Comparative Enzymatic and Gene Expression Responses in Wheat to DON- and NIV-Producing Fusarium Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Genotypes
2.2. Fungal Isolates and Inoculum Production
2.3. Inoculation Procedures and Plant Sampling
2.4. Determination of Enzymatic Activities
2.5. Quantitative Real-Time PCR (RT-qPCR) Analysis
2.6. Experimental Design and Data Analysis
3. Results
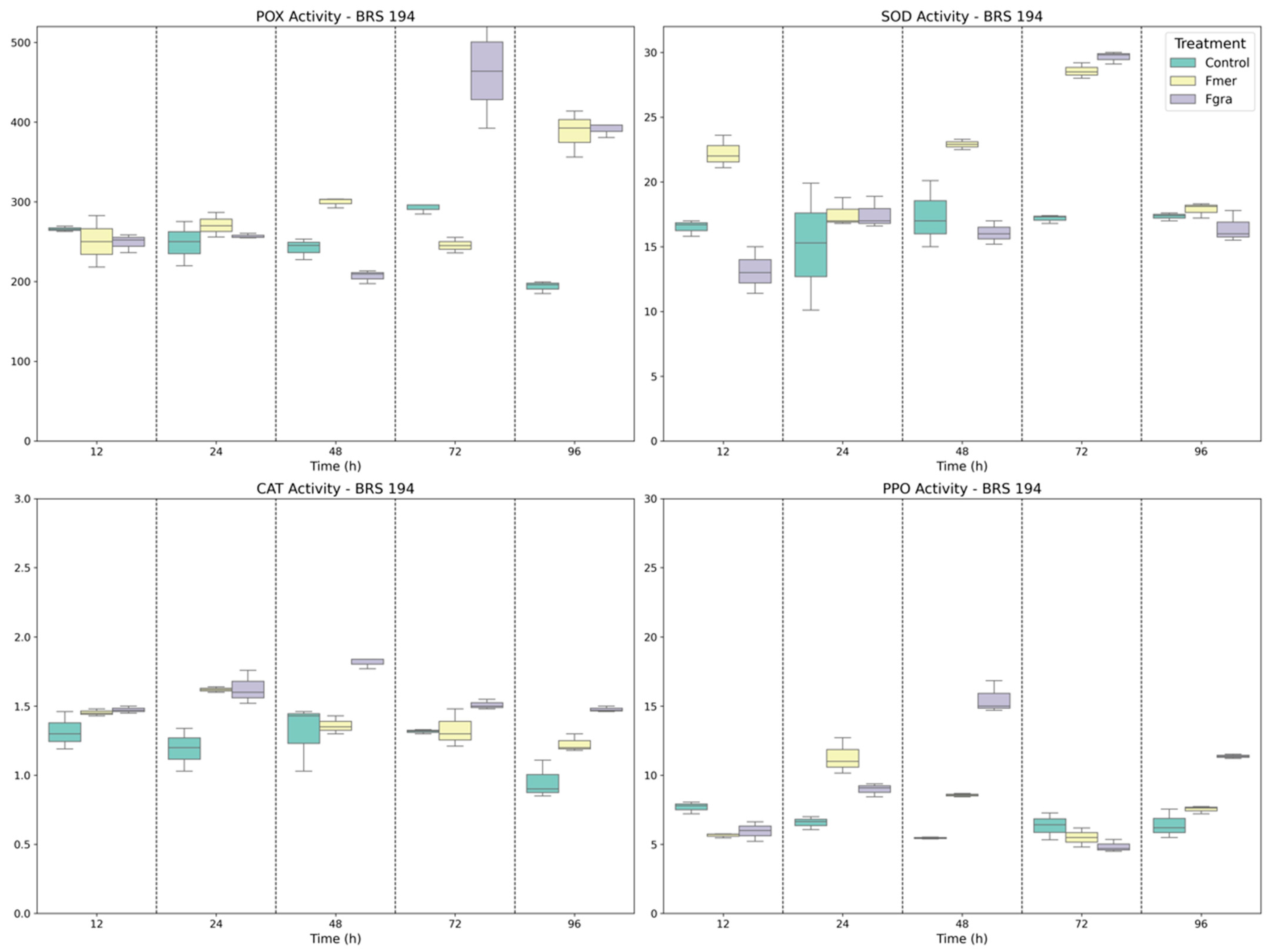
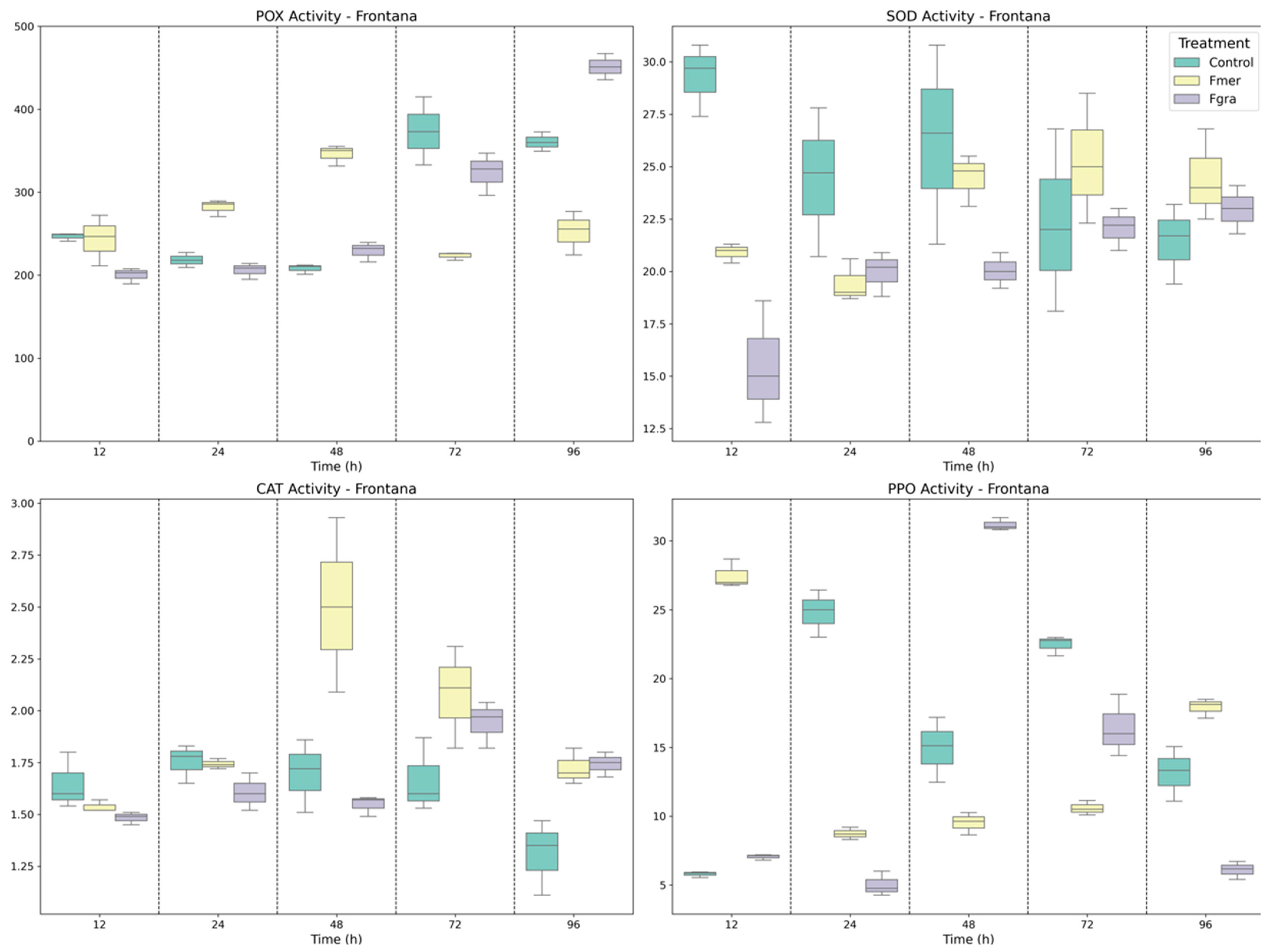
3.1. Antioxidant Enzymes Activity in Wheat-Fusarium Interaction
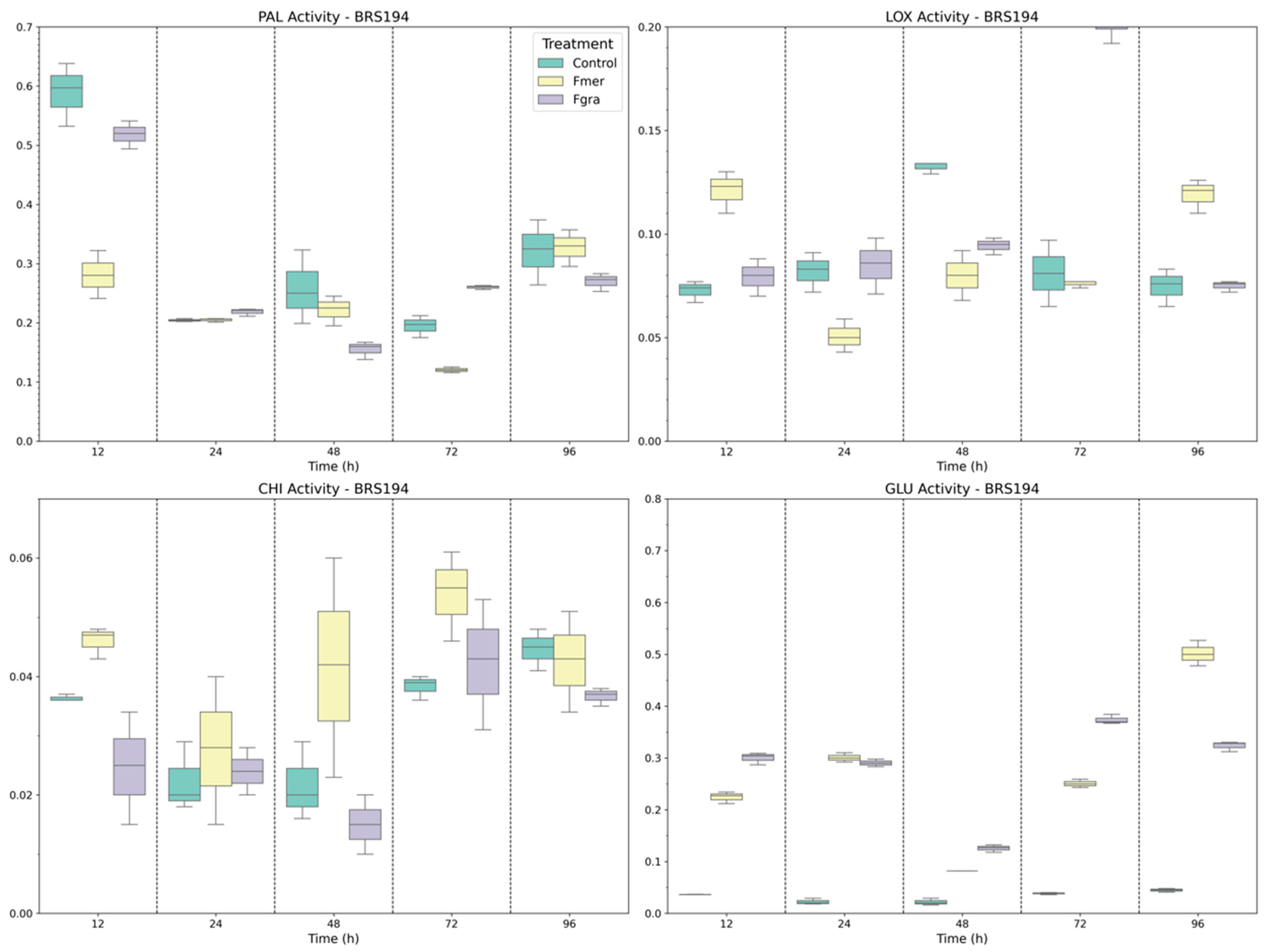
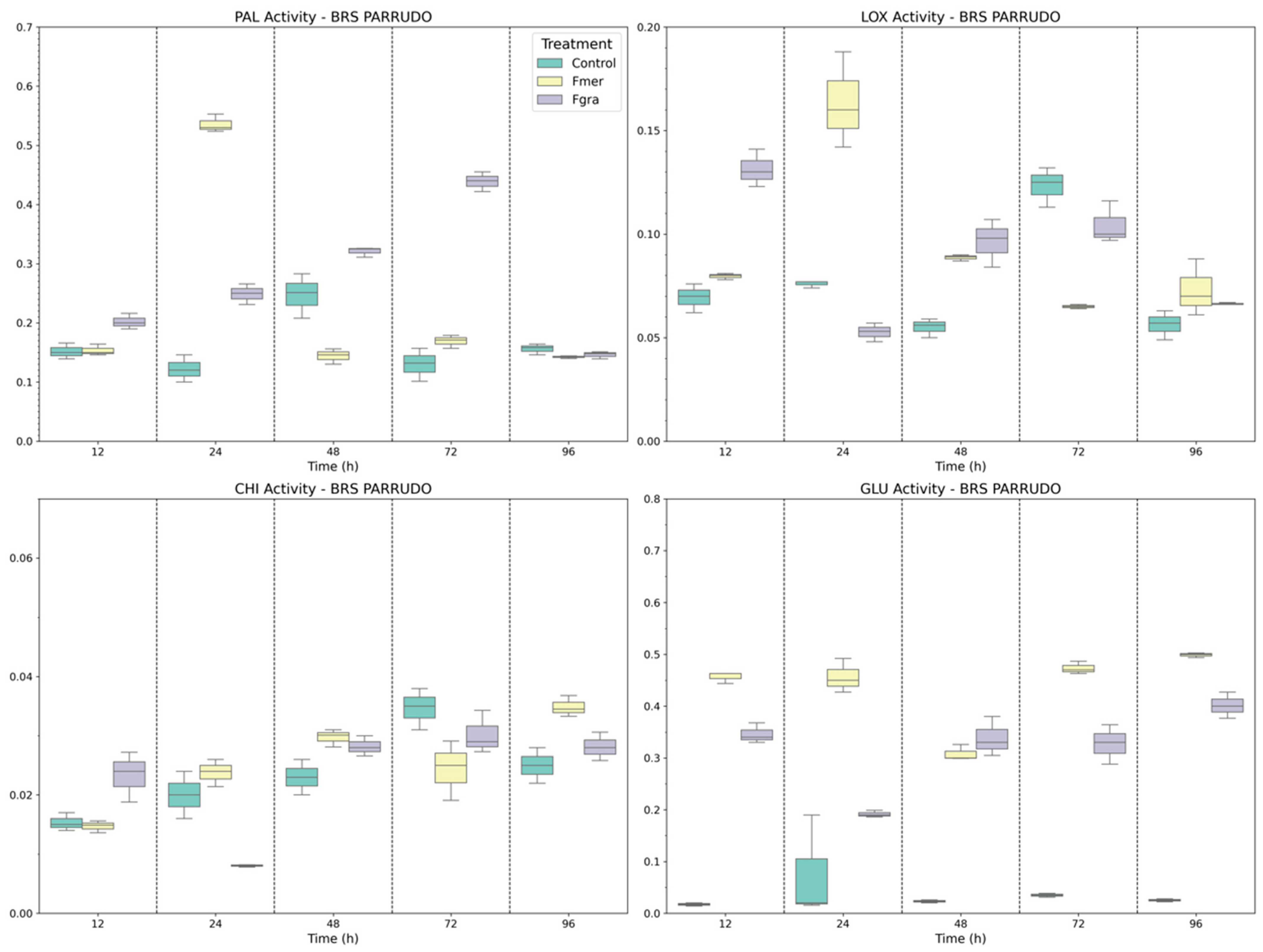
3.2. Defense-Related Enzymes in Phenolic Biosynthesis, Signaling, and Fungal Cell Wall Degradation
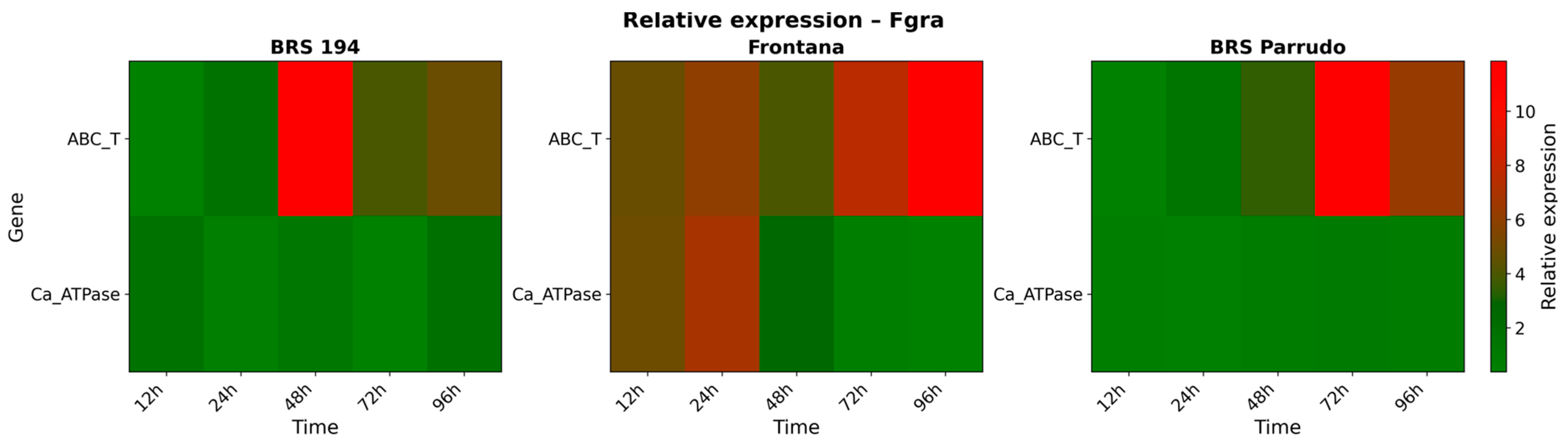
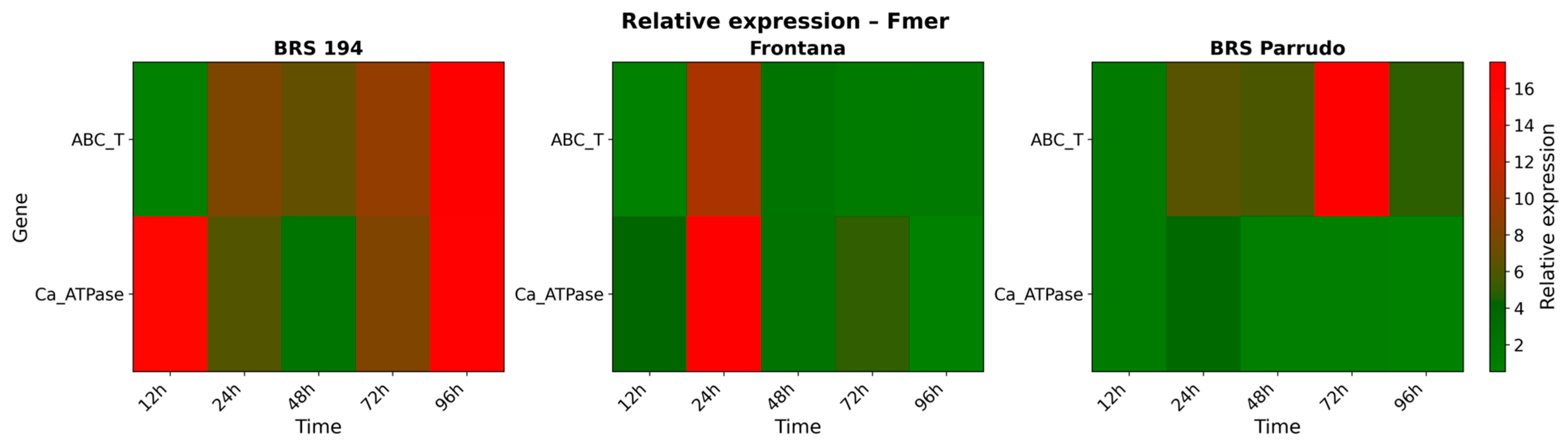
3.3. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAI | Hours after inoculation |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POX | Peroxidase |
| PPO | Polyphenol oxidase |
| PAL | Phenylalanine ammonia-lyase |
| GLU | β-1,3-glucanases |
| QUI | Chitinase |
| LOX | Lipoxygenase |
| PR protein | Pathogenesis-related protein |
| Fgra | Fusarium graminearum (DON producer) |
| Fmer | Fusarium meridionale (NIV producer) |
| DON | Deoxynivalenol |
| NIV | Nivalenol |
| FGSC | F. graminearum species complex |
| ROS | Reactive oxygen species |
| FHB | Fusarium head blight |
Appendix A
| Transcript | Forward Primer | Sequence ID GeneBank | Reverse Primer | Author |
|---|---|---|---|---|
| ABC-Transporter | GCCAACGAATA GATCCTCCA | XM_044491856.1 | ACCACCTTCAGCCT TTTCCT | [38] |
| Ca2+-ATPase | TCCTGATGAAAAGGGCAACC | XM_044539437.1 | GCACAGCACCAGAT ACAAATGC | [46] |
