Population Dynamics of Bigeye Grunt Brachydeuterus auritus (Valenciennes, 1831) in the Coastal Waters of Sierra Leone: A Near-Threatened Species on the IUCN Red List
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
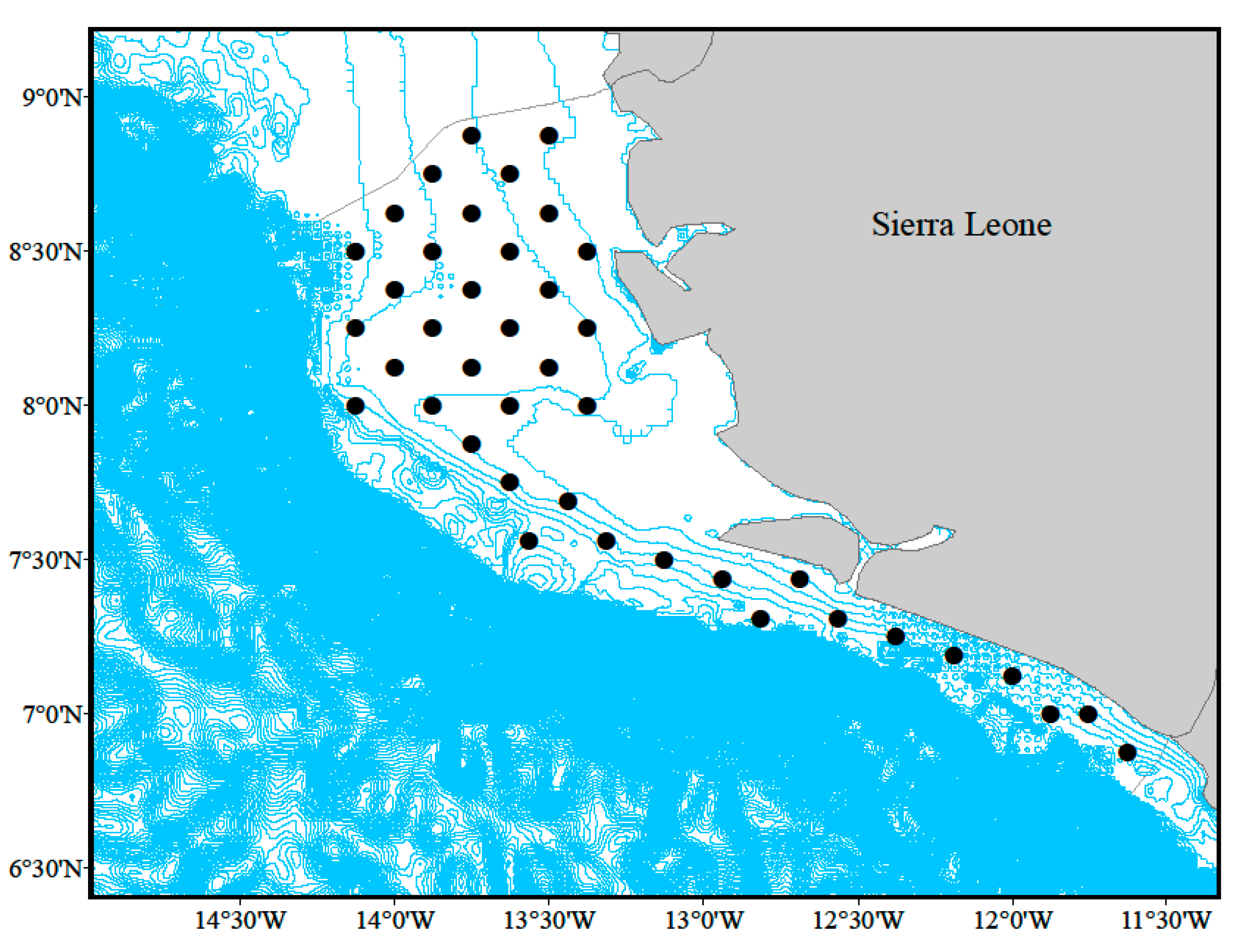
2.1. Surveys
2.2. Data Analysis
2.3. LBB Modeling
2.4. Generalized Additive Model (GAM)
3. Results
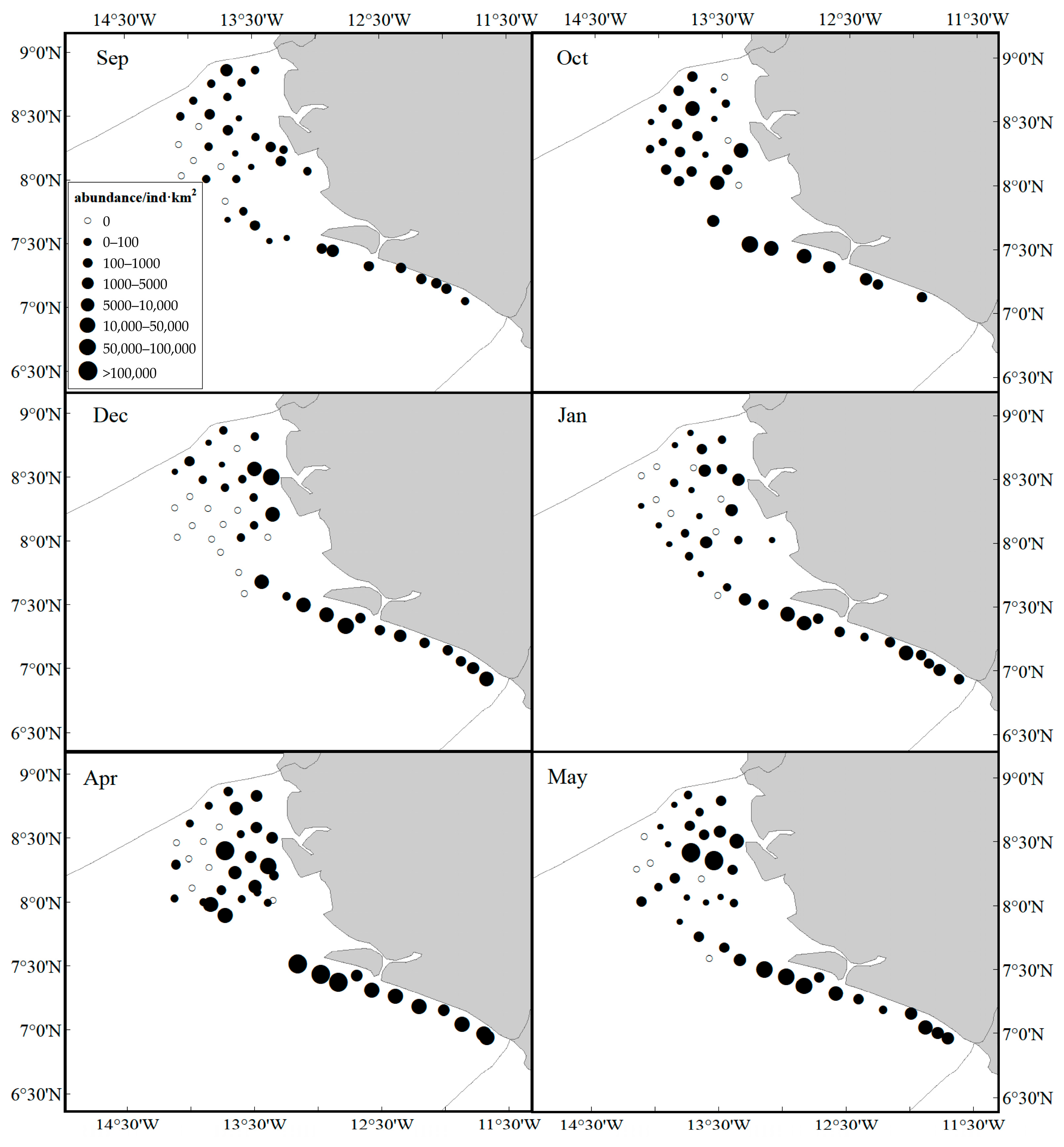
