Evidence for Semantic Communication in Alarm Calls of Wild Sichuan Snub-Nosed Monkeys
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species
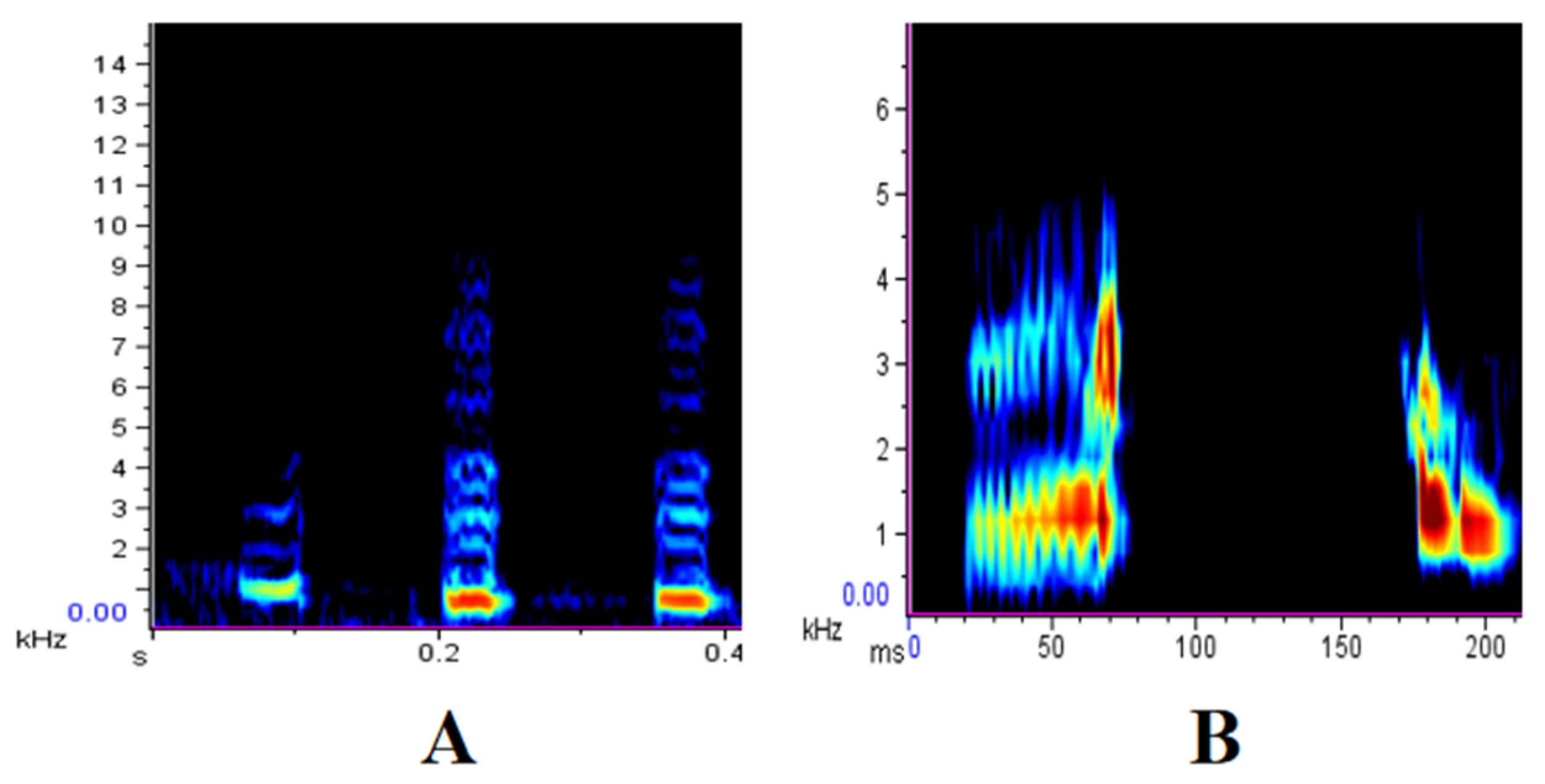
2.2. Data Collection and Playback Stimuli
2.3. Statistical Analysis
2.4. Ethics Statement
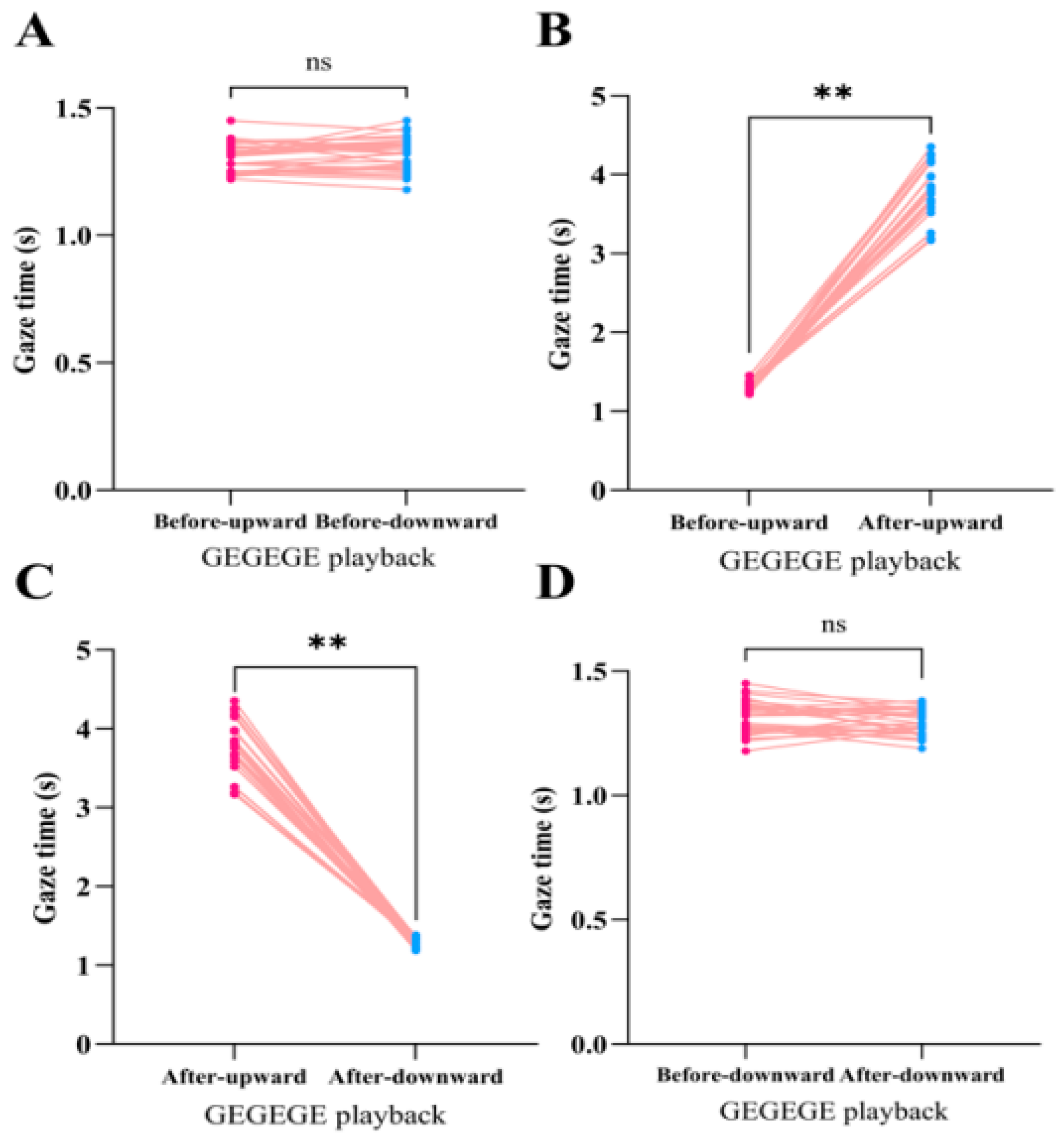
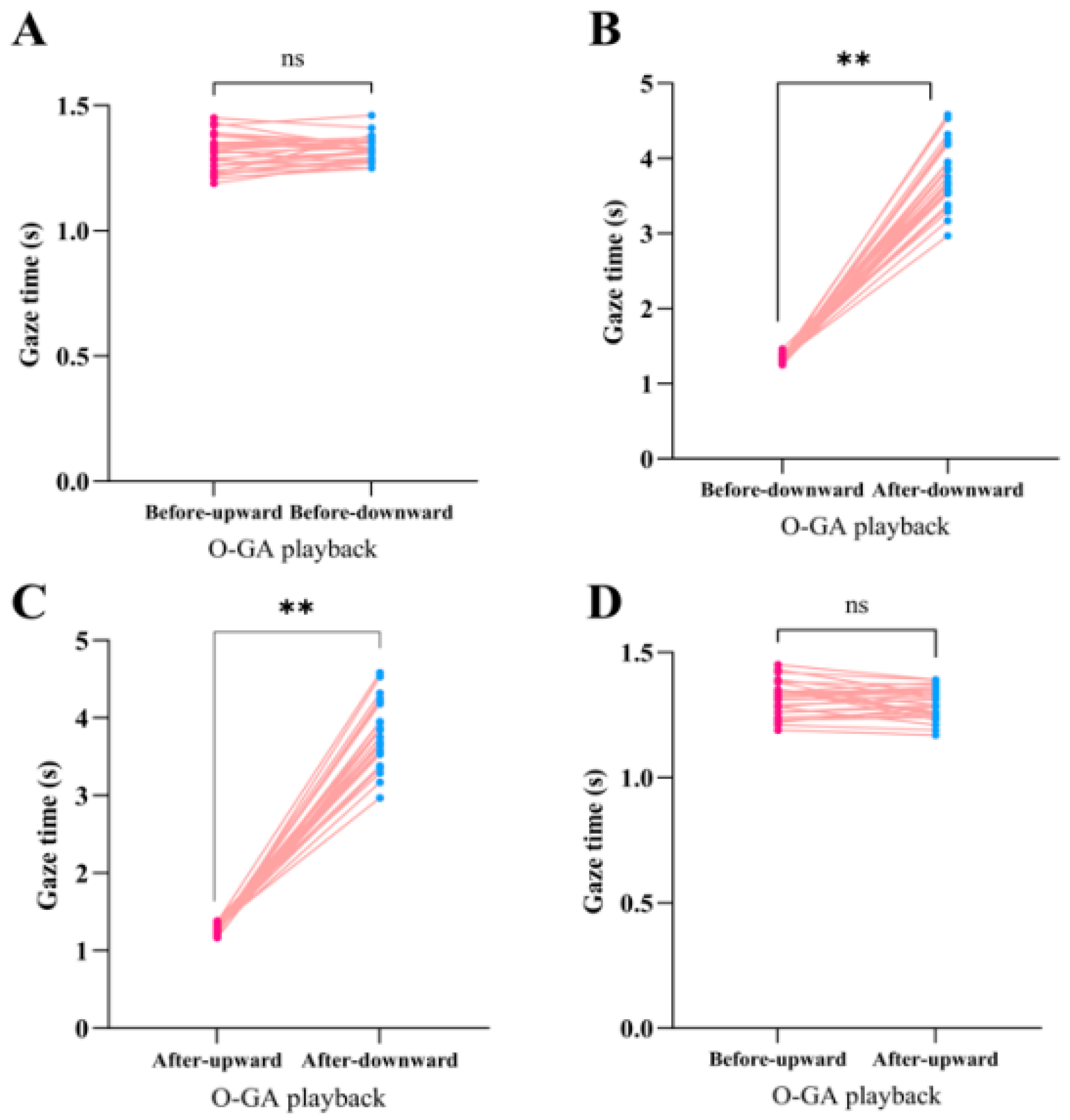
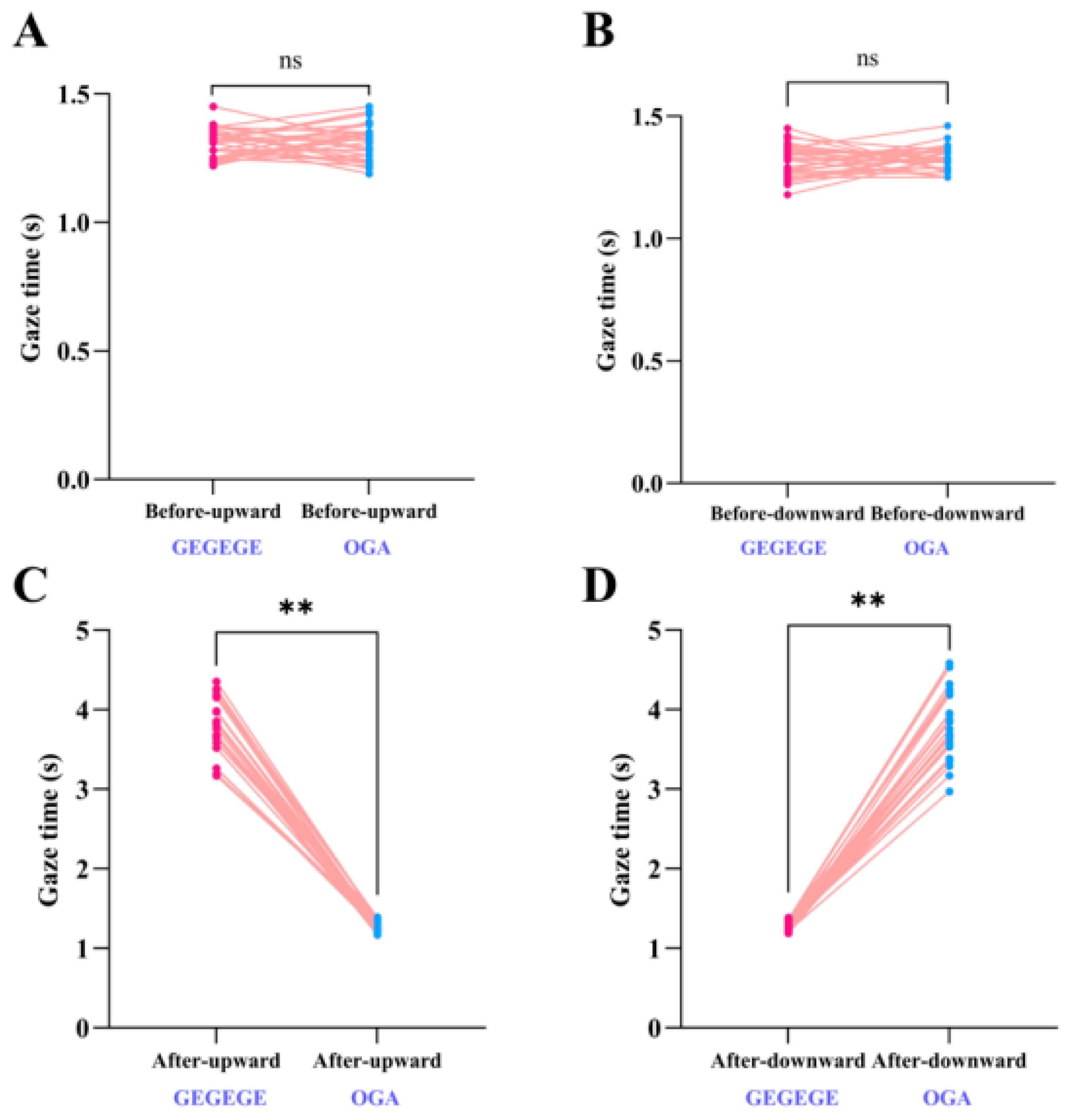
3. Results
3.1. Gaze Duration
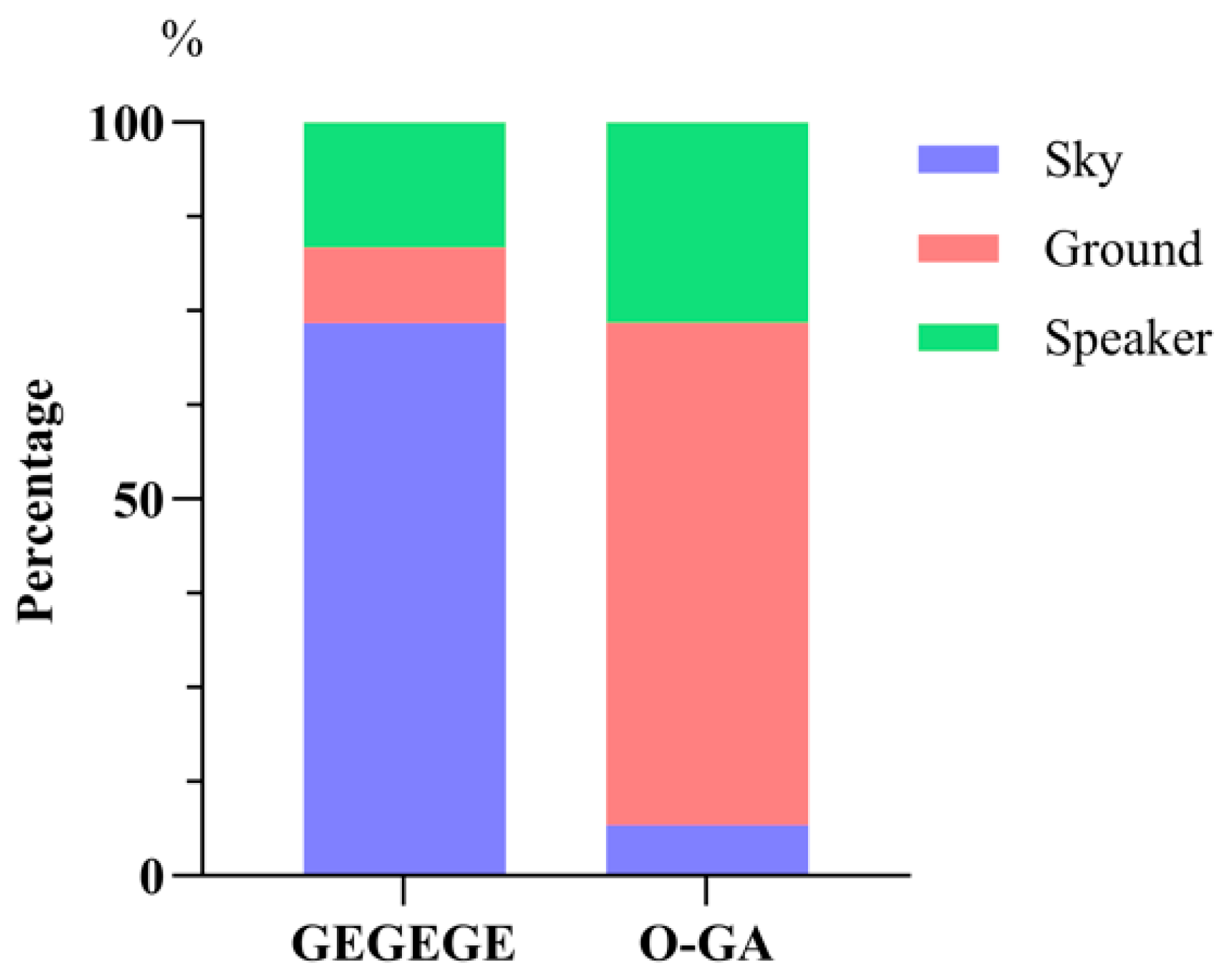
3.2. Direction of First Gaze
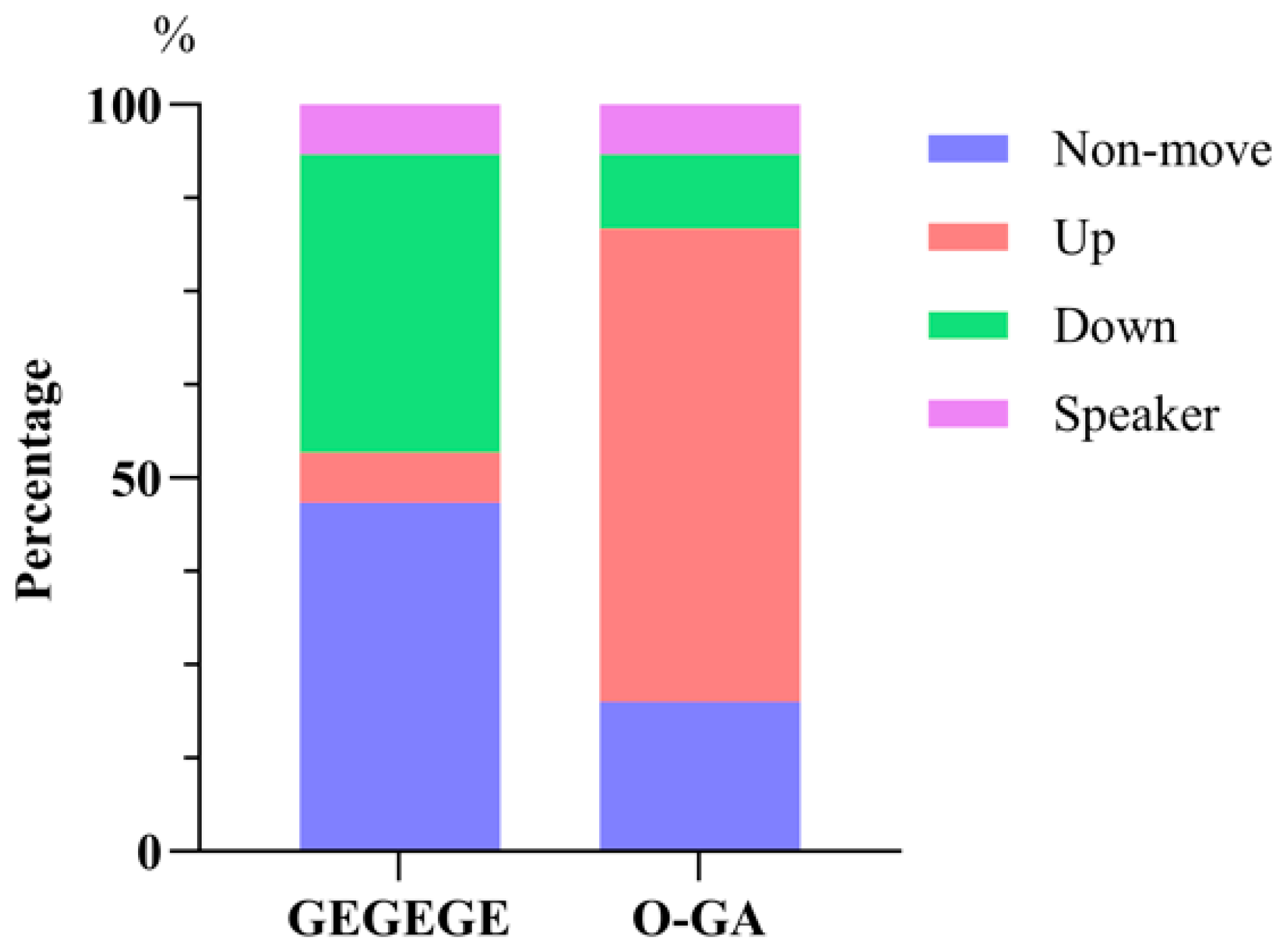
3.3. Locomotor Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyfarth, R.M.; Cheney, D.L.; Marler, P. Monkey responses to three different alarm calls: Evidence of predator classification and semantic communication. Science 1980, 210, 801–803. [Google Scholar] [CrossRef]
- Templeton, C.N.; Greene, E.; Davis, K. Allometry of alarm calls: Black-capped chickadees encode information about predator size. Science 2005, 308, 1934–1937. [Google Scholar] [CrossRef]