| Ubiquitin | CCCTGGAGGTG GAGTCATCTGA | AK446922.1 | GCGGCCATCCTCAA GCTGCTTA | [40] |
| RNA-helicase | GCACAGGGAAT CGTCAAAGT | XM_044542377.1 | CACACATAGCTTCA TTCGTTCA | [38] |
| Source of Variation | DF | F Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| POX | PPO | CAT | SOD | LOX | PAL | GLU | QUI | ||
| F1 | 2 | 8.3 * | 406.3 * | 151.1 * | 75.6 * | 20 * | 212.5 * | 200 * | 0 * |
| F2 | 2 | 8.2 * | 4.9 * | 0.9 ns | 24.2 * | 1 ns | 0.6 ns | 0 ns | 15 * |
| F1 × F2 | 4 | 6.5 * | 31.7 * | 15.9 * | 18.8 * | 20 * | 62.5 * | 130 * | 15 * |
| F3 | 4 | 31.1 * | 53.9 * | 10.4 * | 53.7 * | 20 * | 50 * | 50 * | 50 * |
| F1 × F3 | 8 | 22.7 * | 21.4 * | 13.5 * | 3.5 * | 30 * | 68.7 * | 60 * | 5 * |
| F2 × F3 | 8 | 13.1 * | 63.7 * | 7.9 * | 3.2 * | 40 * | 47.5 * | 30 * | 4.5 * |
| F1 × F2 × F3 | 16 | 23.4 * | 88.3 * | 7.4 * | 10.7 * | 30 * | 22.5 * | 40 * | 4.5 * |
References
- Petronaitis, T.; Simpfendorfer, S.; Hüberli, D. Importance of Fusarium Spp. in Wheat to Food Security: A Global Perspective. In Plant Diseases and Food Security in the 21st Century; Springer: Cham, Switzerland, 2021; pp. 127–159. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium graminearum Species Complex: A Bibliographic Analysis and Web-Accessible Database for Global Mapping of Species and Trichothecene Toxin Chemotypes. Phytopathology® 2022, 112, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Gupta, A.; Pandey, S.; Paul, V.; Saurabh, V.; Pandey, A.K.; Selvakumar, R.; Barua, S.; Kapri, M.; et al. Nivalenol Mycotoxin Concerns in Foods: An Overview on Occurrence, Impact on Human and Animal Health and Its Detection and Management Strategies. Toxins 2022, 14, 527. [Google Scholar] [CrossRef]