3.1. Spatiotemporal Distribution
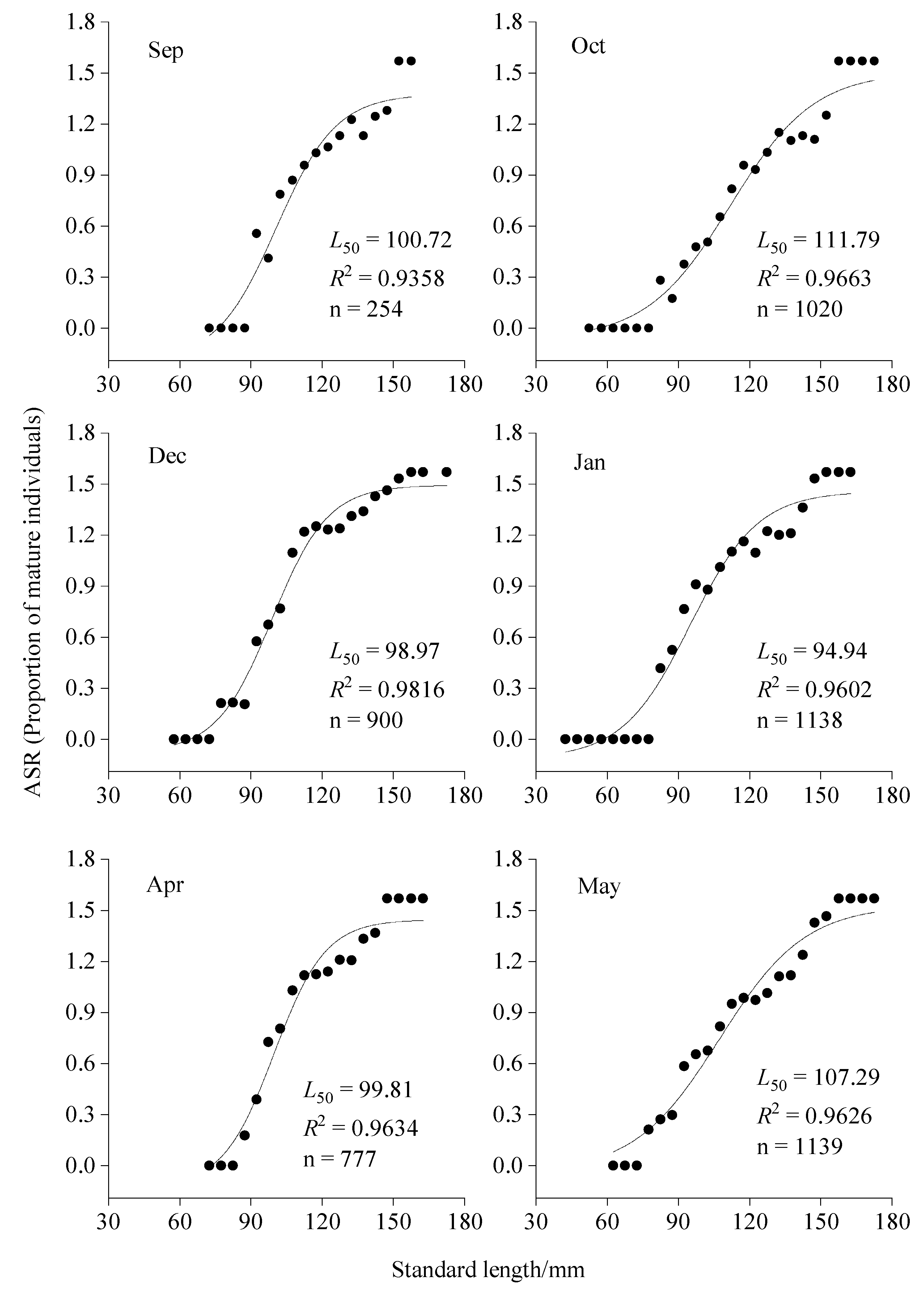
3.2. Variations in Population Parameters
- Sep: W = 3.72 × 10−5L2.94 (R2 = 0.9618, n = 851, p < 0.001);
- Oct: W = 3.46 × 10−5L2.98 (R2 = 0.9735, n = 1062, p < 0.001);
- Dec: W = 6.05 × 10−5L2.84 (R2 = 0.9836, n = 1001, p < 0.001);
- Jan: W = 2.67 × 10−5L3.00 (R2 = 0.9802, n = 1236, p < 0.001);
- Apr: W = 1.07 × 10−5L3.19 (R2 = 0.9866, n = 1231, p < 0.001);
- May: W = 2.45 × 10−5L3.02 (R2 = 0.9910, n = 1261, p < 0.001).
- Sep: Lt = 172 × (1 − e−0.34 × (t + 0.56));
- Oct: Lt = 174.8 × (1 − e−0.29 × (t + 0.66));
- Dec: Lt = 165.5 × (1 − e−0.40 × (t + 0.48));
- Jan: Lt = 166.2 × (1 − e−0.34 × (t + 0.57));
- Apr: Lt = 165.1 × (1 − e−0.38 × (t + 0.51));
- May: Lt = 173.5 × (1 − e−0.35 × (t + 0.54)).