- Mehon, F.G.; Stephan, C. Intentional alarm calling in wild female putty-nosed monkeys (Cercopithecus nictitans). Anim. Behav. Cogn. 2022, 9, 385–395. [Google Scholar] [CrossRef]
- Caesar, C.; Zuberbuehler, K. Referential alarm calling behaviour in New World primates. Curr. Zool. 2012, 58, 680–697. [Google Scholar] [CrossRef]
- Palazzolo, G. A case for animal reference: Beyond functional reference and meaning attribution. Synthese 2024, 203, 59. [Google Scholar] [CrossRef]
- Schel, A.M.; Candiotti, A.; Zuberbühler, K. Predator-deterring alarm call sequences in Guereza colobus monkeys are meaningful to conspecifics. Anim. Behav. 2010, 80, 799–808. [Google Scholar] [CrossRef]
- Adams, D.B.; Kitchen, D.M. Model vs. playback experiments: The impact of sensory mode on predator-specific escape responses in saki monkeys. Ethology 2020, 126, 563–575. [Google Scholar] [CrossRef]
- Clarke, E.; Reichard, U.H.; Zuberbühler, K. The syntax and meaning of wild gibbon songs. PLoS ONE 2006, 1, e73. [Google Scholar] [CrossRef]
- Kirchhof, J.; Hammerschmidt, K. Functionally referential alarm calls in tamarins (Saguinus fuscicollis and Saguinus mystax)–evidence from playback experiments. Ethology 2006, 112, 346–354. [Google Scholar] [CrossRef]
- Wheeler, B.C. Production and perception of situationally variable alarm calls in wild tufted capuchin monkeys (Cebus apella nigritus). Behav. Ecol. Sociobiol. 2010, 64, 989–1000. [Google Scholar] [CrossRef]
- Arnold, K.; Zuberbühler, K. The alarm-calling system of adult male putty-nosed monkeys, Cercopithecus nictitans martini. Anim. Behav. 2006, 72, 643–653. [Google Scholar] [CrossRef]
- Macedonia, J.M.; Evans, C.S. Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 1993, 93, 177–197. [Google Scholar] [CrossRef]