- Pestka, J. Toxicological Mechanisms and Potential Health Effects of Deoxynivalenol and Nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Spolti, P.; Barros, N.C.; Gomes, L.B.; dos Santos, J.; Del Ponte, E.M. Phenotypic and Pathogenic Traits of Two Species of the Fusarium graminearum Complex Possessing Either 15-ADON or NIV Genotype. Eur. J. Plant Pathol. 2012, 133, 621–629. [Google Scholar] [CrossRef]
- Mendes, G.R.L.; Del Ponte, E.M.; Feltrin, A.C.; Badiale-Furlong, E.; de Oliveira, A.C. Common Resistance to Fusarium Head Blight in Brazilian Wheat Genotypes. Sci. Agric. 2018, 75, 426–431. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL Mapping and Marker-Assisted Selection for Fusarium Head Blight Resistance in Wheat: A Review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Mesterházy, A. Types and Components of Resistance to Fusarium Head Blight of Wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Shen, Y.; Sun, Z.; Li, L.; Li, T. Linking Multi-Omics to Wheat Resistance Types to Fusarium Head Blight to Reveal the Underlying Mechanisms. Int. J. Mol. Sci. 2022, 23, 2280. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, Y.; Zhang, S.; Sha, J.; Sun, Y.; Hu, Z.; Gong, L.; Dai, Y.; Gao, Y.; Wang, Y.; et al. Wheat Resistance to Fusarium Head Blight and Breeding Strategies. Crop Health 2025, 3, 9. [Google Scholar] [CrossRef]
- Hu, C.; Chen, P.; Zhou, X.; Li, Y.; Ma, K.; Li, S.; Liu, H.; Li, L. Arms Race between the Host and Pathogen Associated with Fusarium Head Blight of Wheat. Cells 2022, 11, 2275. [Google Scholar] [CrossRef]
