multiplex amplicon sequencing assay. Ticks Tick-Borne Dis. 2023, 14, 102207. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Park, J.S.; Aknazarov, B.; Lee, H.I. Identification of tickborne pathogens in cattle and sheep ticks from kyrgyzstan using next-generation sequencing. Parasites Vectors 2025, 18, 292. [Google Scholar] [CrossRef]
- Marchenko, V.Y.; Kolosova, N.P.; Danilenko, A.V.; Bragina, M.K.; Nhai, T.T.; Ryzhikov, A.B. Diversity of coronaviruses in wild and domestic birds in Vietnam. Asian Pac. J. Trop. Med. 2022, 15, 442–450. [Google Scholar] [CrossRef]
- Santa, M.A.; Rezansoff, A.M.; Chen, R.; Gilleard, J.S.; Musiani, M.; Ruckstuhl, K.E.; Massolo, A. Deep amplicon sequencing highlights low intra-host genetic variability of Echinococcus multilocularis and high prevalence of the European-type haplotypes in coyotes and red foxes in Alberta, Canada. PLoS Neglected Trop. Dis. 2021, 15, e0009428. [Google Scholar] [CrossRef]
- Jakab, S.; Bali, K.; Freytag, C.; Pataki, A.; Fehér, E.; Halas, M.; Jerzsele, Á.; Szabó, I.; Szarka, K.; Bálint, Á.; et al. Deep sequencing of porcine reproductive and respiratory syndrome virus ORF7: A promising tool for diagnostics and epidemiologic surveillance. Animals 2023, 13, 3223. [Google Scholar] [CrossRef]
- Michelet, L.; Tambosco, J.; Biet, F.; Fach, P.; Delannoy, S.; Boschiroli, M.L. Deciphering the evolution of the temporal and geographic distribution of French Mycobacterium bovis genotypes using a high throughput SNP-targeted amplicon sequencing method. Infect. Genet. Evol. 2023, 114, 105497. [Google Scholar] [CrossRef]
- King, J.; Pohlmann, A.; Dziadek, K.; Beer, M.; Wernike, K. Cattle connection: Molecular epidemiology of BVDV outbreaks via rapid nanopore whole-genome sequencing of clinical samples. BMC Vet. Res. 2021, 17, 242. [Google Scholar] [CrossRef]
- de Vries, E.M.; Cogan, N.O.I.; Gubala, A.J.; Mee, P.T.; O’Riley, K.J.; Rodoni, B.C.; Lynch, S.E. Rapid, in-field deployable, avian influenza virus haemagglutinin characterisation tool using minion technology. Sci. Rep. 2022, 12, 11886. [Google Scholar] [CrossRef]
- Briese, T.; Kapoor, A.; Mishra, N.; Jain, K.; Kumar, A.; Jabado, O.J.; Lipkin, W.I. Virome capture sequencing enables sensitive viral diagnosis and comprehensive virome analysis. mBio 2015, 6, e01491-15. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Gorham, J.M.; Mazaika, E.; Parfenov, M.; Dang, X.; DePalma, S.; McKean, D.; Seidman, C.E.; Seidman, J.G.; Koralnik, I.J. ViroFind: A novel target-enrichment deep-sequencing platform reveals a complex JC virus population in the brain of PML patients. PLoS ONE 2018, 13, e0186945. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Li, N.; Luo, Z.; McGeachy, A.; Alcantara, A.F.M.; Ewing, A.; Hyland, F.C. Abstract 1207: Custom next-generation sequencing primer designs for targeted sequencing of multi strain viral targets. Cancer Res. 2020, 80, 1207. [Google Scholar] [CrossRef]
- Tafess, K.; Ng, T.T.L.; Lao, H.Y.; Leung, K.S.S.; Tam, K.K.G.; Rajwani, R.; Tam, S.T.Y.; Ho, L.P.K.; Chu, C.M.K.; Gonzalez, D.; et al. Targeted-sequencing workflows for comprehensive drug resistance profiling of Mycobacterium tuberculosis cultures using two commercial sequencing platforms: Comparison of analytical and diagnostic performance, turnaround time, and cost. Clin. Chem. 2020, 66, 809–820. [Google Scholar] [CrossRef]
- Vasanthaiah, S.; Verma, R.; Kumar, A.; Bandari, A.K.; George, J.; Rastogi, M.; Manjunath, G.K.; Sharma, J.; Kumar, A.; Subramani, J.; et al. Culture-free whole genome sequencing of Mycobacterium tuberculosis using ligand-mediated bead enrichment method. Open Forum Infect. Dis. 2024, 11, ofae320. [Google Scholar] [CrossRef]
- Mighell, T.L.; Nishida, A.; O’Connell, B.L.; Miller, C.V.; Grindstaff, S.; Thornton, C.A.; Adey, A.C.; Doherty, D.; O’Roak, B.J. Cas12a-capture: A novel, low-cost, and scalable method for targeted sequencing. Cris. J. 2022, 5, 548–557. [Google Scholar] [CrossRef]