3.3. Exploitation Status of Bigeye Grunt
3.4. GAM Analysis
3.4.1. GAM Test
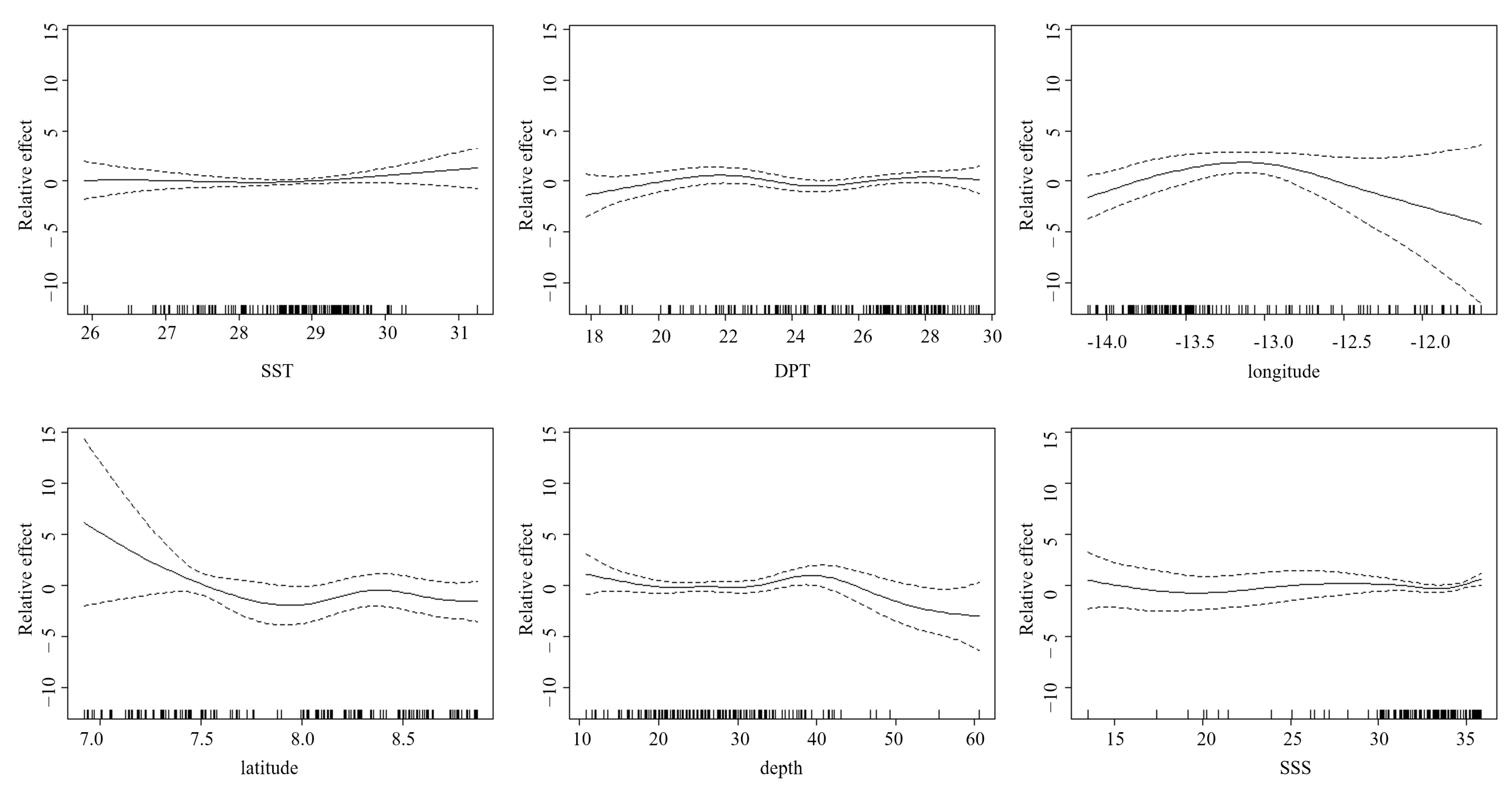
3.4.2. Distribution of Abundance Under Different Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Morais, L.; Sidibé, A.; Sylla, M.; Camara, K.; Carpenter, K.E.; Djiman, R.; Nunoo, F.; Sagna, A.; Williams, A.B.; Montiero, V.; et al. Brachydeuterus auritus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2015; p. e.T194413A. [Google Scholar] [CrossRef]
- Amponsah, S.K.K.; Danson, P.K.O.; Nunoo, F.K. Study of the population parameters of the bigeye grunt, Brachydeuterus auritus (Valenciennes, 1831) in Ghanaian coastal waters and its implications for management. Int. J. Fish. Aquat. Stud. 2016, 4, 413–419. [Google Scholar]
- Abbey, L.D.; Glover-Amengor, M.; Atikpo, M.O.; Howell, N.K. Proximate and biochemical characterization of burrito (Bachydeuterus auritus) and flying gurnard (Dactylopterus volitans). Food Sci. Nutr. 2017, 5, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.E.; De Angelis, N. The Living Marine Resources of the Eastern Central Atlantic; Volume 4: Bony Fishes Part 2 (Perciformes to Tetradontiformes) and Sea Turtles; FAO Species Identification Guide for Fishery Purposes; FAO: Rome, Italy, 2016; pp. 2343–3124. [Google Scholar]
- Konoyima, J.K. Sex ratio, stages of gonad development and growth pattern of Brachydeuterus auritus and Pomadasys jubelini in Sierra Leone, West Africa. Int. J. Basic Appl. Innov. Res. 2020, 9, 43–59. [Google Scholar]
- Bannerman, P.O.; Cowx, I.G. Stock assessment of the big-eye grunt (Brachydeuterus auritus, Val.) fishery in Ghanaian coastal waters. Fish. Res. 2002, 59, 197–207. [Google Scholar] [CrossRef]
- Amponsah, S.K.K.; Abdulhakim, A.; Ofori-Danson, P.K.; Anyan, K.F. Population dynamics of Bigeye grunt, Brachydeuterus auritus (Valenciennes, 1831) in Ghana and management implications. Fish. Aquac. J. 2017, 8, 1000233. [Google Scholar] [CrossRef]
- Zhao, G.; Li, S.; Yang, J.; Rao, X.; Shen, F.; Huang, H.; Li, L. Body weight-length relationship and relative weight of bigeye grunt (Brachydeuterus auritus) in the coastal waters of Sierra Leone. Haiyang Xuebao 2025, 47, 74–83. [Google Scholar]
- Zhao, G.; Huang, H.; Li, L.; Qu, T.; Fan, R.; Feng, C.; Li, S.; Yang, J. Biological charcteristics of Brachydeuterus auritus in the coastal waters off Sierra Leone. Mar. Fish. 2023, 45, 680–690. [Google Scholar] [CrossRef]
- Seto, K.; Belhabib, D.; Mamie, J.; Copeland, D.; Vakily, J.M.; Seilert, H.; Baio, A.; Harper, S.; Zeller, D.; Zylich, K.; et al. War, fish, and foreign fleets: The marine fisheries catches of Sierra Leone 1950–2015. Mar. Policy 2017, 83, 153–163. [Google Scholar] [CrossRef]
- Le Lœuff, P.; von Cosel, R. Biodiversity patterns of the marine benthic fauna on the Atlantic coast of tropical Africa in relation to hydroclimatic conditions and paleogeographic events. Acta Oecol. 1998, 1, 309–321. [Google Scholar] [CrossRef]