- Schad, L.; Dongre, P.; van de Waal, E.; Fischer, J. Loud call production in male vervet monkeys (Chlorocebus pygerythrus) varies with season and signaller rank. Int. J. Primatol. 2025, 46, 538–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Watanabe, K.; Li, B.; Tan, C.L. Social organization of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, Central China. Primates 2006, 47, 374–382. [Google Scholar] [CrossRef]
- Yang, B.; Anderson, J.R.; Mao, M.; Wang, K.; Li, B. Maternal caretaking behavior towards a dead juvenile in a wild, multi-level primate society. Sci. Rep. 2022, 12, 4780. [Google Scholar] [CrossRef]
- Bouchet, H.; Blois-Heulin, C.; Lemasson, A. Social complexity parallels vocal complexity: A comparison of three non-human primate species. Front. Psychol. 2013, 4, 390. [Google Scholar] [CrossRef] [PubMed]
- Gustison, M.L.; le Roux, A.; Bergman, T.J. Derived vocalizations of geladas (Theropithecus gelada) and the evolution of vocal complexity in primates. Philos. Trans. R. Soc. B 2012, 367, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hong, B.; Anderson, J.R.; Fu, W.W.; Ren, Y.; Gou, N.N.; Shen, J.N.; Jia, K.S.; Lei, Y.H.; Wang, K.F.; et al. Dead trees as an indicator in tourism risk monitoring at primate ecotourism sites. Curr. Zool. 2023, 69, 103–105. [Google Scholar] [CrossRef]
- Yang, B.; Anderson, J.R.; Han, M.Y.; Meng, X.Y.; Luo, J.; Jia, K.S.; Chen, Y.F.; Tian, W.Y.; Qiao, B.B.; Zhang, C.; et al. Male aggressive behaviors as an indicator in primate tourism management assessment. Glob. Ecol. Conserv. 2024, 50, e02858. [Google Scholar] [CrossRef]
- Fischer, J.; Noser, R.; Hammerschmidt, K. Bioacoustic field research: A primer to acoustic analyses and playback experiments with primates. Am. J. Primatol. 2013, 75, 643–663. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Lehner, P.N. Sampling methods in behavior research. Poult. Sci. 1992, 71, 643–649. [Google Scholar] [CrossRef]