- Lin, A.; Singh, A.; Allred, A.; Allard, M.; Waltman, D.; Imanian, B.; Ng, J.H.J.; Sanahmadi, Y.; Khaksar, R. Targeted next-generation sequencing assay for direct detection and serotyping of salmonella from enrichment. J. Food Prot. 2024, 87, 100256. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, B.; Li, Y.; He, X.Z.; Li, A.; Wu, W.; Duan, S.X.; Qiu, F.Z.; Wang, J.; et al. VSITA, an improved approach of target amplification in the identification of viral pathogens. Biomed. Environ. Sci. 2018, 31, 272–279. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Beghini, F.; Cumbo, F.; McIver, L.J.; Thompson, K.N.; Zolfo, M.; Manghi, P.; Dubois, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using metaphlan 4. Nat. Biotechnol. 2023, 41, 1633–1644. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the bacterial and viral bioinformatics resource center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2022, 51, D678–D689. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.P.G.; Chorlton, S.D.; Krajden, M.; Manges, A.R. Agnostic sequencing for detection of viral pathogens. Clin. Microbiol. Rev. 2023, 36, e00119-22. [Google Scholar] [CrossRef] [PubMed]
- Getchell, M.; Wulandari, S.; de Alwis, R.; Agoramurthy, S.; Khoo, Y.K.; Mak, T.-M.; Moe, L.; Stona, A.-C.; Pang, J.; Momin, M.H.F.H.A.; et al. Pathogen genomic surveillance status among lower resource settings in Asia. Nat. Microbiol. 2024, 9, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Koudokpon, H.; Lègba, B.; Sintondji, K.; Kissira, I.; Kounou, A.; Guindo, I.; Koné, K.M.; Abdou, M.; Koné, A.; Sambou, C.; et al. Empowering public health: Building advanced molecular surveillance in resource-limited settings through collaboration and capacity-building. Front. Health Serv. 2024, 4, 1289394. [Google Scholar] [CrossRef]
- Agboli, E.; Bitew, M.; Malaka, C.N.; Kallon, T.M.P.S.; Jalloh, A.M.S.; Yankonde, B.; Shempela, D.M.; Sikalima, J.F.M.; Joseph, M.; Kasonde, M.; et al. Building pathogen genomic sequencing capacity in Africa: Centre for epidemic response and innovation fellowship. Trop. Med. Infect. Dis. 2025, 10, 90. [Google Scholar] [CrossRef]
- Marine, R.L.; Ntim, N.A.A.; Castro, C.J.; Attiku, K.O.; Pratt, D.; Duker, E.; Agbosu, E.; Ng, T.F.F.; Gatei, W.; Obodai, E.; et al. Strengthening laboratory surveillance of viral pathogens: Experiences and lessons learned building next-generation sequencing capacity in Ghana. Int. J. Infect. Dis. 2019, 81, 231–234. [Google Scholar] [CrossRef]
- Gaston, D.C.; Miller, H.B.; Fissel, J.A.; Jacobs, E.; Gough, E.; Wu, J.; Klein, E.Y.; Carroll, K.C.; Simner, P.J. Evaluation of metagenomic and targeted next-generation sequencing workflows for detection of respiratory pathogens from bronchoalveolar lavage fluid specimens. J. Clin. Microbiol. 2022, 60, e00526-22. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- Jiang, X.-W.; Huang, T.-S.; Xie, L.; Chen, S.-Z.; Wang, S.-D.; Huang, Z.-W.; Li, X.-Y.; Ling, W.-P. Development of a diagnostic assay by three-tube multiplex real-time PCR for simultaneous detection of nine microorganisms causing acute respiratory infections. Sci. Rep. 2022, 12, 13306. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; Carrasco-González, J.A.; Linhares, D.C.L.; Corzo, C.A.; Campos-Villalobos, J.I.; Henao-Díaz, A.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; González-González, R.B.; Parra-Saldívar, R.; et al. Emergent molecular techniques applied to the detection of porcine viruses. Vet. Sci. 2023, 10, 609. [Google Scholar] [CrossRef]
- Marklewitz, M.; Jaguparov, A.; Wilhelm, A.; Akande, O.W.; Musul, B.; Poates, A.L.; Afrough, B.; Norberg, A.; Hull, N.C.; Ehsani, S.; et al. Genomics costing tool: Considerations for improving cost-efficiencies through cross scenario comparison. Front. Public Health 2025, 12, 1498094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Yordanov, B.; Gaunt, A.; Wang, M.X.; Dai, P.; Chen, Y.-J.; Zhang, K.; Fang, J.Z.; Dalchau, N.; Li, J.; et al. A deep learning model for predicting next-generation sequencing depth from DNA sequence. Nat. Commun. 2021, 12, 4387. [Google Scholar] [CrossRef]
- Alouane, T.; Laamarti, M.; Essabbar, A.; Hakmi, M.; Bouricha, E.M.; Chemao-Elfihri, M.W.; Kartti, S.; Boumajdi, N.; Bendani, H.; Laamarti, R.; et al. Genomic diversity and hotspot mutations in 30,983 SARS-CoV-2 genomes: Moving toward a universal vaccine for the “confined virus”? Pathogens 2020, 9, 829. [Google Scholar] [CrossRef]
- Shang, M.; Guo, J.; Guo, J. Point-of-care testing of infectious diseases: Recent advances. Sens. Diagn. 2023, 2, 1123–1144. [Google Scholar] [CrossRef]