- Barange, M.; Bahri, T.; Beveridge, M.C.M.; Funge-Smith, S.; Poulain, F. Impacts of Climate Change on Fisheries and Aquaculture; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2018; pp. 1–654. [Google Scholar]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Zagaglia, C.R.; Lorenzzetti, J.A.; Stech, J.L. Remote sensing data and longline catches of yellowfin tuna (Thunnus albacares) in the equatorial Atlantic. Remote Sens. Environ. 2004, 93, 267–281. [Google Scholar] [CrossRef]
- Mainuddin, M.; Saiton, K.; Saiton, S. Albacore fishing ground in relation to oceanographic conditions in the western north pacific ocean using remotely sensed satellite data. Fish. Oceanogr. 2008, 17, 61–73. [Google Scholar] [CrossRef]
- Briand, K.; Molony, B.; Lehodey, P. A study on the variability of albacore (Thunnus alalunga) longline catch rates in the southwest Pacific Ocean. Fish. Oceanogr. 2011, 20, 517–529. [Google Scholar] [CrossRef]
- Nikolskiy, G.V. The Ecology of Fishes; Academy Press: London, UK; New York, NY, USA, 1963. [Google Scholar]
- Keys, A.B. The weight-length relation in fishes. Proc. Natl. Acad. Sci. USA 1928, 14, 922–925. [Google Scholar] [CrossRef]
- Huang, Z.; Chang, J. Fractal characteristics of length-weight relationship in fish. Acta Hydrobiol. Sinca 1999, 4, 330–336. [Google Scholar] [CrossRef]
- Hile, R. Age and growth of the cisco, Leucichthys ardeti (Le Sueur), in the lakes of the North-Eastern Highlands. Bull. Bureau Fish. 1936, 48, 211–317. [Google Scholar]
- Blackwell, B.G.; Brown, M.L.; Willis, D.W. Relative Weight (Wr) Status and Current Use in Fisheries Assessment and Management. Rev. Fish. Sci. 2000, 8, 181–214. [Google Scholar] [CrossRef]
- Bertalanffy, L.V. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Pauly, D. Theory and Management of Tropical Multispecies Stocks; A Review, with Emphasis on the Southeast Asian Demersal Fisheries. ICLARM Stud. Rev. 1979, 1, 1–35. [Google Scholar]
- Pauly, D.; Munro, J.L. Once more on the comparison of growth in fish and invertebrates. Fishbyte 1984, 2, 21. [Google Scholar]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO Fisheries Technical Pape No. 234; FAO: Rome, Italy, 1983. [Google Scholar]
- Chen, Y.; Paloheimo, J.E. Estimating fish length and age at 50% maturity using a logistic type model. Aquat. Sci. 1994, 56, 206–219. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Li, Y.; Xing, Y.; Kang, B. Sustainable exploitation of dominant fishes in the largest estuary in southeastern china. Water 2020, 12, 3390. [Google Scholar] [CrossRef]
- Holt, S.J. The Evaluation of Fisheries Resources by the Dynamic Analysis of Stocks, and Notes on the Time Factors Involved; ICNAF Special Publication; International Commission for the Northwest Atlantic Fisheries: Vancouver, BC, Canada, 1958; pp. 77–95. [Google Scholar]
- Froese, R.; Winker, H.; Gascuel, D.; Sumaila, U.R.; Pauly, D. Minimizing the impact offishing. Fish Fish. 2016, 17, 785–802. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment-Part 2-Tables of Yield Functions; FAO Fisheries Technicalo Paper; FAO: Rome, Italy, 1966; Volume 38, p. 67. [Google Scholar]