- Gao, X.; Li, F.; Sun, Y.; Jiang, J.; Tian, X.; Li, Q.; Duan, K.; Lin, J.; Liu, H.; Wang, Q. Basal Defense Is Enhanced in a Wheat Genotype Resistant to Fusarium Head Blight. J. Integr. Agric. 2024, 23, 1238–1258. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kánczugowska, J.; Stepién, L. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Nicholson, P.; Doohan, F.M. Action and Reaction of Host and Pathogen during Fusarium Head Blight Disease. New Phytol. 2010, 185, 54–66. [Google Scholar] [CrossRef]
- Barna, B.; Fodor, J.; Harrach, B.D.; Pogány, M.; Király, Z. The Janus Face of Reactive Oxygen Species in Resistance and Susceptibility of Plants to Necrotrophic and Biotrophic Pathogens. Plant Physiol. Biochem. 2012, 59, 37–43. [Google Scholar] [CrossRef]
- Carmody, M.; Waszczak, C.; Idänheimo, N.; Saarinen, T.; Kangasjärvi, J. ROS Signalling in a Destabilised World: A Molecular Understanding of Climate Change. J. Plant Physiol. 2016, 203, 69–83. [Google Scholar] [CrossRef]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Walker, P.L.; Belmonte, M.F.; McCallum, B.D.; McCartney, C.A.; Randhawa, H.S.; Henriquez, M.A. Dual RNA-Sequencing of Fusarium Head Blight Resistance in Winter Wheat. Front. Plant Sci. 2023, 14, 1299461. [Google Scholar] [CrossRef]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U.; Głowacka, K.; Wiwart, M. The Associations between Leaf Morphology, Phenylalanine Ammonia Lyase Activity, Reactive Oxygen Species, and Fusarium Resistance in Selected Species of Wheat with Different Ploidy Levels. Plants 2019, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jin, X.; Jia, X.; Wang, H.; Cao, A.; Zhao, W.; Pei, H.; Xue, Z.; He, L.; Chen, Q.; et al. Transcriptome-Based Discovery of Pathways and Genes Related to Resistance against Fusarium Head Blight in Wheat Landrace Wangshuibai. BMC Genom. 