- Bateson, M.; Martin, P. Measuring Behaviour: An Introductory Guide; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Fichtel, C.; Kappeler, P.M. Anti-predator behavior of group-living Malagasy primates: Mixed evidence for a referential alarm call system. Behav. Ecol. Sociobiol. 2002, 51, 262–275. [Google Scholar] [CrossRef]
- Deshpande, A.; van de Waal, E.; Zuberbühler, K. Context-dependent alarm responses in wild vervet monkeys. Anim. Cogn. 2023, 26, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Scott-Phillips, T.; Heintz, C. Animal communication in linguistic and cognitive perspective. Annu. Rev. Linguist. 2023, 9, 93–111. [Google Scholar] [CrossRef]
- Fichtel, C.; Van Schaik, C.P. Semantic differences in sifaka (Propithecus verreauxi) alarm calls: A reflection of genetic or cultural variants? Ethology 2006, 112, 839–849. [Google Scholar] [CrossRef]
- Kilpatrick, A.; Ćwiek, A.; Lewis, E.; Kawahara, S. A cross-linguistic, sound symbolic relationship between labial consonants, voiced plosives, and Pokémon friendship. Front. Psychol. 2023, 14, 1113143. [Google Scholar] [CrossRef]
- Dubreuil, C.; Barrett, L.; Henzi, P.S.; Notman, H.; Pavelka, M.S. Age differences in the responses of vervet monkeys, Chlorocebus pygerythrus, to terrestrial alarm calls. Anim. Behav. 2023, 201, 87–100. [Google Scholar] [CrossRef]
- Berthet, M.; Coye, C.; Dezecache, G.; Kuhn, J. Animal linguistics: A primer. Biol. Rev. 2023, 98, 81–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, F.-J.; Anderson, J.R.; Fu, W.-W.; Gou, N.-N.; Shen, J.-N.; Cen, F.-S.; Tu, Y.-R.; Mao, M.; Wang, K.-F.; Yang, B.; et al. Evidence for Semantic Communication in Alarm Calls of Wild Sichuan Snub-Nosed Monkeys. Biology 2025, 14, 1028. https://doi.org/10.3390/biology14081028