- Stephen, K.B.; Manoj, S.; Roberto, F.K.; David, A.; Julia, B.; Martin, C.; Cathy, D.; David, D.; William, M.; Keith, R.; et al. Patterns and practices in fisheries assessment peer review systems. Maine Policy 2020, 117, 103880. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013. [Google Scholar]
- Song, Y.; Wang, T.; Xiong, M.; Yang, S.; Zhang, H.; Ying, J.; Shi, Y.; Zhao, G.; Zhang, X.; Liu, X.; et al. Analysis of the Distribution Characteristics of Jellyfish and Environmental Factors in the Seawater Intake Area of the Haiyang Nuclear Power Plant in China. Biology 2024, 13, 433. [Google Scholar] [CrossRef]
- Dormann, C.G.; Elith, J.; Bacher, S.; Lautenback, S. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Planque, B.; Bellier, E.; Lazure, P. Modelling potential spawning habitat of sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) in the Bay of Biscay. Fish. Oceanogr. 2007, 16, 16–30. [Google Scholar] [CrossRef]
- Fang, J.T.; Zhang, J.; Feng, X.; Chen, Z.Z. Relationship between Sthenoteuthis oualaniensis fishing ground and marine environmental factors in Nansha area. J. Shanghai Ocean. Univ. 2019, 28, 419–426. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Xu, B.; Feng, Y.; Wang, X.; Zhang, X.; Li, F.; Chen, J. Community structure of ichthyoplankton and its relationship with environmental factors in coastal waters of southern Shandong Peninsula. Ecol. Environ. Sci. 2021, 30, 995–1004. [Google Scholar]
- Feng, C.; Huang, H.; Qu, T.; Fan, R.; Coker, I.C.; Seisay, L.D.; Chen, S.; Yang, J.; Rao, X.; Li, L. Temporal and spatial patterns of demersal fish assemblages in the coastal water of Sierra Leone. Reg. Stud. Mar. Sci. 2022, 56, 102674. [Google Scholar] [CrossRef]
- Panfili, J.; Thior, D.; Ecoutin, J.M.; Ndiaye, P.; Albaret, J.J. Influence of salinity on the size at maturity for fish species reproducing in contrasting West African estuaries. J. Fish. Biol. 2006, 69, 95–113. [Google Scholar] [CrossRef]
- Konoyima, K.J.; Seisay, L.D. Aspects of reproductive biology of Pseudupeneus prayensis collected from the coast off Sierra Leone, West Africa. J. Appl. Biosci. 2021, 159, 16371–16381. [Google Scholar] [CrossRef]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; et al. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Ciannelli, L.; Fisher, J.A.; Skern-Mauritzen, M.; Hunsicker, M.E.; Hidalgo, M.; Frank, K.T.; Bailey, K.M. Theory, consequences and evidence of eroding population spatial structure in harvested marine fishes: A review. Mar. Ecol. Prog. Ser. 2013, 480, 227–243. [Google Scholar] [CrossRef]
- Barnett, L.A.; Ward, E.J.; Jannot, J.E.; Shelton, A.O. Dynamic spatial heterogeneity reveals interdependence of marine faunal density and fishery removals. Ecol. Indic. 2019, 107, 105585. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, K.; Li, J.; Xu, Y.; Sun, M.; Xu, S.; Cai, Y.; Qiu, Y.; Chen, Z. Status assessment of the Beibu Gulf coastal fishery ecosystem using a multispecies size-spectrum model. Rev. Fish. Biol. Fisher. 2025, 35, 659–679. [Google Scholar] [CrossRef]