2013, 14, 197. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Wagner, C.; Nosenko, T.; Omony, J.; Steiner, B.; Nussbaumer, T.; Mayer, K.F.X.; Buerstmayr, H. Fusarium Head Blight Resistance in European Winter Wheat: Insights from Genome-Wide Transcriptome Analysis. BMC Genom. 2021, 22, 470. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to Hemi-Biotrophic F. Graminearum Infection Is Associated with Coordinated and Ordered Expression of Diverse Defense Signaling Pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef]
- Sirangelo, T.M. Molecular Investigations to Improve Fusarium Head Blight Resistance in Wheat: An Update Focusing on Multi-Omics Approaches. Plants 2024, 13, 2179. [Google Scholar] [CrossRef]
- Lemmens, M.; Griesser, M.; Scholz, U.; Schondelmaier, J.; Buerstmayr, H.; Steiner, B. Molecular Mapping of Resistance to Fusarium Head Blight in the Spring Wheat Genotype Frontana. Theor. Appl. Genet. 2004, 109, 215–224. [Google Scholar] [CrossRef]
- Caierão, E.; Scheeren, P.L.; Silva, M.S.; Castro, R.L. History of Wheat Genotypes Released by Embrapa in Forty Years of Research. Crop Breed. Appl. Biotechnol. 2014, 14, 216–223. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Spolti, P.; Ward, T.; Gomes, L.B.; Nicolli, C.P.; Kuhnem, P.R.; da Silva, C.N.; Tessmann, D.J. Regional and Field-Specific Factors Affect the Composition of Fusarium Head Blight Pathogens in Subtropical No-till Wheat Agroecosystem of Brazil. Phytopathology 2015, 105, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, C.P.; Spolti, P.; Tibola, C.S.; Fernandes, J.M.C.; Del Ponte, E.M. Fusarium Head Blight and Trichothecene Production in Wheat by Fusarium graminearum and F. meridionale Applied Alone or in Mixture at Post-Flowering. Trop. Plant Pathol. 