| Comparison Item | Cattle [60] | Horse [68] | Felids [66] | Cattle [69] | Feline [70] | Dog [71] |
|---|---|---|---|---|---|---|
| Number of Pathogens Detected | 43 | 62 | 31 | 17 | 6 | 17 |
| Pathogen Types | Bacteria, viruses, parasites, micromycetes | Bacteria, viruses, parasites, micromycetes | Viruses and bacteria | Bacteria, viruses, Protozoa | Bacteria, viruses | Bacteria, viruses, Protozoa |
| Host Species | Cattle, small ruminants | Horses | Domestic cats, bobcats, cougars | Cattle, small ruminants | Feline | |
| Targeted Sequencing Method | tNGS (targeted amplicon sequencing) | tNGS (targeted amplicon sequencing) | TGC-NGS (targeted genome capture sequencing) | tNGS (targeted nanopore sequencing) | tNGS (targeted amplicon sequencing) | tNGS (targeted amplicon sequencing) |
| Sample Types | Milk, nasal swabs, lung tissue, blood, amniotic fluid, etc. | Respiratory, reproductive, nervous, and digestive systems | Blood, serum, tissue, feces, cell culture supernatant | Placenta, amniotic fluid, vaginal swab, semen, fetal tissues | Oropharyngeal/nasal swabs, respiratory tissues | / |
| Multipathogen Detection | High—simultaneous detection of multiple pathogens | Very high—broader pathogen coverage | High—supports cross-species pathogen detection | High—supports cross-species pathogen detection | High | High |
| Low-Abundance Pathogen Detection | LOD = Ct 38 | LOD = Ct 30–35 | Up to 5600-fold enrichment, improves detection sensitivity | LOD = Ct 37 | LOD = Ct 35–37, variability observed in SARS-CoV-2 | LOD = Ct 35–36 |
| Pathogen Typing Capability | Detects virulence, resistance, and toxin genes | Further optimized to identify resistance genes | Enables whole-genome sequencing for pathogen genotyping | Enables pathogen typing | Enables pathogen typing | Enables pathogen typing |
| AMR Gene Detection | Limited | Expanded AMR gene coverage | Focuses on full-genome detection of pathogens | / | / | / |
| Application Scope | Clinical diagnosis of infectious diseases, outbreak surveillance | Equine disease diagnostics, antimicrobial resistance monitoring | Research on felid pathogens, cross-species pathogen studies | Suitable for diagnosis and surveillance in high coinfection bovine cases | Clinical detect | Clinical detect |
| Bioinformatics Complexity | Requires bioinformatics analysis | Requires bioinformatics analysis | Requires advanced data processing and comparison | Open-sourced on GitHub with ready-to-use pipeline | Requires bioinformatics analysis | Requires bioinformatics analysis |
| Limitations | Sample type affects detection; some genes may be missed | Low-abundance detection remains challenging; high data demands | Probe design limits pathogen coverage; partial detection possible | Detection ≠ causation; interpret with clinical context | Inconsistent performance for SARS-CoV-2 detection | May miss ultra-low abundance pathogens; single time-point blood samples might fail PCR/tNGS detection |
| Detection Cost | High, but lower than WGS | High, but lower than WGS | Currently high (USD 450–550/sample), but may decline | Cost-effective if multiple samples are processed within a single flow cell | Cost-effective tNGS for large-scale respiratory screening | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Lu, W.; Liu, R.; Zhang, S.; Cao, J.; Ma, C. From Panels to Pathogen Networks: The Expanding Role of Targeted Sequencing in Veterinary Medicine. Biology 2025, 14, 1075. https://doi.org/10.3390/biology14081075
Luo J, Lu W, Liu R, Zhang S, Cao J, Ma C. From Panels to Pathogen Networks: The Expanding Role of Targeted Sequencing in Veterinary Medicine. Biology. 2025; 14(8):1075. https://doi.org/10.3390/biology14081075
Chicago/Turabian StyleLuo, Jiali, Wentao Lu, Ruiting Liu, Shukai Zhang, Jie Cao, and Chong Ma. 2025. "From Panels to Pathogen Networks: The Expanding Role of Targeted Sequencing in Veterinary Medicine" Biology 14, no. 8: 1075. https://doi.org/10.3390/biology14081075
APA StyleLuo, J., Lu, W., Liu, R., Zhang, S., Cao, J., & Ma, C. (2025). From Panels to Pathogen Networks: The Expanding Role of Targeted Sequencing in Veterinary Medicine. Biology, 14(8), 1075. https://doi.org/10.3390/biology14081075