- Konan, K.J.; Joanny, T.G.T.; Sylla, S.; Lianthuam, L.; Kumar, K.L. Stock assessment of the bigeye grunt Brachydeuterus auritus (Valenciennes, 1832) in the coastal water of Ivory Coast. Int. J. Agric. Pol. Res. 2015, 3, 222–230. [Google Scholar] [CrossRef]
- Adebiyi, F.A. Growth pattern of the big eye grunt Brachydeuterus auritus (Valenciennes, 1832) off Lagos, Nigeria. Indian J. Fish. 2013, 60, 9–12. [Google Scholar]
- Richard, K.; Gao, C.; Pandong, N.; Ma, Q.; Tian, S.; Wu, F.; Ousmane, S. Stock status assessments of five small pelagic species in the atlantic and pacific oceans using the length-based bayesian estimation (LBB) method. Front. Mar. Sci. 2020, 7, 592082. [Google Scholar] [CrossRef]
- Hordyk, A.R.; Prince, J.D.; Carruthers, T.R.; Walters, C.J. Comment on ‘a new approach for estimating stock status from length frequency data’ by froese et al. ICES J. Mar. Sci. 2018, 76, 457–460. [Google Scholar] [CrossRef]





| Month | Total Individuals | Sex Ratio (♀/♂) | Standard Length Range (mm) | Mean Standard Length ± SD (mm) |
|---|---|---|---|---|
| Sep | 851 | 0.89 | 30~162 | 107.95 ± 26.67 |
| Oct | 1062 | 0.81 | 54~172 | 113.03 ± 18.55 |
| Dec | 1001 | 0.99 | 32~164 | 105.23 ± 28.70 |
| Jan | 1236 | 0.87 | 20~164 | 104.1 ± 28.41 |
| Apr | 1231 | 0.82 | 40~163 | 98.19 ± 30.47 |
| May | 1261 | 1.06 | 38~173 | 96.46 ± 31.44 |
| Month | Class Bin (mm) | Linf Prior | Z/K Prior | M/K Prior | F/K Prior | Lc Prior | Alpha Prior |
|---|---|---|---|---|---|---|---|
| Sep | 5 | 16.6 | 1.74 | 1.5 | 0.235 | 8.67 | 8.69 |
| Oct | 5 | 17.5 | 1.42 | 1.5 | 0.3 | 9.69 | 32.5 |
| Dec | 5 | 17.5 | 1.93 | 1.5 | 0.428 | 8.67 | 9.86 |
| Jan | 5 | 16.6 | 1.64 | 1.5 | 0.138 | 8.67 | 10.4 |
| Apr | 5 | 19.7 | 0.663 | 1.5 | 0.3 | 5.61 | 37.5 |
| May | 5 | 19.1 | 0.808 | 1.5 | 0.3 | 5.61 | 26.9 |
| Variable | SST | DPT | Longitude | Latitude | Depth | SSS |
|---|---|---|---|---|---|---|
| VIF value | 1.860 | 2.083 | 4.138 | 4.074 | 1.657 | 1.421 |
| Month | Probability of Occurrence (%) | Average Biomass ± SE (kg/km2) | Average Abundance ± SE (ind/km2) | Contribution to the Total Weight (%) | Contribution to the Total Number (%) |
|---|---|---|---|---|---|
| Sep | 84.62 | 38.18 ± 8.74 | 1133.24 ± 241.77 | 3.33 | 4.39 |
| Oct | 90.32 | 228.18 ± 70.38 | 6998.36 ± 2520.58 | 11.6 | 20.29 |
| Dec | 68.29 | 139.73 ± 39.92 | 7819.81 ± 2779.11 | 7.93 | 21.8 |
| Jan | 80.95 | 84.32 ± 24.85 | 3434.13 ± 1070.49 | 4.87 | 11.65 |
| Apr | 82.93 | 272.39 ± 82.10 | 28,597.5 ± 11,969.11 | 10.17 | 34.7 |
| May | 85.37 | 258.6 ± 76.67 | 17,057.22 ± 6241.67 | 7.4 | 24.11 |
| Month | Sep | Oct | Dec | Jan | Apr | May |
|---|---|---|---|---|---|---|
| a | 3.72 × 10−5 | 3.46 × 10−5 | 6.05 × 10−5 | 2.67 × 10−5 | 1.07 × 10−5 | 2.45 × 10−5 |
| b | 2.94 | 2.98 | 2.84 | 3.00 | 3.19 | 3.03 |
| Linf | 165 | 174.8 | 165.5 | 166.2 | 165.1 | 173.5 |
| K | 0.30 | 0.29 | 0.40 | 0.34 | 0.38 | 0.35 |
| Z | 0.91 | 0.57 | 1.11 | 0.68 | 1.09 | 0.98 |
| M | 0.47 | 0.44 | 0.60 | 0.52 | 0.56 | 0.53 |
| F | 0.44 | 0.13 | 0.51 | 0.16 | 0.53 | 0.45 |
| E | 0.49 | 0.22 | 0.46 | 0.23 | 0.48 | 0.46 |
| t0 | −0.65 | −0.66 | −0.48 | −0.57 | −0.51 | −0.54 |