2015, 40, 134–140. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; Pascholati, S.F.; Fortunato, A.A.; Camargo, L.E.A. Comparison of Root and Foliar Applications of Potassium Silicate in Potentiating Post-Infection Defences of Melon against Powdery Mildew. Plant Pathol. 2015, 64, 1085–1093. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of Antioxidant Enzymes to Transfer from Elevated Carbon Dioxide to Air and Ozone Fumigation, in the Leaves and Roots of Wild-Type and a Catalase-Deficient Mutant of Barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Zucker, M. Induction of Phenylalanine Deaminase by Light and Its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965, 40, 779–784. [Google Scholar] [CrossRef]
- Lage, A.; Camila, C.; Resende, R.S.; Rodrigues, F.A.; Ferraz, H.G.M.; Moreira, W.; Oliveira, J.R.; Mariano, R.L. Silicon Reduces Bacterial Speck Development on Tomato Leaves. Trop. Plant Pathol. 2013, 38, 436–442. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from Soybeans. Methods Enzymol. 1981, 71, 441–451. [Google Scholar]
- Dallagnol, L.J.; Rodrigues, F.A.; DaMatta, F.M.; Mielli, M.V.; Pereira, S.C. Deficiency in Silicon Uptake Affects Cytological, Physiological, and Biochemical Events in the Rice--Bipolaris Oryzae Interaction. Phytopathology 2011, 101, 92–104. [Google Scholar] [CrossRef]