Cao F-J, Anderson JR, Fu W-W, Gou N-N, Shen J-N, Cen F-S, Tu Y-R, Mao M, Wang K-F, Yang B, et al. Evidence for Semantic Communication in Alarm Calls of Wild Sichuan Snub-Nosed Monkeys. Biology. 2025; 14(8):1028. https://doi.org/10.3390/biology14081028
Chicago/Turabian StyleCao, Fang-Jun, James R. Anderson, Wei-Wei Fu, Ni-Na Gou, Jie-Na Shen, Fu-Shi Cen, Yi-Ran Tu, Min Mao, Kai-Feng Wang, Bin Yang, and et al. 2025. "Evidence for Semantic Communication in Alarm Calls of Wild Sichuan Snub-Nosed Monkeys" Biology 14, no. 8: 1028. https://doi.org/10.3390/biology14081028
APA StyleCao, F.-J., Anderson, J. R., Fu, W.-W., Gou, N.-N., Shen, J.-N., Cen, F.-S., Tu, Y.-R., Mao, M., Wang, K.-F., Yang, B., & Li, B.-G. (2025). Evidence for Semantic Communication in Alarm Calls of Wild Sichuan Snub-Nosed Monkeys. Biology, 14(8), 1028. https://doi.org/10.3390/biology14081028