| ϕ′ | 1.91 | 1.95 | 2.03 | 1.97 | 2.01 | 2.02 |
| L50 | 100.72 | 111.79 | 98.97 | 94.94 | 99.81 | 107.29 |
| Month | Sep | Oct | Dec | Jan | Apr | May |
|---|---|---|---|---|---|---|
| Linf | 16.7 | 17.2 | 16.5 | 16.9 | 16.5 | 17.5 |
| Lopt | 11 | 11 | 14 | 11 | 15 | 14 |
| Lc-opt | 9.3 | 7.8 | 10 | 8.7 | 11 | 10 |
| M/K | 1.44 | 1.66 | 0.585 | 1.54 | 0.352 | 0.699 |
| F/K | 1.3 | 0.226 | 0.154 | 0.831 | 0.0766 | 0.109 |
| Z/K | 2.7 | 1.9 | 0.747 | 2.36 | 0.431 | 0.809 |
| F/M | 0.908 | 0.136 | 0.263 | 0.532 | 0.22 | 0.158 |
| B/B0 | 0.514 | 0.835 | 0.725 | 0.591 | 0.758 | 0.791 |
| B/Bmsy | 1.4 | 2.0 | 1.7 | 1.6 | 1.7 | 1.9 |
| Lc | 12.9 | 10.1 | 8.84 | 6.61 | 5.23 | 5.7 |
| L95th/Linf | 0.98 | 0.99 | 1 | 1 | 1 | 1 |
| Status | healthy | healthy | healthy | healthy | healthy | healthy |
| GAM | R2 | AIC | Explanation Rate (%) |
|---|---|---|---|
| log(abundance) ~ s(SST) | 0.0357 | 809.4270 | 4.71 |
| log(abundance) ~ s(SST) + s(DPT) | 0.0861 | 803.7746 | 11.8 |
| log(abundance) ~ s(SST) + s(DPT) + s(longitude) | 0.357 | 744.7764 | 38.9 |
| log(abundance) ~ s(SST) + s(DPT) + s(longitude) + s(latitude) | 0.402 | 736.9369 | 45.1 |
| log(abundance) ~ s(SST) + s(DPT) + s(longitude) + s(latitude) + s(depth) | 0.429 | 732.3952 | 48.9 |
| log(abundance) ~ s(SST) + s(DPT) + s(longitude) + s(latitude) + s(depth) + s(SSS) | 0.452 | 730.8089 | 53 |
| Parameter | df | F | p Value |
|---|---|---|---|
| SST | 2.085 | 2.585 | 0.0392 |
| DPT | 4.004 | 2.402 | 0.0413 |
| longitude | 4.187 | 16.3 | 2 × 10−16 |
| latitude | 6.167 | 7.691 | 2 × 10−16 |
| depth | 6.857 | 3.029 | 0.00304 |
| SSS | 1 | 0.663 | 2 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Feng, C.; Liu, H.; Qu, T.; Fan, R.; Coker, I.C.R.; Seisay, L.D.; Huang, H.; Li, L. Population Dynamics of Bigeye Grunt Brachydeuterus auritus (Valenciennes, 1831) in the Coastal Waters of Sierra Leone: A Near-Threatened Species on the IUCN Red List. Biology 2025, 14, 1037. https://doi.org/10.3390/biology14081037
Zhao G, Feng C, Liu H, Qu T, Fan R, Coker ICR, Seisay LD, Huang H, Li L. Population Dynamics of Bigeye Grunt Brachydeuterus auritus (Valenciennes, 1831) in the Coastal Waters of Sierra Leone: A Near-Threatened Species on the IUCN Red List. Biology. 2025; 14(8):1037. https://doi.org/10.3390/biology14081037
Chicago/Turabian StyleZhao, Guoqing, Chunlei Feng, Hewei Liu, Taichun Qu, Ruiliang Fan, Ivorymae C. R. Coker, Lahai Duramany Seisay, Hongliang Huang, and Lingzhi Li. 2025. "Population Dynamics of Bigeye Grunt Brachydeuterus auritus (Valenciennes, 1831) in the Coastal Waters of Sierra Leone: A Near-Threatened Species on the IUCN Red List" Biology 14, no. 8: 1037. https://doi.org/10.3390/biology14081037
APA StyleZhao, G., Feng, C., Liu, H., Qu, T., Fan, R., Coker, I. C. R., Seisay, L. D., Huang, H., & Li, L. (2025). Population Dynamics of Bigeye Grunt Brachydeuterus auritus (Valenciennes, 1831) in the Coastal Waters of Sierra Leone: A Near-Threatened Species on the IUCN Red List. Biology, 14(8), 1037. https://doi.org/10.3390/biology14081037