- da Cruz, M.F.A.; Rodrigues, F.Á.; Polanco, L.R.; Curvêlo, C.R.D.S.; Nascimento, K.J.T.; Moreira, M.A.; Barros, E.G. Inducers of Resistance and Silicon on the Activity of Defense Enzymes in the Soybean-Phakopsora pachyrhizi Interaction. Bragantia 2013, 72, 162–172. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Walter, S.; Kahla, A.; Arunachalam, C.; Perochon, A.; Khan, M.R.; Scofield, S.R.; Doohan, F.M. A Wheat ABC Transporter Contributes to Both Grain Formation and Mycotoxin Tolerance. J. Exp. Bot. 2015, 66, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, S.; Samans, B.; Lück, S.; Friedt, W. Jasmonate and Ethylene Dependent Defence Gene Expression and Suppression of Fungal Virulence Factors: Two Essential Mechanisms of Fusarium Head Blight Resistance in Wheat? BMC Genom. 2012, 13, 369. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A Free, Open-Source System for Microarray Data Management and Analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef]
- Wang, W.; Xia, M.X.; Chen, J.; Yuan, R.; Deng, F.N.; Shen, F.F. Gene Expression Characteristics and Regulation Mechanisms of Superoxide Dismutase and Its Physiological Roles in Plants under Stress. Biochemistry 2016, 81, 465–480. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J. Factors Affecting Resistance of Wheat to Scab Caused by Gibberella Zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Poudel, B.; Mullins, J.; Puri, K.D.; Leng, Y. Molecular Mapping of Quantitative Trait Loci for Fusarium Head Blight Resistance in the Brazilian Spring Wheat Genotype “Surpresa”. Front. Plant Sci. 2022, 12, 778472. [Google Scholar] [CrossRef]
- Qi, P.-F.; Zhang, Y.-Z.; Liu, C.-H.; Zhu, J.; Chen, Q.; Guo, Z.-R.; Wang, Y.; Xu, B.-J.; Zheng, T.; Jiang, Y.-F.; et al. Fusarium graminearum ATP-Binding Cassette Transporter Gene FgABCC9 Is Required for Its Transportation of Salicylic Acid, Fungicide Resistance, Mycelial Growth and Pathogenicity towards Wheat. Int. J. Mol. Sci. 2018, 19, 2351. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cheng, B.; Chen, T.; Sui, J.; Wu, W.; Xu, Q.; Wang, W. Genetic mapping of the Fusarium head blight resistance gene in wheat Guixie 3. J. Appl. Genet. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, G.d.R.L.; Pazdiora, P.C.; Viana, V.E.; Dallagnol, L.J.; Calgaro, L.C.; da Silva, G.J.; Del Ponte, E.M.; Oliveira, A.C.d. Comparative Enzymatic and Gene Expression Responses in Wheat to DON- and NIV-Producing Fusarium Species. Biology 2025, 14, 1063. https://doi.org/10.3390/biology14081063
Mendes GdRL, Pazdiora PC, Viana VE, Dallagnol LJ, Calgaro LC, da Silva GJ, Del Ponte EM, Oliveira ACd. Comparative Enzymatic and Gene Expression Responses in Wheat to DON- and NIV-Producing Fusarium Species. Biology. 2025; 14(8):1063. https://doi.org/10.3390/biology14081063
Chicago/Turabian StyleMendes, Gabriela da Rocha Lemos, Paulo Cesar Pazdiora, Vivian Ebeling Viana, Leandro José Dallagnol, Laura Christina Calgaro, Glacy Jaqueline da Silva, Emerson Medeiros Del Ponte, and Antônio Costa de Oliveira. 2025. "Comparative Enzymatic and Gene Expression Responses in Wheat to DON- and NIV-Producing Fusarium Species" Biology 14, no. 8: 1063. https://doi.org/10.3390/biology14081063
APA StyleMendes, G. d. R. L., Pazdiora, P. C., Viana, V. E., Dallagnol, L. J., Calgaro, L. C., da Silva, G. J., Del Ponte, E. M., & Oliveira, A. C. d. (2025). Comparative Enzymatic and Gene Expression Responses in Wheat to DON- and NIV-Producing Fusarium Species. Biology, 14(8), 1063. https://doi.org/10.3390/biology14081063








